Recent Advances in Aerobic Photo-Oxidation over Small-Sized IB Metal Nanoparticles
Abstract
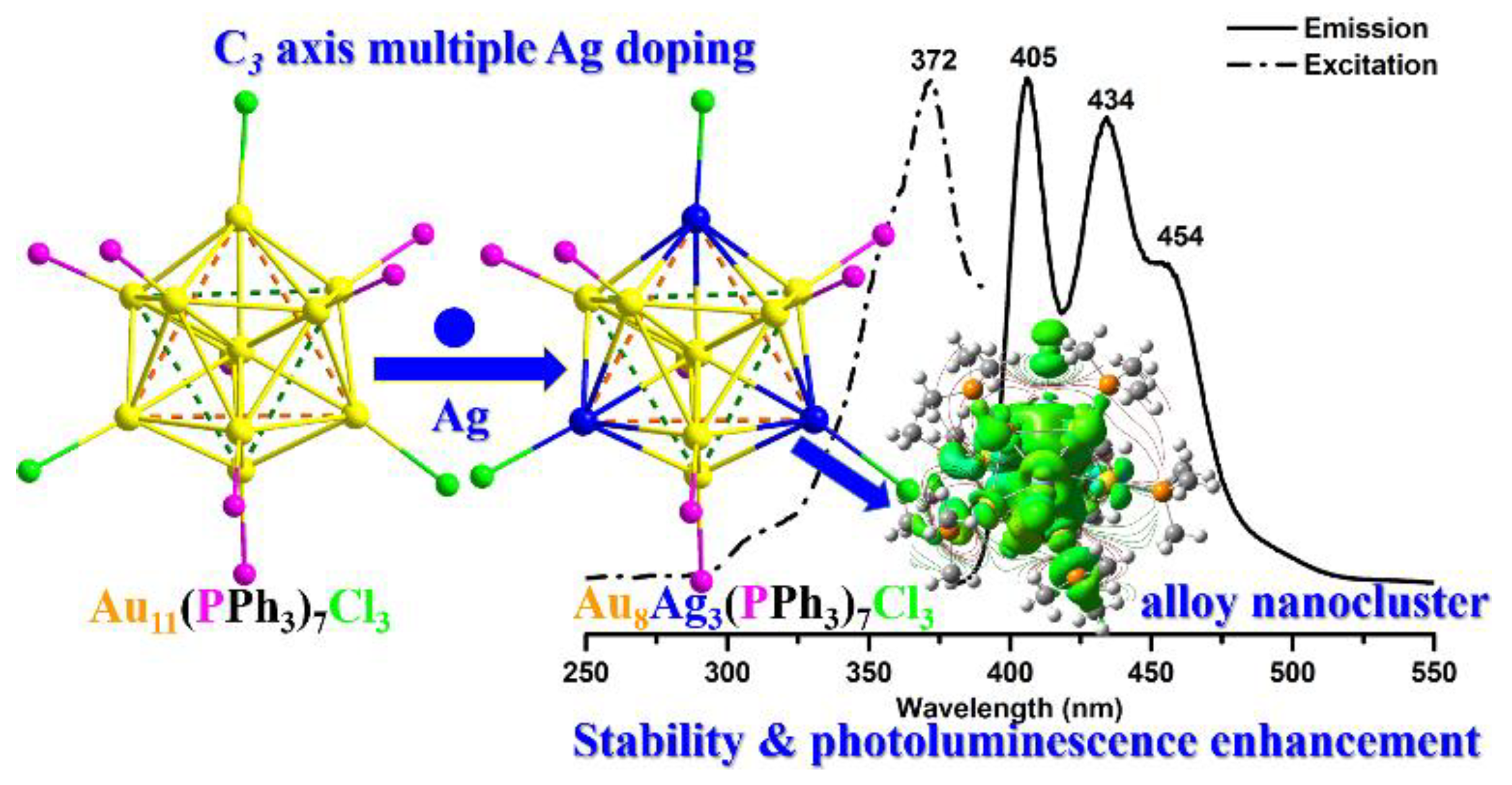
:1. Introduction
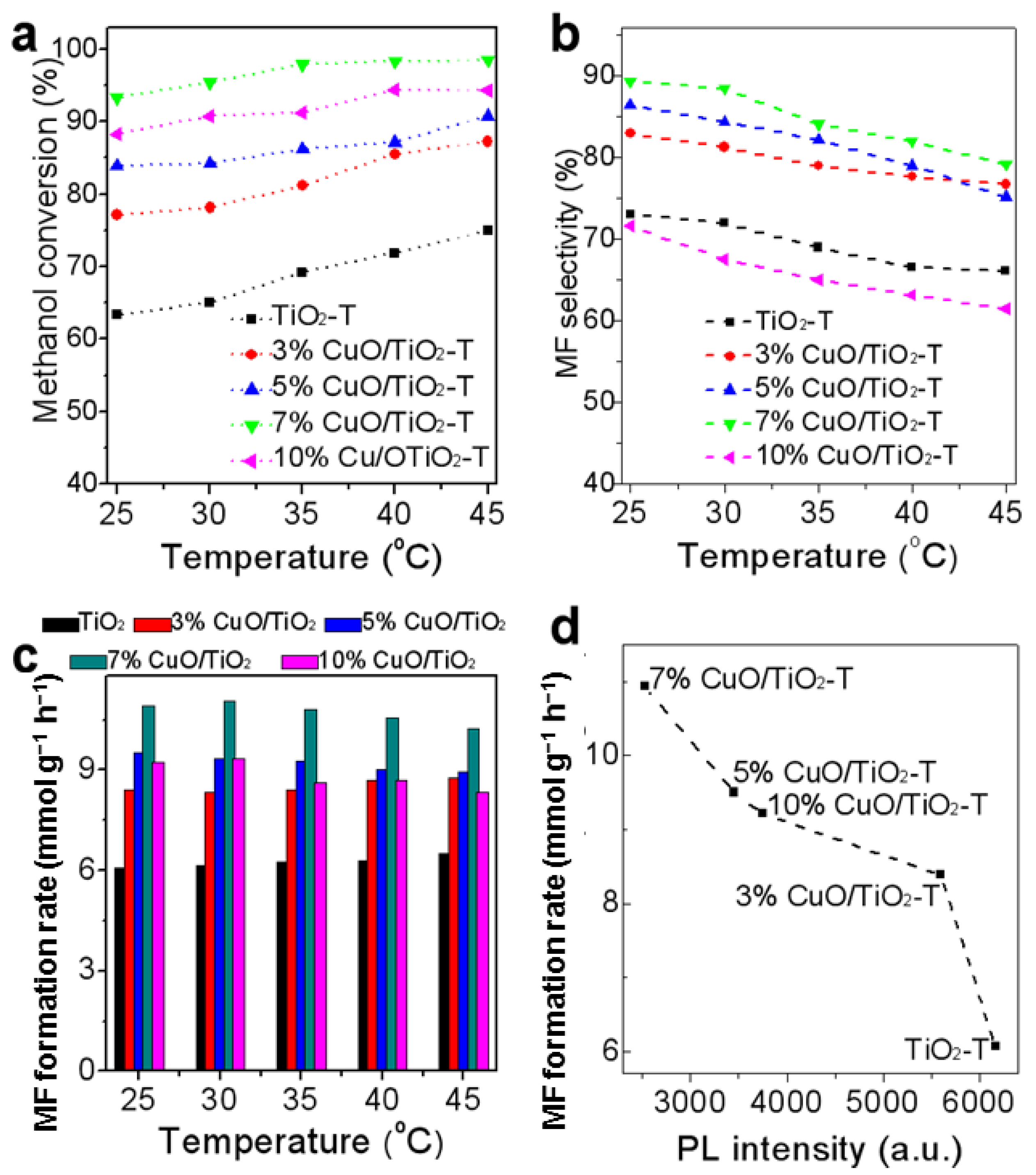
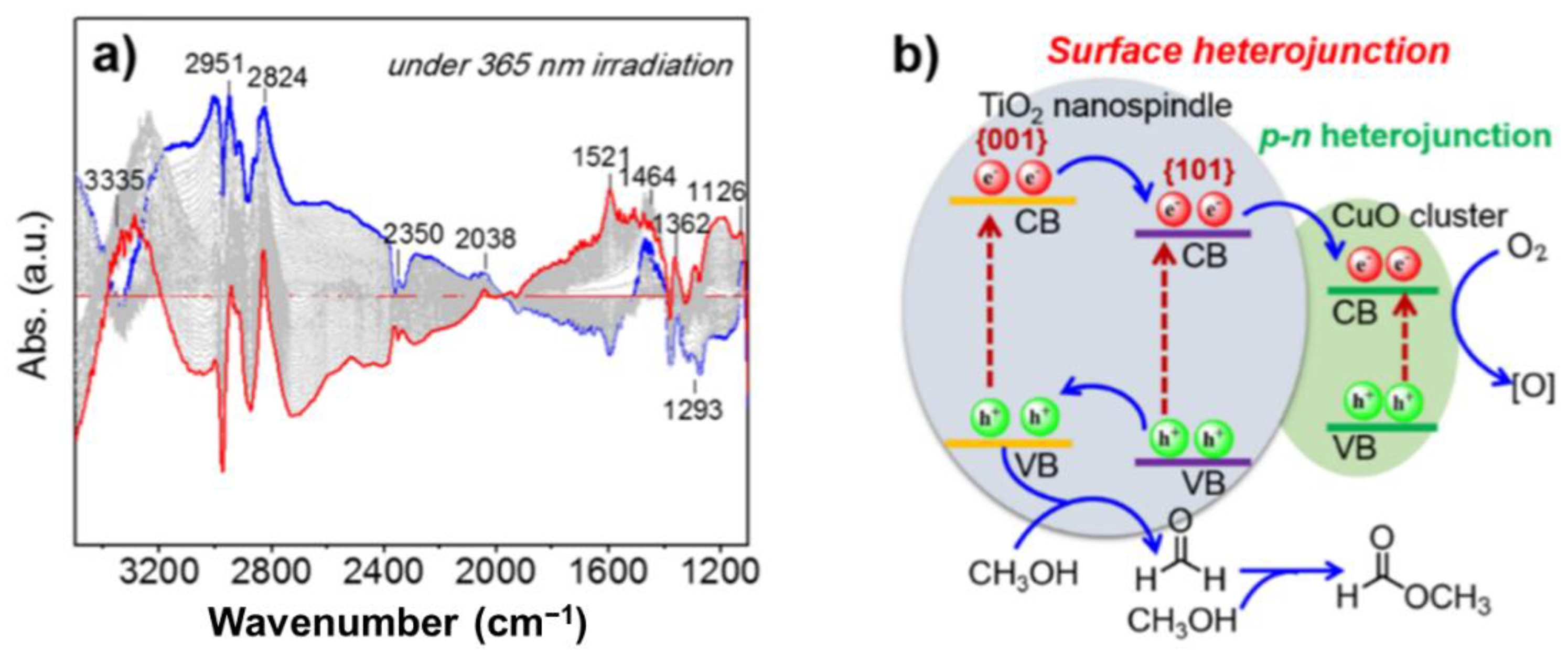
2. Methanol Conversion to Methyl Formate
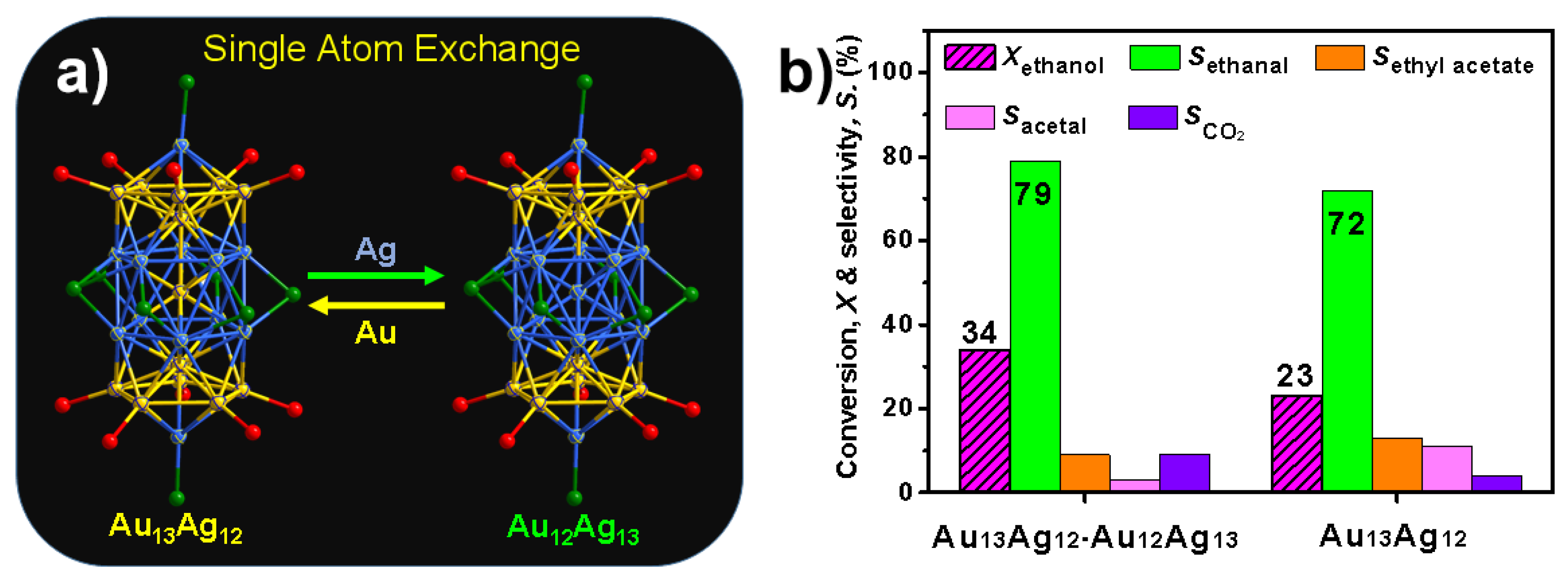
3. Ethanol Conversion
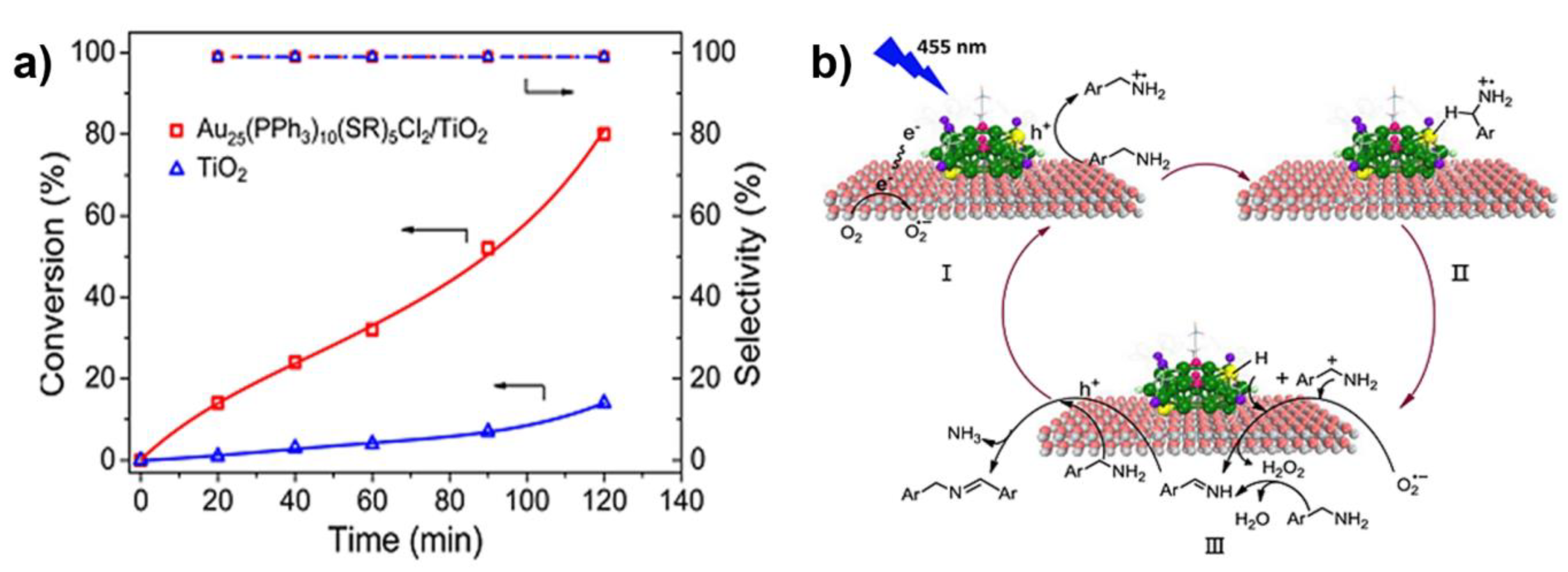
4. Benzylamine to Imine
5. Sulfide to Sulfoxide
6. Conclusions, Challenges, and Future Perspective
- (1)
- Four different types of photo-oxidations were achieved over the TiO2-supported metal nanoclusters and nanoparticles presented