Photocatalyzed Production of Urea as a Hydrogen–Storage Material by TiO2–Based Materials
Abstract
1. Introduction
- -
- High storage capacity, a minimum of 6.5 wt.% of H2 abundance, and 50 g L−1 of H2 availability in the material;
- -
- Low cost, less than USD 266 per kilogram of hydrogen;
- -
- Operating ambient temperature between −40 °C and 60 °C;
- -
- Low toxicity, non–explosive, and possibly inert storage for water and oxygen.
2. Industrial Synthesis of Urea and Alternative Syntheses
3. Developments in the Photocatalyzed Production of Urea Using TiO2–Based Materials
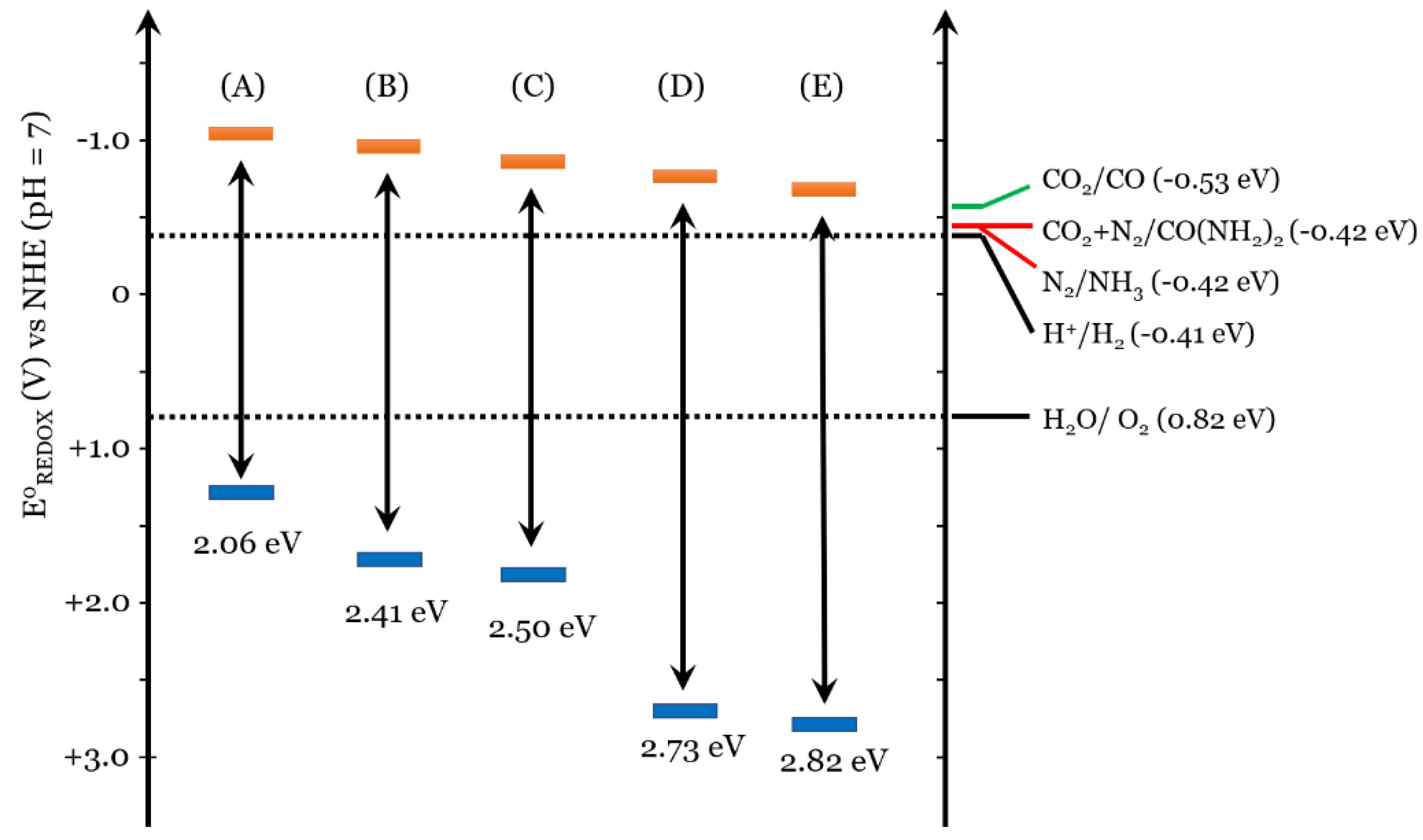
4. Use of Titanium Dioxide in Urea Photocatalyzed Synthesis
5. Proposed Reactions and Mechanism for Photocatalyzed Urea Synthesis
6. Distribution of Reported Products
7. Energy Consumption and Technology Scaling
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, D.; Zhao, S.; Zheng, N.; Beckers, Y.; Wang, J. Urea Metabolism and Regulation by Rumen Bacterial Urease in Ruminants—A Review. Ann. Anim. Sci. 2018, 18, 303–318. [Google Scholar] [CrossRef]
- Patra, A.K.; Aschenbach, J.R. Ureases in the Gastrointestinal Tracts of Ruminant and Monogastric Animals and Their Implication in Urea-N/Ammonia Metabolism: A Review. J. Adv. Res. 2018, 13, 39–50. [Google Scholar] [CrossRef] [PubMed]
- De Ventura, T.; Zanirato, V. Recent Advances in the Synthesis of Sulfonylureas. Eur. J. Org. Chem. 2021, 2021, 1201–1214. [Google Scholar] [CrossRef]
- Berrada, H.; Font, G.; Moltó, J.C. Determination of Urea Pesticide Residues in Vegetable, Soil, and Water Samples. Crit. Rev. Anal. Chem. 2003, 33, 19–41. [Google Scholar] [CrossRef]
- Amine-Khodja, A.; Boulkamh, A.; Boule, P. Photochemical Behaviour of Phenylurea Herbicides. Photochem.Photobiol.Sci. 2004, 3, 145–156. [Google Scholar] [CrossRef]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating Worldwide Use of Urea—A Global Change Contributing to Coastal Eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Lee, J.-M. Conventional and New Materials for Selective Catalytic Reduction (SCR) of NOx. ChemCatChem 2018, 10, 1499–1511. [Google Scholar] [CrossRef]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle Emissions Trapping Materials: Successes, Challenges, and the Path Forward. Appl. Catal. B Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy Storage Technologies and Real Life Applications—A State of the Art Review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef]
- Olabi, A.G.; Onumaegbu, C.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Al-Alami, A.H. Critical Review of Energy Storage Systems. Energy 2021, 214, 118987. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering Smart Grid: A Comprehensive Review of Energy Storage Technology and Application with Renewable Energy Integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Elishav, O. The Nitrogen Economy: Economic Feasibility Analysis of Nitrogen-Based Fuels as Energy Carriers. Appl. Energy 2017, 185, 183–188. [Google Scholar] [CrossRef]
- Ye, K.; Wang, G.; Cao, D.; Wang, G. Recent Advances in the Electro–Oxidation of Urea for Direct Urea Fuel Cell and Urea Electrolysis. Top Curr. Chem. 2018, 376, 42. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a Hydrogen Carrier: A Perspective on Its Potential for Safe, Sustainable and Long-Term Energy Supply. Energy Environ. Sci. 2011, 4, 1216. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Z.; Tao, S. Urea-Based Fuel Cells and Electrocatalysts for Urea Oxidation. Energy Technol. 2016, 4, 1329–1337. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and Related Chemicals as Potential Indirect Hydrogen Storage Materials. Int. J. Hydrog. Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- U.S. Drive Hydrogen Production Tech Team Roadmap 2017. Available online: https://www.energy.gov/sites/prod/files/2017/11/f46/HPTT%20Roadmap%20FY17%20Final_No,202017 (accessed on 23 March 2022).
