Abstract
Titanium dioxide (TiO2) is one of the stable and potential metal oxide semiconductor nanomaterials with flexible properties which allows them to be used in a variety of applications (i.e., environmental remediation, energy storage and production, and also as a pigment in personal care products, etc.). However, its low surface area, poor adsorption capacity and high bandgap energy (~3.2 eV) prevents its full potency. Especially, TiO2 with high bandgap (~3.2 eV) reduces its visible light absorption capacity and catalytic efficiency. Various modification processes (i.e., metal and non-metal doping, composite materials (mixed metal oxide, high surface area adsorbents), and dye sensitization etc.) have been accomplished for stimulating the characteristics of TiO2 and the associated catalytic efficiency. Among the modifications, the non-metal doping process in TiO2, specifically nitrogen doping, is one of the efficient dopants for enhancing the photocatalytic efficiency of TiO2 in the presence of visible light irradiation. However, the morphology of TiO2, structural changes in TiO2 during N-doping, properties (e.g., morphology and electronic) of N-doped TiO2 and also reaction operational parameters (e.g., doping concentration) hold a greater impact for enhancing the photocatalytic properties of TiO2 either positively or negatively. Furthermore, the synthesis methodologies have a major influence on the synthesis of stable N-TiO2 with pronounced photocatalytic efficiencies. Nevertheless, the methodologies for highly stable N-TiO2 synthesis, properties evaluation and their correlation with photocatalytic efficiencies are still not appropriately stabilized to accomplish the commercial utilization of N-TiO2. Therefore, this review article focuses on the synopsis of various synthesis methodologies and either their efficiencies or inefficiencies, the mechanism involved in the doping processes, changes in the structural, electronic and morphological properties observed due to the N-doping along with the photocatalytic capacity. Furthermore, the opportunities, challenges and future requirements linked to the development of durable N-doped TiO2-based semiconductor nanomaterials for efficient catalytic performance is also represented.
1. Introduction
Since the discovery of water splitting using TiO2 by Fujishima and Honda [1], TiO2 is a well-accepted stable and widely utilized metal-oxide-based semiconductor nanomaterial as photocatalyst for the decomposition of environmental pollutants (water, air and soil) and renewable energy (e.g., hydrogen) production. This could be ascribed to the unique features of TiO2 materials such as low toxicity, chemical and biological stability, long durability, high photocatalytic activity, strength against photocorrosion, cost-effectiveness and abundant availability. Furthermore, due to these unique characteristics of TiO2, it has been widely used as a pigment in various products (i.e., toothpastes, paints, medicines, plastics, sunscreens and textiles, etc.) preparation. Furthermore, the high hydrophilicity of TiO2 received significant industrial application, such as preparation of self-cleaning surfaces and antifogging mirrors, and it also possesses outstanding anticorrosion properties. In addition, TiO2 could be supported on various solid materials which encourage its repeated usage [2,3,4,5,6,7]. Therefore, TiO2 has been extensively used for various commercial/practical applications. Nevertheless, the practical application of TiO2 materials in environmental pollutants degradation and renewable energy production is still limited. TiO2 material could only be activated in the presence of ultraviolet (UV) light irradiation (high bandgap energy, ~3.2 eV) and could not be effectively used in solar light irradiation containing 2–3% UV light, 40–43% visible light and 50–55% near infrared light; as well as indoor room light (fluorescent, contains a small amount of UV light) irradiation. In addition, the low surface area and poor adsorption capacity of TiO2 and high rate of recombination of photogenerated charge carriers on TiO2 surface prevent its full potency in practical application [8,9,10].
In order for effective utilization of the solar spectrum by TiO2, to decrease the recombination rate of photogenerated charge carriers and to enhance the associated photocatalytic property, the research on narrowing/lowering of the TiO2 bandgap energy has been carried out by metal ion and non-metal ion doping, bi or tri-metal co-doping, surface sensitization, introduction of oxygen (O) defects and making composites (binary or tertiary) by coupling with other semiconductors and graphene-based materials, respectively [6,9,11,12,13,14,15,16]. In addition, the particle size of the TiO2 is a crucial factor that influences its surface structure and properties resulting in the improved photocatalytic performance of the TiO2. Similarly, the surface area and the adsorption capacity of TiO2 is improved by coupling with high surface area adsorbent (carbon (activated carbon, biochar, nanotube), biochar, zeolite, silica and clays, etc.) materials and thus resulting in various catalytic performances [17,18,19,20,21,22,23,24,25].
Among the modifications, metal and non-metal doping is being considered as an efficient process by the introduction of new energy levels between the conduction and valence band of the TiO2 leading to the efficient visible absorption of light, charge carriers separation and migration properties, and change in particle size and morphology of TiO2 and other surface characteristics that improved the photocatalytic activities of TiO2 catalysts. However, the additional high cost associated with the preparation of metal-doped TiO2 lowers its full commercial potency. Some of the metal-doped TiO2 have not been applied/used for real scale applications because they showed poor photocatalytic capacity and reproducibility. Therefore, the non-metal (N, S, P, B, C, F) doping on TiO2 has been studied. Among these non-metals, it is observed that the nitrogen doping into TiO2 (N-TiO2), provides visible light responsive photocatalysts and received significant attention because of their comparable atomic size, low ionization energy, high electronegativity, stability and cost-effectiveness [13,26,27]. Further, the nitrogen doping improves the photogenerated charge carrier’s separation and migration rates, which enhances the visible light photocatalytic efficiency. For the first time, Sato prepared NOx-doped TiO2 and evaluated its catalytic activity by the oxidation of carbon monoxide and ethane and by oxygen isotope equilibration under the irradiation of visible light [28]. Later, Asahi et al. synthesized TiO2−xNx thin films, proving that nitrogen doping is a substitutional one that showed higher photocatalytic ability than the undoped TiO2 under the irradiation of visible light, however, it exhibited similar activity under UV light [29]. Subsequently, significant research has been carried out on the development of nitrogen-doped TiO2 using various synthesis methodologies, studying the doping mechanism, characteristics and stability of the catalyst, and also providing evidences for improved visible light photocatalytic performance of N-TiO2. Web of science bibliometrics analysis for the last 20 years (2001–2021) showed the increasing trend of research publication on N-TiO2 (Figure 1). In the years from 2001 to 2010, the research communications on N-TiO2 is 1106 which is significantly enhanced to 4109 in the years from 2011 to 2021. This shows the importance of research on the development of N-TiO2 materials. Despite the various studies available on N-TiO2, the optimized synthesis methodologies, nitrogen doping mechanism, the stability and reusability of the materials are still unclear, and controversies regarding their commercial application also existed. Therefore, this review article describes the synthesis methodologies and mechanism of nitrogen doping, changes in the structural, morphological, electronic characteristics and photocatalytic performance of the N-doped TiO2. This review also provides a comprehensive overview of enhancement in the catalytic reaction parameters (e.g., lifetime of the photoproduced charge carriers and reactive radical species) and the associated visible light photocatalytic application of N-doped TiO2. Furthermore, future suggestions and prospects on the synthesis of highly durable nitrogen-doped TiO2 and possibilities for their commercial usability are also summarized and discussed.
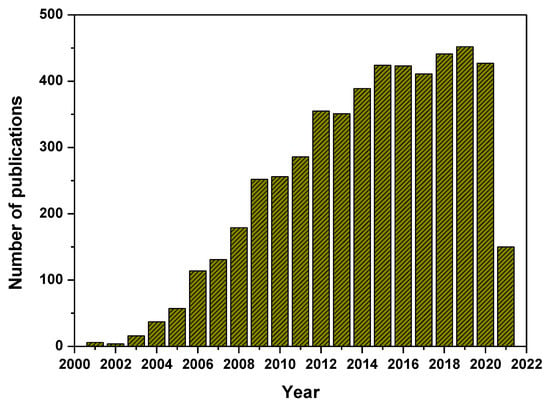
Figure 1.
Number of publications on N-doped TiO2 photocatalysts (source: Web of Science, keywords for searching: N-doped TiO2, nitrogen-doped TiO2, N-TiO2 and TiO2−xNx).
2. Principles of TiO2 Photocatalysis
Photocatalysis is one of the advanced oxidation processes, and involves production of electron-hole pairs upon irradiation of suitable light on the surface of semiconductor nanomaterials (e.g., TiO2) followed by production of reactive radical species occurring for redox reactions with pollutants (Equations (1)–(11), Figure 2a). When the TiO2 material is irradiated with light energy equal or greater than its bandgap energy, it excites the electrons (e−) in the valence band (VB) to conduction band (CB), leaving behind holes (h+) in the valence band (VB) of the semiconductor nanomaterial. Furthermore, these charge carriers react with the adsorbed water molecules or hydroxyl (−OH) groups on the nanomaterial’s surface or dissolved oxygen in the reaction medium. Subsequently, it generates reactive radical species, such as superoxide radical anion (O2•−) and hydroxyl (•OH) radicals, which degrade and mineralize the pollutants adsorbed on the nanomaterial’s surface into water, carbon dioxide and other inorganic anions. In addition, the holes in the VB directly oxidize the pollutants adsorbed on the nanomaterial’s surface. Furthermore, the electrons in the CB indirectly reduce the pollutants using hydroxyl (•OH) radicals formed during cleavage of in situ formed hydrogen peroxide (H2O2), which is formed by reaction between the superoxide radical anion (O2−) and proton (H+) [30]. In the case of N-TiO2, the isolated N 2p narrow band forms above the O 2p in the VB which is responsible for the visible light response of N-TiO2 (Figure 2b). In addition, the recombination of photogenerated charge carriers might occur, which liberates heat that decreases the photocatalytic efficiency of TiO2. Therefore, the photogenerated charge carrier’s generation, separation and transfer efficiency are the vital parameters for efficient photocatalytic activity.
TiO2 + hʋ (λ ≥ band gap) → TiO2 (h + vB) + TiO2 (e− cB)
(h + vB) + H2O → TiO2 + H+ + •OH
TiO2 (h + vB) + −OH → TiO2 + •OH
TiO2 (e−cB) + O2 → TiO2 + O2−
2O2− + 2H+ → H2O2
O2− + H+ → HO2•
HO2• + HO2• → H2O2
H2O2 + hʋ → 2 •OH
HO2• + H+ + TiO2 (e−CB) → TiO2 + OH− + •OH
TiO2 (h + vB) + pollutants → oxidation products
OH + O2− + water or air pollutants → intermediate products → CO2 + H2O + inorganic ions
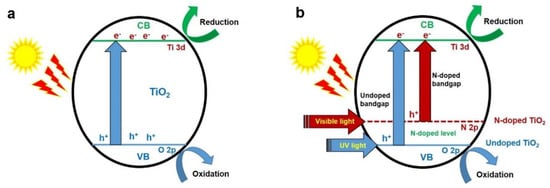
Figure 2.
Schematic representation of the principle of photocatalysis on (a) TiO2 and (b) N-TiO2 materials.
Similarly, the photo-produced electron-hole pairs on the semiconductor’s (e.g., TiO2) surface have great potency in the production of H2 by water splitting and reduction of CO2 into fuels and various value-added chemicals. Further, the VB and CB band edge position of semiconductor nanomaterials are essential which gives information on oxidative and reductive power of photo-produced holes and elections. The CB and VB edge potential/band position must be sufficient/higher than the redox potential required for generation of higher concentration of •OH (−OH/•OH = +2.4 V/NHE) and O2− (O2/O2− = −0.33 V/NHE) radicals which could be effectively used for hydrogen production and CO2 reduction reactions (Figure 3). Nevertheless, the conduction (−0.29 eV) and valence band (+2.92 eV) edge position of TiO2 are not convincing enough to produce required concentrations of charge carriers [31]. Furthermore, the wide bandgap energy of TiO2 (~3.2 eV) limit its light absorption to the ultraviolet light region only (~5% in solar light) and inefficient in absorption of freely available solar spectrum (~45% visible light and ~50% near infrared light). Furthermore, the poor adsorption capacity (low surface area), higher recombination rate of photo-produced charge carriers on the TiO2’s surface, the morphology and the phase composition of TiO2 (anatase, rutile and brookite) have significant influence on the catalytic efficiency of TiO2. To overcome these, significant research efforts have been put forwarded for modification of TiO2 properties by various processes which are described in the next section.
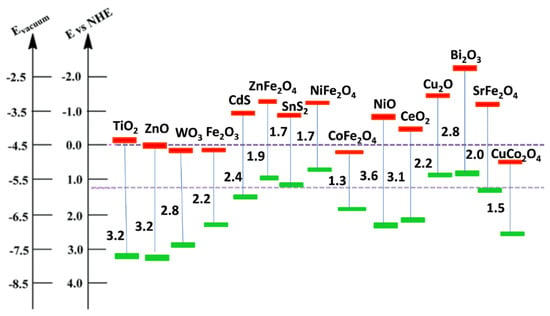
Figure 3.
Band position of different semiconductors. Reproduced with permission from [31].
3. Modification of TiO2 Materials and the Nitrogen Doping
Utilization of TiO2 materials for complete harvesting of renewable solar (~5% UV light, ~45% visible and ~50% near infrared light) and indoor light could be performed by tuning its bandgap. In addition, the photocatalytic efficiency of TiO2 could be improved by decreasing the possibility of recombination of photoproduced charge carriers, improving the surface to volume ratio, changing the ratio of anatase to rutile phase, particle size, morphology, creation and modification in the oxygen vacancy, surface modifications and trapping charge carriers and reactive radical species and other physicochemical characteristics. Thus, the tuning of bandgap and tailoring the properties of TiO2 is performed by various strategies like doping with metal ions/non-metal ions, incorporation with other semiconductors and high surface area adsorbents, co-doping and surface sensitization with dyes or metal complexes or acid.
Among the modifications, non-metal ion doping into TiO2, especially nitrogen-doped TiO2 (N-TiO2), has received significant attention because of its capability to change the surface-electronic properties that favors efficient photocatalysis. The comparable atomic size of nitrogen (155 pm) with oxygen (152 pm) can substitute the oxygen atoms from the TiO2 lattice. Similarly, the ionization energy of nitrogen (first ionization energy 1402.3 kJ/mol) is comparable to the oxygen ionization energy (first ionization energy 1313.9 kJ/mol) that modifies the surface and electronic properties of TiO2 and results in the enhanced photocatalytic efficiencies. Therefore, significant research has been carried out on the modifications of TiO2 and various research and review articles were published on N-doped TiO2 as well [9,13,32,33,34,35,36]. In order to obtain desired physicochemical and electronic properties various methodologies and nitrogen sources (Figure 4) have been applied for the synthesis of N-doped TiO2, which are described in the forthcoming section.
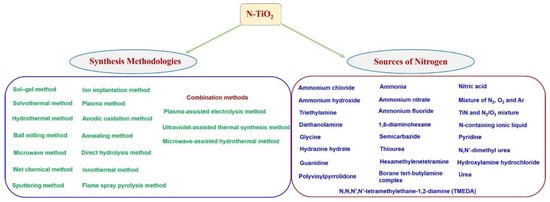
Figure 4.
Methodologies and the nitrogen sources used for N-TiO2 synthesis.
4. Synthesis Methodologies of N-Doped TiO2
4.1. Sol–Gel Method
The sol–gel method is often regarded as a facile technique for the preparation of doped TiO2 catalysts because of its process simplicity. Various types of sol–gel exist depending on the method of application. In the sol–gel method, during hydrolysis, the hydroxyl bridge is formed between the nitrogen and metal center resulting in the formation of biphasic gel structure that increases with the increase in the share of nitrogen. The main advantage of this method is the low temperature of the process, which results in the reduction of production costs as well as it does not demand any special equipment for production. Typically used precursor solution as a source of titanium includes titanium sulphate or titanium trichloride. However, other commonly used precursors for the synthesis of N-doped TiO2 materials include titanium hydroxide, titanium tetrachloride and titanium isopropoxide. The sources of nitrogen include urea, hydrazine, guanidine hydrochloride, triethylamine and ammonium chloride. Avisar et al. prepared N-doped TiO2 thin films by sol–gel technique using isopropanol, ammonium hydroxide, triethanolamine and tetrabutylorthotitanate precursor solutions. The sol was prepared by means of thin dip coating on the glass surface followed by air drying and calcination resulted in a crystalline thin film with anatase phase [37]. Caratto et al. synthesized N-doped TiO2 nanocomposites by sol–gel method using isopropanol, titanium isopropoxide (TTIP) and ammonia solution and the presence of N3− ions were confirmed through various physicochemical characterization techniques. Further, the resultant N-doped TiO2 is found to show lower surface area, however, the nanocomposite is found to be more stable in the visible range [38].