in this paper, including the photo-oxidation of methanol to methyl formate, and ethanol conversion, a benzylamine to imine conversion, and sulfide to sulfoxide conversion, which give some new exploits in photocatalysis.
- (2)
- Copper oxide nanoclusters supported onto the TiO2 semiconductors with a different morphology of nanosheets, nanospindles, and nanotubes have been prepared to investigate the performance of photocatalytic oxidation reactions. The morphology (exposed facets) effects have been well studied.
- (3)
- The active centre for photocatalytic aerobic oxidation was identified and created as an example. The CuOx/TiO2{101} interface was considered to be a key point in the photo-oxidation of methanol.
- (4)
- Single-atom-exchanging in the metal clusters has a big influence on the catalytic activity but cannot affect product selectivity. However, the detailed mechanism is still unable to reveal this.
- (5)
- Via the characterization of ATR-IR spectra to dig out the possible reaction intermediates [63], we have clearly mapped out the whole conversion pathway and worked out the controversy in this photocatalysis.
- (1)
- More efforts must be put into the creation of novel photo/catalysts with specific active reaction sites for a high-efficient conversion [64,65,66], and the catalytic efficiency should be further enhanced by the design of the novel metal nanoclusters or nanoparticles on other light-absorbing nanomaterials (e.g., ZnO, g-C3N4, BiOX, Mxenes, etc.). The photocatalysis also needs to be developed using UV/VIS light, which is close to the real applications.
- (2)
- In order to reveal the reaction mechanisms, DFT studies and in-situ characterizations should be used in this catalytic reaction system, and, in turn, it will be more helpful in obtaining new photocatalysts with high efficiency.