- Grinberg Dana, A.; Shter, G.E.; Grader, G.S. Nitrogen-Based Alternative Fuels: Progress and Future Prospects. Energy Technol. 2016, 4, 7–18. [Google Scholar] [CrossRef]
- Rafiee, A.; Rajab Khalilpour, K.; Milani, D.; Panahi, M. Trends in CO2 Conversion and Utilization: A Review from Process Systems Perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Srinivas, B.; Kumari, V.D.; Sadanandam, G.; Hymavathi, C.; Subrahmanyam, M.; De, B.R. Photocatalytic Synthesis of Urea from in Situ Generated Ammonia and Carbon Dioxide. Photochem. Photobiol. 2012, 88, 233–241. [Google Scholar] [CrossRef]
- Xiang, X.; Guo, L.; Wu, X.; Ma, X.; Xia, Y. Urea Formation from Carbon Dioxide and Ammonia at Atmospheric Pressure. Environ. Chem. Lett. 2012, 10, 295–300. [Google Scholar] [CrossRef]
- Yahya, N.; Qureshi, S.; ur Rehman, Z.; Alqasem, B.; Fai Kait, C. Green Urea Synthesis Catalyzed by Hematite Nanowires in Magnetic Field. J. Magn. Magn. Mater. 2017, 428, 469–480. [Google Scholar] [CrossRef]
- Alqasem, B.; Sikiru, S.; Ali, E.M.; Elraies, K.A.; Yahya, N.; Rostami, A.; Ganeson, M.; Nyuk, C.M.; Qureshi, S. Effect of Electromagnetic Energy on Net Spin Orientation of Nanocatalyst for Enhanced Green Urea Synthesis. J. Mater. Res. Technol. 2020, 9, 16497–16512. [Google Scholar] [CrossRef]
- Yahya, N.; Alqasem, B.; Irfan, M.; Qureshi, S.; Rehman, Z.U.; Shafie, A.; Soleimani, H. The Effect of Saturation Magnetization of Nanocatalyst and Oscillating Magnetic Field for Green Urea Synthesis. Phys. B Condens. Matter 2017, 507, 95–106. [Google Scholar] [CrossRef]
- Díaz, D.J.; Darko, A.K.; McElwee-White, L. Transition Metal-Catalyzed Oxidative Carbonylation of Amines to Ureas. Eur. J. Org. Chem. 2007, 2007, 4453–4465. [Google Scholar] [CrossRef]
- Shi, F.; Deng, Y.; SiMa, T.; Peng, J.; Gu, Y.; Qiao, B. Alternatives to Phosgene and Carbon Monoxide: Synthesis of Symmetric Urea Derivatives with Carbon Dioxide in Ionic Liquids. Angew. Chem. Int. Ed. 2003, 42, 3257–3260. [Google Scholar] [CrossRef]
- Nguyen, D.S.; Cho, J.K.; Shin, S.-H.; Mishra, D.K.; Kim, Y.J. Reusable Polystyrene-Functionalized Basic Ionic Liquids as Catalysts for Carboxylation of Amines to Disubstituted Ureas. ACS Sustain. Chem. Eng. 2016, 4, 451–460. [Google Scholar] [CrossRef]
- He, X.; Li, X.-Y.; Song, Y.; Xia, S.-M.; Lang, X.-D.; He, L.-N. Synthesis of Urea Derivatives Using Carbon Dioxide as Carbonylation Reagent in Ionic Liquids. Curr. Organocatalysis 2017, 4, 112–121. [Google Scholar] [CrossRef]
- Andraos, J. A Green Metrics Assessment of Phosgene and Phosgene-Free Syntheses of Industrially Important Commodity Chemicals. Pure Appl. Chem. 2012, 84, 827–860. [Google Scholar] [CrossRef]
- Shibata, M.; Yoshida, K.; Furuya, N. Electrochemical Synthesis of Urea on Reduction of Carbon Dioxide with Nitrate and Nitrite Ions Using Cu-Loaded Gas-Diffusion Electrode. J. Electroanal. Chem. 1995, 387, 143–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhao, Y.; Chen, S.; Mahmood, I.B. Stabilization of Source-Separated Human Urine by Chemical Oxidation. Water Sci. Technol. 2013, 67, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Senecal, J.; Vinnerås, B. Urea Stabilisation and Concentration for Urine-Diverting Dry Toilets: Urine Dehydration in Ash. Sci. Total Environ. 2017, 586, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.; Lomstein, B.A.; Blackburn, T.H. Evidence for Bacterial Urea Production in Marine Sediments. FEMS Microbiol. Ecol. 1993, 12, 51–59. [Google Scholar] [CrossRef]
- Tuchman, M.; Rajagopal, B.S.; McCann, M.T.; Malamy, M.H. Enhanced Production of Arginine and Urea by Genetically Engineered Escherichia Coli K-12 Strains. Appl. Environ. Microbiol. 1997, 63, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Alfian, M.; Purwanto, W.W. Multi-objective Optimization of Green Urea Production. Energy Sci. Eng. 2019, 7, 292–304. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Multi-Objective Optimization and Analysis of a Solar Energy Driven Steam and Autothermal Combined Reforming System with Natural Gas. J. Nat. Gas Sci. Eng. 2019, 69, 102927. [Google Scholar] [CrossRef]
- Ishaq, H.; Siddiqui, O.; Chehade, G.; Dincer, I. A Solar and Wind Driven Energy System for Hydrogen and Urea Production with CO2 Capturing. Int. J. Hydrog. Energy 2021, 46, 4749–4760. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Van herle, J.; Maréchal, F.; Desideri, U. Techno-Economic Comparison of 100% Renewable Urea Production Processes. Appl. Energy 2021, 284, 116401. [Google Scholar] [CrossRef]