4.2. Solvothermal or Hydrothermal Method
Solvothermal or hydrothermal methods are carried out under specific pressure and temperature in a hydrothermal reactor (commonly autoclave). In general, both of these methods were used to synthesize mesoporous N-doped TiO2 nanocomposites. Initially, titanium and nitrogen precursors will be dissolved in either organic or aqueous medium and the resultant mixture is kept in the hydrothermal condition for several hours, thereby ending up in the formation of varied N-doped TiO2 nanocomposites. Pan et al. prepared the N-TiO2 hollow microspheres by the dissolution of TTIP into a propanol solution and the obtained clear solution was transformed into the Teflon-lined stainless-steel autoclave set at a temperature of 200 °C with a process time of 1 day. The resultant N-doped TiO2 hollow microspheres are found to contain numerous nanothorns and small fragments of spherical shells that could be attributed to the structural collapse that might have occurred during the calcination process or could be due to the disassembly of the solvothermal reactions. The synthesized microsphere is found to have large surface area with outstanding light harvesting properties. Further, it exhibits high photocatalytic activity due to the presence of large accessible surface area that ultimately enhances the decomposition of organic molecules [39]. Similarly, Chainarong et al. synthesized N-TiO2 powders by hydrothermal method using hydrogen titanate. The mixture of TiO2 powder and sodium hydroxide solution were poured into the Teflon-lined stainless-steel autoclave and maintained at 130 °C for 1 day. The nitrogen was doped into resultant synthesized TiO2 by impregnation method followed by heat treatment (400 °C for 2 h). The morphology of the synthesized N-TiO2 is cubic nanoparticles with length in the range of 50–500 nm. The vibration at 1430 cm−1 of the FT-IR spectra of the synthesized N-TiO2 powders shows the presence of an O-N band that confirms that the N atom is embedded in the TiO2 network. The surface area of the synthesized nitrogen-doped TiO2 is found to be less than P25-Degussa and hydrothermal method synthesized TiO2 (HM-TiO2), however the photocatalytic activity is found to be higher under visible light than the latter two [40].
4.3. Ball Milling Method
The ball milling technique, often regarded as a mechano-chemical synthesis method, is a process where the kinetic energy of the moving balls is used to mill the material, which breaks the existing chemical bonds by fracturing the material therein resulting in the formation of fresh surface. The resultant newly formed surface containing dangling bonds is reported to be chemically reactive. Sometimes, this process could also create a high pressure/temperature exceeding several GPa/1000 °C depending upon the type of material. For instance, Yin et al. synthesized N-doped TiO2 powder using hexamethylenetetramine and urea by mechanochemical method (using a planetary ball mill). As a result, it was observed that the use of mechanical treatment with high energy enhances phase conversion of anatase to rutile phase. Further, high-temperature calcination was needed to eliminate the organics from the obtained product [41,42]. Techitdheera et al. developed N-doped TiO2 powders by ball milling method with variation in operating time and annealing temperatures in N2 atmosphere. The results of SEM analysis of N-doped TiO2 powder show high surface area with the increase in the milling time. After milling treatment and annealing with nitrogen, the crystalline structure of TiO2 was identical to the structure of the TiO2 precursor, which was evident from the XRD analysis. Further, the impurity phase in the TiO2 precursor is found to reduce while adopting the ball milling technique with NH3 (solution) and annealing with N2 [43].
4.4. Microwave Method
The microwave method involves the use of microwave irradiation, an alternative energy source for catalyst preparation, under varying frequency range between 0.3 and 300 GHz (with wavelength range of 1 mm–1 m) [44]. Microwave irradiation upon a material causes disruption by thermal/athermal means. In the former, microwaves heat the material by means of molecular interaction, whereas in the latter, microwaves interact with the polar molecules therein causing disruption by various physical, chemical and biological reactions [45]. Kadam et al. carried out synthesis of N-doped TiO2 nanostructure by microwave assisted method under a microwave irradiation exposure time of 20 min (900 W, 250 MHz). The synthesized N-doped TiO2 nanoparticle is found to have a surface area of 140 m2/g with high thermal stability up to 800 °C with retention of anatase phase confirmed by XRD and TEM analyses [46]. Azami et al. prepared N-doped TiO2 powder with urea as N source by using microwave irradiation technique under a microwave power of 800 W with an exposure time of 30 min. The bandgap energy of the obtained N-doped TiO2 was found to be 2.9 eV by UV-Vis/DRS spectrum. The results of the study reveal that N-TiO2 can be formed at 230 °C with the use of microwave irradiation heating that cannot be obtained under normal conducting heating [47]. Thus, this method has desirable effect on producing dielectric heating that produces heat by means of dipole molecule rotation within the dielectric. Hence, uniform heating of the material is obtained in this technique. However, the use of this technique requires a special reactor that is needed for a microwave generator, hence it entails high instrument cost. The advantage is still the lower reaction time in comparison with the hydrothermal methods.
4.5. Wet Chemical Method
The wet chemical method involves the use of nitrogen atoms containing precursors to dope the N into TiO2 for the preparation of highly active N-doped TiO2 powders with visible responsible capability. Existing studies have experimented with a wide range of reagents, such as ammonia, urea, ammonium hydroxide, triethylamine, etc. of varying strength under different operating conditions. Lin et al. (2015) carried out the synthesis of N-TiO2 by hydrolysis of TTIP using an ammonium hydroxide solution (precursor) at different concentrations with composite calcination temperatures (ranging between 300 and 600 °C) and carried out the photocatalytic activity experimentations under visible light. The results of XPS reveal that the N doped into the TiO2 lattice and N doping is found to retard the conversion from anatase to rutile phase. Further, the maximum photocatalytic activity was observed with an ammonium hydroxide concentration of 150 mL and at a calcination temperature of 500 °C. The enhanced photoactivity relies primarily on the synthesis condition. Furthermore, the surface area of the resultant N-TiO2 during photocatalyst preparation depends upon the nitrogen precursor used [48]. For instance, Makropoulou et al. synthesized N-doped TiO2 by using TTIP with varying nitrogen precursors, such as ammonia, triethylamine and urea. The synthesized N-TiO2 powder is found to have a specific surface area of 29 m2/g while using NH3 as precursor whereas the surface area is as low as 12 m2/g while using triethylamine as precursor. However, the surface area of the N-TiO2 powder synthesized using urea precursor is found to be the same as that of the self-prepared TiO2 alone (79 m2/g). However, the resultant N-doped TiO2 catalysts are reported to be effective in inactivating the bacterial contaminants from the water [49].
4.6. Sputtering Method
The sputtering process is a dry process mainly adopted to prepare thin films of N-TiO2 by means of depositing the thin film of sputtered atoms (nitrogen) onto the TiO2 surface [50]. The type of sputtering process can be categorized according to the source of sputtering, such as ion beam, electron cyclotron resonance, direct current, etc. For the synthesis of N-TiO2 thin films, the commonly used sputtering method includes direct current sputtering (if target is electrically conductive) and radio frequency sputtering (employed irrespective of whether the target material is electrically conductive or not) [51,52]. The sputtered atoms resulting from the sputtering process are usually ejected into the gas phase that gets deposited onto the surface within the vacuum chamber. In general, the sputtered atoms explode collision cascades in the target where an atom will be ejected if the leftover energy of the sputtered atom is greater than the binding energy of the target surface and thus sputtering happens. Dobromir et al. developed N-doped TiO2 thin film by radio frequency (RF) sputtering method. In the study, pure Ti (99.7%) of 3.0″ diameter was used as a sputtering target under a highly pure gaseous mixture (Ar + N2 + O2). The prepared thin films were then subjected to an annealing treatment by successive oven heating at 400–600 °C. After this thermal treatment, the rutile crystalline phase formation was observed with the decrease in the nitrogen dopant concentration that is bounded to titanium by the substitution of oxygen that was removed by re-oxidation of the surface. Further, the results of VB XPS analysis suggest the reduction in the bandgap of N-TiO2 with the increase of the concentration of the dopant [53]. Chan et al. prepared the N-doped TiO2 films by reactive sputtering method on both glass and silicon substrates with air as the reactive gas to conduct the procedure at high base pressures (of about 1.3 × 10−2 Pa) in order to reduce the process time. The phase transformation of the prepared films from mixed to anatase was observed with the increase in the air/Ar flow ratio. The Tauc plots show the optimal bandgap energy of the prepared TiO2−xNx films from 3.05 to 3.11 eV and falls under the visible light regime. It was observed that there is an almost linear drop in the bandgap with the rise in the N concentration in the films that is useful to improve the photocatalytic property under visible light [54].
4.7. Plasma Method
The plasma method involves the use of plasma produced by applying energy to a gas to alter the electronic structure of the atoms and to produce excited ions and species. The atmospheric plasma sources include direct current and low frequency discharge, microwave discharges, and plasma undergo ignition by means of radio frequency waves. Plasma treatments can be applied for the preparation of N-TiO2 photocatalyst. In this method, the Ti-precursor solution is initially vaporized and transported by inert gas into the plasma reactor to react with excited nitrogen and thus being implanted to the vaporized TiO2 particles [55]. In another way, in the plasma method, the pristine TiO2 particles can be treated with the N2/H2 mixture or nitrogen gas to produce a yellow powder [56,57]. Yamada et al. prepared N-TiO2 (surface doped) by plasma surface modification technique with argon/nitrogen plasmas, in series. The TiO2 film containing Ti-N bonds produced by the substitutional N-doping demonstrates visible light activity, which is revealed by the decomposition of methylene blue while evaluating the photocatalytic activity [58]. Chen et al. synthesized N-TiO2 photocatalyst by applying the atmospheric pressure plasma enhanced nanoparticle synthesis (APPENS) method. The advantage of this APPENS over other plasma techniques is operation under normal pressure and temperature and also possessing a higher rate of film deposition at comparatively low power consumption. Further, the resultant N-doped TiO2 photocatalyst was more effective in toluene and isopropanol removal than the commercial P25 and ST01 (TiO2 powder, Ishihara Sangyo Kaisha, Osaka, Japan) photocatalyst, and undoped photocatalysts under visible light. Thus, the APPENS process could potentially be used to control indoor and outdoor air pollutants [59].
4.8. Ion Implantation Method
The ion implantation method employs the implantation of N impurity into lattice oxygen sites of the TiO2 crystal lattice, which modifies the electronic arrangement by the presence of localized states at the top of the valence band. This change would reduce the bandgap therefore enhancing the photocatalytic activity under visible light. Panepinto et al. prepared N-TiO2 by N ion implantation by a dose less than 1016 ions/cm2 in order to control the modification of the morphological and crystalline properties of the irradiated anatase thin films [60]. Borras et al. (2007) carried out N ion implantation by bombarding the surface of anatase TiO2 thin film (at ion energy of 50 keV for varying doses of 1.2 × 1017, 6 × 1016 and 3 × 1016 ions/cm2) by using metal organic chemical vapor deposition (MOCVD) method. The MOCVD samples, under visible light, revealed a sharp drop in wetting contact angle from 80° to 55°. Both the films (MOCVD and non-MOCVD) attain total hydrophilicity under posterior UV irradiation [61]. Ghicov et al. (2006) synthesized N-TiO2 nanotube film by N ion implantation technique by bombardment at 60 keV with a nominal dose of 1 × 1016 ions/m2. The resultant N-doped TiO2 nanotube film of crystalline anatase structure is found to have improved photocurrent response in both visible and UV range. Apart from the enhanced properties, the bombardment at this condition led to the phase conversion from anatase to an amorphous structure and hence demands subsequent thermal annealing process. This recuperates the anatase phase as well as enhances the photocurrent in both ultraviolet and visible range [62].
4.9. Direct Hydrolysis of Organic/Inorganic Salts
Direct hydrolysis involves the preparation of N-doped TiO2 using an organic/inorganic salt, and usually the air/moisture sensitive alkoxide precursors under visible light. Khan et al. (2021) synthesized N-TiO2 powder by a co-precipitation method using tri-thiocyanuric acid with P25 and then subsequently calcinated for 4 h at 550 °C in a nitrogen atmosphere. It was found that the N-doping increases the absorption of visible light and also enhances the transfer/separation of photo-excited charge carriers [63]. Similarly, Ji et al. (2019) prepared N-TiO2 composite fibers (PAN-COOH fibers) by surface hydrolysis using PAN fibers in a sulfuric acid solution. The resultant composite fiber is found to exhibit enriched photocatalytic capacity under UV-Vis light. However, the resultant composite fibers were found to contain mostly amorphous carbon, however there is no evidence of crystalline phase except anatase phase. Further the resultant N-TiO2 composite fibers were found to have a surface area of 116.41 m2/g, still slightly lower than that of the ordered mesoporous black TiO2 with the surface area of 124 m2/g [64].
In most of the direct hydrolysis methods, N-TiO2 is either synthesized by heat treatment of pure TiO2 in an NH3 atmosphere or air/moisture-sensitive titanium precursors are used, which are costly and hard to handle. However, Japa et al. (2020) carried out preparation of N-doped TiO2 by a thermal hydrolysis technique from TiOSO4 using ammonium hydroxide (NH4OH) as a source of nitrogen and a precipitating agent. In the study, authors used a cost effective, stable under air/moisture and easy to handle inorganic titanium salt (TiOSO4) instead of these sensitive alkoxide precursors and hence merit real scale applications. The resultant N-doped TiO2 is found to show its potency toward application in photocatalytic synthesis of fine organic chemicals in visible light. Further, the higher activity of N-TiO2 in comparison with bare TiO2 could be attributed to the increase in the absorption in the visible light, enhanced photoinduced charge transfer and separation [65].
4.10. Anodic Oxidation Method
The preparation of N-doped TiO2 by anodic oxidation method involves two steps. Initially, the formation of oxide occurs at the metal interface by oxygen anions. Subsequently, the dissolution reaction is being initiated by the F− ions in the electrolyte. During oxide formation, the anions develop on the interface of the electrolyte through water electrolysis and these anions come into contact with the surface of the metal by diffusion. Concurrently, Ti4+ ions passage in the opposite direction through the oxide layer therefore reacting with oxygen anions, the TiO2 formation ultimately occurs (Equation (12)). The reaction of F− ions with Ti4+ at the metal/oxide surface are shown in Equations (13) and (14).
Ti + 2H2O → TiO2 + 4H+ + 4 e−
TiO2 + 4H+ + 6 F− → (TiF6)2− + H2O
Ti4+ + 6 F− → (TiF6)2−
Le et al. (2018) carried out N-doping of TiO2 nanotube arrays (TNA) by a two-step anodization method with NH4F and glycerol-water electrolyte. The N-doping concentration varied between 0 and 9.47% by controlling the flow rate of nitrogen gas between 0 and 500 cc/min during thermal annealing at 450 °C for 3 h. The enhanced catalytic activity was detected for N-TNAs while compared to TNAs. While experimenting methylene blue degradation, it was observed that the reaction rate constant reached 0.26/h for N-TNAs, which is about 125% higher than that obtained with TNAs that showed about 0.115/h [66]. Furthermore, recent studies show that the two-step anodization method could be able to prepare highly ordered TNAs with diverse top layer morphology with increased photocatalytic and photoelectrochemical activity [67,68,69]. Mazierski et al. (2016) synthesized N-doped TiO2 nanotube arrays by anodizing titanium foils in an organic electrolyte. The results conclude that the nanotubes synthesized with varying nitrogen concentrations of approximately the same length show differences in the crystallite size, bandgap value and Ti3+ state that imparts deviations in photoactivity [70].
4.11. Annealing Method
Annealing involves the thermal oxidation process at varying temperatures under an air/Ar/N2 atmosphere to synthesize N-doped TiO2 nanomaterials. Lai et al. (2010) developed N-TiO2 nanotubes by immersion in 1 M NH3.H2O solution and subsequently annealed to attain the crystalline phase of N-doped TNA electrode. The resultant N-TiO2 nanotubes are found to have large absorption areas with suitable potential for degradation of environmental pollutants. Further, the as-developed TiO2 thin film shows an amorphous structure and hence annealing is necessary for phase transformation of the amorphous TiO2 thin film into a crystallized anatase phase [71]. Furthermore, Wan et al. (2007) fabricated rutile phase N-TiO2 thin films by thermal oxidation followed by annealing TiN at a temperature above 700 °C under air atmosphere [72]. Liu et al. (2009) synthesized visible light active N-anatase TiO2 sheets with dominant (001) facets derived from annealing of as-prepared TiO2 nanosheet under NH3 atmosphere at 400 °C. The activity observed during rhodamine B degradation with the synthesized N-TiO2 nanosheet is higher than the commercial P25 TiO2 particles [73].
