Catalytic Composite Systems Based on N-Doped TiO2/Polymeric Materials for Visible-Light-Driven Pollutant Degradation: A Mini Review
Abstract
:1. Introduction
- (a)
- the Eg of anatase TiO2 is 3.2 eV, i.e., it absorbs light in the UV region, so that only a small portion (5%) of sunlight can be used for a photocatalytic process. This is a great limitation in its use as a photocatalyst for the conversion of solar energy into chemical energy [20];
- (b)
- (c)
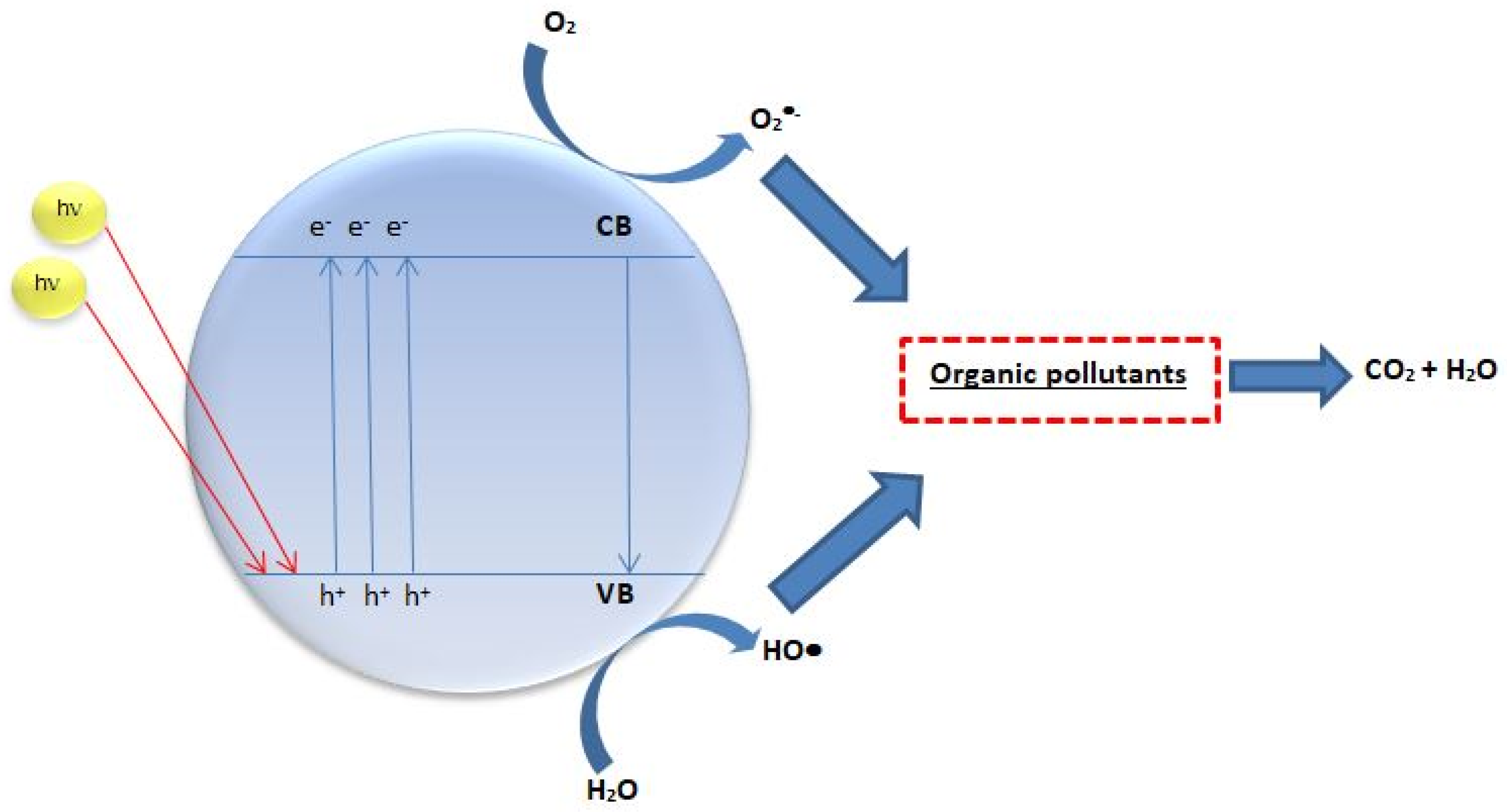
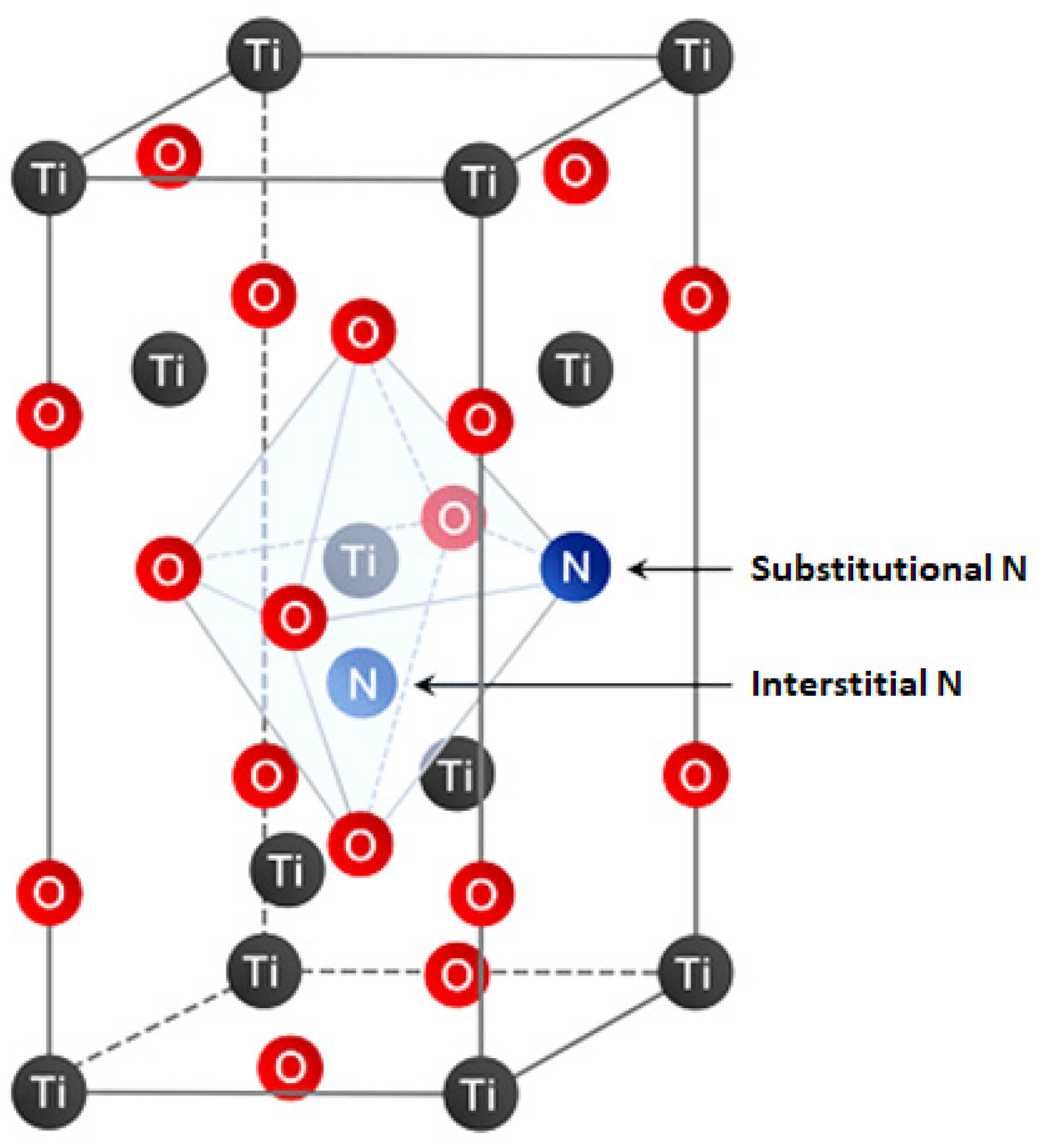
2. Why N-Doped TiO2 for Heterogeneous Photocatalysis?
3. Immobilization of N-Doped TiO2 on Polymeric Supports
4. General Overview on Methods for the Immobilization of N-Doped TiO2 on Polymeric Supports
5. Applications of N-Doped TiO2 on Polymeric Supports as Visible-Light-Active Photocatalytic Systems
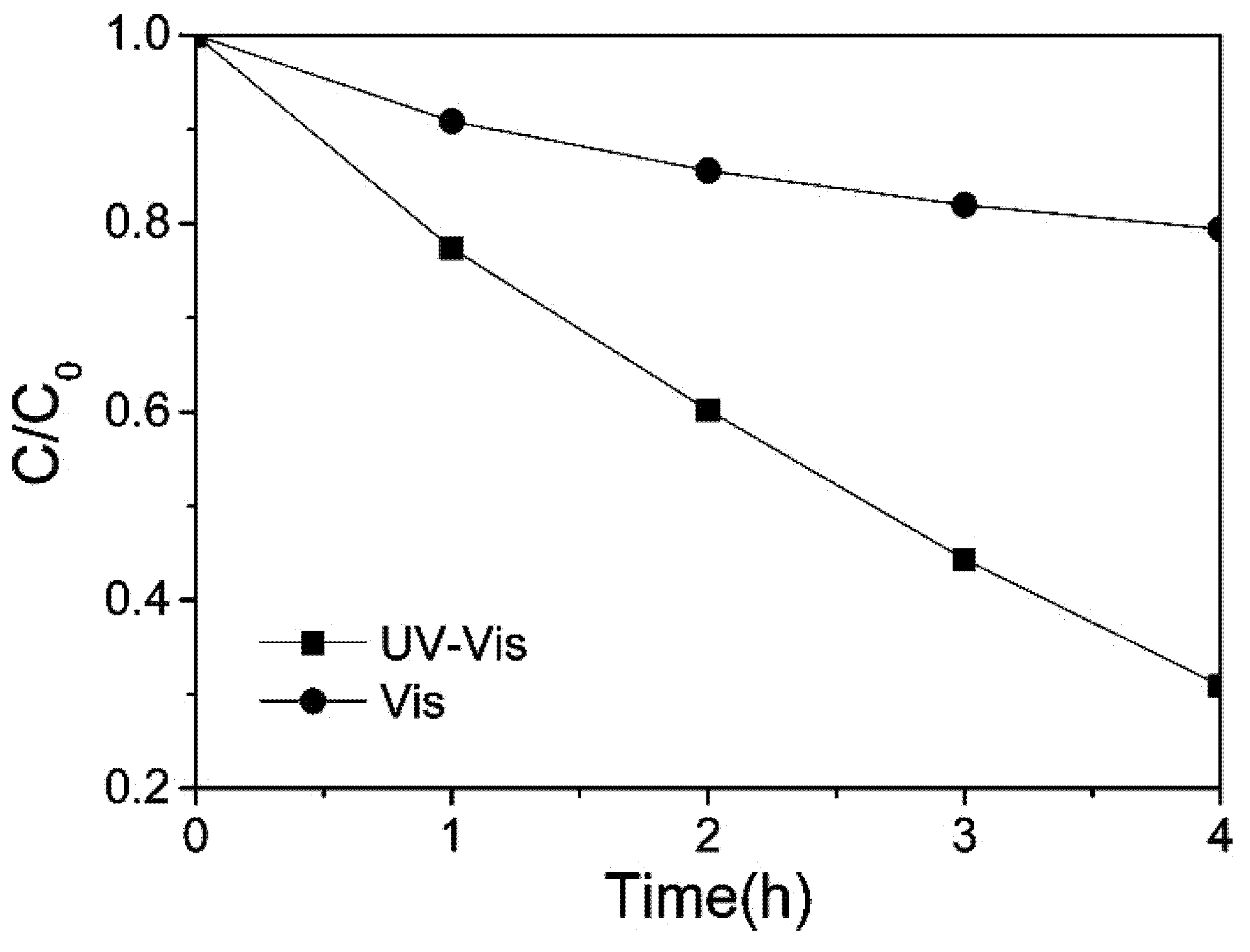
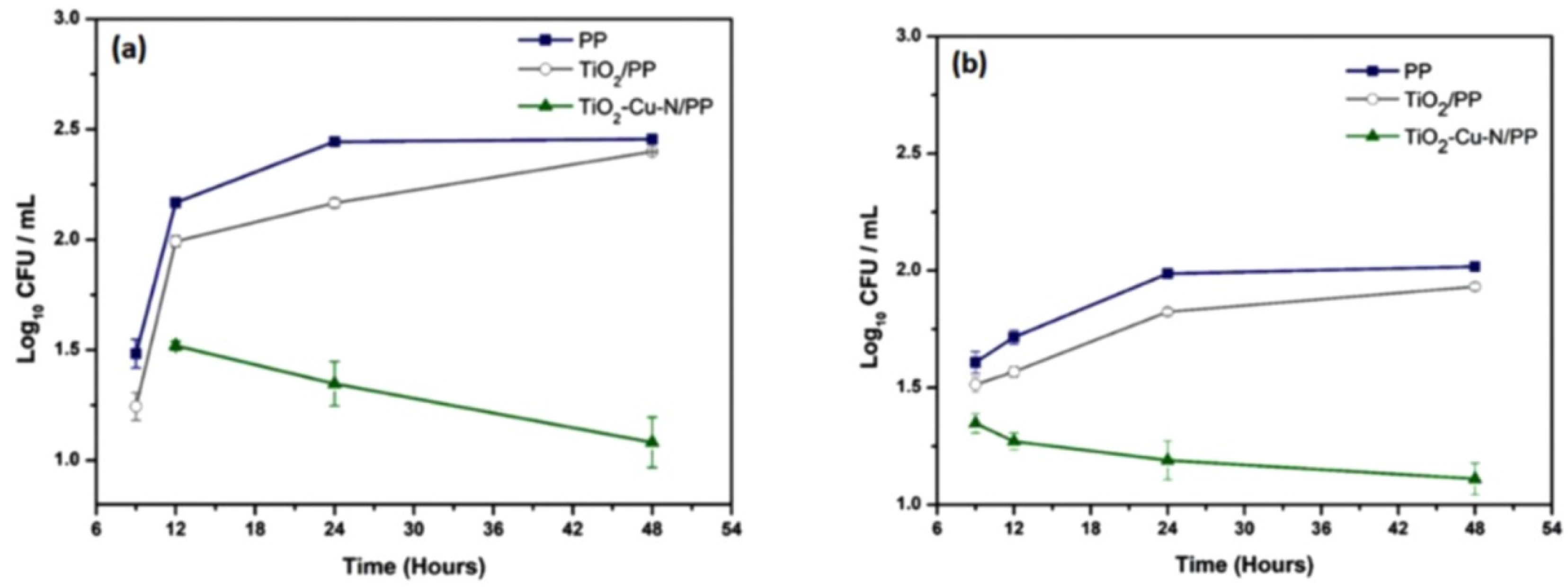
5.1. N-Doped TiO2/PP (Polypropylene)
5.2. N-Doped TiO2/PS (Polystyrene)
5.2.1. N-Doped TiO2/Syndiotactic Polystyrene (s-PS) Monolithic Aerogels
5.3. N-Doped TiO2/PET (Polyethylene Terephthalate)
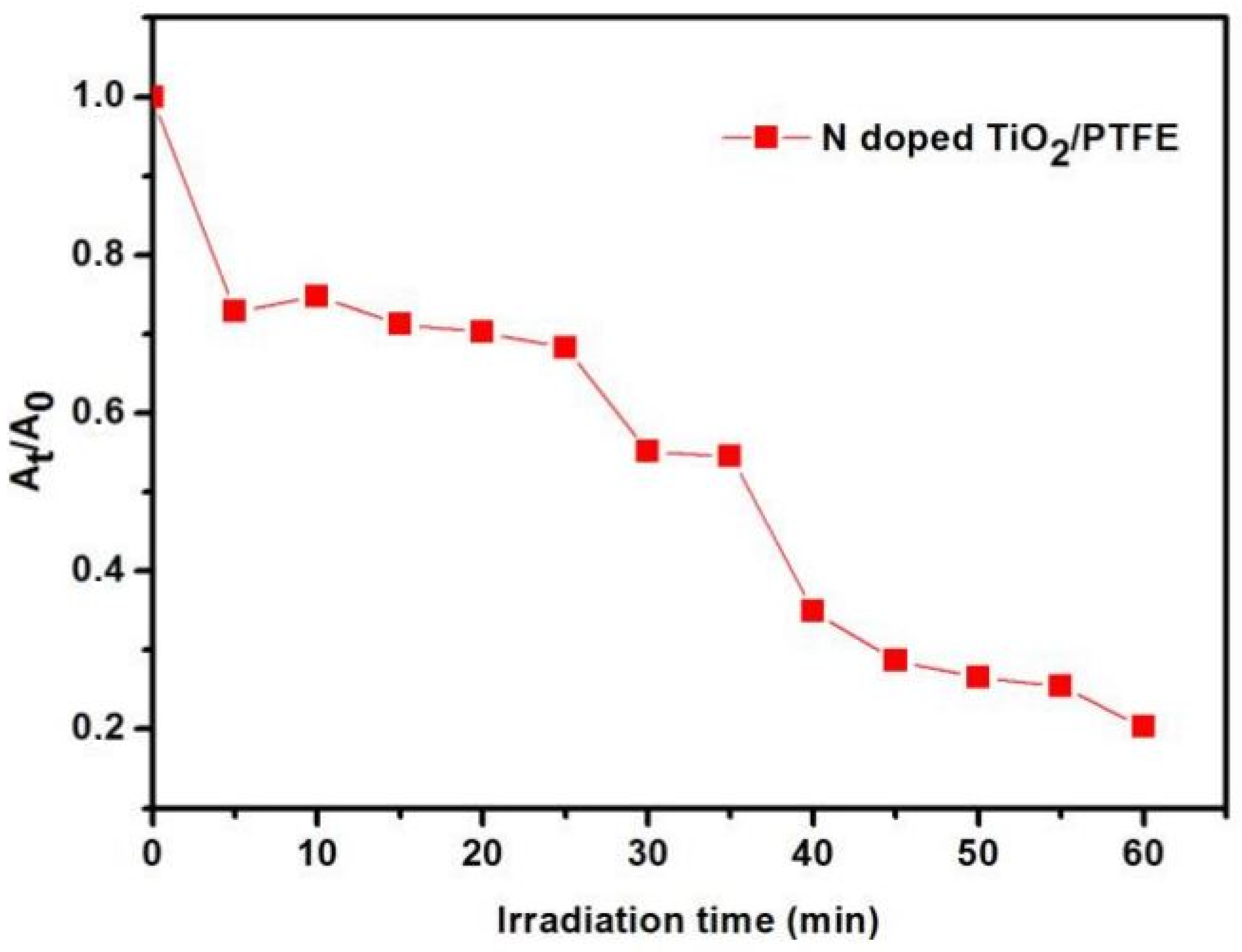
5.4. N-Doped TiO2/PTFE (Polytetrafluoroethylene)
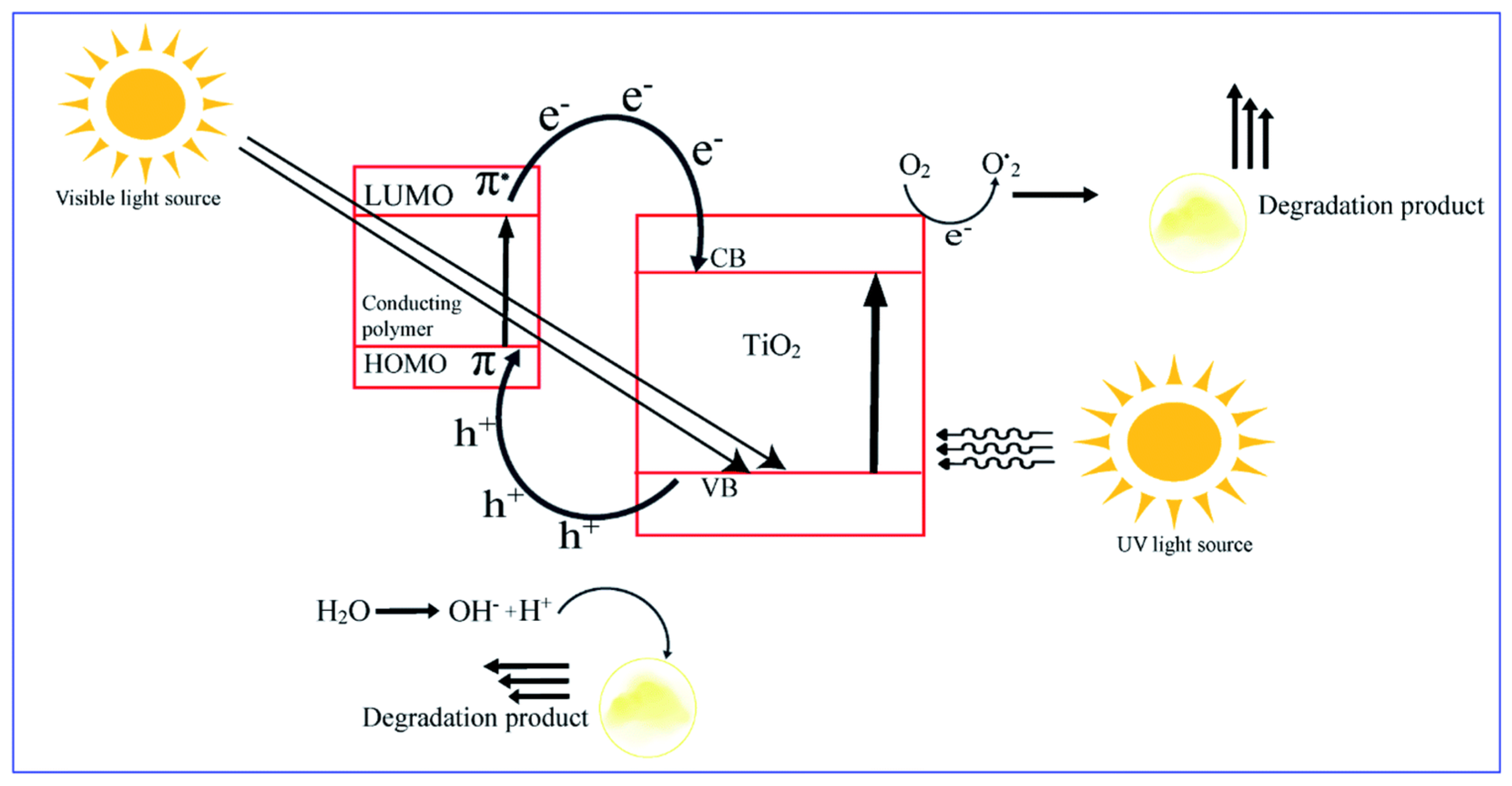
5.5. Conducting-Polymer-Based Photocatalysis
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- World Wide Fund (WWF) Organization. Available online: http://www.worldwildlife.org/threats/water-scarcity (accessed on 8 August 2021).
- Fiorentino, A.; Ferro, G.; Alferez, M.C.; Polo-López, M.I.; Fernández-Ibañez, P.; Rizzo, L. Inactivation and regrowth of multidrug resistant bacteria in urban wastewater after disinfection by solar-driven and chlorination processes. J. Photochem. Photobiol. B 2015, 148, 43–50. [Google Scholar] [CrossRef]
- Alhaji, M.; Sanaullah, K.; Khan, A.; Hamza, A.; Muhammad, A.; Ishola, M.; Rigit, A.; Bhawani, S. Recent developments in immobilizing titanium dioxide on supports for degradation of organic pollutants in wastewater-A review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052. [Google Scholar] [CrossRef]
- Schiavello, M. Heterogeneous Photocatalysis, 1st ed.; Wiley: New Jersey, NJ, USA, 1997. [Google Scholar]