- (3)
- (4)
- Alloy metal nanoparticle catalysts still need to be further and wildly used in catalytic biomass conversions; as the electronic property can be well modified to tune the catalytic performance, it is a kind of excellent method for designing suitable catalysts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jin, R.; Li, G.; Sharma, S.; Li, Y.; Du, X. Toward Active-Site Tailoring in Heterogeneous Catalysis by Atomically Precise Metal Nanoclusters with Crystallographic Structures. Chem. Rev. 2021, 121, 567–648. [Google Scholar] [CrossRef]
- He, W.; Ma, G.; Shen, Q.; Tang, Z. Engineering Gold Nanostructures for Cancer Treatment: Spherical Nanoparticles, Nanorods, and Atomically Precise Nanoclusters. Nanomaterials 2022, 12, 1738. [Google Scholar] [CrossRef]
- Yin, C.; Liu, S.; Qin, Z.; Zhang, Y.; Li, G.; Zhao, Z. Butterfly-Like Tetranuclear Copper(I) Clusters for Efficient Alkyne Homocoupling Reactions. Eur. J. Inorgan. Chem. 2021, 392–397. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, Q.; Xie, J. Atom-Precision Engineering Chemistry of Noble Metal Nanoparticles. Ind. Eng. Chem. Res. 2022, 61, 7594–7612. [Google Scholar] [CrossRef]
- Li, G.; Jin, R. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Qin, Z.; Xu, H.; Li, G. Heterogeneous Cross-Coupling over Gold Nanoclusters. Nanomaterial 2019, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, Y.; Wang, H.; Li, Z.; Zheng, K.; Li, S.; Li, G. Transition-Metal-Mediated Catalytic Properties of CeO2-Supported Gold Clusters in Aerobic Alcohol Oxidation. Nano Res. 2018, 11, 2139–2148. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Chen, W.; Xu, W.W.; Han, Z.-k.; Waheed, A.; Ye, Z.; Li, G.; Baiker, A. Continuous Dimethyl Carbonate Synthesis from CO2 and Methanol over BixCe1−xOδ Monoliths: Effect of Bismuth Doping on Population of Oxygen Vacancies, Activity, and Reaction Pathway. Nano Res. 2022, 15, 1366–1374. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, Z.; Sharma, S.; Li, G. Recent progress in heterogeneous catalysis over atomically and structurally precise metal nanoclusters. Chem. Rec. 2021, 21, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Diez-Pascual, A.M.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Rajput, N.S.; El Moutaouakil, A.; Jouiad, M. Recent Advances in the Design of Plasmonic Au/TiO2 Nanostructures for Enhanced Photocatalytic Water Splitting. Nanomaterials 2020, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Kiss, J.; Sapi, A.; Toth, M.; Kukovecz, A.; Konya, Z. Rh-induced Support Transformation and Rh Incorporation in Titanate Structures and Their Influence on Catalytic Activity. Catalysts 2020, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Patial, S.; Hasija, V.; Raizada, P.; Singh, P.; Singh, A.A.P.K.; Asiri, A.M. Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and future scenario. J. Environ. Chem. Eng. 2020, 8, 103791. [Google Scholar] [CrossRef]
- Xia, C.; Nguyen, T.H.C.; Nguyen, X.C.; Kim, S.Y.; Nguyen, D.L.T.; Raizada, P.; Singh, P.; Nguyen, V.-H.; Nguyen, C.C.; Hoang, V.C.; et al. Emerging cocatalysts in TiO2-based photocatalysts for light-driven catalytic hydrogen evolution: Progress and perspectives. Fuel 2022, 307, 121745. [Google Scholar] [CrossRef]
- Raza, A.; Altaf, S.; Ali, S.; Ikram, M.; Li, G. Recent Advances in Carbonaceous Sustainable Nanomaterials for Wastewater Treatments. Sustain. Mater. Technol. 2022, 32, e00406. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Khan, A.A.P.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering nanostructures of CuO-based photocatalysts for water treatment: Current progress and future challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Jiang, Q.; Guo, S.; Baiker, A.; Li, G. Ternary CuCrCeOx Solid Solution Enhances N2-Selectivity in the NO Reduction with CO in the Presence of Water and Oxygen. ChemCatChem 2022, 14, e202200203. [Google Scholar]