- Xia, M.; Mao, C.; Gu, A.; Tountas, A.A.; Qiu, C.; Wood, T.E.; Li, Y.F.; Ulmer, U.; Xu, Y.; Viasus, C.J.; et al. Solar Urea: Towards a Sustainable Fertilizer Industry. Angew. Chem. Int. Ed. 2022, 61, e202110158. [Google Scholar] [CrossRef]
- Kuwabata, S.; Yamauchi, H.; Yoneyama, H. Urea Photosynthesis from Inorganic Carbon and Nitrogen Compounds Using TiO 2 as Photocatalyst. Langmuir 1998, 14, 1899–1904. [Google Scholar] [CrossRef]
- Liu, B.-J.; Torimoto, T.; Yoneyama, H. Photocatalytic Reduction of Carbon Dioxide in the Presence of Nitrate Using TiO2 Nanocrystal Photocatalyst Embedded in SiO2 Matrices. J. Photochem. Photobiol. A Chem. 1998, 115, 227–230. [Google Scholar] [CrossRef]
- Torimoto, T.; Liu, B.-J.; Yoneyama, H. Effect of Solvents on Photocatalytic Reduction of Carbon Dioxide Using Semiconductor Photocatalysts. Stud. Surf. Sci. Catal. 1998, 114, 553–556. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Urea Photosynthesis inside Polyelectrolyte Capsules: Effect of Confined Media. Langmuir 2005, 21, 5582–5587. [Google Scholar] [CrossRef] [PubMed]
- Ustinovich, E.A.; Shchukin, D.G.; Sviridov, D.V. Heterogeneous Photocatalysis in Titania-Stabilized Perfluorocarbon-in-Water Emulsions: Urea Photosynthesis and Chloroform Photodegradation. J. Photochem. Photobiol. A Chem. 2005, 175, 249–252. [Google Scholar] [CrossRef]
- Shchukin, D.; Sviridov, D. Photocatalytic Processes in Spatially Confined Micro- and Nanoreactors. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 23–39. [Google Scholar] [CrossRef]
- Maimaiti, H.; Xu, B.; Sun, J.; Feng, L. Photocatalytic Synthesis of Urea (CO2/N2/H2O) on Coal–Based Carbon Nanotubes with the Fe-Core-Supported Ti3+–TiO2 Composite Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 6991–7002. [Google Scholar] [CrossRef]
- Sun, B.; Liu, Y.; Lou, F.; Chen, P. White-Light-Controlled Resistive Switching Chearacteristics of TiO2/Cu2O Composite Nanorods Array. Chem. Phys. 2015, 457, 28–31. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C.S. Selective Photocatalytic Reduction of CO2 into CH4 over Pt-Cu2O TiO2 Nanocrystals: The Interaction between Pt and Cu2O Cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. IJERPH 2022, 19, 5681. [Google Scholar] [CrossRef]
- Shand, M.; Anderson, J.A. Aqueous Phase Photocatalytic Nitrate Destruction Using Titania Based Materials: Routes to Enhanced Performance and Prospects for Visible Light Activation. Catal. Sci. Technol. 2013, 3, 879. [Google Scholar] [CrossRef]
- Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 Pigment Technology: A Review. Prog. Org. Coat. 1992, 20, 105–138. [Google Scholar] [CrossRef]
- Hua, Z.; Dai, Z.; Bai, X.; Ye, Z.; Wang, P.; Gu, H.; Huang, X. Copper Nanoparticles Sensitized TiO2 Nanotube Arrays Electrode with Enhanced Photoelectrocatalytic Activity for Diclofenac Degradation. Chem. Eng. J. 2016, 283, 514–523. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile? - Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Ramkumar, J.; Babu, S.M. Semiconductor Nanoparticles Sensitized TiO2 Nanotubes for High Efficiency Solar Cell Devices. Renew. Sustain. Energy Rev. 2016, 57, 1307–1321. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A Review of TiO2 Nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Nair, R.V.; Gummaluri, V.S.; Matham, M.V.; C, V. A Review on Optical Bandgap Engineering in TiO2 Nanostructures via Doping and Intrinsic Vacancy Modulation towards Visible Light Applications. J. Phys. D Appl. Phys. 2022, 55, 313003. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-Doped TiO2 Systems with Improved Photocatalytic Activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Zahid, R.; Manzoor, M.; Rafiq, A.; Ikram, M.; Nafees, M.; Butt, A.R.; Hussain, S.G.; Ali, S. Influence of Iron Doping on Structural, Optical and Magnetic Properties of TiO2 Nanoparticles. Electron. Mater. Lett. 2018, 14, 587–593. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The Roles of Metal Co-Catalysts and Reaction Media in Photocatalytic Hydrogen Production: Performance Evaluation of M/TiO2 Photocatalysts (M = Pd, Pt, Au) in Different Alcohol–Water Mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A Review on Catalytic Applications of Au/TiO2 Nanoparticles in the Removal of Water Pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-Doped Titanium Dioxide (N-Doped TiO2) for Visible Light Photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Y.; Miao, S.; Gong, M.; Liu, X. In Situ Synthesis of Carbon Doped TiO2 Nanotubes with an Enhanced Photocatalytic Performance under UV and Visible Light. Carbon 2017, 125, 544–550. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly Photoactive, Low Bandgap TiO2 Nanoparticles Wrapped by Graphene. Adv. Mater. 2012, 5. [Google Scholar]