- Yang, Z.; Qi, Y.; Wang, F.; Han, Z.; Jiang, Y.; Han, H.; Liu, J.; Zhang, X.; Ong, W.J. State-of-the-art advancements in photo-assisted CO 2 hydrogenation: Recent progress in catalyst development and reaction mechanisms. J. Mater. Chem. A 2020, 8, 24868–24894. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Dunnill, C.W.; Parkin, I.P. Nitrogen-doped TiO2 thin films: Photocatalytic applications for healthcare environments. Dalton Trans. 2011, 40, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Petronella, F.; Truppi, A.; Dell’Edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable synthesis of mesoporous TiO2 for environmental photocatalytic applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef] [Green Version]
- Mansurov, R.; Safronov, A.; Lakiza, N.; Beketov, I. Photocatalytic Activity of Titanium Dioxide Nanoparticles Immobilized in the Polymer Network of Polyacrylamide Hydrogel. Russ. J. Appl. Chem. 2017, 90, 1712–1721. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Vaiano, V.; Sacco, O.; Matarangolo, M. Photocatalytic degradation of paracetamol under UV irradiation using TiO2-graphite composites. Catal. Today 2018, 315, 230–236. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D.; Picca, R.; Cioffi, N. Ag modified ZnS for photocatalytic water pollutants degradation: Influence of metal loading and preparation method. J. Colloid Interface Sci. 2019, 537, 671–681. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Matarangolo, M. ZnO supported on zeolite pellets as efficient catalytic system for the removal of caffeine by adsorption and photocatalysis. Sep. Purif. Technol. 2018, 193, 303–310. [Google Scholar] [CrossRef]
- Fessi, N.; Nsib, M.F.; Chevalier, Y.; Guillard, C.; Dappozze, F.d.r.; Houas, A.; Palmisano, L.; Parrino, F. Pickering emulsions of fluorinated TiO2: A new route for intensification of photocatalytic degradation of nitrobenzene. Langmuir 2020, 36, 13545–13554. [Google Scholar] [CrossRef]
- Žerjav, G.; Scandura, G.; Garlisi, C.; Palmisano, G.; Pintar, A. Sputtered vs. sol-gel TiO2-doped films: Characterization and assessment of aqueous bisphenol A oxidation under UV and visible light radiation. Catal. Today 2020, 357, 380–391. [Google Scholar] [CrossRef]
- Uribe-López, M.; Hidalgo-López, M.; López-González, R.; Frías-Márquez, D.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.; Jaramillo-Páez, C.; Sánchez-Cid, P.; Hidalgo, M. Microwave-assisted sol-gel synthesis of TiO2 in the presence of halogenhydric acids. Characterization and photocatalytic activity. J. Photochem. Photobiol. A Chem. 2020, 394, 112457. [Google Scholar] [CrossRef]