4.12. Ionothermal Method
In 2004, Cooper et al. used the ionothermal method for the first time to synthesize crystalline zeolites using ionic liquid (1-Methyl 3-ethyl imidazolium bromide) and eutectic mixture (choline chloride/urea) as solvents and templates. The ionothermal method depends on the solvents which are predominantly ionic, because sufficient quantities of molecular water disrupt the reaction, preventing the formation of zeolites. They then termed this procedure as ionothermal synthesis to distinguish it from hydrothermal preparations, which take place in a predominantly molecular solvent [74]. Pipi et al. ionothermally prepared N-TiO2 using titanium butoxide as a titanium source and choline chloride and urea (molar ratio 1:2) eutectic mixture as a solvent and source of nitrogen. Different compositions of N-TiO2 were prepared by varying the percentages of the eutectic mixture, titanium butoxide, water, temperature and reaction time, and in both reflux and autoclave methods. The photocatalytic efficiency of the N-TiO2 nanoparticles was determined by the oxidation of N, N-dimethyl-4-nitrosoaniline (RNO) dye in the presence of UV and visible light irradiation. Photocatalyst synthetized by the reflux ionothermal method revealed higher photocatalytic activity because of the presence of mixed phase (anatase/brookite) in N-TiO2 [75].
4.13. Flame Spray Pyrolysis (FSP) Method
Flame spray pyrolysis has been used mostly for the large-scale manufacture of ceramic commodities such as titania, fumed silica, alumina and zinc oxide, respectively. FSP describes the formation of fine particles from gases in flames. Whereas, in the conventional spray pyrolysis, the aerosol droplets formation takes place by atomization of the solution in a hot wall reactor, undergoes evaporation and solute concentration within the droplet, drying, thermolysis of the precipitate particle at higher temperature to form a microporous particle, and finally a dense particle by sintering process. The advantages of FSP are the ability to dissolve the precursor directly in the fuel, simplicity in introduction of the precursor into the hot reaction zone (e.g., a flame) and flexibility in using the high-velocity spray jet for rapid quenching of aerosol formation. The schematic representation of particle formation in FSP is shown in Figure 5. Initially, the precursor material is injected into the burner as a gas, droplets or solid particles. The solid or liquid precursors are exposed to high flame temperature and rapidly evaporate into vapors that react and form intermediates, product molecules and clusters that quickly grow to nanosize particles by either coagulation, surface reactions, or both. Once the aerosol stream leaves the high-temperature zone and the temperature slowly cools down to low temperature for particle collection, particle growth takes place continuously by coagulation, though complete particle coalescence no longer occurs resulting in aggregates of primary particles [76,77].
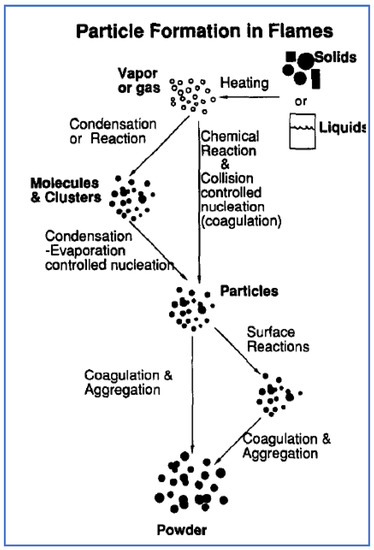
Figure 5.
Schematic representation of particle formation by FSP process. Reprinted with permission from [76].
Boningari et al. developed a visible light active N-TiO2 by single-step flame spray pyrolysis (FSP) method. XPS analysis revealed that the FSP method directs the nitrogen doping predominantly in the form of interstitial nitrogen (Ti–O−N) rather than substitutional nitrogen (Ti–N). Further XRD analysis demonstrated that N-TiO2 showed the distortion and strain in the crystal structure instigated by the incorporation of the nitrogen atoms. In addition, the growth or expansion of crystal lattice is due to that the atomic radius of nitrogen atoms (r = 1.71 Å) being larger than oxygen (r = 1.40 Å). The nitrogen atoms doping into the crystal structure of TiO2 modifies the electronic band structure of TiO2, leading to the formation of new mid-gap N 2p energy band levels above the O 2p valance band which suppress the recombination of photogenerated charge carriers. The increased separation efficiency of charge carriers on the N-TiO2 showed higher visible photocatalytic activity than the pure TiO2 in degradation of phenol as a model pollutant [78].
4.14. Combination Methods
4.14.1. Plasma-Assisted Electrolysis
The aforementioned methods are generally used for the synthesis of TiO2 and N-TiO2 with required crystal structure/phase, morphology, surface properties and particle size. However, the reaction operational parameters such as the amount of precursors, pH, nitrogen sources, reaction time and temperature must be controlled to obtain the desired properties. Furthermore, it requires high temperature and longer duration for reaction and drying the developed TiO2 and N-TiO2 nanoparticles, which limits the mass production of the materials and affects the practical applicability. To overcome these, Kim et al. (2018) developed a simple plasma enhanced electrolysis where the N-TiO2 directly synthesized using small amount of electrolyte (nitric acid) and titanium (Ti) metal as precursors (Figure 6). The developed N-TiO2 materials were calcined at different temperatures for transforming the amorphous TiO2 into the crystalline TiO2 anatase phase and the N-doping is an interstitial N doping state in TiO2 lattices. N-TiO2 nanoparticles showed superior photocatalytic ability than TiO2 nanoparticle in MO dye degradation under the irradiation of visible light [79].
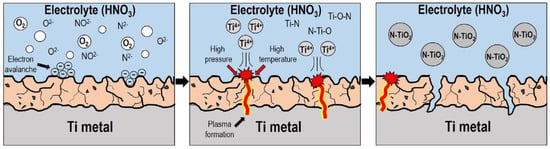
Figure 6.
Schematic diagram of mechanism of N-TiO2 formation via plasma-assisted electrolysis. Reproduced with permission from [79].
4.14.2. Ultraviolet-Assisted Thermal Synthesis
In this method, the ultraviolet (UV) light is used in the thermal method to assist the preparation of the photocatalytic nanomaterials in a shorter reaction time at ambient temperature and pressure. UV light is capable of initiating reactions similar to those that occur at high temperature, and the UV light energy can overwhelm the obstacle of the activation energy to disconnect the bonds and form new products. It is well known that, as per the Arrhenius law, increase in the temperature of the reactants increases the constant rate of the reaction. Further, it is well understood from the literature that the electron irradiation increases the speed of the synthetic process at low temperatures. Moreover, the UV photons change the molecule’s structure by splitting of bonds and exciting the molecules into a higher level of energy, or ionization of molecules [80].
4.14.3. Microwave-Assisted Hydrothermal Method
As describe above, the hydrothermal method is a material synthesis method at specific temperature pressure and time, and an effective method for some materials that are insoluble at normal temperature and pressure. Furthermore, it is efficient in controlling the crystal phase, morphology and particle size of the products; therefore, it is a simple, cost-effective and environmentally friendly method. However, the hydrothermal method consumes significant amounts of time and energy. Therefore, microwave assistance, such as microwave-assisted hydrothermal method, has drawn notable attention because of its advantages, such as low reaction time, lower energy usage and materials with unique physicochemical and electronic properties. Yin et al. (2008) developed N-doped TiO2 (TiO2−xNy) nanoparticles with monoclinic single phase using the microwave-assisted hydrothermal technique with hexamethylenetetramine and TiCl3 as a nitrogen and titanium sources along with hydrothermal reaction temperature of 160–230 °C for 5–60 min using a microwave system (1000 W). TiO2−xNy showed higher photocatalytic efficiency in annihilation of nitrogen monoxide in the presence of both visible-light (λ > 510 nm) and UV light (λ > 290 nm) due to the high specific surface area and fine particle size [81]. Similarly, Peng et al. (2010) prepared N-doped titanate nanotubes using the microwave-hydrothermal method at 450 W and 130 °C for 2 h followed by sintering in 20% O2/80% N2 atmosphere at various temperatures (250 °C, 350 °C and 450 °C for 2 h) for conversion of nanotube layers into anatase. Absorption spectra revealed that N-doped titanate nanotubes exhibit an increased absorption in the visible light regions than the bare titanate nanotube observed from the appearance of light-yellow color of the materials. This validates the N-doping results in the creation of new energy levels above the titanate nanotube VB and decreases the bandgap of the materials. N-doped titantate nanotubes showed improved photocatalytic ability over the titanate nanotube in degradation of methyl orange dye under 15 W commercial fluorescent lamps [82].
5. Possible Mechanism for N Doping in TiO2
Generally, the doping of nitrogen onto TiO2 in two ways, namely, substitutional or interstitial, that creates electronic mid-gaps between the band structure of TiO2 that reduces the bandgap energy of TiO2 and enhances the visible light photocatalytic capacity. Substitutional doping modifies the surface characteristics of TiO2 whereas interstitial doping modifies the lattice structure of TiO2. The pictorial presentation of the N doping (both substitutional and interstitial) is shown in Figure 7.
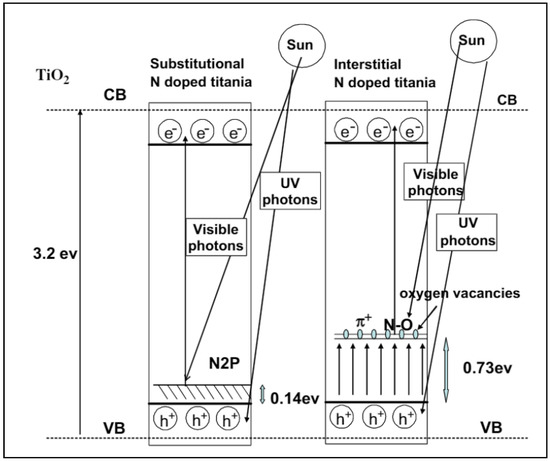
Figure 7.
Electronic band structure of N-doped TiO2 (both substitutional and interstitial doping). Reprinted with permission from [83].
However, the state of the N-doping into TiO2 lattice and the corresponding bandgap reduction mechanism is still debatable, so, hereafter, we will describe some of the literature evidence for the possible mechanism of N doping into TiO2. Asahi et al. demonstrated the state of N in TiO2−xNx by XPS analysis, revealing that three peaks at binding energy values of 396, 400 and 402 eV, attributed to the atomic β-N (396 eV) and molecularly chemisorbed γ-N2 (400 and 402 eV) [29]. Irie et al. observed N 1s peak at 396 eV, corresponded to Ti-N bonds, discovered that the oxygen sites in the TiO2 lattice were substituted by the nitrogen atoms and forms an isolated narrow band above the VB and reduced the bandgap energy [84]. Similarly, Chen and Burda (2004) investigated the nitrogen incorporation into TiO2 lattice using XPS analysis, revealing the O-Ti-N bond formation by nitrogen doping that was confirmed by shifting of binding energies (Ti 2p3/2 and O 1s) to low binding energy in the XPS was observed. Ti2p3/2 of TiO2 observed at 459.7 eV and it was lowered to 458.8 eV after N doping, the lower binding energy (0.9 eV lower than the TiO2) validated the N incorporation into the TiO2 lattice. Similarly, in O 1s spectra, an additional peak at 532 eV is observed for TiO2−xNx attributed to the creation of oxidized Ti-N, which leads to the Ti-O-N structure, confirming nitrogen substitution in TiO2. Furthermore, N 1s (400 eV, 405 eV) and O 1s (533.5 eV) peaks for nitrogen oxides (NO and NO2) are not observed on the TiO2 surface, validating the formation of the Ti-O-N structure [85]. Similarly, Sathish et al. synthesized spherical shape N-TiO2 through a chemical method. XRD analysis revealed an increase in the particle size and no variation in the “d” spacing values was perceived after N doping, validating that N is doped into the TiO2 lattice without modifying the average unit cell dimension. In XPS, the Ti 2p3/2 level peak for TiO2 and N-TiO2 materials was observed at 459.3 and 458.5 eV; the low binding energy value after N doping exhibited that the electronic interactions of Ti with N anions and the oxygen site is substituted by the N and modified the TiO2 lattice. Furthermore, for N-TiO2, the N 1s level exhibited a single peak at 398.2 eV corresponding to the N− anion incorporation into the TiO2 lattice as N-Ti-O. Thus, it could be concluded that the status of N is anion-like (N−), as well as the N-Ti-O structure in the TiO2 lattice [86]. Likewise, significant research activities have been performed, however, the disputes involved in the status of N in the N-TiO2 still remaining debatable because of the change in the synthesis methodologies, nitrogen sources and other reaction operational parameters.
6. Property Evaluation of N-Doped TiO2 and Its Photocatalytic Efficiency
The photocatalytic efficiency of the TiO2 depends on various properties, such as crystal structure, defects, phase compositions, morphology, particle size, chemical state, bandgap values, surface area and adsorption capacity, nature of the irradiation source, electron-hole recombination behaviors on the TiO2’s surface and reactive radicals’ generation. It is well known that the higher bandgap values of TiO2 and the physicochemical and electronic properties do not fulfil the requirements for obtaining an efficient photocatalytic activity. In order to obtain the desired properties, significant research activities are undertaken and still in progress utilizing different synthesis methodologies, various nitrogen sources and modifications in the reaction operational parameters. Herein, we tried to describe a few literature evidences for evaluating changes in the properties of TiO2 after N doping and their influence on the photodegradation efficiency. For instance, Irie et al. prepared TiO2−xNx powders with homogeneous anatase (TiO2) phase by annealing the TiO2 under NH3 flow at different temperatures (550, 575 and 600 °C for 3 h). XPS results showed the presence of Ti-N bonds, which come from the substitution of oxygen sites by nitrogen atoms and confirmed that O-Ti-N bonds are formed. Furthermore, the TiO2−xNx (x = 0.0050, 0.011 and 0.019) showed significant optical absorption in the visible light region. The increase in the x value results in the incremental value of visible light absorption (>550 nm), attributed to Ti3+ because at 550 °C the NH3 decomposes into N2 and H2 and the H2 acts as a reducing gas. TiO2−xNx samples completely degraded the isopropanol in either UV or visible light. Furthermore, the quantum efficiency (QY) is decreased when the x value is increased, which affects the photocatalytic degradation efficiency. Therefore, irradiating the TiO2−xNx with UV light generated higher QY values than irradiating it with visible light. This is because the oxygen sites in the TiO2 lattice were substituted by N atoms forming a narrow band above the valence band. Thus, irradiating the TiO2−xNx under UV light excites the electrons from both the VB and the narrow band, but in the presence of visible light electrons are excited from the newly formed narrow band only. Annealing of TiO2 in the presence of NH3 atmosphere partially replaced the oxygen sites with nitrogen atoms and simultaneously reduced the N-TiO2 that causes an increase in oxygen vacancy (Vo) and the amount of Ti3+. Thus, the Vo is generated below the lower end of the conduction band (0.75–1.18 eV) of anatase TiO2 that acts as a recombination center for charge carriers [84]. Lee et al. developed a N-nanoporous TiO2 (N-nTiO2) with the mixture of anatase and brookite crystalline phases and high surface area by plasma treatment method. XRD revealed that the N-nTiO2 bicrystalline structure with different bandgap energy draws attention for interfacial charge transfer (i.e., junction effect). Furthermore, the size of N-nTiO2 (~18 nm) is smaller than the as-synthesized nTiO2 (~28.3 nm), which could be due to the degradation of the polymer (hexadecyltrimethylammonium bromide, HTAB) network during plasma treatment. In addition, the interconnected nTiO2 nanoparticles generate the nanoporous network wall, which leads to the high surface area (392.8 m2/g: nTiO2; 375.9 m2/g: N-nTiO2), pore diameter and pore volume as compared to standard TiO2 and as-grown N-TiO2. The surface energy determination through contact angle measurements revealed that the surface energy of N-nTiO2 (245.61 mJ m−2) was greater than that (78.48 mJ m−2, 313%) of developed nTiO2. Furthermore, the oxygen in the TiO2 lattice is substituted by N through both interstitial and substitutional doping, and the photoproduced charge carrier’s recombination is significantly suppressed by N doping, confirmed through photoluminescence analysis. For N-nTiO2, the N-doping-induced charge-transfer transition from Vo to new energy levels occurred, which was confirmed through the shifting of emission signal to a higher wavelength (~650 nm). These improved characteristics of TiO2 after N-doping showed significant enrichment in the photocatalytic ability in degradation of RhB dye (within 70 min time) and antibacterial performance (sterilization of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), Figure 8) as compared to standard TiO2, as developed nTiO2 and Ar plasma treated nTiO2 [87].
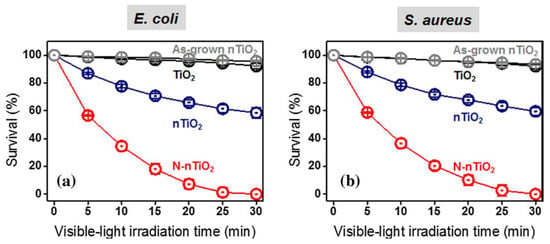
Figure 8.