- Li, Q.; Shang, J.K. Self-Organized Nitrogen and Fluorine Co-Doped Titanium Oxide Nanotube Arrays with Enhanced Visible Light Photocatalytic Performance. Environ. Sci. Technol. 2009, 43, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Andoshe, D.M.; Yim, K.; Sohn, W.; Kim, C.; Kim, T.L.; Kwon, K.C.; Hong, K.; Choi, S.; Moon, C.W.; Hong, S.-P.; et al. One-Pot Synthesis of Sulfur and Nitrogen Codoped Titanium Dioxide Nanorod Arrays for Superior Photoelectrochemical Water Oxidation. Appl. Catal. B Environ. 2018, 234, 213–222. [Google Scholar] [CrossRef]
- Yin, H.; Wang, X.; Wang, L.; Nie, Q.; Zhang, Y.; Wu, W. Cu2O/TiO2 Heterostructured Hollow Sphere with Enhanced Visible Light Photocatalytic Activity. Mater. Res. Bull. 2015, 72, 176–183. [Google Scholar] [CrossRef]
- Frank, A.J.; Kopidakis, N.; van de Lagemaat, J. Electrons in Nanostructured TiO2 Solar Cells: Transport, Recombination and Photovoltaic Properties. Coord. Chem. Rev. 2004, 248, 1165–1179. [Google Scholar] [CrossRef]
- Liu, G.; Hoivik, N.; Wang, K.; Jakobsen, H. Engineering TiO2 Nanomaterials for CO2 Conversion/Solar Fuels. Sol. Energy Mater. Sol. Cells 2012, 105, 53–68. [Google Scholar] [CrossRef]
- Wei, L.; Yu, C.; Zhang, Q.; Liu, H.; Wang, Y. TiO2–Based Heterojunction Photocatalysts for Photocatalytic Reduction of CO 2 into Solar Fuels. J. Mater. Chem. A 2018, 6, 22411–22436. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic Reduction of Nitrate: Fundamentals to Full-Scale Water Treatment Applications. Appl. Catal. B Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Lee, A.F. Synthetic Strategies to Nanostructured Photocatalysts for CO2 Reduction to Solar Fuels and Chemicals. J. Mater. Chem. A 2015, 3, 14487–14516. [Google Scholar] [CrossRef]
- Doudrick, K.; Yang, T.; Hristovski, K.; Westerhoff, P. Photocatalytic Nitrate Reduction in Water: Managing the Hole Scavenger and Reaction by-Product Selectivity. Appl. Catal. B Environ. 2013, 136–137, 40–47. [Google Scholar] [CrossRef]
- Hérissan, A.; Meichtry, J.M.; Remita, H.; Colbeau-Justin, C.; Litter, M.I. Reduction of Nitrate by Heterogeneous Photocatalysis over Pure and Radiolytically Modified TiO2 Samples in the Presence of Formic Acid. Catal. Today 2017, 281, 101–108. [Google Scholar] [CrossRef]
- Kobwittaya, K.; Sirivithayapakorn, S. Photocatalytic Reduction of Nitrate over Fe-Modified TiO2. APCBEE Procedia 2014, 10, 321–325. [Google Scholar] [CrossRef][Green Version]
- Tugaoen, H.O.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in Photocatalytic Reduction of Nitrate as a Water Treatment Technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef]
- Matamala-Troncoso, F.; Ky Nguyen, C.; MacFarlane, D.R.; Isaacs, M.; Sáez-Navarrete, C. Facile Methodology to Generate Cu2O/TiO2 Heterojunction on FTO Electrode for Photoelectroreduction of Nitrate. Mater. Lett. 2021, 293, 129663. [Google Scholar] [CrossRef]
- Freire, J.M.A.; Matos, M.A.F.; Abreu, D.S.; Becker, H.; Diógenes, I.C.N.; Valentini, A.; Longhinotti, E. Nitrate Photocatalytic Reduction on TiO2: Metal Loaded, Synthesis and Anions Effect. J. Environ. Chem. Eng. 2020, 8, 103844. [Google Scholar] [CrossRef]
- Liu, L.-F.; Zhang, Y.; Yang, F.-L.; Chen, G.; Yu, J.C. Simultaneous Photocatalytic Removal of Ammonium and Nitrite in Water Using Ce3+–Ag+ Modified TiO2. Sep. Purif. Technol. 2009, 67, 244–248. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Viswanathan, B. Photocatalytic Reduction of Nitrite and Nitrate Ions over Doped TiO2 Catalysts. J. Photochem. Photobiol. A Chem. 1997, 107, 215–220. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Viswanathan, B. Photocatalytic Reduction of Nitrite and Nitrate Ions to Ammonia on M/TiO2 Catalysts. J. Photochem. Photobiol. A Chem. 1997, 108, 73–78. [Google Scholar] [CrossRef]