- Laguna, O.H.; Murcia, J.J.; Rojas, H.; Jaramillo-Paez, C.; Navío, J.A.; Hidalgo, M.C. Differences in the catalytic behavior of Au-metalized TiO2 systems during phenol photo-degradation and CO oxidation. Catalysts 2019, 9, 331. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Zhang, Y.; Hou, R.; Zhang, X.; Xue, C.; Wang, S.; Zhu, B.; Li, N.; Shao, G. Photogenerated electron transfer process in heterojunctions: In situ irradiation XPS. Small Methods 2020, 4, 2000214. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Pisano, D.; Sannino, D.; Ciambelli, P. From the design to the development of a continuous fixed bed photoreactor for photocatalytic degradation of organic pollutants in wastewater. Chem. Eng. Sci. 2015, 137, 152–160. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Sannino, D. Main parameters influencing the design of photocatalytic reactors for wastewater treatment: A mini review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO 2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Yusuf, A.; Oladipo, H.; Ozer, L.Y.; Garlisi, C.; Loddo, V.; Abu-Zahra, M.R.; Palmisano, G. Modelling of a recirculating photocatalytic microreactor implementing mesoporous N-TiO2 modified with graphene. Chem. Eng. J. 2020, 391, 123574. [Google Scholar] [CrossRef]
- Kim, T.H.; Go, G.-M.; Cho, H.-B.; Song, Y.; Lee, C.-G.; Choa, Y.-H. A novel synthetic method for N doped TiO2 nanoparticles through plasma-assisted electrolysis and photocatalytic activity in the visible region. Front. Chem. 2018, 6, 458. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Zhang, J.; Chen, F. New approaches to prepare nitrogen-doped TiO2 photocatalysts and study on their photocatalytic activities in visible light. Appl. Catal. B Environ. 2009, 89, 563–569. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Mrowetz, M.; Balcerski, W.; Colussi, A.; Hoffmann, M.R. Oxidative power of nitrogen-doped TiO2 photocatalysts under visible illumination. J. Phys. Chem. B 2004, 108, 17269–17273. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 photocatalysts: A review of their characteristics and capacity for emerging contaminants removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO 2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-x N x powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Zhao, A.; Masa, J.; Xia, W.; Maljusch, A.; Willinger, M.-G.; Clavel, G.; Xie, K.; Schlögl, R.; Schuhmann, W.; Muhler, M. Spinel Mn–Co oxide in N-doped carbon nanotubes as a bifunctional electrocatalyst synthesized by oxidative cutting. J. Am. Chem. Soc. 2014, 136, 7551–7554. [Google Scholar] [CrossRef] [PubMed]