Antibacterial activities of TiO2, as-grown TiO2, nTiO2 and N-nTiO2 against (a) E. coli and (b) S. aureus under visible-light irradiation. Reprinted with permission from [87].
Cong et al. developed N-TiO2 by a microemulsion−hydrothermal technique using triethylamine, urea, thiourea, and hydrazine hydrate as nitrogen sources. Photocatalytic activity demonstrated that the triethylamine as optimum nitrogen sources for N-TiO2 synthesis and offered higher photocatalytic ability in degradation of RhB dye and 2,4-dichlorophenol than the other nitrogen-sources-based N-TiO2 and undoped TiO2. The N doping does not have influence on the crystal structure (i.e., retaining of anatase phase) and size, however, the particle size is reduced which was confirmed by Raman spectroscopy analysis. Furthermore, there is no change in the d-spacing value after N doping specified that N has been incorporated into the TiO2 lattice without altering the average unit cell dimension. Raman spectra further revealed that the presence of peaks corresponds to vibrational characteristic band of Ti−N that validated that nitrogen substitutes some oxygen atoms in the TiO2 lattice. Further, the decrease in the binding energy of Ti2p3/2 after N doping indicates that different electronic interactions of Ti with anions occurred, leading to the partial electron transformation from the N to the Ti and an increase of the electron density on Ti was obtained because of the lower electronegativity of nitrogen compared to oxygen, which validates that N doped into the lattice and substitutes for oxygen. Thus, the chemical states of the N doped into TiO2 coexist in the form of either N−Ti−O, Ti−O−N, or both. Similarly, the bandgap of TiO2 is decreased from 3.04 to 2.70 eV after N-doping, which enhances the visible light absorption characteristics. The recombination of photoproduced charge carriers is significantly quenched after N doping, attributed to either trapping of electron and hole by the oxygen vacancy and the doped nitrogen or the transfer of excited electron from the valence band to the new levels introduced by nitrogen doping on the upper of the conduction band. Therefore, N doping altered the local structure of TiO2 and improved the visible light absorption and separation and transfer rate of photoproduced charge carriers leading to the higher photocatalytic activity in the degradation of RhB dye and 2,4-dichlorophenol (Figure 9) [88].
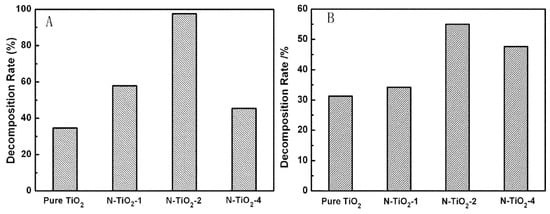
Figure 9.
Decomposition efficiency of TiO2 and N-TiO2 with different N/Ti ratios in (A) RhB dye and (B) 2,4-DCP degradation in the presence of visible light irradiation for 1 h and 5 h time periods. Reprinted with permission from [88].
Hu et al. synthesized N-TiO2 by the sol–gel method using trimethylamine as N precursor and studied the influence of calcination temperature (250, 300 and 350 °C for 3 h) on the degradation of methyl orange dye. The TiO2 structure changes from amorphousness to crystalline and the average nanocrystal size was increased with increasing calcination temperatures. The increase in average nanocrystal size is due to the agglomeration of nanoparticles observed during the calcination process. Furthermore, the pure anatase phase of TiO2 was obtained at 300 and 350 °C calcination and the colors of these samples changes from tan (N-TiO2-250) to light gray-yellow (N-TiO2-350). The N-TiO2 calcined at 250 and 300 °C temperature showed higher visible light absorption due to the presence of surface organic residues whereas visible light absorption was decreased with increase in the calcination temperature. In addition, the N species doped onto the interstitial sites of the TiO2 lattice, and the loss of doped N and amount of organic residues was observed with increasing the calcination temperature and the oxidized N was formed. The N-TiO2 calcined at 300 °C showed low loss of doped N, small particle size, high N-doping level and low concentration of surface organic species that significantly improved the visible light photocatalytic activity in degradation of MO dye [89].
Similarly, Suwannaruang et al. observed that the high photocatalytic activity of N-TiO2 depends upon the selection of nitrogen sources, and prepared a N-TiO2 with nanorice grain morphology and high purity anatase phase by hydrothermal method using urea as a nitrogen precursor. When the nitrogen concentration was up to 7.5%, the nanorice grain morphology and particle size of the N-TiO2 was unaltered and it was decreased to smaller size when the nitrogen concentration increased to 10 and 12.5%. The decrease in size is due to the presence of higher concentration of nitrogen in the TiO2 lattice that increases the nucleation agent in the system and creates strain or stress in the system that suppresses the grain growth process. Similarly, increase in the nitrogen concentration increased the specific surface area and the average pore diameter of the N-TiO2 that enhances the surface reactions. Further, the change in the shape of the pore from cylinder-shaped pore (long nanorice) to bottle shaped or slit-shaped pore (short nanorice), and the pore size distribution from broad to narrow distribution occurred. In addition, anatase to rutile phase transformation is restrained in N-TiO2 that was calcined at 800 °C, whereas complete transformation to rutile phase occurred in bare TiO2 materials. N doping inhibited the phase transformation (anatase to rutile) and increased the crystallite size that could be due to three reasons, namely, the use of urea delays the phase change, the three Ti-O bonds were reduced by the doped nitrogen that destroyed the connection of rutile structure and the substitution of oxygen atom in the TiO2 lattice by doped nitrogen decreases the lattice distortion and accumulates strain energy indicating that the nitrogen atoms can lock the Ti-O species at the interface of the TiO2. Similarly, the bandgap values of N-TiO2 are increased (from 3.11 to 3.17 eV) with increase in the nitrogen concentration and lower than the TiO2 material (3.20 eV). Furthermore, either substitutional (Ti–N–O…Ti (N) linkage), interstitial (Ti–O–N…Ti (N) linkage), or both N dopings occurred in the anatase TiO2 lattice. The aforementioned changes in the properties of TiO2 after N doping are the reasons for higher photocatalytic efficiency of N-TiO2 (12.5%N) in photodegradation of ciprofloxacin under UV light as compared to other doped and commercial standard samples (anatase, rutile and Degussa P-25 TiO2). The changes in the properties of N-TiO2 leads to the formation of higher concentration of hydroxyl radicals, determined using fluorescence spectroscopy during the photodegradation of ciprofloxacin using coumarin molecules as a scavenger [90]. Recently, Mirzaei et al. (2021) developed a multi-homojunction with nitrogen gradient-doped TiO2 by pulsed laser deposition (PLD) for enhanced light absorption in the visible range and improved the charge carrier’s separation and transfer efficiency through the generation of an extended band bending and an oriented electric field. Gradient nitrogen-doped TiO2 (g-N-TiO2) was fabricated by layer-by-layer deposition of N-TiO2 with different concentrations of doped nitrogen on fluorine-doped tin oxide (FTO) and polished Si (100) and the thickness of each layer (~90 nm) was controlled by varying the pulse number during laser deposition. XPS analysis demonstrated that slight shift in the Ti2p peak’s position attributed to the partial replacement of nitrogen with oxygen occurred, which increases the electron density on Ti. Furthermore, it is demonstrated that increasing the N2:O2 ratio during PLD revealed substitutional N doping. The developed g-N-TiO2 is a complete anatase crystal structure, with no additional peaks that could be due to the controlled substitutional N doping without either phase change, the low concentration of N doping, or both, in the structure. Higher visible light absorption (lower energy) was observed in higher concentration of the N doping (50–90%) with substitutional doping and the bandgap of TiO2 was decreased to 2.6 eV. Furthermore, the g-N-TiO2 showed slight lower light absorption than the N-TiO2 sample; however, the g-N-TiO2 exhibited higher photocatalytic activity compared to TiO2 and N-TiO2 samples. This enhancement in the photocatalytic activity is mainly due to the enhanced separation and transfer efficiency of photo-generated charge carriers by the formation of homojunction with terraced band bending. Therefore, it is confirmed that the doping of N introduces an acceptor level above the valence band of TiO2 and lowers the Fermi level (EF) of TiO2 and the band bending occurs when different layers (with different EF) are brought into contact. However, it is limited in non-gradient-doped N-TiO2 and showed low separation efficiency. Furthermore, the formation of terraced valence band levels for different layers in g-N-TiO2 occurred that trigger a built-in electric field perpendicular to the substrate that enables the transfer of charge carriers by repelling them in different directions along the vertical direction of columnar-like structure of the g-N-TiO2. Therefore, the g-N-TiO2 demonstrated a superior photocatalytic performance in sulfamethoxazole removal compared to the pristine and conventional nitrogen-doped TiO2 (non-gradient) [91]. Similarly, significant research has been performed on the synthesis of N-doped TiO2 and the evaluation of structural, textural and electronic properties and their influence on photocatalytic efficiency.
7. Co-Doping into N-Doped TiO2
Co-doping is another approach for enhancing the visible light photocatalytic capacity of N-doped TiO2 by coupling with other metal or non-metal ions and semiconductor nanomaterials for improving visible light absorption and adsorption capacity and suppressing the recombination of photogenerated charge carriers. Generally, the improvement in the visible light absorption and suppression of charge carrier recombination after co-doping can occur via two pathways, namely, generation of new energy levels in between the Ti 3d states of the conduction band and the O 2p states of the valence band or the co-doping compensates the addition charge created by the presence of N3− in TiO2, thereby reducing the charge carrier’s recombination centers and increasing the amount of dopant. Significant studies have been carried out on the co-doping of N-TiO2 and showed efficient photocatalytic activity. However, it is rather difficult to conclude which co-dopants are better at improving the photocatalytic efficiency of N-TiO2 because of the variations in the synthesis and experimental operational parameters, and the properties of N-TiO2. Therefore, this section describes the various metals, non-metals and nanomaterial-based co-dopants for improving the photocatalytic activity of N-TiO2.
Rani et al. prepared Ni-N-TiO2 nanoparticles using the sol-gel method. They observed that the introduction of dopants (nickel and nitrogen) generates impurity levels of Ni 3d below the Ti 3d conduction band and N 2p above the O 2p valence band that causes significant effort in decreasing the bandgap of TiO2, which leads to the transfer of electrons from VB to CB taking place (Figure 10). In addition, the average crystalline size of the materials is decreased which enhances the surface area of the doped materials. The enhancement in the surface area and decrease in the bandgap energy showed significant visible light catalytic activity in congo red (CR) and methyl orange (MO) dye degradation [92].
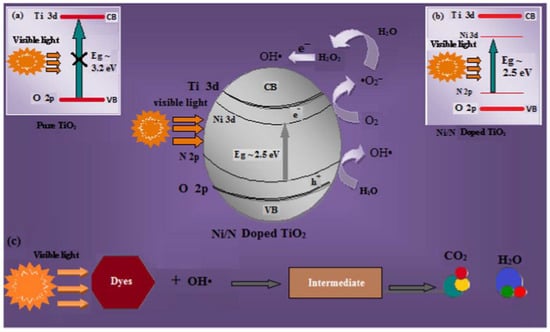
Figure 10.
Schematic representation of photogenerated electron-hole pairs separation and transport process on (a) pure TiO2 and (b) Ni-N-co-doped TiO2 nanoparticles under visible light irradiation for (c) degradation of dyes. Reprinted with permission from [92].
Alotaibi et al. developed Zn and N co-doped TiO2 thin films on glass substrates using aerosol-assisted chemical vapor deposition and evaluated their influence on superoxide formation, catalytic activity and bactericidal properties. Superoxide formation from metal oxides was determined using 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium sodium salt (XTT) and an increased concentration of superoxide formation from the Zn, N: TiO2 thin films. Similarly, the transient absorption spectroscopy study revealed that photogenerated charge carrier populations were increased when films were co-doped with Zn and N and showed efficient photocatalytic activity and bactericidal activity in degradation of stearic acid and E. Coli under UVA and fluorescent light (Figure 11, asterisk symbol) [93].
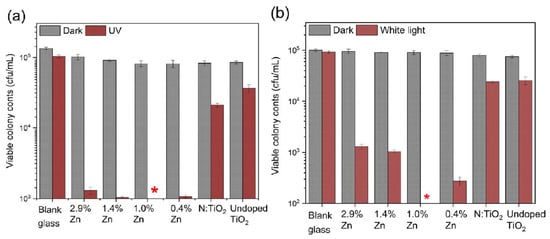
Figure 11.
Bactericidal activity of the undoped TiO2, N:TiO2 and the series Zn, N:TiO2 catalyst in the presence of (a) UVA (8 h) and (b) fluorescent light (18 h) irradiation. Reprinted with permission from [93]. Asterisk showed the highest bactericidal activity of Zn, N: TiO2 thin films (1%) under both UVA and fluorescent light irradiation.
Selcuk et al. synthesized N-TiO2 by hydrolysis method and the metals, such as Fe, Cr, Ni and Pt, were deposited onto the N-TiO2 by reduction of the respective metal salts. The hydrogen production reaction revealed that both Pt-N-TiO2 (11,620 μmol) and Ni-N-TiO2 (2946 μmol) showed higher water splitting efficiency than the other metal-doped N-TiO2, N-TiO2 and TiO2 materials in aqueous methanol solution. Subsequently, Ni–N–TiO2 was selected as a suitable alternative to Pt-N-TiO2 and a detailed study was carried out on the Ni co-doped N-TiO2 with different nickel concentrations. Ni–N–TiO2 with 10 μmol Ni/g N–TiO2 exhibited higher water splitting efficiency (490 micromoles H2 g−1cat h−1) than the other Ni concentration doped N-TiO2 [94]. Similarly, Lin and Shih (2015) developed metal ions (M = Cr, Ni, Cu, Nb) and nitrogen co-doped TiO2 photocatalysts through one-pot microwave-assisted hydrothermal method. They observed that the doping of metal ions introduced donor levels in the bandgap of TiO2−xN, which mainly occurs through the hybridization between 3d orbital of metal dopants, 2p orbital of nitrogen and associated charge compensation vacancies in M-doped TiO2−xNy. Among the co-dopants, Cu/TiO2−xN exhibited higher photocatalytic activity in hydrogen production under UV (27.4 mmol g−1 h−1) and visible light (283 μmol g−1 h−1) irradiation from aqueous methanol solution. The higher photocatalytic activity is due to the enhanced visible light absorption (introduction of new donor levels), uniform dispersion of copper ions inside the mesoporous TiO2−xN network and the photogenerated charge carrier’s separation and transfer efficiency [95]. Abdelraheem et al. hydrothermally synthesized anatase nitrogen and boron co-doped TiO2 (N-B-TiO2) using borane tert-butylamine complex as a source for N and B for degradation of bisphenol A (BPA) in the presence of visible light irradiation. All N-B-TiO2-doped samples exhibited higher photocatalytic activity than the pure TiO2 and N-TiO2 materials and fitted with pseudo-first-order kinetics. The results demonstrated that 2% loaded showed higher photocatalytic performance than the catalytic system used in the study (Figure 12). Furthermore, the influence of various operational parameters (initial BPA concentration, calcination temperature, dopant concentration) on the degradation of BPA were also studied [96].
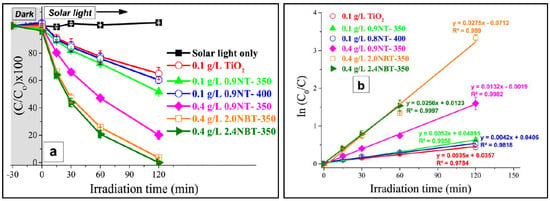
Figure 12.
Photocatalytic degradation efficiency (a) and pseudo-first-order kinetics fitting (b) of BPA degradation. Reprinted with permission from [96].
Similarly, they used the same materials for degradation of pollutants such as BPA, ibuprofen (IBP), triclosan (TCS), diclofenac (DCF) and estrone (E1) spiked in Milli-Q water and different wastewater (microfiltration effluent, secondary-treated wastewater and reverse osmosis permeate from GWRS facility at Orange County, CA, USA) samples in the presence of visible light irradiation. The degradation reaction was performed using individual compounds and quinary mixture of pollutants. The results revealed that N-TiO2 showed higher degradation efficiency of pollutants in a single system compared to the quinary degradation system. The order of degradation efficiency is DCF > E1 > BPA~IBP > TCS, the difference in efficiency is attributed to their different affinities towards reactive oxygen-based species (ROS, 1O2, O2•−, HOO• and •OH), especially •OH. Therefore, the role of ROS in pollutant decomposition is higher than the affinity of pollutants towards N-B-TiO2 catalyst surface because of the low adsorption of pollutants onto the N-B-TiO2 surface. They also studied the influence of the presence of inorganic ions (i.e., NO3−, Cl−, Br−, HCO3−) on the degradation efficiency and identified the possible intermediates formed during degradation of pollutants [97]. Similarly, significant research activities have been performed, therefore Table 1 summarized a few of the co-doped N-TiO2 photocatalytic systems.