- Zhang, F.; Pi, Y.; Cui, J.; Yang, Y.; Zhang, X.; Guan, N. Unexpected Selective Photocatalytic Reduction of Nitrite to Nitrogen on Silver-Doped Titanium Dioxide. J. Phys. Chem. C 2007, 111, 3756–3761. [Google Scholar] [CrossRef]
- Huang, S.-R.; Huang, P.-J. Visible-Light Driven Graphene Oxide/Titanium Dioxide Hydrogels for Photocatalytic Reduction of Nitrite. J. Environ. Chem. Eng. 2022, 10, 106902. [Google Scholar] [CrossRef]
- Rao, N.N.; Dube, S.; Manjubala; Natarajan, P. Photocatalytic Reduction of Nitrogen over (Fe, Ru or Os)/TiO2 Catalysts. Appl. Catal. B Environ. 1994, 5, 33–42. [Google Scholar] [CrossRef]
- Krasae, N.; Wantala, K. Enhanced Nitrogen Selectivity for Nitrate Reduction on Cu–NZVI by TiO2 Photocatalysts under UV Irradiation. Appl. Surf. Sci. 2016, 380, 309–317. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Liu, H.; Zhang, X.; Hocking, R.K.; Sun, C. First Principles Study of Single Fe Atom Supported on TiO2(001) for Nitrogen Reduction to Ammonia. Appl. Surf. Sci. 2022, 572, 151417. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Viswanathan, B. Photocatalytic Reduction of Dinitrogen to Ammonia. Indian J. Chem. 1996, 35 A, 443–453. [Google Scholar]
- Sayão, F.A.; Nuñez, L.; Zanoni, M.V.B. Efficient Photoelectrochemical Reduction of Nitrite to Ammonium and Nitrogen Containing Gaseous Species Using Ti/TiO2 Nanotube Electrodes. J. Braz. Chem. Soc. 2014. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Zou, J. Nitrogen Reduction Utilizing Solvated Electrons Produced by Thermal Excitation of Trapped Electrons in Reduced Titanium Oxide. New J. Chem. 2018, 42, 6084–6090. [Google Scholar] [CrossRef]
- Yamazoe, S.; Okumura, T.; Tanaka, T. Photo-Oxidation of NH3 over Various TiO2. Catal. Today 2007, 120, 220–225. [Google Scholar] [CrossRef]
- Al Sawah, M.A.; Richard, D.; De Bellefon, C.; Chovelon, J.-M.; Ferronato, C. Dégradation photocatalytique des ions ammonium en présence de TiO2 dopé. Comptes Rendus Chim. 2010, 13, 502–507. [Google Scholar] [CrossRef]
- Bahadori, E.; Conte, F.; Tripodi, A.; Ramis, G.; Rossetti, I. Photocatalytic Selective Oxidation of Ammonia in a Semi-Batch Reactor: Unravelling the Effect of Reaction Conditions and Metal Co-Catalysts. Catalysts 2021, 11, 209. [Google Scholar] [CrossRef]
- Meessen, J. Urea Synthesis. Chem. Ing. Tech. 2014, 86, 2180–2189. [Google Scholar] [CrossRef]
- Li, X.; Tung, C.; Wu, L. Quantum Dot Assembly for Light-Driven Multielectron Redox Reactions, Such as Hydrogen Evolution and CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 10804–10811. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Mendive, C.B.; Dillert, R.; Bahnemann, D.W. Kinetic and Mechanistic Investigations of Multielectron Transfer Reactions Induced by Stored Electrons in TiO2 Nanoparticles: A Stopped Flow Study. J. Phys. Chem. A 2011, 115, 2139–2147. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, S. Enhanced Photocatalytic Activity of Mesoporous TiO2 Aggregates by Embedding Carbon Nanotubes as Electron-Transfer Channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef]
- Ahmmad, B.; Kusumoto, Y.; Somekawa, S.; Ikeda, M. Carbon Nanotubes Synergistically Enhance Photocatalytic Activity of TiO2. Catal. Commun. 2008, 9, 1410–1413. [Google Scholar] [CrossRef]
- Guo, M.Y.; Liu, F.; Leung, Y.H.; Ng, A.M.C.; Djurišić, A.B.; Chan, W.K. TiO2–Carbon Nanotube Composites for Visible Photocatalysts—Influence of TiO2 Crystal Structure. Curr. Appl. Phys. 2013, 13, 1280–1287. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent Progress in Enhancing Photocatalytic Efficiency of TiO2–Based Materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Kominami, H.; Furusho, A.; Murakami, S.; Inoue, H.; Kera, Y.; Ohtani, B. Effective Photocatalytic Reduction of Nitrate to Ammonia in an Aqueous Suspension of Metal-Loaded Titanium(IV) Oxide Particles in the Presence of Oxalic Acid. Catal. Lett. 2001, 76, 31–34. [Google Scholar] [CrossRef]
- Kominami, H.; Nakaseko, T.; Shimada, Y.; Furusho, A.; Inoue, H.; Murakami, S.; Kera, Y.; Ohtani, B. Selective Photocatalytic Reduction of Nitrate to Nitrogen Molecules in an Aqueous Suspension of Metal-Loaded Titanium(Iv) Oxide Particles. Chem. Commun. 2005, 2933–2935. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Yates, J.T. TiO2-Based Photocatalysis: Surface Defects, Oxygen and Charge Transfer. Top Catal. 2005, 35, 197–210. [Google Scholar] [CrossRef]