- Beranek, R.; Kisch, H. Surface-modified anodic TiO2 films for visible light photocurrent response. Electrochem. Commun. 2007, 9, 761–766. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Tennakone, K.; Tilakaratne, C.; Kottegoda, I. Photocatalytic degradation of organic contaminants in water with TiO2 supported on polythene films. J. Photochem. Photobiol. A Chem. 1995, 87, 177–179. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Buoyant photocatalyst with greatly enhanced visible-light activity prepared through a low temperature hydrothermal method. Ind. Eng. Chem. Res. 2009, 48, 2891–2898. [Google Scholar] [CrossRef]
- Tavares, C.; Marques, S.; Lanceros-Méndez, S.; Rebouta, L.; Alves, E.; Barradas, N.; Munnik, F.; Girardeau, T.; Rivière, J.-P. N-doped photocatalytic titania thin films on active polymer substrates. J. Nanosci. Nanotechnol. 2010, 10, 1072–1077. [Google Scholar] [CrossRef]
- Betancur-Henao, C.; Hernández-Montes, V.; Santa-Marín, J.; Buitrago-Sierra, R. Polypropylene modified with Cu–N-doped titanium dioxide forantibacterial applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035016. [Google Scholar] [CrossRef]
- Aini, F.; Hastuti, S.; Wahyuningsih, S. Synthesis N doped TÍO2/PTFE by solvothermal method and their self cleaning performance. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 858, p. 012022. [Google Scholar]
- Vaez, M.; Alijani, S.; Omidkhah, M.; Zarringhalam Moghaddam, A. Synthesis, characterization and optimization of N-TiO2/PANI nanocomposite for photodegradation of acid dye under visible light. Polym. Compos. 2018, 39, 4605–4616. [Google Scholar] [CrossRef]
- Ata, R.; Sacco, O.; Vaiano, V.; Rizzo, L.; Tore, G.Y.; Sannino, D. Visible light active N-doped TiO2 immobilized on polystyrene as efficient system for wastewater treatment. J. Photochem. Photobiol. A Chem. 2017, 348, 255–262. [Google Scholar] [CrossRef]
- Kowalska, K.; Maniakova, G.; Carotenuto, M.; Sacco, O.; Vaiano, V.; Lofrano, G.; Rizzo, L. Removal of carbamazepine, diclofenac and trimethoprim by solar driven advanced oxidation processes in a compound triangular collector based reactor: A comparison between homogeneous and heterogeneous processes. Chemosphere 2020, 238, 124665. [Google Scholar] [CrossRef] [PubMed]