Table 1.
Summary of the few co-doped N-TiO2 based photocatalytic systems for degradation of pollutants.
8. N-TiO2 Based Z-Scheme Heterojunction System
In order to enhance the photocatalytic activity of N-TiO2, the Z-scheme type heterojunction system was developed by integrating the N-TiO2 with another semiconductor nanomaterial. The Z-scheme type materials classified as direct system where the redox-mediator is not used, whereas in the case of indirect system the Z-scheme is formed through various redox-mediators (e.g., IO3−/I−, Fe3+/Fe2+). The Z-scheme system increases the visible light response of N-TiO2 and the photogenerated electron and hole pairs are efficiently separated in the CB and VB of the two different semiconductors which leads to enhancement in the photocatalytic efficiency. The Z-scheme system has been used for the degradation of water pollutants, however, the experimental evidence for the Z-scheme mechanisms is ambiguous and has not been investigated deeply. Furthermore, the Z-scheme describes only thermodynamically uphill reactions (e.g., water splitting reaction), whereas the photocatalytic degradation of pollutants is thermodynamically downhill reactions. Therefore, the usage of Z-scheme in photocatalytic degradation application might create scientific misconception and detailed explanation is given in references [109,110]. Therefore, the water splitting application of N-TiO2-based Z-scheme-type systems is described hereafter. Nakada et al. developed a Z-scheme system using RuO2-modified rutile TiO2:Ta/N (as an O2 evolution photocatalyst), Ru-loaded SrTiO3:Rh (as an H2 evolution photocatalyst) and an Fe3+/Fe2+ redox couple for water splitting in an aqueous NaIO3 solution under visible light (λ > 420 nm) and AM 1.5G simulated sunlight irradiation. In the presence of visible light irradiation, Z-scheme transfer of photogenerated charge carriers occurred between two different photocatalysts that leads to the continuous and simultaneous evolution of O2 and H2 (Figure 13) [111].
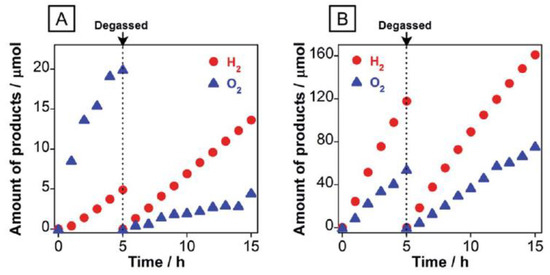
Figure 13.
Photocatalytic H2 and O2 evolution using RuO2/TiO2:Ta/N and Ru/SrTiO3:Rh in an aqueous solution containing (A) NaIO3 (1 mM) and (B) FeCl3 (1 mM) under visible-light irradiation. Reprinted with permission from [111].
Similarly, they prepared a co-catalyst-free visible-light-driven Z-scheme water-splitting system by combining N/F-co-doped rutile TiO2 (as an O2-evolution photocatalyst) with Ru/SrTiO3:Rh (as an H2-evolution photocatalyst) and [Co(bpy)3]3+/2+ (bpy = 2,2′-bipyridine) redox mediator. In the initial stage of light irradiation, O2 evolution occurred because of the absence of [Co(bpy)3]3+ and the continuation of irradiation leads to formation of [Co(bpy)3]3+ which leads to the steady O2 evolution on N/F-co-doped rutile TiO2 and the H2 evolution on Ru/SrTiO3:Rh materials. The photogenerated charge carriers are efficiently separated in the CB and VB of the two different semiconductors through the redox mediators, eventually the continuous evolution of O2 and H2 was observed and showed two times higher activity than the RuO2-modified rutile TiO2:Ta/N system [112].
9. Visible Light Photocatalysis of N-Doped TiO2
Nitrogen doping into TiO2 effectively increases the visible light absorption (i.e., reduces the bandgap energy) and suppresses the rapid recombination of photogenerated electron hole pairs that significantly improves the photocatalytic capacity of TiO2 in the presence of visible light irradiation. In addition, the phase composition, surface area, morphology and defects in N-TiO2 also play a vital role in enhancing the visible light photocatalytic activity. Therefore, various literature is available on the development of N-TiO2 that has mainly been used for the degradation of pollutants, renewable energy production (i.e., water splitting), CO2 reduction, antibacterial, photoreforming of wastewater, sensors, solar cells and organic transformation applications. This review article mainly focuses on summarizing the photocatalytic application of N-doped TiO2 towards pollutant degradation and water splitting applications.
9.1. Pollutants Degradation
Photocatalytic degradation capacity of N-TiO2 has been majorly studied for the degradation of pollutants in both the aqueous and gaseous phase under visible light irradiation. Photocatalytic degradation application of N-doped TiO2 was first evaluated by Sato; NOx-doped TiO2 was prepared by calcination of titanium hydroxide and utilized for oxidation of carbon monoxide and ethane and by oxygen isotope equilibration in the presence of visible light irradiation [28]. Asahi et al. prepared TiO2−xNx thin films by sputtering method and evaluated its catalytic activity by degradation of methylene blue and gaseous acetaldehyde in the presence of visible light irradiation (wavelength <500 nm). TiO2−xNx exhibited higher photocatalytic activity than the bare TiO2 under visible light irradiation, however, it exhibited similar activity under UV light [29]. Burda et al. reported a room temperature synthesis of TiO2−xNx by direct amination of TiO2 nanoparticles using triethylamine, the color of TiO2 changed to yellow after nitrogen doping, which showed significant optical response in the visible light range. The photocatalytic activity demonstrated that TiO2−xNx showed higher catalytic activity than the TiO2 system in the degradation of MB dye under irradiation of 390 and 540 nm visible light using a laser system (Clark MXR 2001 femtosecond) [113]. Nosaka et al. prepared N-TiO2 by calcination of TiO2 powder with organic nitrogen compounds (urea, guanidine hydrochloride, guanidine carbonate) at various temperatures (350, 450 and 550 °C). Two kinds of N signals were obtained in XPS analysis of N-doped TiO2, which contributes to the visible light absorbance. XPS signals at 396 eV and 400 eV assigned to Ti-N (substituted N atom for the O site in TiO2 lattice) and N–O, respectively. The results demonstrated that the absorption edge of TiO2 powder is significantly extended towards visible light irradiation, and N-TiO2 prepared by guanidine showed higher visible light absorbance than urea, showing that a higher amount of nitrogen was doped with guanidine. However, the N-TiO2 prepared by urea exhibited higher degradation efficiency in degradation of 2-propanol than that prepared with guanidine hydrochloride which indicates that high visible light absorption does not correlate with the photocatalytic activity. So, the higher photocatalytic activity is due to the substituted N atom for the O site in TiO2 lattice and N–O have negative impacts on visible light photocatalytic activity [114]. Kalantar et al. utilized the ultrasonic assisted direct impregnation method for preparation of N-TiO2 using urea as nitrogen source. Crystallite size of TiO2 (21 nm) is decreased after nitrogen doping (19 nm, N-TiO2), which results in higher surface area (63 m2/g) than the TiO2 nanoparticle (50 m2/g). Furthermore, the ionic radius of N3− (0.171 nm), which is close to that of O2− (0.144 nm), could be incorporated into the TiO2 lattice that weakens the crystalline phase stabilization, causes lattice distortion that leads to decrease in the crystallite size and enhancement in the surface area. The increase in the surface area is due to the ultrasonic irradiation that produces cracks and creates porous structure on the TiO2 particle and also prevents the agglomeration of nanoparticles. This showed higher visible light activity than the TiO2 in oxidative desulfurization of dibenzothiophene in a diesel fuel model under visible light irradiation and air bubbling. Furthermore, the enhancement in the photocatalytic activity is because of the effective separation of photogeneration of electron hole pairs through new N 2p energy levels formed above the valence band of the TiO2 [115]. Ramezani Sani et al. developed N-TiO2 nanowires using an annealing method under nitrogen atmosphere at different temperatures. N-TiO2 nanowires exhibited the presence of both anatase and rutile phases of TiO2 and significant enhancement in the visible light absorption was observed leading to higher visible light photocatalytic activity in methyl orange dye degradation than the TiO2 nanowires [116]. Bakre et al. studied the influence of nitrogen sources on the photocatalytic activity of N-TiO2 under natural sunlight irradiation. N-TiO2 prepared by decomposition of urea, semicarbazide and N, N′-dimethyl urea precursors, and the nitrogen doping occurred on the TiO2 lattice. The N-TiO2 prepared using semicarbazide precursors showed higher photocatalytic activity in degradation of MB and RhB dye compared to other precursor-based N-TiO2 and the standard Degussa P-25 TiO2 catalyst. The optimal nitrogen doping, anatase–rutile coupling, smaller size, large surface area, efficient suppression of charge carrier’s recombination and the presence of more surface hydroxyl groups are responsible for the higher photocatalytic degradation efficiency [117]. Huang et al. developed a N-TiO2 material with abundant and tunable oxygen vacancies using a hydrothermal method followed by calcination in NH3 atmosphere. The photocatalytic efficiency of the N-TiO2 was evaluated by the degradation of rhodamine B dye (10 mg/L) and tetracycline antibiotic (20 mg/L) in the presence of visible light irradiation. The N-TiO2 showed superior visible light photocatalytic activity than the bare TiO2. The superior activity is attributed to the existence of new N 2p energy levels formed between the CB and VB after N doping and the rich oxygen vacancies that enhance the visible light absorption and inhibit the recombination of photogenerated electron hole pairs (Figure 14) [118].
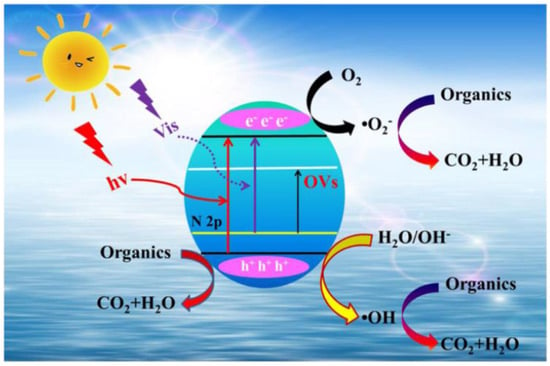
Figure 14.
Schematic representation of photocatalytic mechanism of RhB and TC degradation using N-TiO2. Reprinted with permission from [118].
Similarly, Zeng et al. prepared N-TiO2 using precipitation combined with a thermal decomposition method using urea (CO(NH2)2, U), ammonium chloride (NH4Cl, AC) and ammonium nitrate (NH4NO3, AN) as a nitrogen source, for the degradation of flumequine (FLU) antibiotic under visible light irradiation. All three nitrogen-source-based N-TiO2 showed higher photocatalytic performance than the pure TiO2; the order is TiO2/AN > TiO2/AC > TiO2/U (Figure 15a). The apparent first-order rate constants (kapp) for FLU degradation in the presence of TiO2/AN catalyst are about 35, 16 or 1.4 times higher than that of pure TiO2, TiO2/U or TiO2/AC, respectively (Figure 15b). The superior photocatalytic activity of TiO2/AN sample attributed to the presence of two different valence states of nitrogen (N5+ and N3−) in this catalyst captures the photoproduced electrons, resulting in the reduction of recombination rate of the photogenerated electron and hole pairs [119].
Figure 15.
Photocatalytic FLU degradation efficiency (a) and its kinetic constant value (b) using TiO2, TiO2/AN, TiO2/AC and TiO2/U catalyst under simulated sunlight irradiation. Reprinted with permission from [119].
In addition to properties of TiO2, the nature of the irradiation sources also have significant influence on enhancement in the photocatalytic activity of N-TiO2 photocatalyst. Tryba et al. prepared N-TiO2 photocatalyst by a sol–gel method using ammonia as nitrogen source and the synthesized materials are calcined at 200 to 500 °C for 1 h. The photocatalytic activity was evaluated by the degradation of acetaldehyde under UV and visible light (fluorescent lamp) irradiation. The results demonstrated that all the N-TiO2 samples showed lower catalytic activity than the undoped TiO2 and the N-TiO2 calcined at 300 °C exhibited higher efficiency among the calcined materials. The fluorescent lamp comprises of large region in the visible spectrum but the presence of small part of UV light caused TiO2 samples to not have any significant visible light photocatalytic activity. The blank test in the presence of a fluorescent lamp exhibited 8.5% acetaldehyde decomposition, which clearly shows that UV activity of TiO2 was greater than the visible light activity [120].
Similarly, after Sato and Asahi et al., inventive N-TiO2 were also used for the degradation of various gaseous pollutants in the presence of visible light irradiation. For example, Li et al. synthesized yellow colored N-TiO2 by spray pyrolysis method using mixed solution of TiCl4 and nitrogen precursors (urea, guanidine or ammonium fluoride). Among the precursors used, N-TiO2 prepared from ammonium fluoride exhibited higher photocatalytic activity towards acetaldehyde and trichloroethylene degradation than the other nitrogen-sources-based N-TiO2 and standard Degussa P25 TiO2 under visible light irradiation. The enhancement in the photocatalytic activity is attributed to high surface area, co-doping of F along with N doping onto TiO2 that enhances the visible light absorption and surface acidity. In addition, N doping created oxygen vacancies that enhance the visible photocatalytic activity, however, the comparison showed that the degradation efficiency is less significant than the co-doping of F, surface acidity and area [121]. Aoki et al. developed N-TiO2 by annealing the anatase TiO2 and urea in air, followed which the N-TiO2 was coated onto the glass plate for the degradation of formaldehyde and toluene mixtures in the presence of visible light. The N-TiO2 surface showed hydrophilic nature exhibiting preferential adsorption of hydrophilic formaldehyde than the hydrophobic toluene and revealed higher degradation efficiency towards formaldehyde than the toluene in the mixtures. Thus, when the N-TiO2 is applied for purification of indoor air, the degradation of hydrophilic pollutants is suppressed by the presence of hydrophobic pollutants [122]. Thus, the presence of toluene did not affect the degradation of formaldehyde, whereas, the formaldehyde presence decreased the toluene deficiency. Jo and Kim developed N-TiO2 by calcination of TiO2 nanoparticles with organic nitrogen compounds (urea, guanidine hydrochloride and guanidine carbonate) as a nitrogen source, applied to the degradation of indoor level volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene, m, p-xylene and o-xylene in the presence of visible light irradiation. For all VOCs, the N-TiO2 exhibited higher degradation efficiency than the unmodified TiO2, whereas the degradation efficiency depends highly on the stream flow rate, hydraulic diameter and relative humidity [123]. Similarly, He et al. synthesized N-TiO2 nanoparticles with anatase-brookite mixed-phase TiO2 by solvothermal method using different nitrogen sources (ammonia, hydrazine hydrate or ammonium nitrate). N-TiO2 prepared with hydrazine hydrate as precursor showed higher photocatalytic activity in degradation of gaseous benzene under UV light irradiation and the degradation efficiency was not changed after 15 experiment cycles [124]. Priya and Philip prepared N-TiO2 and evaluated its catalytic efficiency by degradation of VOCs (methanol, acetone, dichloromethane (DCM), benzene and toluene) present in pharmaceutical wastewater under visible and solar light irradiation. The degradation rate followed an order of benzene > toluene > DCM > methanol > acetone and showed faster degradation rate under solar light irradiation [125]. Khan et al. prepared N-TiO2 by co-precipitation of tri-thiocyanuric acid (TCA) with Degussa P25 TiO2 followed by calcination under N2 atmosphere (550 °C, 4 h). Results showed both the interstitial position of nitrogen and nitrogen substitutional doping on the oxygen site into the TiO2 lattice. N-doping improved the visible-light absorption and enhanced the charge carrier’s separation/transfer efficiency by capturing the holes by reduced titanium ions and showed higher photocatalytic activity in degradation of NOx under UV and visible light irradiation [63]. Recently, TiO2 and N-TiO2 were derived by combustion/calcination of metal organic frameworks (MOFs). Zhao et al. prepared N-TiO2 with anatase-rutile phase by calcining the titanium metal organic frameworks (MIL-125 (Ti)) and melamine mixture at different temperatures in air. Results revealed that N doping is a substitutional one and replaced the O atoms in the TiO2 lattice, and the N-TiO2 synthesized at 500 °C showed higher visible light photocatalytic activity than the N-TiO2 synthesized at other temperature and Degussa P-25 TiO2 in degradation of gaseous benzene [126]. Similarly, various research studies are available on N-TiO2 that show the importance of N-TiO2 and their significant visible light photocatalytic activities towards degradation of pollutants in the aqueous and gaseous phase (Table 2).

Table 2.
Photocatalytic degradation of water and gaseous pollutants using N-TiO2 materials.