- Wang, Y.; Jiang, Q.; Xu, L.; Han, Z.-K.; Guo, S.; Li, G.; Baiker, A. Effect of Configuration of Copper Oxide-Ceria Catalysts in NO Reduction with CO: Superior Performance of Copper-Ceria Solid Solution. ACS Appl. Mater. Interfaces 2021, 13, 61078–61087. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.; Guo, S.; Han, Z.-K.; Ta, N.; Li, G.; Baiker, A. NO reduction with CO over CuOx/CeO2 Nanocomposites: Influence of Oxygen Vacancies and Lattice Strain. Catal. Sci. Technol. 2021, 11, 6543–6552. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhan, E.S.; Cai, W.J.; Li, J.; Shen, W.J. Methanol Selective Oxidation to Methyl Formate over ReOx/CeO2 Catalysts. Catal. Lett. 2008, 120, 274–280. [Google Scholar] [CrossRef]
- Kaiser, D.; Beckmann, L.; Walter, J.; Bertau, M. Conversion of Green Methanol to Methyl Formate. Catalysts 2021, 11, 869. [Google Scholar] [CrossRef]
- Shi, Q.; Wei, X.; Raza, A.; Li, G. Recent Advances in Aerobic Photo-Oxidation of Methanol to Valuable Chemicals. ChemCatChem 2021, 13, 3381–3395. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.; Li, G. Recent Progress in Green Conversion of Biomass Alcohol to Chemicals via Aerobic Oxidation. Biomass 2022, 2, 103–115. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Ping, G.C.; Wang, X.J.; Xu, H.; Li, J.M.; Cui, J.Q.; Abroshan, H.D.; Ding, H.J.; Li, G. CuO/TiO2 heterojunction composites: An efficient photocatalyst for selective oxidation of methanol to methyl formate. J. Mater. Chem. A 2019, 7, 2253–2260. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous Gold Catalysts for Selective Gas-Phase Oxidative Coupling of Methanol at Low Temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Zhang, X.; Ali, S.; Cao, C.; Rafi, A.A.; Li, G. Photoelectrochemical Energy Conversion over 2D Materials. Photochem 2022, 2, 272–298. [Google Scholar] [CrossRef]
- Liu, J.; Han, C.H.; Yang, X.Z.; Gao, G.J.; Shi, Q.Q.; Tong, M.; Liang, X.Y.; Li, C.F. Methyl formate synthesis from methanol on titania supported copper catalyst under UV irradiation at ambient condition: Performance and mechanism. J. Catal. 2016, 333, 162–170. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.H.; Fang, Q.H.; Abroshan, H.D.; Kim, H.; Haruta, M.; Li, G. Gold-Palladium Nanoalloys Supported by Graphene Oxide and Lamellar TiO2 for Direct Synthesis of Hydrogen Per-oxide. ACS Appl. Mater. Interfaces 2018, 10, 40599–40607. [Google Scholar] [CrossRef]
- Kollhoff, F.; Schneider, J.; Li, G.; Sami, B.; Shen, W.J.; Berger, T.; Diwald, O.; Libuda, J. Anchoring of carboxyl-functionalized porphyrins on MgO, TiO2, and Co3O4 nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 24858–24868. [Google Scholar] [CrossRef]
- Shao, B.; Zhao, W.N.; Miao, S.; Huang, J.H.; Wang, L.L.; Li, G.; Shen, W.J. Facet-dependent anchoring of gold nanoparticles on TiO2 for CO oxidation. Chin. J. Catal. 2019, 40, 1534–1539. [Google Scholar] [CrossRef]
- Waheed, A.; Shi, Q.; Maeda, N.; Meier, D.M.; Qin, Z.; Li, G.; Baiker, A. Strong Activity Enhancement of the Photocatalytic Degradation of an Azo Dye on Au/TiO2 Doped with FeOx. Catalysts 2020, 10, 933. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Qin, Z.X.; Yu, C.L.; Waheed, A.; Xu, H.; Gao, Y.; Abroshan, H.; Li, G. Experimental and mechanistic understanding of photo-oxidation of methanol catalyzed by CuO/TiO2-spindle nano-composite: Oxygen vacancy engineering. Nano Res. 2020, 13, 939–946. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Shi, Q.; Ping, G.; Xu, H.; Waleed, A.; Li, G. CuO Nanoparticle-Decorated TiO2-Nanotube Heterojunctions for Direct Synthesis of Methyl Formate via Photo-Oxidation of Methanol. ACS Omega 2020, 5, 15942–15948. [Google Scholar] [CrossRef]
- Fang, Q.; Qin, Z.; Shi, Y.; Liu, F.; Barkaoui, S.; Abroshan, H.; Li, G. Au/NiO Composite: A Catalyst for One-Pot Cascade Conversion of Furfural. ACS Appl. Energy Mater. 2019, 2, 2654–2661. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Huang, J.; Zhang, C.; Hong, F.; Zhou, Y.; Li, G.; Haruta, M. Efficient Aerobic Oxidation of Glucose to Gluconic Acid over Activated Carbon-Supported Gold Clusters. ChemSuSChem 2017, 10, 1976–1980. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, J.; Zhao, D.; Yang, Y.; Li, Z.; Li, G. Motif Mediated Au25(SPh)5(PPh3)10X2 Nanorod of Conjugated Electron Delocalization. Nano Res. 2019, 12, 501–507. [Google Scholar] [CrossRef]