- Sacco, O.; Sannino, D.; Vaiano, V. Packed bed photoreactor for the removal of water pollutants using visible light emitting diodes. Appl. Sci. 2019, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Di Capua, G.; Femia, N.; Sannino, D. Use of visible light modulation techniques in urea photocatalytic degradation. Water 2019, 11, 1642. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Ju, J.; He, H.; Li, F.; Tao, L.; Dong, Y.; Cheng, B. Photocatalytic Degradation Performance of TiO2/PTFE Membrane Catalyst to Methylene Blue. Chem. Lett. 2016, 45, 1440–1443. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Heimfarth, T.; Mulato, M. Influence of the physical–chemical properties of polyaniline thin films on the final sensitivity of varied field effect sensors. Mater. Chem. Phys. 2015, 160, 257–263. [Google Scholar] [CrossRef]
- Zhang, H.; Han, Y.; Yang, L.; Guo, X.; Wu, H.; Mao, N. Photocatalytic activities of PET filaments deposited with N-doped TiO2 nanoparticles sensitized with disperse blue dyes. Catalysts 2020, 10, 531. [Google Scholar] [CrossRef]
- Dahal, P.; Kim, Y.C. Preparation and characterization of modified polypropylene by using electron beam irradiation. J. Ind. Eng. Chem. 2013, 19, 1879–1883. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P.; Longo, S.; Venditto, V.; Guerra, G. N-doped TiO2/s-PS aerogels for photocatalytic degradation of organic dyes in wastewater under visible light irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1175–1181. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Removal of phenol in aqueous media by N-doped TiO2 based photocatalytic aerogels. Mater. Sci. Semicond. Process. 2018, 80, 104–110. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462, 178–195. [Google Scholar] [CrossRef]
- Langlet, M.; Kim, A.; Audier, M.; Herrmann, J. Sol-gel preparation of photocatalytic TiO 2 films on polymer substrates. J. Sol-Gel Sci. Technol. 2002, 25, 223–234. [Google Scholar] [CrossRef]