9.2. Water Splitting (H2 Production)
In order to fulfill the increasing energy demands and eradicate environmental problems, the generation of energy from renewable resources is receiving significant attention. Splitting of water into hydrogen (H2) and oxygen (O2) is one of the sustainable processes for the production of renewable energy. For higher H2 production efficiency, the CB and VB position of semiconductors must be higher than the H2 (H+/H2, 0 V) and O2 (O2/H2O, 1.23 V) evolution potentials. Furthermore, the water splitting reactions are majorly performed in the pure water medium and addition of the sacrificial agents, and also photoreforming of wastewater (simultaneous production of H2 and degradation of pollutants). Various semiconductor nanomaterials have been utilized for the H2 production reaction, in which N-TiO2 is one of the catalytic systems effectively used for this purpose under the irradiation of visible light [15,143,144,145,146,147].
Babu et al. developed a rice-grain-like N-TiO2 nanostructure morphology using combined sol–gel and electrospinning followed by an annealing process. N doping did not change the morphology, crystallinity and d-spacing of the TiO2 fibers. No variation in the d-spacing of the nanostructure’s anatase (101) plane shows that the N has been doped into the TiO2 lattice (substitutional N-doping) without altering the unit cell dimension, which was confirmed by XPS analysis. N-TiO2 (28 µmol/h) exhibited enhanced water splitting efficiency compared to TiO2 fibers (2 µmol/h). Likewise, N-TiO2 fibers showed higher methylene blue dye degradation efficiency [148]. Similarly, N-TiO2 was developed by the controlled oxidation of titanium nitride at different temperatures (350 °C, 400 °C and 450 °C) and that the color changes from black to yellow confirmed the formation of N-TiO2. The nitrogen doping occurred on the TiO2 lattice showed shifting of binding energy values of Ti 2p to higher values in the XPS. The N-TiO2 sample (N-TiO2-450) showed the higher H2-evolution potential than the Degussa P-25 TiO2 in a Na2S-Na2SO3 sacrificial system under UV-Vis light irradiation [149]. Wang et al. prepared N-doped TiO2 on quartz glass with preferred (112) and (211) facet orientation by RF magnetron sputtering method using a mixture of N2, O2 and Ar gas. XPS analysis indicated that the binding energy value at 396.5 eV validated the substitutional integration of N into the TiO2 film. Furthermore, by increasing the N2 gas flow rate, the growth of preferred (211) facet orientation is increased, which leads to higher surface energies of anatase facets ((112) and (211)) than the (101) facet of anatase TiO2. The huge percentage of exposed facets, especially (211) facets, showed higher photocatalytic water splitting efficiency (4500 µmol H2 h−1 m−2) than the undoped TiO2 film. In addition, the high surface energy (211) facets would destabilize the O 2p states on the surface where the water molecules are easily absorbed and dissociated, which leads to higher water splitting efficiency [150]. Similarly, Fakhouri et al. developed pure and N-TiO2 thin films through RF reactive magnetron sputtering method, and observed both substitutional and interstitial N doping onto TiO2 thin films. Photocatalytic ability was evaluated by degradation of N-methyl-2-pyrrolidone (NMP) and water splitting reaction under light irradiation (both UV and visible). Both the film morphology and N doping sites have significant effect on the photocatalytic ability. The increase in the interstitial N-doping sites enhanced the photocatalytic activity; whereas increases in the substitutional N-doping sites decrease the activity under UV light irradiation but not under visible light. This could be attributed to the recombination of photo-generated charge carriers when the films have more substitutional nitrogen and decreased activity under UV light. Furthermore, TiO2 film with dominant interstitial N doping sites progress photocatalytic activity, due to the separation of photoproduced charge separation on the porous films having higher surface area than the anticipated surface [151]. Similarly, Sun et al. prepared surface heterojunction N-TiO2 nanobelt structures with co-exposed (001) and (101) facets and showed diverse transmission properties and reactivity for photogenerated charge carriers and enhanced photocatalytic ability. N-TiO2 nanobelts form the surface heterojunction that induces an electrostatic force, in which the electrons in the (001) facet are immediately attracted to the (101) facet, transferred to the surface (active reaction sites) and combine with H+ and form H2. Water splitting reaction revealed N-TiO2 nanobelts with co-exposed (001) and (101) facets showed higher H2 production (670 μmol h−1 g−1) rate than the other N-TiO2 nanobelts with a single facet exposed [152]. Recently, Esmat et al. developed an oxygen vacancy (Vo)-bearing N-doped anatase TiO2 through the calcination of layered titanate nanosheets comprising N, N-dimethylformamide (DMF), an organic N source, in air. XPS analysis demonstrated that N 1s shows two peaks at 402.2 eV and 400.1 eV, corresponded to the nitrogen in the adsorbed DMF and interstitial doping of nitrogen in TiO2 lattices validating the presence of Ti−O−N bond and the concomitant creation of Vo. N doping and the formation of Vo significantly enhanced the light absorption of TiO2 (up to 500 nm). Furthermore, the introduction of Vo donates the electrons in TiO2, facilitating the photogenerated charge carrier separation and transfer which leads to more enhanced water splitting efficiency (1035 μmol h−1 g−1) than the undoped TiO2 (~300 μmol h−1 g−1) [153]. Similarly, various research studies are available on N-TiO2 photocatalyst for hydrogen production application, which are summarized in Table 3.

Table 3.
Photocatalytic splitting of water into H2 and O2 using N-TiO2 materials.
10. Stability, Recovery and Reusability of N-TiO2 Photocatalyst
The stability of photocatalyst is one of the important factors for up-scaling the developed photocatalytic systems for field level applications. Generally, the stability of the photocatalyst was determined by performing the catalytic recycle experiment. Initially the catalytic materials recovered from the reaction mixture by either centrifugation, filtration, or both processes, are then subjected to various chemical and physical methods for purifying/cleaning the catalytic materials and reused for the same catalytic reaction. For example, Zeng et al. developed a N-TiO2 by combination of precipitation with thermal decomposition method using urea, ammonium chloride and ammonium nitrate as nitrogen sources, for the degradation of flumequine (FLU) antibiotic under visible light irradiation. The N-TiO2 prepared with ammonium nitrate showed higher photocatalytic activity than the pure TiO2. The stability and reusability of the N-TiO2 was evaluated by degradation of FLU in four recycling experiments. After the first cycle, the catalyst was recovered by centrifugation and washed three times with ultrapure water, then the fresh FLU solution was added and the degradation experiment was performed. The same procedure was repeated for three cycles (Figure 16). The results demonstrated that after the first cycle the FLU was completely removed and afterwards the FLU removal efficiency remained at 91.8% after four cycles of experiment. The degradation efficiency is slightly affected during four consecutive cycles which may be due to the loss of materials during the washing process, and also indicates that the N-TiO2 prepared with ammonium nitrate showed good stability [119].
Figure 16.
The stability of N-TiO2 (prepared with ammonium nitrate) for FLU degradation under visible light irradiation. Reprinted with permission from [119].
Similarly, Mirzaei et al. (2021) prepared a gradient nitrogen-doped TiO2 (g- N-TiO2) by PLD method for the removal of sulfamethoxazole (SMX) antibiotic in water in the presence of simulated solar light irradiation. The g-N-TiO2 showed superior photocatalytic activity in degradation of SMX compared to pure TiO2 and N-TiO2. The stability and reusability of the materials was evaluated by performing three consecutive runs, revealing that maximum ~12% loss in the SMX removal was obtained and the photocatalyst was very stable (Figure 17) [91].
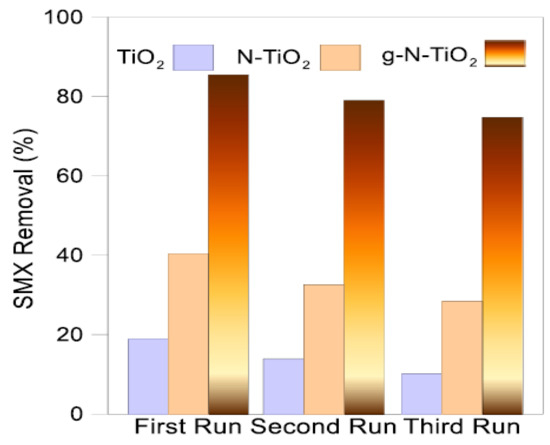
Figure 17.
Recycling test of SMX degradation using TiO2, N-TiO2 and g-N-TiO2 photocatalysts. Reprinted with permission from [91].
From this catalytic recovery process, it is understood that there is a material loss during either centrifugation, filtration, or both; therefore, decrease in the photocatalytic catalytic activity was observed after the second or third cycles of experiment. Therefore, recently, the immobilization of catalytic materials on the magnetic support have been performed which helps to easily collect and separate the materials by applying an external magnetic field. Zangeneh et al. prepared visible light active magnetic N-TiO2/ZnFe2O4 and CN-co-doped TiO2/ZnFe2O4 nanocomposite materials by sol–gel–hydrothermal method using urea and L-asparagine as nitrogen source. The photocatalytic activity evaluation results demonstrated that 2 wt.% of L-asparagine derived CN–TiO2/ZnFe2O4 (97%) showed superior visible light photocatalytic activity than the CN–TiO2 (90.5%), N–TiO2/ZnFe2O4 (70%), N–TiO2 (53%) and pure TiO2 (30%) in degradation of direct red 16 (DR16) dye. After the first cycle of the experiment, the photocatalyst was recovered by the external magnetic field, washed with distilled water, irradiated under visible light (3 h) for degradation of adsorbed pollutants and dried (100 °C). Thereafter, the degradation experiment was repeated for six cycles, up to four cycles no loss in the photoactivity of the materials was noted and then 7% loss in degradation efficiency was observed. The results revealed that the photocatalyst can be completely recovered by applying an external magnetic field, which was confirmed by vibrating sample magnetometer (VSM) analysis results [161].
11. Opportunity, Challenges and Future Requirement/Prospects
TiO2 is a potential semiconducting nanomaterial for the environmental remediation, energy production, sensors, solar cells, self-cleaning of tiles, glass and windows and antifogging applications, etc., and the same has also been demonstrated at commercial scale and well-documented. Furthermore, clean solar energy can be harnessed for development of sustainable and economically viable effluent treatment technologies. Therefore, N-doped TiO2 is one of the efficient visible light responsive photocatalysts for harvesting solar light and utilizing it for various applications. As described above, various literature evidences revealed the various synthesis methodologies for preparation of efficient N-TiO2 and evaluated its physicochemical and electronic properties. However, challenges still remain with respect to the understanding of nitrogen doping mechanisms, influence of nitrogen concentration on its position in the TiO2 lattice, photogenerated charge carrier dynamics, influence of heating on phase transformation, reactive radical’s generation and their concentration on catalytic efficiency. Therefore, the following research gaps/opportunities are available and need to be considered to fulfil the future direction of development of effective N-doped TiO2 photocatalytic systems for the environmental remediation, energy harvesting and production applications.
- (a)
- The defects in TiO2, electronic structure of disordered TiO2, physicochemical properties and their influence on the catalytic efficiency needs to be investigated.
- (b)
- Each methodology involves different operational parameters and uses different nitrogen sources which limit the synthesis optimizing N doping and also upgradation of large-scale synthesis of efficient N-TiO2 for practical implementation. Therefore, there is an urgent need for an optimized synthesized methodology and nitrogen sources for preparation of N-TiO2 and this could be a promising photoresponsive nanomaterials research.
- (c)
- The stability of N-TiO2 is still questionable, thus the co-doping of N-TiO2 with other metals, non-metals and semiconductors could improve its stability and reusability, thus boosting the practical applicability of the materials. However, the study of the practical degradation ability of the N-TiO2 systems in realistic wastewater is needed.
- (d)
- The recovery and reusability of N-TiO2 could be improved by integrating/supporting N-TiO2 on magnetic supports. However, there is still a decrease in photocatalytic efficiency due to the loss of materials after several cycles and also the energy requirement for post-treatment of recovered catalyst is still on the high side. Therefore, the immobilization of photocatalytic materials on various solid supports followed by fabrication of different geometry of reactors have been performed, and the catalytic materials are easily recovered after the catalytic reaction and reused for multiple cycles of experiments while retaining photocatalytic efficiency. However, the upscaling of the process for real scale application depends upon the various operational parameters (e.g., coating techniques, reproducibility in coating thickness, etc.). Therefore, there is an opportunity to optimize the various operational parameters for designing effective immobilized photocatalytic reactor systems.
- (e)
- The N doping/incorporation into TiO2 is still debatable and it depends upon the sources of nitrogen and the synthesis methodologies adopted. Furthermore, controlling the N doping concentration in the TiO2 lattice is essential for efficient catalytic efficiency. Therefore, the characterization of N-TiO2 using combined analytical techniques (e.g., transient optical spectroscopy) along with theoretical study is needed to control the doping concentration and create awareness of the structural features of the N-TiO2.
- (f)
- Theoretical analysis and computational modeling of the experimental data is needed that offers clear representation on the required nitrogen source type, synthesis methodology for development of durable N-doped TiO2, structure, doping mechanism and the catalytic efficiency of N-TiO2.
12. Summary and Conclusions
N-TiO2 is one of the efficient non-metal-doped visible light active photocatalysts for the removal of environmental pollutants and renewable energy production. Nitrogen doping enhances visible light absorption through narrowing the bandgap energy and improves the photoproduced charge separation and transfer efficiency. In this review, we summarize the various synthesis methodologies adopted and nitrogen sources used for preparation of N-TiO2 and also their influence on the catalytic efficiency. Further, the possible mechanisms for N doping, changes in the properties and their visible-light catalytic applications in pollutants degradation and water splitting reactions are discussed. It could be understood that each method is unique in their approach for preparation of N-TiO2 with excellent photocatalytic efficiency. The modifications in the reaction operational parameters and the co-doping offers excellent structural, textural and electronic properties to N-doped TiO2, which leads to enhanced visible-light photocatalytic efficiency. In addition, the above-mentioned research gaps need to be focused on for the development of efficient renewable solar-energy-based photocatalytic systems. Thus, N-TiO2 could be considered as an efficient counterpart to noble metal and other metal-doped TiO2 systems.