- Yamakata, A.; Vequizo, J.J.M. Curious Behaviors of Photogenerated Electrons and Holes at the Defects on Anatase, Rutile, and Brookite TiO2 Powders: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 234–243. [Google Scholar] [CrossRef]
- Lv, C.; Zhong, L.; Liu, H.; Fang, Z.; Yan, C.; Chen, M.; Kong, Y.; Lee, C.; Liu, D.; Li, S.; et al. Selective Electrocatalytic Synthesis of Urea with Nitrate and Carbon Dioxide. Nat. Sustain. 2021, 4, 868–876. [Google Scholar] [CrossRef]
- Kermeli, K.; Worrell, E.; Graus, W.; Corsten, M. Energy Efficiency and Cost Saving Opportunities for Ammonia and Nitrogenous Fertilizer Production; United States Environmental Protection Agency: Washington, DC, USA, 2017; p. 163. [Google Scholar]
- International Fertilizer Industry Association Fertilizers. Climate Change and Enhancing Agricultural Productivity Sustainably; International Fertilizer Industry Association: Paris, France, 2009. [Google Scholar]
- Jo, W.-K.; Tayade, R.J. New Generation Energy-Efficient Light Source for Photocatalysis: LEDs for Environmental Applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Khademalrasool, M.; Farbod, M.; Talebzadeh, M.D. The Improvement of Photocatalytic Processes: Design of a Photoreactor Using High-Power LEDs. J. Sci. Adv. Mater. Devices 2016, 1, 382–387. [Google Scholar] [CrossRef]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.T.; Allsopp, D.W.E.; Bowen, C.R.; van Grieken, R.; Marugán, J. Design and Validation of a LED-Based High Intensity Photocatalytic Reactor for Quantifying Activity Measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. Recent Developments in Photocatalytic Dye Degradation upon Irradiation with Energy-Efficient Light Emitting Diodes. Chin. J. Catal. 2014, 35, 1781–1792. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar Energy: Potential and Future Prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Prăvălie, R.; Patriche, C.; Bandoc, G. Spatial Assessment of Solar Energy Potential at Global Scale. A Geographical Approach. J. Clean. Prod. 2019, 209, 692–721. [Google Scholar] [CrossRef]

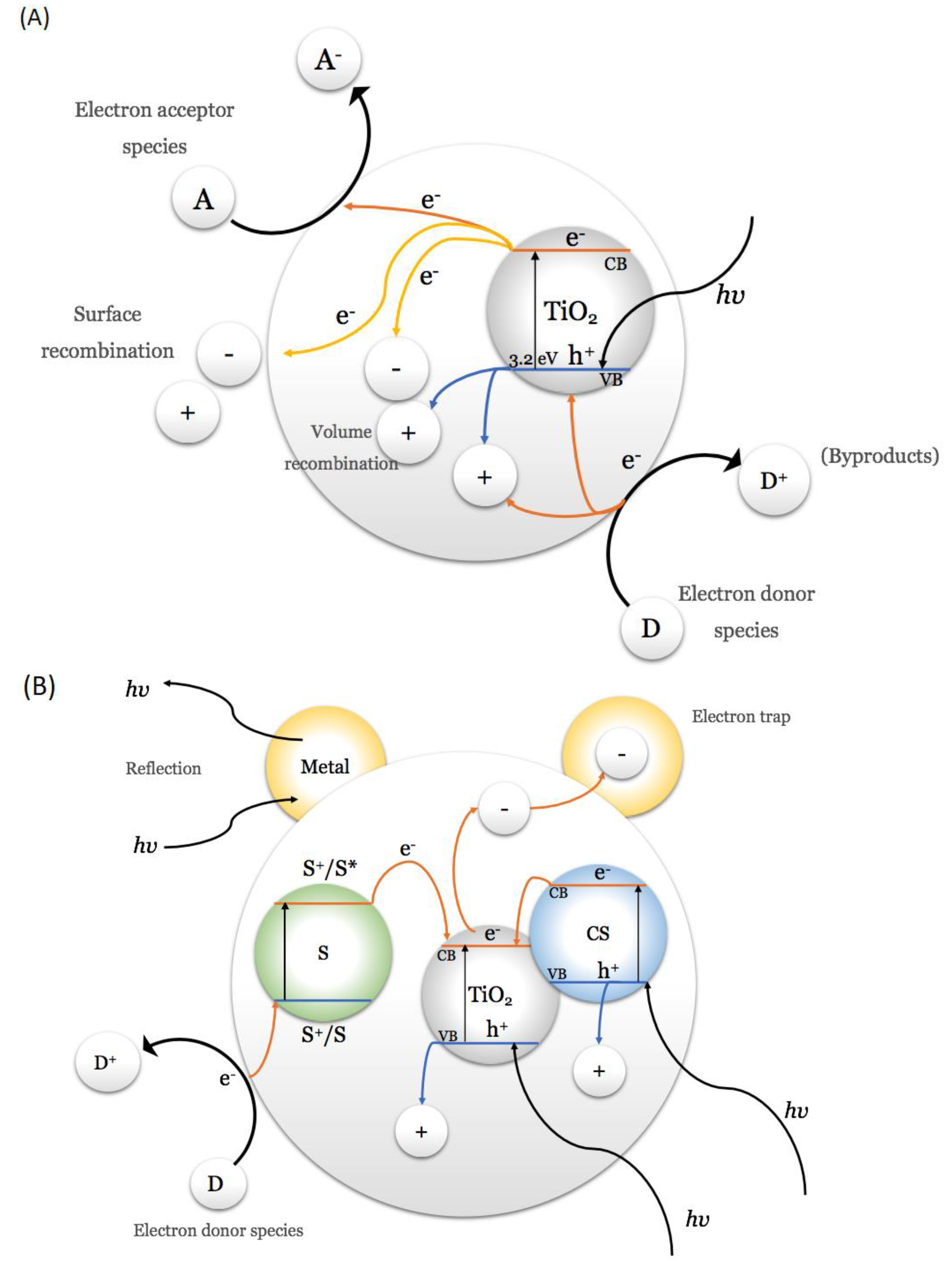
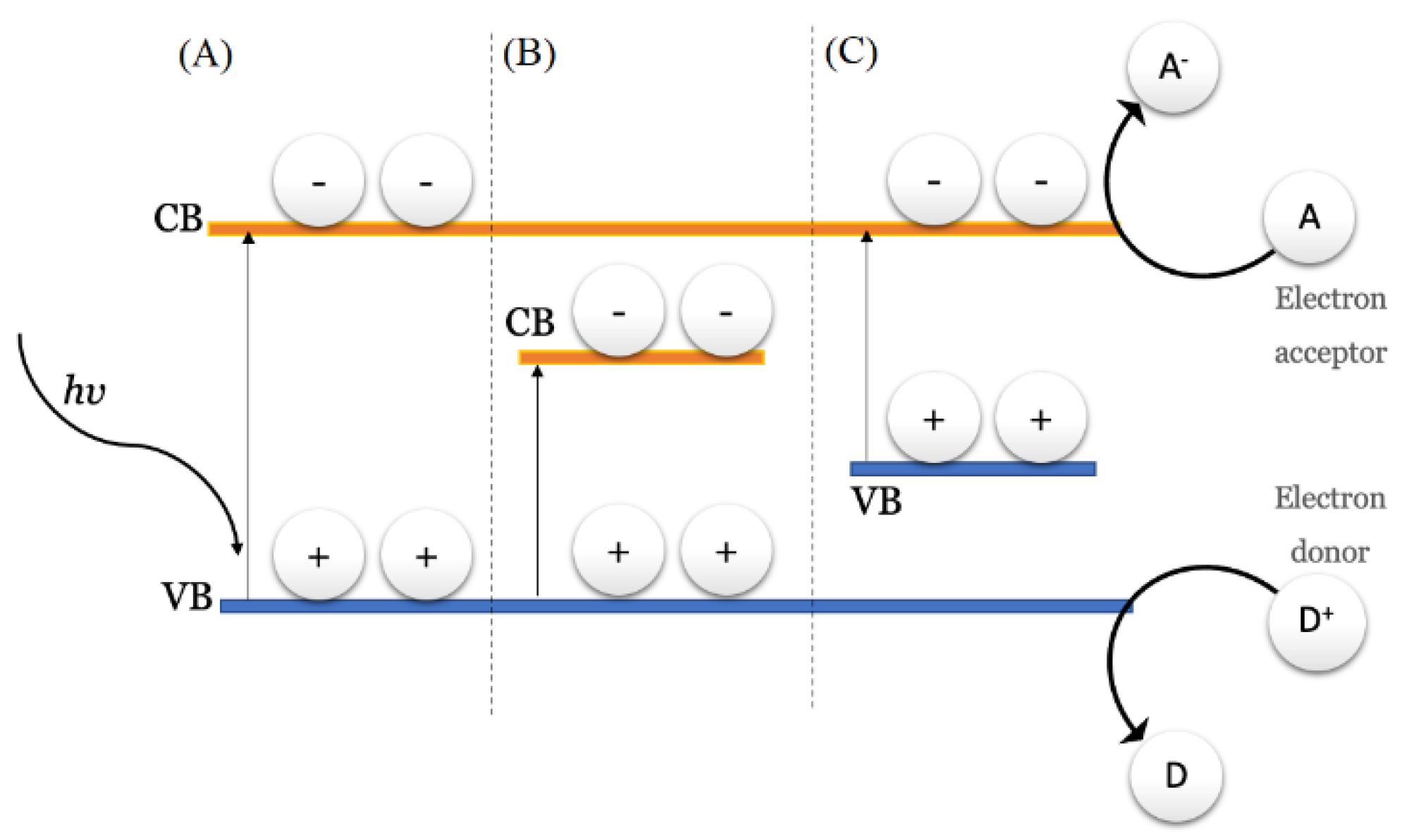
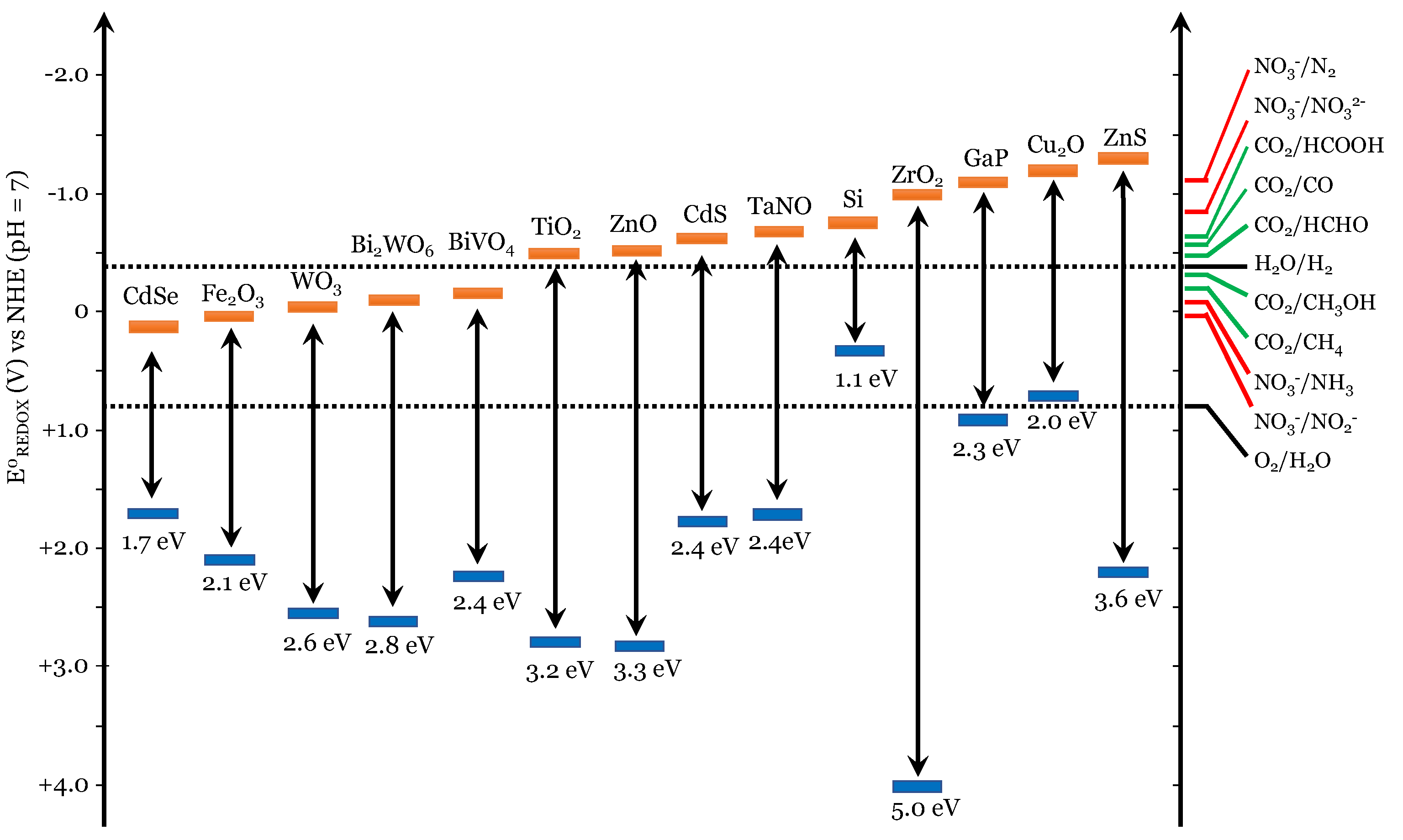
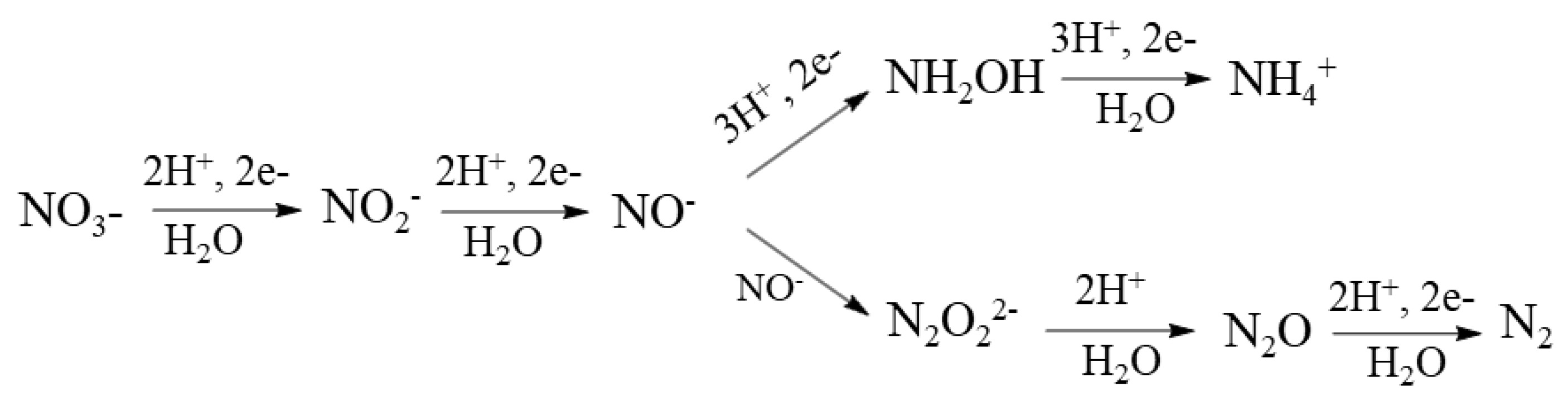

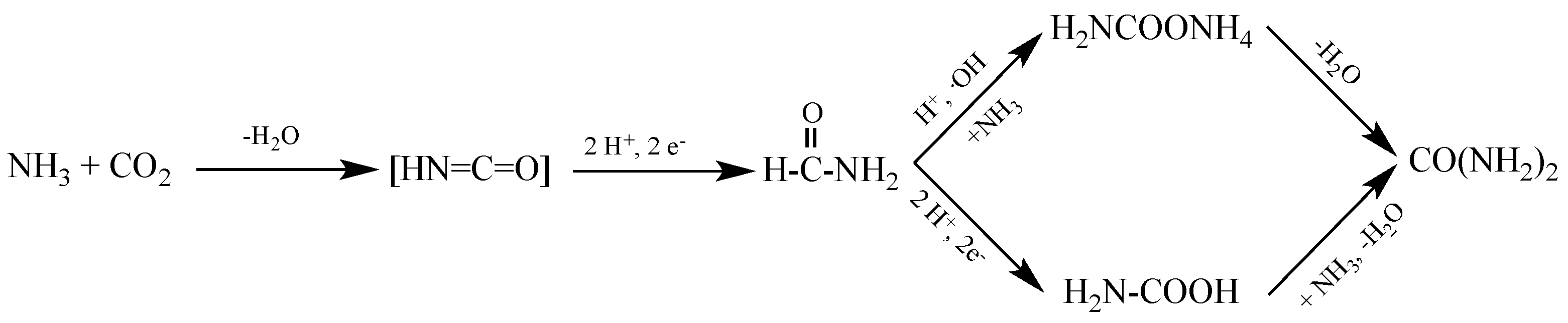
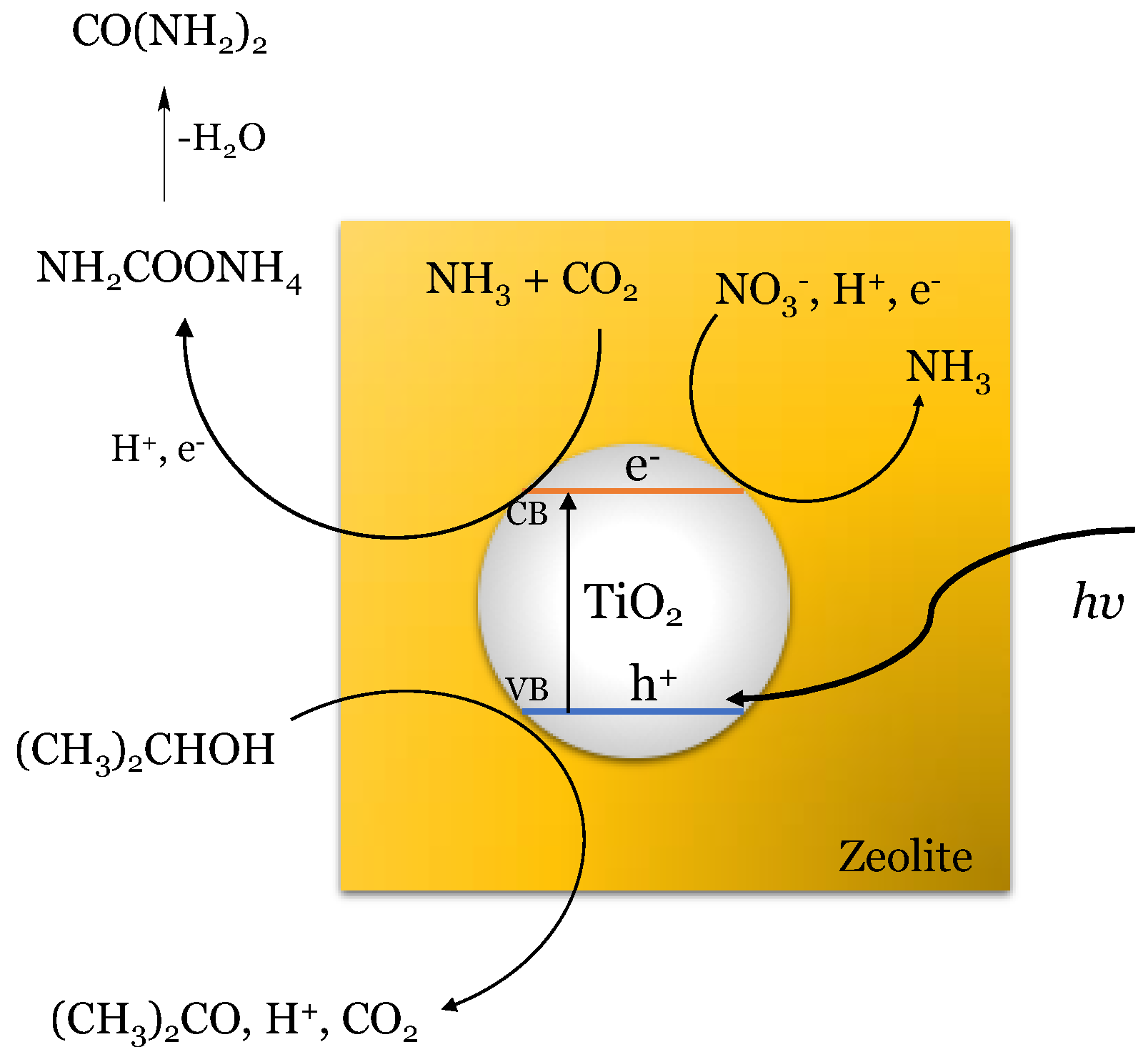
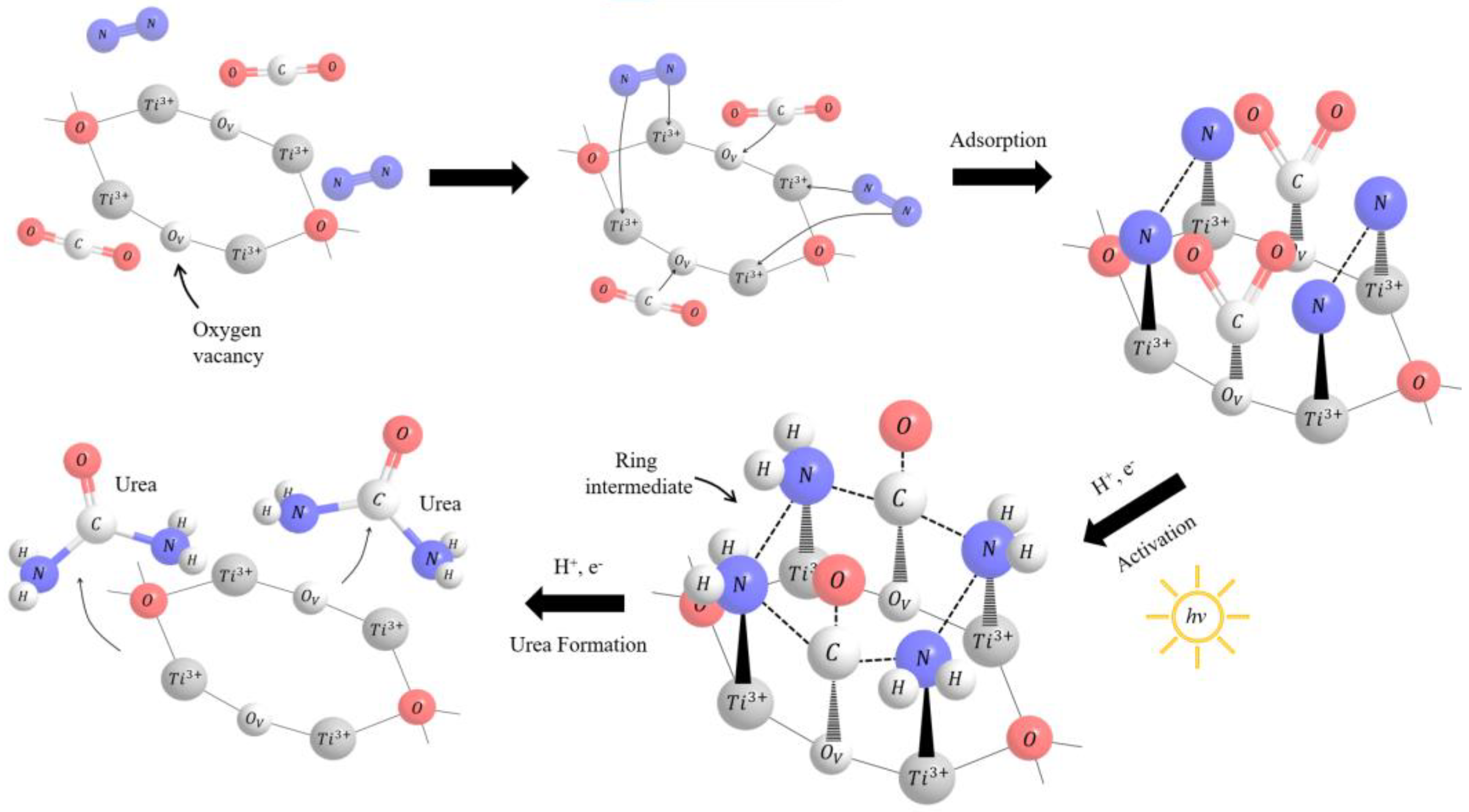
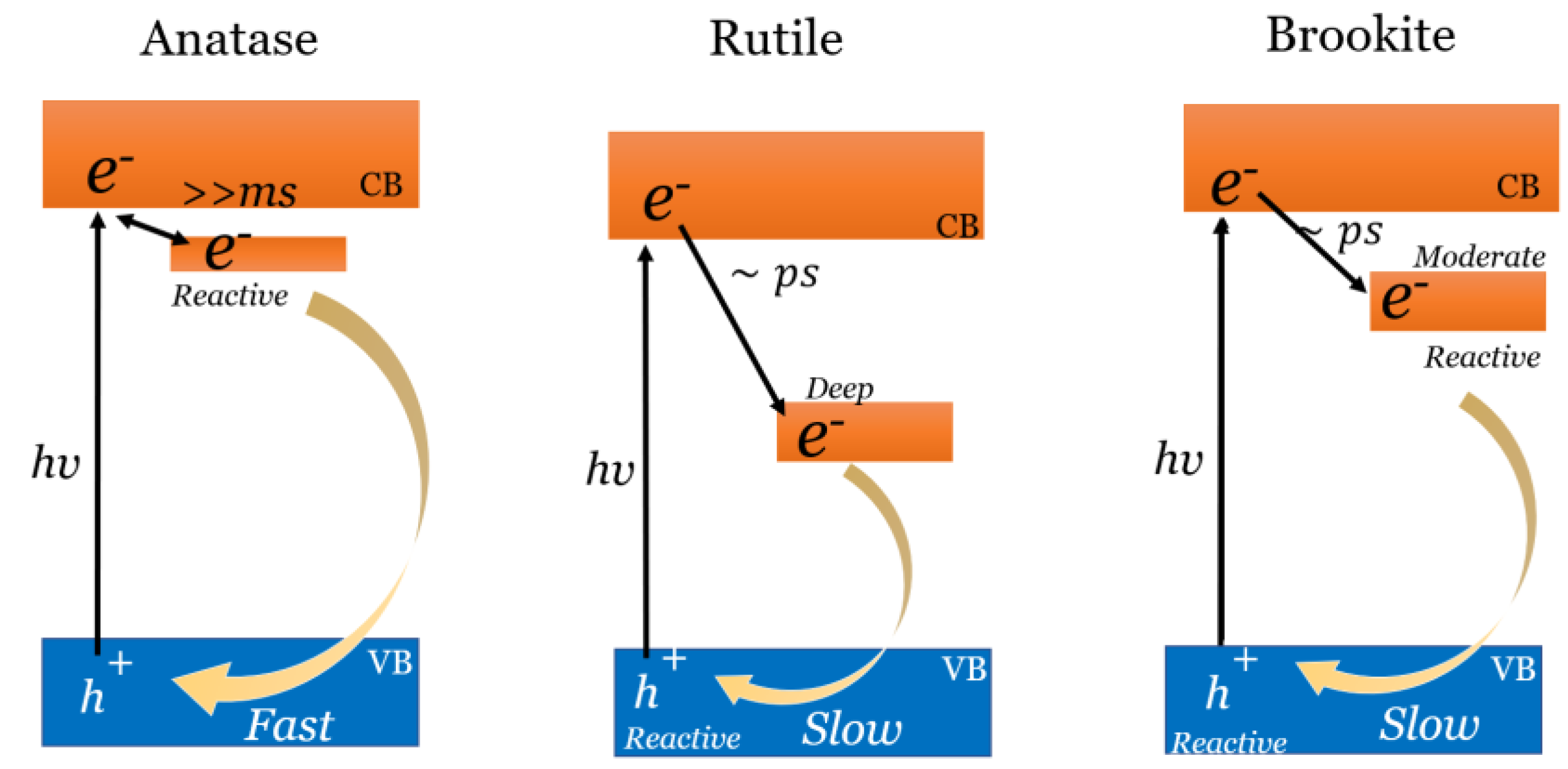
| Catalyst | Solvent | Amount of Products (µmol) | |||||
|---|---|---|---|---|---|---|---|
| Urea | Acetone | Methanol | NH4+ | H2 | Ti3+ | ||
| Q–TiO2/PVPD | PC | 5.6 | 61.0 | 1.2 | 2.7 | 0.18 | 0.14 |
| Q–TiO2 coloidal | PC | 0.22 | 2.5 | 0.12 | 0 | 0 | 0 |
| P–25 TiO2/PVPD | PC | 2.3 | 30.1 | 0.04 | 1.2 | 0.08 | – |
| P–25 Coloidal | PC | 0.1 | 1.1 | 0.05 | 0 | 0.06 | – |
| P–25 TiO2/PVPD | H2O | 0 | 0.07 | 0 | 0 | 0.01 | – |
| Solvent | Dielectric Constant, | Amount of Products (mM) (a) | |||
|---|---|---|---|---|---|
| Urea | NH3 | HCO2− | CO | ||
| Ethylene glycol monoethyl ether | 29.6 | 1.00 | 0.20 | 0.80 | 0.50 |
| Acetonitrile | 37.5 | 1.15 | 0.15 | 0.70 | 0.20 |
| Sulfolane | 43.0 | 1.00 | 0.20 | 0.40 | 0.25 |
| PC | 69.0 | 0.85 | 0.25 | 0.10 | 0.05 |
| Water | 78.5 | 2.75 | 0.75 | 0.10 | 0.05 |
| Catalyst | Amount of Products (mMh) (a) | Urea–Formate Ratio | |
|---|---|---|---|
| Urea | Formate | ||
| TiO2 | 0.28 | 0.11 | 2.5 |
| TiO2/Cu | 0.40 | 0.050 | 8.0 |
| PFD:TiO2 | 0.58 | 0.15 | 3.9 |
| PFD:TiO2/Cu | 1.12 | 0.025 | 45 |
| Author | Catalyst | C Source | N Source | Solvent | Illumination Time, h | Urea, mM | Urea, mM h−1 |
|---|---|---|---|---|---|---|---|
| S. Kuwabata et al. [41] | Q–TiO2/PVPD | CO2, sat. | NH2OH 0.020 M (a) | PC | 1 | 5.7 (a,c) | 5.7 |
| B.-J. Liu et al. [42] | Q–TiO2/SiO2 | CO2, sat. | LiNO3 0.020 M | H2O | 5 | 2.75 (b) | 0.55 |
| D.G. Shchukin et al. [44] | Cu/TiO2–PVA–PAH/PSS (2.2 μm diameter) | CO2, sat. | NaNO3 0.1 M | H2O | 5 | 1.72 (c) | 0.34 |
| E.A. Ustinovich et al. [45] | Cu/TiO2:PFD | CO2, sat. | NaNO3 1.0 M | PDF:H2O | 1 | 1.1 (c,d) | 1.1 |
| B. Srinivas et al. [21] | Fe2TiO5(10wt%)/HZSM–5 | 2–propanol 1 v/v% | KNO3 0.016 M | H2O | 6 | 0.31 (e) | 0.052 |
| H. Maimaiti et al. [47] | Ti3+–TiO2/Fe–CNTs | CO2 (100 mL min−1 flow rate) | N2 (100 mL min−1 flow rate) | H2O | 4 | 0.710 (f) | 0.178 |
| Amount of Products, mM | |||||||||||||||