- Qin, Z.; Sharma, S.; Wan, C.-Q.; Malola, S.; Xu, W.-W.; Häkkinen, H.; Li, G. A Homoleptic Alkynyl-Ligated [Au13Ag16L24]3− Cluster as a Catalytically Active Eight-Electron Superatom. Angew. Chem. Int. Ed. 2020, 59, 970–975. [Google Scholar]
- Qin, Z.; Zhang, J.; Wan, C.-Q.; Liu, S.; Abroshan, H.; Jin, R.; Li, G. Atomically Precise Nanoclusters with Reversible Isomeric Transformation for Rotary Nanomotors. Nat. Commun. 2020, 11, 6019. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, S.; Han, W.; Li, Z.; Xu, W.W.; Zhang, J.; Li, G. Tailoring optical and photocatalytic properties by single-Ag-atom exchange in Au13Ag12(PPh3)10Cl8 nanoclusters. Nano Res. 2022, 15, 2971–2976. [Google Scholar] [CrossRef]
- Zhu, H.; Yuan, X.; Yao, Q.; Xie, J. Shining photocatalysis by gold-based nanomaterials. Nano Energy 2021, 88, 106306. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Abroshan, H.; Ge, Q.; Yang, X.; Xu, H.; Li, G. Catalytic CO Oxidation Using Bimetallic MxAu25-x Clusters: A Combined Experimental and Computational Study on Doping Effects. J. Phys. Chem. C 2016, 120, 10261–10267. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, X.; Shi, Q.; Li, J.; Ping, G.; Xu, H.; Ding, H.; Li, G. Synergistic Effects of PtFe/CeO2 Catalyst afford high Catalytic Performance in selective hydrogenation of cinnamaldehyde. J. Rare Earths, 2022; in press. [Google Scholar] [CrossRef]
- Du, X.; Huang, Y.; Pan, X.; Han, B.; Su, Y.; Jiang, Q.; Li, M.; Tang, H.; Li, G.; Qiao, B. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 5811. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Shi, Q.; Waheed, A.; Cao, Y.; Li, G. Cascade aldol condensation of an aldehyde via the aerobic oxidation of ethanol over an Au/NiO composite. Nanoscale Adv. 2019, 1, 3654–3659. [Google Scholar] [CrossRef] [Green Version]
- Mallat, T.; Baiker, A. Potential of Gold Nanoparticles for Oxidation in Fine Chemical Synthesis. Annu. Rev. Chem. Biomol. 2012, 3, 11–28. [Google Scholar] [CrossRef]
- Naya, S.; Kimura, K.; Tada, H. One-Step Selective Aerobic Oxidation of Amines to Imines by Gold Nanoparticle-Loaded Rutile Titanium(IV) Oxide Plasmon Photocatalyst. ACS Catal. 2013, 3, 10–13. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, J.; Sharma, S.; Malola, S.; Wu, K.; Häkkinen, H.; Li, G. Photo-Induced Cluster-to-Cluster Transformation of [Au37−xAgx(PPh3)13Cl10]3+ into [Au25−yAgy(PPh3)10Cl8]+: Fragmentation of A Trimer of 8-Electron Superatoms by Light. J. Phys. Chem. Lett. 2021, 12, 10920–10926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qin, Z.; Liu, S.; Zhu, M.; Li, G. On the Redox Property of Ag16Au13 Clusters and Its Catalytic Application in the Photooxidation. J. Chem. Phys. 2021, 154, 164308. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Luo, N.; Wang, Y.; Abroshan, H.; Li, G.; Wang, F. Visible Light Gold Nanocluster Photocatalyst: Selective Aerobic Oxidation of Amines to Imines. ASC Catal. 2017, 7, 3632–3638. [Google Scholar] [CrossRef]
- Liu, C.; Abroshan, H.; Yan, C.; Li, G.; Haruta, M. One-pot synthesis of Au11(PPh2Py)7Br3 for the highly chemoselective hydrogenation of nitrobenzadehyde. ACS Catal. 2016, 6, 92–99. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Abroshan, H.; Wang, J.; Li, Z.; Li, G.; Haruta, M. Phosphine/Phenylacetylide-Ligated Au Cluster for Multicomponent Coupling Reaction. J. Catal. 2016, 337, 287–294. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, D.; Zhao, L.; Xiao, Q.; Wu, T.; Zhang, J.; Wan, C.; Li, G. Tailoring the Stability, Photocatalysis and Photoluminescence Properties of Au11 Nanocluster via Doping Engineering. Nanoscale Adv. 2019, 1, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Mulliken, R.S. Interpretation of the Atmospheric Oxygen Bands; Electronic Levels of the Oxygen Molecule. Nature 1928, 122, 505. [Google Scholar] [CrossRef]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549. [Google Scholar] [CrossRef]