Author Contributions
T.S.N.: conceptualization, methodology, writing—original draft, writing—review and editing, formal analysis; V.M.: conceptualization, methodology, writing—original draft, writing—review and editing, formal analysis; R.J.T.: conceptualization, writing—review and editing, investigation and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the CSIR for financial support through MLP project (MLP-19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
CSIR-CLRI Communication No. 1599. The authors also thank the Director, CSIR-CLRI and CSIR-CSMCRI for their continuous support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fujishima:, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic Degradation of Aqueous Nitrobenzene by Nanocrystalline TiO2. Ind. Eng. Chem. Res. 2006, 45, 922–927. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef]
- Natarajan, K.; Natarajan, T.S.; Kureshy, R.I.; Bajaj, H.C.; Jo, W.K.; Tayade, R.J. Photocatalytic H2 Production using Semiconductor Nanomaterials via Water Splitting—An Overview. In Advanced Materials Research; Al-Ahmed, A., Hossain, M.K., Afzaal, M., Bahaidarah, H.M., Eds.; Trans Tech Publications, Ltd.: Stafa-Zurich, Switzerland, 2015; Volume 1116, pp. 130–156. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Tayade, R.J. Photocatalysis: Present, past and future. In Inorganic Pollutants in Wastewater: Method of Analysis, Removal and Treatment; Inamuddin, Mohammad, A., Asiri, A.M., Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2017; Volume 16, pp. 1–63. [Google Scholar]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Transition metal ion impregnated mesoporous TiO2 for photocatalytic degradation of organic contaminants in water. Ind. Eng. Chem. Res. 2006, 45, 5231–5238. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Enhanced photocatalytic activity of bismuth-doped TiO2 nanotubes under direct sunlight irradiation for degradation of Rhodamine B dye. J. Nanoparticle Res. 2013, 15, 1–18. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansaric, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium dioxide: An overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Enhanced Photocatalytic Activity of TiO2-Coated NaY and HY Zeolites for the Degradation of Methylene Blue in Water. Ind. Eng. Chem. Res. 2007, 46, 369–376. [Google Scholar] [CrossRef]
- Palanivelu, K.; Im, J.S.; Lee, Y.-S. Carbon Doping of TiO2 for Visible Light Photo Catalysis—A review. Carbon Sci. 2007, 8, 214–224. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Lazar, M.A.; Jasra, R.V. Enhanced Photocatalytic Activity by Silver Metal Ion Exchanged NaY Zeolite Photocatalysts for the Degradation of Organic Contaminants and Dyes in Aqueous Medium. Ind. Eng. Chem. Res. 2008, 47, 7545–7551. [Google Scholar] [CrossRef]
- Jo, W.-K.; Won, Y.; Hwang, I.; Tayade, R.J. Enhanced Photocatalytic Degradation of Aqueous Nitrobenzene Using Graphitic Carbon−TiO2 Composites. Ind. Eng. Chem. Res. 2014, 53, 3455–3461. [Google Scholar] [CrossRef]
- Mogilevsky, G.; Hartman, O.; Emmons, E.D.; Balboa, A.; DeCoste, J.B.; Schindler, B.J.; Iordanov, I.; Karwacki, C.J. Bottom-Up Synthesis of Anatase Nanoparticles with Graphene Domains. ACS Appl. Mater. Interfaces 2014, 6, 10638–10648. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Palmyra tuber peel derived activated carbon and anatase TiO2 nanotube based nanocomposites with enhanced photocatalytic performance in rhodamine 6G dye degradation. Process. Saf. Environ. Prot. 2016, 104, 346–357. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Lee, J.Y.; Bajaj, H.C.; Jo, W.K.; Tayade, R.J. Synthesis of multiwall carbon nanotubes/TiO2 nanotube composites with enhanced photocatalytic decomposition efficiency. Catal. Today 2017, 282, 13–23. [Google Scholar] [CrossRef]
- Thomas, M.; Natarajan, T.S. TiO2-High Surface Area Materials Based Composite Photocatalytic Nanomaterials for Degradation of Pollutants: A Review. In Photocatalytic Nanomaterials for Environmental Applications; Tayade, R.J., Gandhi, V., Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2018; Volume 27, pp. 48–96. [Google Scholar]
- Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Yalçın, Y.; Kılıç, M.; Çınar, Z. The Role of Non-Metal Doping in TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2016, 13, 281–296. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 294, 269–271. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Acharya, R.; Naik, B.; Parida, K. Cr(VI) remediation from aqueous environment through modified-TiO2-mediated photocatalytic reduction. Beilstein J. Nanotechnol. 2018, 9, 1448–1470. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Rivas, J.; Solis, R.R.; Gimeno, O.; Sagasti, J. Photocatalytic elimination of aqueous 2-methyl-4-chlorophenoxyacetic acid in the presence of commercial and nitrogen-doped TiO2. Int. J. Environ. Sci. Technol. 2015, 12, 513–526. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Gimeno, O.; Pérez-Bote, J.L. Photocatalytic ozonation of pyridine-based herbicides by N-doped titania. J. Chem. Technol. Biotechnol. 2016, 91, 1998–2008. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Martínez-Piernas, A.; Agüera, A. Ozonation, photocatalysis and photocatalytic ozonation of diuron. Intermediates identification. Chem. Eng. J. 2016, 292, 72–81. [Google Scholar] [CrossRef]
- Avisar, D.; Horovitz, I.; Lozzi, L.; Ruggieri, F.; Baker, M.; Abel, M.-L.; Mamane, H. Impact of water quality on removal of carbamazepine in natural waters by N-doped TiO2 photo-catalytic thin film surfaces. J. Hazard. Mater. 2013, 244–245, 463–471. [Google Scholar] [CrossRef]
- Caratto, V.; Setti, L.; Campodonico, S.; Carnasciali, M.M.; Botter, R.; Ferretti, M. Synthesis and characterization of nitrogen-doped TiO2 nanoparticles prepared by sol–gel method. J. Sol. Gel. Sci. Technol. 2012, 63, 16–22. [Google Scholar] [CrossRef]
- Pan, J.H.; Han, G.; Zhou, R.; Zhao, X.S. Hierarchical N-doped TiO2 hollow microspheres consisting of nanothorns with exposed anatase {101} facets. Chem. Commun. 2011, 47, 6942–6944. [Google Scholar] [CrossRef]
- Chainarong, S.; Sikong, L.; Pavasupree, S.; Niyomwas, S. Synthesis and Characterization of Nitrogen-doped TiO2 Nanomaterials for Photocatalytic Activities under Visible Light. Energy Procedia 2011, 9, 418–427. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, Q.; Saito, F.; Sato, T. Preparation of visible light-activated titania photocatalyst by mechanochemical method. Chem. Lett. 2003, 32, 358–359. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Komatsu, M.; Zhang, Q.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Preparation of nitrogen-doped titania with high visible light induced photocatalytic activity by mechanochemical reaction of titania and hexamethylenetetramine. J. Mater. Chem. 2003, 13, 2996–3001. [Google Scholar] [CrossRef]
- Techitdheera, W.; Rattanarak, J.; Mekprasart, W.; Pecharapa, W. Influence of milling time, NH3 additive and annealing temperature on physical properties of modified commercial TiO2 powders via ball milling process. Energy Procedia 2014, 56, 667–672. [Google Scholar] [CrossRef][Green Version]
- Kassim, K.; Hamali, M.A.; Yamin, B. A new alternative synthesis of salicylaldazine via microwave irradiation method. J. Chem. 2019, 2019, 9546373. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Terzian, N.; Kennedy, K.J.; Droste, R.L.; Hamoda, M. Athermal microwave effects for enhancing digestibility of waste activated sludge. Water Res. 2007, 41, 2457–2466. [Google Scholar] [CrossRef]
- Kadam, A.N.; Dhabbe, R.S.; Kokate, M.R.; Gaikwad, Y.B.; Garadkar, K.M. Preparation of N doped TiO2 via microwave-assisted method and its photocatalytic activity for degradation of Malathion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 669–676. [Google Scholar] [CrossRef]
- Azami, M.S.; Nawawi, W.I.; Jawad, A.H.; Ishak, M.A.M.; Ismail, K. N-doped TiO2 Synthesised via Microwave Induced Photocatalytic on RR4 Dye Removal under LED Light Irradiation. Sains Malays. 2017, 46, 1309–1316. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Weng, C.-H.; Srivastav, A.L.; Lin, Y.-T.; Tzeng, J.-H. Facile Synthesis and Characterization of N-Doped TiO2 Photocatalyst and Its Visible-Light Activity for Photo-Oxidation of Ethylene. J. Nanomater. 2015, 2015, 807394. [Google Scholar] [CrossRef]
- Makropoulou, T.; Panagiotopoulou, P.; Venieri, D. N-doped TiO2 photocatalysts for bacterial inactivation in water. J. Chem. Technol. Biotechnol. 2018, 93, 2518–2526. [Google Scholar] [CrossRef]
- Irie, H.; Washizuka, S.; Watanabe, Y.; Kako, T.; Hashimoto, K. Photoinduced hydrophilic and electrochemical properties of nitrogen-doped TiO2 films. J. Electrochem. Soc. 2005, 152, E351. [Google Scholar] [CrossRef]
- Kitano, M.; Funatsu, K.; Matsuoka, M.; Ueshima, M.; Anpo, M. Preparation of nitrogen-substituted TiO2 thin film photocatalysts by the radio frequency magnetron sputtering deposition method and their photocatalytic reactivity under visible light irradiation. J. Phys. Chem. B 2006, 110, 25266–25272. [Google Scholar] [CrossRef]
- Obata, K.; Irie, H.; Hashimoto, K. Enhanced photocatalytic activities of Ta, N co-doped TiO2 thin films under visible light. Chem. Phys. 2007, 339, 124–132. [Google Scholar] [CrossRef]
- Dobromir, M.; Manole, A.V.; Nica, V.; Apetrei, R.; Neagu, M.; Luca, D. Analyzing the Development of N-Doped TiO2 Thin Films Deposited by RF Magnetron Sputtering. Sens. Lett. 2013, 11, 675–678. [Google Scholar] [CrossRef]
- Chan, M.-H.; Lu, F.-H. Characterization of N-doped TiO2 films prepared by reactive sputtering using air/Ar mixtures. Thin Solid Film. 2009, 518, 1369–1372. [Google Scholar] [CrossRef]
- Chen, X.; Ma, E.; Liu, G.; Yin, M. Excited-state dynamics of Er3+ in Gd2O3 nanocrystals. J. Phys. Chem. C 2007, 111, 9638–9643. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Photophysical and photocatalytic properties of AgInW2O8. J. Phys. Chem. B 2003, 107, 14265–14269. [Google Scholar] [CrossRef]
- Matsubara, K.; Danno, M.; Inoue, M.; Honda, Y.; Abe, T. Characterization of nitrogen-doped TiO2 powder prepared by newly developed plasma-treatment system. Chem. Eng. J. 2012, 181–182, 754–760. [Google Scholar] [CrossRef]
- Yamada, K.; Nakamura, H.; Matsushima, S.; Yamane, H.; Haishi, T.; Ohira, K.; Kumada, K. Preparation of N-doped TiO2 particles by plasma surface modification. Comptes Rendus Chim. 2006, 9, 788–793. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chang, S.M.; Chang, C.; Den, W. Preparation of N-doped TiO2 photocatalyst by atmospheric pressure plasma process for VOCs decomposition under UV and visible light sources. J. Nanopart. Res. 2007, 9, 365–375. [Google Scholar] [CrossRef]
- Panepinto, A.; Cossement, D.; Snyders, R. Experimental and theoretical study of the synthesis of N-doped TiO2 by N ion implantation of TiO2 thin films. Appl. Surf. Sci. 2021, 541, 148493. [Google Scholar] [CrossRef]
- Borras, A.; Lopez, C.; Rico, V.; Gracia, F.; Gonzalez-Elipe, A.R.; Richter, E.; Battiston, G.; Gerbasi, R.; McSporran, N.; Sauthier, G.; et al. Effect of visible and UV illumination on the water contact angle of TiO2 thin films with incorporated nitrogen. J. Phys. Chem. C 2007, 111, 1801–1808. [Google Scholar] [CrossRef]
- Ghicov, A.; Macak, J.M.; Tsuchiya, H.; Kunze, J.; Haeublein, V.; Frey, L.; Schmuki, P. Ion Implantation and Annealing for an Efficient N-Doping of TiO2 Nanotubes. Nano Lett. 2006, 6, 1080–1082. [Google Scholar] [CrossRef]
- Khan, T.T.; Bari, G.A.K.M.R.; Kang, H.-J.; Lee, T.-G.; Park, J.-W.; Hwang, H.J.; Hossain, S.M.; Mun, J.S.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-Doped TiO2 for Efficient Photocatalytic Degradation of Atmospheric NOx. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, S.; Liu, X.; Gong, M.; Xu, T. Synthesis of carbon- and nitrogen-doped TiO2/carbon composite fibers by a surface-hydrolyzed PAN fiber and their photocatalytic property. J. Mater. Sci. 2020, 55, 2471–2481. [Google Scholar] [CrossRef]
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple preparation of nitrogen-doped TiO2 and its performance in selective oxidation of benzyl alcohol and benzylamine under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125743. [Google Scholar] [CrossRef]
- Le, P.; Hieu, L.; Lam, T.N.; Hang, N.; Truong, N.; Tuyen, L.; Phong, P.T.; Leu, J. Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process. Micromachines 2018, 9, 618. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Wang, D.; Yu, B.; Wang, C.; Zhou, F.; Liu, W. A novel protocol toward perfect alignment of anodized TiO2 nanotubes. Adv. Mater. 2009, 21, 1964–1967. [Google Scholar] [CrossRef]
- Pishkar, N.; Ghoranneviss, M.; Ghorannevis, Z.; Akbari, H. Study of the highly ordered TiO2 nanotubes physical properties prepared with two-step anodization. Results Phys. 2018, 9, 1246–1249. [Google Scholar] [CrossRef]
- Mazierski, P.; Nischk, M.; Gołkowska, M.; Lisowski, W.; Gazda, M.; Winiarski, M.J.; Klimczuk, T.; Zaleska-Medynska, A. Photocatalytic activity of nitrogen doped TiO2 nanotubes prepared by anodic oxidation: The effect of applied voltage, anodization time and amount of nitrogen dopant. Appl. Catal. B Environ. 2016, 196, 77–88. [Google Scholar] [CrossRef]
- Lai, Y.-K.; Huang, J.-Y.; Zhang, H.-F.; Subramaniam, V.-P.; Tang, Y.-X.; Gong, D.-G.; Sundar, L.; Sun, L.; Chen, Z.; Lin, C.-J. Nitrogen-doped TiO2 nanotube array films with enhanced photocatalytic activity under various light sources. J. Hazard. Mater. 2010, 184, 855–863. [Google Scholar] [CrossRef]
- Wan, L.; Li, J.F.; Feng, J.Y.; Sun, W.; Mao, Z.Q. Improved optical response and photocatalysis for N-doped titanium oxide (TiO2) films prepared by oxidation of TiN. Appl. Surf. Sci. 2007, 253, 4764–4767. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Pan, J.; Lu, G.Q.M.; Cheng, H.-M. Visible light responsive nitrogen doped anatase TiO2 sheets with dominant {001} facets derived from TiN. J. Am. Chem. Soc. 2009, 131, 12868–12869. [Google Scholar] [CrossRef]
- Cooper, E.; Andrews, C.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Pipi, A.; Byzynski, G.; Ruotolo, L. Photocatalytic activity and RNO dye degradation of nitrogen-doped TiO2 prepared by ionothermal synthesis. Mater. Res. 2017, 20, 628–638. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Flame aerosol synthesis of ceramic powders. Prog. Energy Combust. 1998, 24, 197–219. [Google Scholar] [CrossRef]
- Madler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel one-step synthesis of nitrogen-doped TiO2 by flame aerosol technique for visible-light photocatalysis: Effect of synthesis parameters and secondary nitrogen (N) source. Chem. Eng. J. 2018, 350, 324–334. [Google Scholar] [CrossRef]
- Kim, T.H.; Go, G.-M.; Cho, H.-B.; Song, Y.; Lee, C.-G.; Choa, Y.-H. A Novel Synthetic Method for N Doped TiO2 Nanoparticles Through Plasma-Assisted Electrolysis and Photocatalytic Activity in the Visible Region. Front. Chem. 2018, 6, 458. [Google Scholar] [CrossRef]
- Nasirian, M.; Mehrvar, M. Photocatalytic degradation of aqueous Methyl Orange using nitrogen-doped TiO2 photocatalyst prepared by novel method of ultraviolet-assisted thermal synthesis. J. Environ. Sci. 2018, 66, 81–93. [Google Scholar] [CrossRef]
- Yin, S.; Liu, B.; Sato, T. Microwave-Assisted Hydrothermal Synthesis of Nitrogen-Doped Titania Nanoparticles. Funct. Mater. Lett. 2008, 1, 173–176. [Google Scholar] [CrossRef]
- Peng, Y.P.; Lo, S.L.; Ou, H.H.; Lai, S.W. Microwave-assisted hydrothermal synthesis of N-doped titanate nanotubes for visible-light-responsive photocatalysis. J. Hazard. Mater. 2010, 183, 754–758. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Yu, H.; Wang, H.; Yang, J. Synthesis and characterization of substitutional and interstitial nitrogen-doped titanium dioxides with visible light photocatalytic activity. J. Solid State Chem. 2008, 181, 130–136. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2−xNx Powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. Photoelectron Spectroscopic Investigation of Nitrogen-Doped Titania Nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2 Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, Y.C.; Lee, S.C.; Park, S.Y.; Son, B.; Lee, J.W.; Lim, C.H.; Choi, C.J.; Choi, M.H.; Lee, S.Y.; et al. Visible-light-responsive bicrystalline (anatase/brookite) nanoporous nitrogen-doped TiO2 photocatalysts by plasma treatment. Chem. Eng. J. 2014, 254, 268–275. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and Characterization of Nitrogen-Doped TiO2 Nanophotocatalyst with High Visible Light Activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Kong, X.; Guo, X. Effect of calcination on the visible light photocatalytic activity of N-doped TiO2 prepared by the sol-gel method. J. Nanosci. Nanotechnol. 2014, 14, 3532–3537. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Mirzaei, A.; Eddah, M.; Roualdes, S.; Ma, D.; Chaker, M. Multiple-homojunction gradient nitrogen doped TiO2 for photocatalytic degradation of sulfamethoxazole, degradation mechanism, and toxicity assessment. Chem. Eng. J. 2021, 422, 130507. [Google Scholar] [CrossRef]