| Photocatalyst | Time, h | C Source | N Source | Electron Donor | Solvent | Urea | NH3 | NH4+ | NO2− | (CH3)2CO | HCOO− | CH3OH | CO | H2 | Ref |
| Q–TiO2/PVPD | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 1.87 (a) | – | 0.90 (a) | – | 20.34 (a) | – | 0.40 (a) | – | 0.06 (a) | [41] |
| Q–TiO2 colloid | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 0.07 (a) | – | – | – | 0.83 (a) | – | 0.04 (a) | – | – | [41] |
| P–25 TiO2/PVPD | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 0.77 (a) | – | 0.40 (a) | – | 10.03 (a) | – | 0.01 (a) | – | 0.03 (a) | [41] |
| P–25 colloid | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 0.03 (a) | – | – | – | 0.04 (a) | – | 0.02 (a) | – | 0.02 (a) | [41] |
| P–25 TiO2/PVPD | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | H2O | – | – | – | – | 0.02 (a) | – | – | – | 0.003 (a) | [41] |
| Q–TiO2/PVPD | 5 (f) | CO sat. | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 0.77 (a) | – | 1.4 (a) | – | 14.70 (a) | – | 0.97 (a) | – | 0.04 (a) | [41] |
| Q–TiO2/PVPD | 5 (f) | HCOOH 0.16 M | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 1.9 (a) | – | 0.90 (a) | – | 19.03 (a) | – | – | – | 0.07 (a) | [41] |
| Q–TiO2/PVPD | 0.5 (f) | CO2 sat. | NH2OH 0.020 M | 2–propanol 1.0 M | PC | 2.99 (a,c) | – | 2.04 (a,c) | – | 8.88 (a,c) | – | 0.47 (a,c) | – | – | [41] |
| Q–TiO2/PVPD | 2 (f) | CO2 sat. | NO (gas) | 2–propanol 1.0 M | PC | 4.56 (a,c) | – | 0.41 (a,c) | – | 28.40 (a,c) | – | 0.34 (a) | – | – | [41] |
| Q–TiO2/SiO2 | 7 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | Ac | 1.52 (b) | 0.24 (b) | – | – | 13.30 (b) | 0.84 (b) | – | 0.32 (b) | 0.004 (a,b) | [42] |
| Q–TiO2/SiO2 | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | Eg | 1.00 (b) | 0.20 (b) | – | – | – | 0.80 (b) | – | 0.50 (b) | – | [42] |
| Q–TiO2/SiO2 | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | Ac | 1.15 (b) | 0.15 (b) | – | – | – | 0.70 (b) | – | 0.20 (b) | – | [42] |
| Q–TiO2/SiO2 | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | Sf | 1.0 (b) | 0.20 (b) | – | – | – | 0.40 (b) | – | 0.25 (b) | – | [42] |
| Q–TiO2/SiO2 | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | PC | 0.85 (b) | 0.25 (b) | – | – | – | 0.10 (b) | – | 0.05 (b) | – | [42] |
| Q–TiO2/SiO2 | 5 (f) | CO2 sat. | LiNO3 0.020 M | 2–propanol 1.0 M | H2O | 2.75 (b) | 0.75 (b) | – | – | – | 0.10 (b) | – | 0.05 (b) | – | [42] |
| TiO2 | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 0.31 (c) | – | 0.04 (c) | – | – | 0.13 (c) | – | – | – | [44] |
| TiO2–PVA–PAH/PSS (8.1 μm capsule) | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 0.43 (c) | – | 0.08 (c) | – | – | 0.11 (c) | – | – | – | [44] |
| TiO2–PVA–PAH/PSS (4.2 μm capsule) | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 0.65 (c) | – | 0.15 (c) | – | – | 0.09 (c) | – | – | – | [44] |
| TiO2–PVA–PAH/PSS (2.2 μm capsule) | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 1.12 (c) | – | 0.38 (c) | – | – | 0.04 (c) | – | – | – | [44] |
| TiO2/Cu | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 0.49 (c) | – | 0.09 (c) | – | – | 0.21 (c) | – | – | – | [44] |
| TiO2/Cu–PVA–PAH/PSS (8.1 μm capsule) | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 0.70 (c) | – | 0.16 (c) | – | – | 0.19 (c) | – | – | – | [44] |
| TiO2/Cu–PVA–PAH/PSS (4.2 μm capsule) | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 1.05 (c) | – | 0.32 (c) | – | – | 0.23 (c) | – | – | – | [44] |
| TiO2/Cu–PVA–PAH/PSS (2.2 μm capsule) | 5 (g) | CO2 sat. | NaNO3 0.1 M | PVA | PVA | 1.72(c) | – | 0.67 (c) | – | – | 0.21 (c) | – | – | – | [44] |
| TiO2 | 1 (h) | CO2 sat. | NaNO3 1.0 M | 2–propanol 1.0 M | PFD–H2O | 0.3 (c,d) | – | – | – | 0.1 (c,d) | – | – | [45] | ||
| TiO2/Cu | 1 (h) | CO2 sat. | NaNO3 1.0 M | 2–propanol 1.0 M | PFD–H2O | 0.4 (c,d) | – | – | – | 0.1 (c,d) | – | – | [45] | ||
| PFD:TiO2 | 1 (h) | CO2 sat. | NaNO3 1.0 M | 2–propanol 1.0 M | PFD–H2O | 0.6 (c,d) | – | – | – | – | 0.2 (c,d) | – | – | [45] | |
| PFD:TiO2/Cu | 1 (h) | CO2 sat. | NaNO3 1.0 M | 2–propanol 1.0 M | PFD–H2O | 1.1 (c,d) | – | – | – | – | 0.03 (c,d) | – | – | [45] | |
| TiO2 | 6 (i) | Oxalic acid 1% w/v | KNO3 0.016 M | Oxalic acid 1% w/v | H2O | 0.18 (e) | 0.12 (e) | – | – | – | – | – | – | – | [21] |
| TiO2 | 6 (i) | Oxalic acid 1% w/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.20 (e) | 0.15 (e) | – | – | – | – | – | – | – | [21] |