- Sivlim, T.; Akkan, Ş.; Altın, İ.; Koç, M.; Sökmen, M. TiO2 immobilized biodegradable polymer for photocatalytic removal of chlorophenol. Water Air Soil Pollut. 2012, 223, 3955–3964. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Highly robust and selective system for water pollutants removal: How to transform a traditional photocatalyst into a highly robust and selective system for water pollutants removal. Nanomaterials 2019, 9, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Imai, H.; Hirashima, H.; Tsukuma, K. Low-temperature synthesis of anatase thin films on glass and organic substrates by direct deposition from aqueous solutions. Thin Solid Film. 1999, 351, 220–224. [Google Scholar] [CrossRef]
- Sacco, O.; Venditto, V.; Fittipaldi, R.; Vaiano, V.; Daniel, C. Composite Polymeric Films with Photocatalytic Properties. Chem. Eng. Trans. 2021, 86, 571–576. [Google Scholar]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227, 185–194. [Google Scholar] [CrossRef]
- Kasanen, J.; Suvanto, M.; Pakkanen, T.T. Self-cleaning, titanium dioxide based, multilayer coating fabricated on polymer and glass surfaces. J. Appl. Polym. Sci. 2009, 111, 2597–2606. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Isotactic polypropylene modified with sorbitol-based derivative and siloxane-silsesquioxane resin. Eur. Polym. J. 2016, 85, 62–71. [Google Scholar] [CrossRef]
- Fabiyi, M.; Skelton, R. Photocatalytic mineralisation of methylene blue using buoyant TiO2-coated polystyrene beads. J. Photochem. Photobiol. A Chem. 2000, 132, 121–128. [Google Scholar] [CrossRef]
- Altın, I.; Sökmen, M. Preparation of TiO2-polystyrene photocatalyst from waste material and its usability for removal of various pollutants. Appl. Catal. B Environ. 2014, 144, 694–701. [Google Scholar] [CrossRef]
- Magalhães, F.; Lago, R. Floating photocatalysts based on TiO2 grafted on expanded polystyrene beads for the solar degradation of dyes. Sol. Energy 2009, 83, 1521–1526. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Navarra, W.; Daniel, C.; Venditto, V. Influence of aggregate size on photoactivity of N-doped TiO2 particles in aqueous suspensions under visible light irradiation. J. Photochem. Photobiol. A Chem. 2017, 336, 191–197. [Google Scholar] [CrossRef]
- Dijkstra, M.; Buwalda, H.; De Jong, A.; Michorius, A.; Winkelman, J.; Beenackers, A. Experimental comparison of three reactor designs for photocatalytic water purification. Chem. Eng. Sci. 2001, 56, 547–555. [Google Scholar] [CrossRef]