- Kawasaki, H.; Kumar, S.; Li, G.; Zeng, C.; Kauffman, D.R.; Yoshimoto, J.; Iwasaki, Y.; Jin, R. Generation of singlet oxygen by photoexcited Au25(SR)18 clusters. Chem. Mater. 2014, 26, 2777–2788. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, C.; Abroshan, H.; Kauffman, D.R.; Li, G. Au38S2(SAdm)20 Photocatalyst for One-Step Selective Aerobic Oxidations. ACS Catal. 2017, 7, 3368–3374. [Google Scholar] [CrossRef]
- Liu, C.; Yan, C.; Lin, J.; Yu, C.; Huang, J.; Li, G. One-Pot Synthesis of Au144(SCH2Ph)60 Nanoclusters and Catalytic Application. J. Mater. Chem. A 2015, 3, 20167–20173. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhou, Y.; Zheng, K.; Abroshan, H.; Kauffman, D.R.; Sun, J.; Li, G. Diphosphine-induced chiral propeller arrangement of gold nanoclusters for singlet oxygen photogeneration. Nano Res. 2018, 11, 5787–5798. [Google Scholar] [CrossRef]
- Waheed, A.; Cao, C.; Zhang, Y.; Zheng, K.; Li, G. Insight into Au/ZnO Catalyzed Aerobic Benzyl Alcohol Oxidation by Modulation-Excitation Attenuated Total Reflection IR Spectroscopy. New J. Chem. 2022, 46, 5361–5367. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Liu, X.; Xu, L.; Liu, B.; Zhang, J.; Xu, H.; Han, Z.; Li, G. In-situ exfoliation and assembly of 2D/2D g-C3N4/TiO2(B) hierarchical microflower: Enhanced photo-oxidation of benzyl alcohol under visible light. Carbon 2022, 195, 401–409. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Liu, X.; Li, J.; Xu, H.; Ding, H.; Li, G. Facile assembly of InVO4/TiO2 heterojunction for enhanced photo-oxidation of benzyl alcohol. Nanomaterials 2022, 12, 1544. [Google Scholar] [CrossRef]
- Shi, Q.; Raza, A.; Xu, L.; Li, G. Bismuth oxyhalide quantum dots modified titanate-necklaces with exceptional population of oxygen vacancies and photocatalytic activity. J. Colloid Interface Sci. 2022, 625, 750–760. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Xu, L.; Yang, X.; Han, Z.; Cao, R.; Li, G. Ionic liquids modified oxygen vacancy-rich amorphous FeNi hydroxide nanoclusters on carbon-based materials as an efficient electrocatalyst for electrochemical water oxidation. J. Energy Chem. 2022, 71, 167–173. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, S.; Yu, C.; Zhang, J.; Pan, X.; Li, G. Ionic liquid-assisted one-step preparation of ultrafine amorphous metallic hydroxide nanoparticles for the highly efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 15767–15773. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Z.; Cui, C.; Luo, Z.; Yang, B.; Jiang, Y.; Lai, C.; Wang, Z.; Wang, X.; Fang, X.; et al. In-Situ Generation and Global Property Profiling of Metal nanoclusters by Ultraviolet Laser Dissociation-Mass Spectrometry. Sci. China Chem. 2022, 65. [Google Scholar] [CrossRef]
- Raza, A.; Ikram, M.; Guo, S.; Baiker, A.; Li, G. Green Synthesis of Dimethyl Carbonate from CO2 and Methanol: New Strategies and Industrial Perspective. Adv. Sustain. Syst. 2022, 6, 2200087. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Pei, W.; Li, G.; Liu, W.; Du, P.; Wang, Z.; Qin, Z.; Qi, H.; Liu, X.; et al. Crystal Phase Mediated Restructuring of Pt on TiO2 with Tunable Reactivity: Redispersion versus Reshaping. ACS Catal. 2022, 12, 3634–3643. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Li, Z.; Yang, X.; Meng, F.; Liang, H.; Lei, Y.; Wu, H.; Zhang, J.; Li, G.; et al. Surface Isolation of Single Metal complexes or Clusters by a Coating Sieving Layer via Atomic Layer Deposition. Cell Rep. Phys. Sci. 2022, 3, 100787. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, M.; Li, G. Recent Advances in Aerobic Photo-Oxidation over Small-Sized IB Metal Nanoparticles. Photochem 2022, 2, 528-538. https://doi.org/10.3390/photochem2030037
Zhang Y, Wang M, Li G. Recent Advances in Aerobic Photo-Oxidation over Small-Sized IB Metal Nanoparticles. Photochem. 2022; 2(3):528-538. https://doi.org/10.3390/photochem2030037
Chicago/Turabian StyleZhang, Yifei, Meng Wang, and Gao Li. 2022. "Recent Advances in Aerobic Photo-Oxidation over Small-Sized IB Metal Nanoparticles" Photochem 2, no. 3: 528-538. https://doi.org/10.3390/photochem2030037
APA StyleZhang, Y., Wang, M., & Li, G. (2022). Recent Advances in Aerobic Photo-Oxidation over Small-Sized IB Metal Nanoparticles. Photochem, 2(3), 528-538. https://doi.org/10.3390/photochem2030037