| TiO2 | 6 (i) | – | KNO3 0.016 M | – | H2O | – | 0.05 (e) | – | Trace | – | – | – | – | – | [21] |
| TiO2 (5% wt)–Zeolite | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.07 (e) | 0.21 (e) | – | 0.1 (e) | – | – | – | – | – | [21] |
| TiO2 (10% wt)–Zeolite | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.10 (e) | 0.11 (e) | – | 0.14 (e) | – | – | – | – | – | [21] |
| TiO2 (15% wt)–Zeolite | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.05 (e) | 0.05 (e) | – | 0.08 (e) | – | – | – | – | – | [21] |
| Fe2TiO5 | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.20 (e) | 0.05 (e) | – | 0.002 (e) | – | – | – | – | – | [21] |
| Fe2Ti2O7 | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.09 (e) | 0.12 (e) | – | 0.01 (e) | – | – | – | – | – | [21] |
| Fe2TiO5 (10%wt)/HZSM–5 | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.31 (e) | 0.15 (e) | – | 0.22 (e) | – | – | – | – | – | [21] |
| Fe2TiO5 (10%wt)/HZSM–5 | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | Oxalic acid 1% w/v | H2O | 0.15 (e) | 0.09 (e) | – | 0.20 (e) | – | – | – | – | – | [21] |
| Fe2Ti2O7 (10%wt)/HZSM–5 | 6 (i) | 2–propanol 1% v/v | KNO3 0.016 M | 2–propanol 1% v/v | H2O | 0.12 (e) | 0.01 (e) | – | 0.03 (e) | – | – | – | – | – | [21] |
| Ti3+–TiO2 (200 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.0152 (j) | 0.0176 (j) | – | – | – | – | – | – | – | [47] |
| Ti3+–TiO2 (300 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.0298 (j) | 0.027 (j) | – | – | – | – | – | – | – | [47] |
| Ti3+–TiO2 (400 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.1543 (j) | 0.0778 (j) | – | – | – | – | – | – | – | [47] |
| Ti3+–TiO2 (500 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.0836 (j) | 0.2227 (j) | – | – | – | – | – | – | – | [47] |
| Ti3+–TiO2 (600 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.0631 (j) | 0.4529 (j) | – | – | – | – | – | – | – | [47] |
| 70%–Ti3+–TiO2/CNTs (400 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.3332 (j) | 0.0704 (j) | – | – | – | – | – | – | – | [47] |
| 75%–Ti3+–TiO2/CNTs (400 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.4079 (j) | 0.064.5 (j) | – | – | – | – | – | – | – | [47] |
| 80%–Ti3+–TiO2/CNTs (400 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.7101 (j) | 0.0727 (j) | – | – | – | – | – | – | – | [47] |
| 85%–Ti3+–TiO2/CNTs (400 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.414 (j) | 0.01621 (j) | – | – | – | – | – | – | – | [47] |
| 90%–Ti3+–TiO2/CNTs (400 °C) | 4 | – | N2 100 mL min−1 | CO2 100 mL min−1 | H2O | 0.1142 (j) | 0.03663 (j) | – | – | – | – | – | – | – | [47] |
| Authors | Catalyst | Wattage Light Source, W | Irradiance, W cm−2 | Irradiated Effective Area, cm2 (a) | Illumination Time, h | Amount of Urea Produced, mg (e) | Energy Consumption, MWh cm−2 ton−1 | Energy Consumption, MWh ton−1 |
|---|---|---|---|---|---|---|---|---|
| S. Kuwabata et al. [41] | Q–TiO2/PVPD | 500 | 0.36 | 2.5 (b) | 1 | 1.03 (f) | 350 | 876 |
| B.-J. Liu et al. [42] | Q–TiO2/SiO2 | 500 | 1.00 | 2.0 (c) | 5 | 1.49 (g) | 6.05 × 103 | 1.21 × 104 |
| D.G. Shchukin et al. [44] | Cu/TiO2–PVA–PAH/PSS (2.2 μm diametre) | 120 | 0.02 | 0.79 (d) | 5 | 0.52 (g) | 194 | 152 |
| E.A. Ustinovich et al. [45] | Cu/TiO2:PFD | 120 | 20 | ND | 1 | 1.06 (h) | 1.89 × 104 | – |
| B. Srinivas et al. [21] | Fe2TiO5(10wt%)/HZSM–5 | 250 | ND | ND | 6 | 1.86 (i) | – | – |
| H. Maimaiti et al. [47] | 80%–Ti3+–TiO2 | 300 | ND | ND | 4 | 0.7101 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matamala-Troncoso, F.; Isaacs, M.; Sáez-Navarrete, C. Photocatalyzed Production of Urea as a Hydrogen–Storage Material by TiO2–Based Materials. Photochem 2022, 2, 539-562. https://doi.org/10.3390/photochem2030038
Matamala-Troncoso F, Isaacs M, Sáez-Navarrete C. Photocatalyzed Production of Urea as a Hydrogen–Storage Material by TiO2–Based Materials. Photochem. 2022; 2(3):539-562. https://doi.org/10.3390/photochem2030038
Chicago/Turabian StyleMatamala-Troncoso, Felipe, Mauricio Isaacs, and César Sáez-Navarrete. 2022. "Photocatalyzed Production of Urea as a Hydrogen–Storage Material by TiO2–Based Materials" Photochem 2, no. 3: 539-562. https://doi.org/10.3390/photochem2030038
APA StyleMatamala-Troncoso, F., Isaacs, M., & Sáez-Navarrete, C. (2022). Photocatalyzed Production of Urea as a Hydrogen–Storage Material by TiO2–Based Materials. Photochem, 2(3), 539-562. https://doi.org/10.3390/photochem2030038