- El-Kalliny, A.S.; Ahmed, S.F.; Rietveld, L.C.; Appel, P.W. Immobilized photocatalyst on stainless steel woven meshes assuring efficient light distribution in a solar reactor. Drink. Water Eng. Sci. 2014, 7, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Li, J.; Liu, A.; Chen, C. Carbon Gels-Modified TiO2: Promising Materials for Photocatalysis Applications. Materials 2020, 13, 1734. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Zhang, R.; Ma, M.; Zhou, Y. Monolithic aerogel photocatalysts: A review. J. Mater. Chem. A 2018, 6, 754–775. [Google Scholar] [CrossRef]
- Daniel, C.; Navarra, W.; Venditto, V.; Sacco, O.; Vaiano, V. Nanoporous Polymeric Aerogels—Based Structured Photocatalysts for the Removal of Organic Pollutant from Water under Visible or Solar Light. Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–120. [Google Scholar]
- de Barros, A.L.; Domingos, A.A.Q.; Fechine, P.B.A.; de Keukeleire, D.; do Nascimento, R.F. PET as a support material for TiO2 in advanced oxidation processes. J. Appl. Polym. Sci. 2014, 131, 107792. [Google Scholar] [CrossRef]
- Heredia, M.; Duffy, J. Photocatalytic destruction of water pollutants using a TiO2 film in pet bottles. In Proceedings of the Solar Conference; University of Massachusetts: Boston, MA, USA, January 2007; p. 277. [Google Scholar]
- Kumar, R.; Travas-Sejdic, J.; Padhye, L.P. Conducting polymers-based photocatalysis for treatment of organic contaminants in water. Chem. Eng. J. Adv. 2020, 4, 100047. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Jouyandeh, M.; Yazdi, M.K.; Zarrintaj, P.; Saeb, M.R.; Lima, E.C.; Gupta, V.K. Conductive polymers in water treatment: A review. J. Mol. Liq. 2020, 312, 113447. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Reddy, K.R.; Karthik, K.; Prasad, S.B.; Soni, S.K.; Jeong, H.M.; Raghu, A.V. Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Lee, S.; Cho, I.-S.; Lee, D.K.; Kim, D.W.; Noh, T.H.; Kwak, C.H.; Park, S.; Hong, K.S.; Lee, J.-K.; Jung, H.S. Influence of nitrogen chemical states on photocatalytic activities of nitrogen-doped TiO2 nanoparticles under visible light. J. Photochem. Photobiol. A Chem. 2010, 213, 129–135. [Google Scholar] [CrossRef]


| Polymers | Physical–Chemical Properties | Ref. |
|---|---|---|
| PTFE | Hydrophobic, narrow pore size, better porosity and excellent mechanical strength | [51] |
| PANI | Environmentally stable, nontoxic and low-cost | [52] |
| PVDF | Electroactive polymer | [42] |
| PET | Good mechanical and thermal durability, inherently non-absorptive to water, resilient to wrinkling as well as shrinking and resistant to most chemicals | [53] |