- Rani, A.; Dhiman, R.L.; Singh, V.; Kumar, S.; Kumar, S. Photocatalytic study of Ni-N-codoped TiO2 nanoparticles under visible light irradiation. Nano Express 2021, 2, 030002. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Promdet, P.; Hwang, G.B.; Li, J.; Nair, S.P.; Sathasivam, S.; Kafizas, A.; Carmalt, C.J.; Parkin, I.P. Zn and N Codoped TiO2 Thin Films: Photocatalytic and Bactericidal Activity. ACS Appl. Mater. Interfaces 2021, 13, 10480–10489. [Google Scholar] [CrossRef]
- Selcuk, M.Z.; Boroglu, M.S.; Boz, I. Hydrogen production by photocatalytic water-splitting using nitrogen and metal co-doped TiO2 powder photocatalyst. Reac. Kinet. Mech. Catal. 2012, 106, 313–324. [Google Scholar] [CrossRef]
- Lin, H.; Shih, C. Efficient one-pot microwave-assisted hydrothermal synthesis of M (M=Cr, Ni, Cu, Nb) and nitrogen co-doped TiO2 for hydrogen production by photocatalytic water splitting. J. Mol. Catal. A Chem. 2016, 411, 128–137. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; Patil, M.K.; Nadagouda, M.N.; Dionysiou, D.D. Hydrothermal synthesis of photoactive nitrogen- and boron- codoped TiO2 nanoparticles for the treatment of bisphenol A in wastewater: Synthesis, photocatalytic activity, degradation byproducts and reaction pathways. Appl. Catal. B Environ. 2019, 241, 598–611. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; Nadagouda, M.N.; Dionysiou, D.D. Solar light-assisted remediation of domestic wastewater by NB-TiO2 nanoparticles for potable reuse. Appl. Catal. B Environ. 2020, 269, 118807. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, Photocatalytic Activity, and Mechanism of Nano-TiO2 Co-Doped with Nitrogen and Iron (III). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and Nitrogen Co-doped TiO2 with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Yu, T.; Tan, X.; Zhao, L.; Yin, Y.; Chen, P.; Wei, J. Characterization, activity and kinetics of a visible light driven photocatalyst: Cerium and nitrogen co-doped TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 86–92. [Google Scholar] [CrossRef]
- Thind, S.S.; Wu, G.; Chen, A. Synthesis of mesoporous nitrogen–tungsten co-doped TiO2 photocatalysts with high visible light activity. Appl. Catal. B Environ. 2012, 111–112, 38–45. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Krause, R.W.M.; Mamba, B.B. Nitrogen/Palladium-Codoped TiO2 for Efficient Visible Light Photocatalytic Dye Degradation. J. Phys. Chem. C 2011, 115, 22110–22120. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhu, Z.; Yan, N.; Liu, Q. Preparation and properties of sliver and nitrogen co-doped TiO2 photocatalyst. Mater. Res. Bull. 2013, 48, 4872–4876. [Google Scholar] [CrossRef]
- Jaiswal, R.; Bharambe, J.; Patel, N.; Dashora, A.; Kothari, D.C.; Miotello, A. Copper and Nitrogen co-doped TiO2 photocatalyst with enhanced optical absorption and catalytic activity. Appl. Catal. B Environ. 2015, 168–169, 333–341. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Visible light activated carbon and nitrogen co-doped mesoporous TiO2 as efficient photocatalyst for degradation of ibuprofen. Sep. Purif. Technol. 2017, 173, 258–268. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; George, S.; Saharan, V.K.; Gogate, P.R.; Pandit, A.B. Synthesis and characterization of samarium and nitrogen doped TiO2 photocatalysts for photo-degradation of 4-acetamidophenol in combination with hydrodynamic and acoustic cavitation. Sep. Purif. Technol. 2019, 209, 254–269. [Google Scholar] [CrossRef]
- Sharotri, N.; Sharma, D.; Sud, D. Experimental and theoretical investigations of Mn-N-co-doped TiO2 photocatalyst for visible light induced degradation of organic pollutants. J. Mater. Res. Technol. 2019, 8, 3995–4009. [Google Scholar] [CrossRef]
- Park, I.-S.; Chung, K.-H.; Kim, S.-C.; Kim, S.-J.; Park, Y.-K.; Jung, S.-C. Photocatalytic degradation of 1,4-dioxane and hydrogen production using liquid phase plasma on N- and Ni- codoped TiO2 photocatalyst. Mater. Lett. 2021, 283, 128751. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thampi, K.R.; Tayade, R.J. Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B Environ. 2018, 227, 296–311. [Google Scholar] [CrossRef]
- Nakada, A.; Nishioka, S.; Vequizo, J.J.M.; Muraoka, K.; Kanazawa, T.; Yamakata, A.; Nozawa, S.; Kumagai, H.; Adachi, S.; Ishitani, O.; et al. Solar-driven Z-scheme water splitting using tantalum/nitrogen co-doped rutile titania nanorod as an oxygen evolution photocatalyst. J. Mater. Chem. A 2017, 5, 11710–11719. [Google Scholar] [CrossRef]
- Miyoshi, A.; Kato, K.; Yokoi, T.; Wiesfeld, J.J.; Nakajima, K.; Yamakara, A.; Maeda, K. Nano vs. bulk rutile TiO2:N,F in Z-scheme overall water splitting under visible light. J. Mater. Chem. A 2020, 8, 11996–12002. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced Nitrogen Doping in TiO2 Nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A.Y. Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Synthesis and characterization of N-doped TiO2 nanoparticles and their application in photocatalytic oxidation of dibenzothiophene under visible light. Ceram. Int. 2016, 42, 14834–14842. [Google Scholar] [CrossRef]
- RamezaniSani, S.; Rajabi, M.; Mohseni, F. Influence of nitrogen doping on visible light photocatalytic activity of TiO2 nanowires with anatase-rutile junction. Chem. Phys. Lett. 2020, 744, 137217. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S.G.; Shirsat, R.N. Influence of N sources on the photocatalytic activity of N-doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651. [Google Scholar] [CrossRef]
- Huang, J.; Dou, L.; Li, J.; Zhong, J.; Li, M.; Wang, T. Excellent visible light responsive photocatalytic behavior of N-doped TiO2 toward decontamination of organic pollutants. J. Hazard. Mater. 2021, 403, 123857. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, X.; Wang, Y. Photocatalytic degradation of flumequine by N-doped TiO2 catalysts under simulated sunlight. Environ. Eng. Res. 2021, 26, 200524. [Google Scholar] [CrossRef]
- Tryba, B.; Wozniak, M.; Zolnierkiewicz, G.; Guskos, N.; Morawski, A.; Colbeau-Justin, C.; Wrobel, R.; Nitta, A.; Ohtani, B. Influence of an Electronic Structure of N-TiO2 on Its Photocatalytic Activity towards Decomposition of Acetaldehyde under UV and Fluorescent Lamps Irradiation. Catalysts 2018, 8, 85. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven nitrogen-doped TiO2 photocatalysts: Effect of nitrogen precursors on their photocatalysis for decomposition of gas-phase organic pollutants. Mater. Sci. Eng. B 2005, 117, 67–75. [Google Scholar] [CrossRef]
- Aoki, K.; Morikawa, T.; Ohwaki, T.; Taga, Y. Photocatalytic Degradation of Formaldehyde and Toluene Mixtures in Air with a Nitrogen-doped TiO2 Photocatalyst. Chem. Lett. 2006, 35, 616–617. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kim, J.-T. Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 2009, 164, 360–366. [Google Scholar] [CrossRef]
- He, F.; Ma, F.; Li, T.; Li, G. Solvothermal synthesis of N-doped TiO2 nanoparticles using different nitrogen sources, and their photocatalytic activity for degradation of benzene. Chin. J. Catal. 2013, 34, 2263–2270. [Google Scholar] [CrossRef]
- Priya, V.S.; Philip, L. Photocatalytic Degradation of Aqueous VOCs Using N Doped TiO2: Comparison of Photocatalytic Degradation under Visible and Sunlight Irradiation. Int. J. Environ. Sci. Dev. 2015, 6, 286–291. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Chen, X.; Chu, H.; Fu, H.; Wang, C.-C. Robust photocatalytic benzene degradation using mesoporous disk-like N-TiO2 derived from MIL-125(Ti). Chin. J. Catal. 2020, 41, 1186–1197. [Google Scholar] [CrossRef]
- Suda, Y.; Kawasaki, H.; Ueda, T.; Ohshima, T. Preparation of high quality nitrogen doped TiO2 thin film as a photocatalyst using a pulsed laser deposition method. Thin Solid Film. 2004, 453–454, 162–166. [Google Scholar] [CrossRef]
- Joung, S.-K.; Amemiya, T.; Murabayashi, M.; Itoh, K. Relation between photocatalytic activity and preparation conditions for nitrogen-doped visible light-driven TiO2 photocatalysts. Appl. Catal. A Gen. 2006, 312, 20–26. [Google Scholar] [CrossRef]
- Fang, J.; Wang, F.; Qian, K.; Bao, H.; Jiang, Z.; Huang, W. Bifunctional N-Doped Mesoporous TiO2 Photocatalysts. J. Phys. Chem. C 2008, 112, 18150–18156. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F. New approaches to prepare nitrogen-doped TiO2 photocatalysts and study on their photocatalytic activities in visible light. Appl. Catal. B Environ. 2009, 89, 563–569. [Google Scholar] [CrossRef]
- Tang, Y.C.; Huang, X.H.; Yu, H.Q.; Tang, L.H. Nitrogen-Doped Photocatalyst Prepared by Mechanochemical Method: Doping Mechanisms and Visible Photoactivity of Pollutant Degradation. Int. J. Photoenergy 2011, 2012, 960726. [Google Scholar]
- Powell, M.J.; Dunnill, C.W.; Parkin, I.P. N-doped TiO2 visible light photocatalyst films via a sol–gel route using TMEDA as the nitrogen source. J. Photochem. Photobiol. A Chem. 2014, 281, 27–34. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170–171, 153–161. [Google Scholar] [CrossRef]
- Huang, W.C.; Ting, J.M. Novel nitrogen-doped anatase TiO2 mesoporous bead photocatalysts for enhanced visible light response. Ceram. Int. 2017, 43, 9992–9997. [Google Scholar] [CrossRef]
- Calisir, M.D.; Gungor, M.; Demir, A.; Kilic, A.; Khan, M.M. Nitrogen-doped TiO2 fibers for visible-light-induced photocatalytic activities. Ceram. Int. 2020, 46, 16743–16753. [Google Scholar] [CrossRef]
- Divyasri, Y.V.; Reddy, N.L.; Lee, K.; Sakar, M.; Rao, V.N.; Venkatramu, V.; Shankar, M.V.; Reddy, N.C.G. Optimization of N doping in TiO2 nanotubes for the enhanced solar light mediated photocatalytic H2 production and dye degradation. Environ. Pollut. 2021, 269, 116170. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.S.; Jadhav, T.; Bhosale, M.; Jadhav, C.H.; Pawar, V.C. Structural and optical properties of N-doped TiO2 nanomaterials. Mater. Today Proc. 2021, 43, 2763–2767. [Google Scholar] [CrossRef]
- Fiorenza, R.; Mauro, A.D.; Cantarella, M.; Gulino, A.; Spitaleri, L.; Privitera, V.; Impellizzeri, G. Molecularly imprinted N-doped TiO2 photocatalysts for the selective degradation of o-phenylphenol fungicide from water. Mater. Sci. Semicond. Process. 2020, 112, 105019. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Peng, D.; Sheng, D.; Di Ma, Y.T.; Zhang, Y. Nitrogen-doped mixed-phase TiO2 with controllable phase junction as superior visible-light photocatalyst for selective oxidation of cyclohexane. Appl. Surf. Sci. 2021, 536, 147953. [Google Scholar] [CrossRef]
- Tzeng, J.-H.; Weng, C.-H.; Yen, L.-T.; Gaybullaev, G.; Chang, C.-J.; de Luna, M.D.G.; Lin, Y.-T. Inactivation of pathogens by visible light photocatalysis with nitrogen-doped TiO2 and tourmaline-nitrogen co-doped TiO2. Sep. Purif. Technol. 2021, 274, 118979. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Wang, Y.; Shi, G.; Xie, X.; Sun, J. Anti-oxidative microstructure design of ultra-stable N-TiO2 composite for the gaseous photodegradation reactions. Chem. Eng. J. 2021, 408, 127257. [Google Scholar] [CrossRef]
- Li, H.; Yin, S.; Wang, Y.; Sato, T. Persistent Fluorescence-Assisted TiO2−xNy-Based Photocatalyst for Gaseous Acetaldehyde Degradation. Environ. Sci. Technol. 2012, 46, 7741–7745. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Samokhvalov, A. Hydrogen by photocatalysis with nitrogen codoped titanium dioxide. Renew. Sustain. Energy Rev. 2017, 72, 981–1000. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.-V.N.; Kim, S.Y.; Le, Q.V. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kibria, M.G.; Mullins, C.B. Metal-free photocatalysts for hydrogen evolution. Chem. Soc. Rev. 2020, 49, 1887–1931. [Google Scholar] [CrossRef]
- Babu, V.J.; Kumar, M.K.; Nair, A.S.; Kheng, T.L.; Allakhverdiev, S.I.; Ramakrishna, S. Visible light photocatalytic water splitting for hydrogen production from N-TiO2 rice grain shaped electrospun nanostructures. Int. J. Hydrog. Energy 2012, 37, 8897–8904. [Google Scholar] [CrossRef]
- Zhang, X.; Song, P.; Cui, X. Nitrogen-doped TiO2 Photocatalysts Synthesized from Titanium Nitride: Characterizations and Photocatalytic Hydrogen Evolution Performance. J. Adv. Oxid. Technol. 2013, 16, 131–136. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.-Q.; Huang, J.-Q.; Deng, Z.-H.; Shi, H.-L.; Wu, L.; Liu, Z.-G.; Cao, Y.-G. Effective water splitting using N-doped TiO2 films: Role of preferred orientation on hydrogen production. Int. J. Hydrog. Energy 2014, 39, 1967–1971. [Google Scholar] [CrossRef]
- Fakhouri, H.; Pulpytel, J.; Smith, W.; Zolfaghari, A.; Mortaheb, H.R.; Meshkini, F.; Jafari, R.; Sutter, E.; Arefi-Khonsari, F. Control of the visible and UV light water splitting and photocatalysis of nitrogen doped TiO2 thin films deposited by reactive magnetron sputtering. Appl. Catal. B Environ. 2014, 144, 12–21. [Google Scholar] [CrossRef]
- Sun, S.; Gao, P.; Yang, Y.; Yang, P.; Chen, Y.; Wang, Y. N-Doped TiO2 Nanobelts with Coexposed (001) and (101) Facets and Their Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2016, 8, 18126–18131. [Google Scholar] [CrossRef]
- Esmat, M.; El-Hosainy, H.; Tahawy, R.; Jevasuwan, W.; Tsunoji, N.; Fukata, N.; Ide, Y. Nitrogen doping-mediated oxygen vacancies enhancing co-catalyst-free solar photocatalytic H2 production activity in anatase TiO2 nanosheet assembly. Appl. Catal. B Environ. 2021, 285, 119755. [Google Scholar] [CrossRef]
- Villa, K.; Black, A.; Domènech, X.; Peral, J. Nitrogen doped TiO2 for hydrogen production under visible light irradiation. Sol. Energy 2012, 86, 558–566. [Google Scholar] [CrossRef]
- Lin, H.; Shih, C. Efficient One-Pot Microwave-Assisted Hydrothermal Synthesis of Nitrogen-Doped TiO2 for Hydrogen Production by Photocatalytic Water Splitting. Catal. Surv. Asia 2012, 16, 231–239. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.Q.; Huang, J.Q.; Wu, L.; Deng, Z.H.; Liu, Z.G.; Liu, Y.; Cao, Y.G. Efficient hydrogen production by photocatalytic water splitting using N-doped TiO2 film. Appl. Surf. Sci. 2013, 283, 188–192. [Google Scholar] [CrossRef]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; Loo, S.C.J.; Wong, L.H.; Dash, S.; Tyagi, A.K. Nitrogen doped anatase-rutile heterostructured nanotubes for enhanced photocatalytic hydrogen production: Promising structure for sustainable fuel production. Int. J. Hydrog. Energy 2016, 41, 5865–5877. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kim, K.H.; Kumar, M.K.; Manvitha, C.; Shim, J.J. Novel approach for the synthesis of nitrogen-doped titania with variable phase composition and enhanced production of hydrogen under solar irradiation. J. Ind. Eng. Chem. 2017, 53, 253–260. [Google Scholar] [CrossRef]
- Liu, S.H.; Tang, W.T.; Lin, W.X. Self-assembled ionic liquid synthesis of nitrogen-doped mesoporous TiO2 for visible-light-responsive hydrogen production. Int. J. Hydrog. Energy 2017, 42, 24006–24013. [Google Scholar] [CrossRef]
- Xu, X.L.; Song, W. Enhanced H2 production activity under solar irradiation over N-doped TiO2 prepared using pyridine as a precursor: A typical sample of N-doped TiO2 series. Mater. Technol. 2017, 32, 52–63. [Google Scholar] [CrossRef]
- Zangeneh, H.; Farhadiana, M.; Zinatizadeh, A.A. A reusable visible driven N and C–N doped TiO2 magnetic nanocomposites for photodegradation of direct red 16 azo dye in water and wastewater. Environ. Technol. 2020, 1–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).