| PP | Good mechanical and physical properties, lightweight and high melting point, among others | [54] |
| PS | Cheap, inert, non-toxic and low-density thermoplastic | [46] |
| Polymers | Method of Synthesis | Pollutant | Light Sources | Ref. |
|---|---|---|---|---|
| PTFE | Solvothermal | Methylene blue | Halogen lamp, 300 W | [44] |
| PANI | PPMSG method | Acid Red 73 | Halogen lamp, 300 W | [45] |
| PVDF | Magnetron sputtering | RED 41 | Xenon lamp, 150 W | [42] |
| PET | Hydrothermal process | Methylene blue | Xenon lamp, 300 W | [53] |
| PP | Dip-coating process | E. coli, S. aureus and MRSA | - | [43] |
| PP | Low-temperature hydrothermal method | Methyl orange | Xenon lamp, 150 W | [41] |
| PS | Solvent-casting method | Metlhylene blue, E.coli | - | [46] |
| PS | Solvent-casting method | Carbamazepine, diclofenac and trimethoprim | Solar light | [47] |
| PS | Solvent-casting method | Methylene blue | White LEDs | [48] |
| PS | Solvent-casting method | E.coli | Solar light | [49] |
| PS | Solvent-casting method | Urea | White LEDs | [50] |
| sPS | Gelation of a catalyst in a polymeric substrate and subsequent extraction with supercritical CO2 | Methylene blue, phenol | White LEDs, 5 W | [55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, O.; Venditto, V.; Pragliola, S.; Vaiano, V. Catalytic Composite Systems Based on N-Doped TiO2/Polymeric Materials for Visible-Light-Driven Pollutant Degradation: A Mini Review. Photochem 2021, 1, 330-344. https://doi.org/10.3390/photochem1030021
Sacco O, Venditto V, Pragliola S, Vaiano V. Catalytic Composite Systems Based on N-Doped TiO2/Polymeric Materials for Visible-Light-Driven Pollutant Degradation: A Mini Review. Photochem. 2021; 1(3):330-344. https://doi.org/10.3390/photochem1030021
Chicago/Turabian StyleSacco, Olga, Vincenzo Venditto, Stefania Pragliola, and Vincenzo Vaiano. 2021. "Catalytic Composite Systems Based on N-Doped TiO2/Polymeric Materials for Visible-Light-Driven Pollutant Degradation: A Mini Review" Photochem 1, no. 3: 330-344. https://doi.org/10.3390/photochem1030021
APA StyleSacco, O., Venditto, V., Pragliola, S., & Vaiano, V. (2021). Catalytic Composite Systems Based on N-Doped TiO2/Polymeric Materials for Visible-Light-Driven Pollutant Degradation: A Mini Review. Photochem, 1(3), 330-344. https://doi.org/10.3390/photochem1030021









