Environmental Correlates of Facultative Paedomorphosis in Newts from a Greek Biodiversity Hotspot: Is Staying Young Enough to Stay Alive?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Data Preparation
2.2. Basic Statistics
2.3. Regression Analysis
3. Results
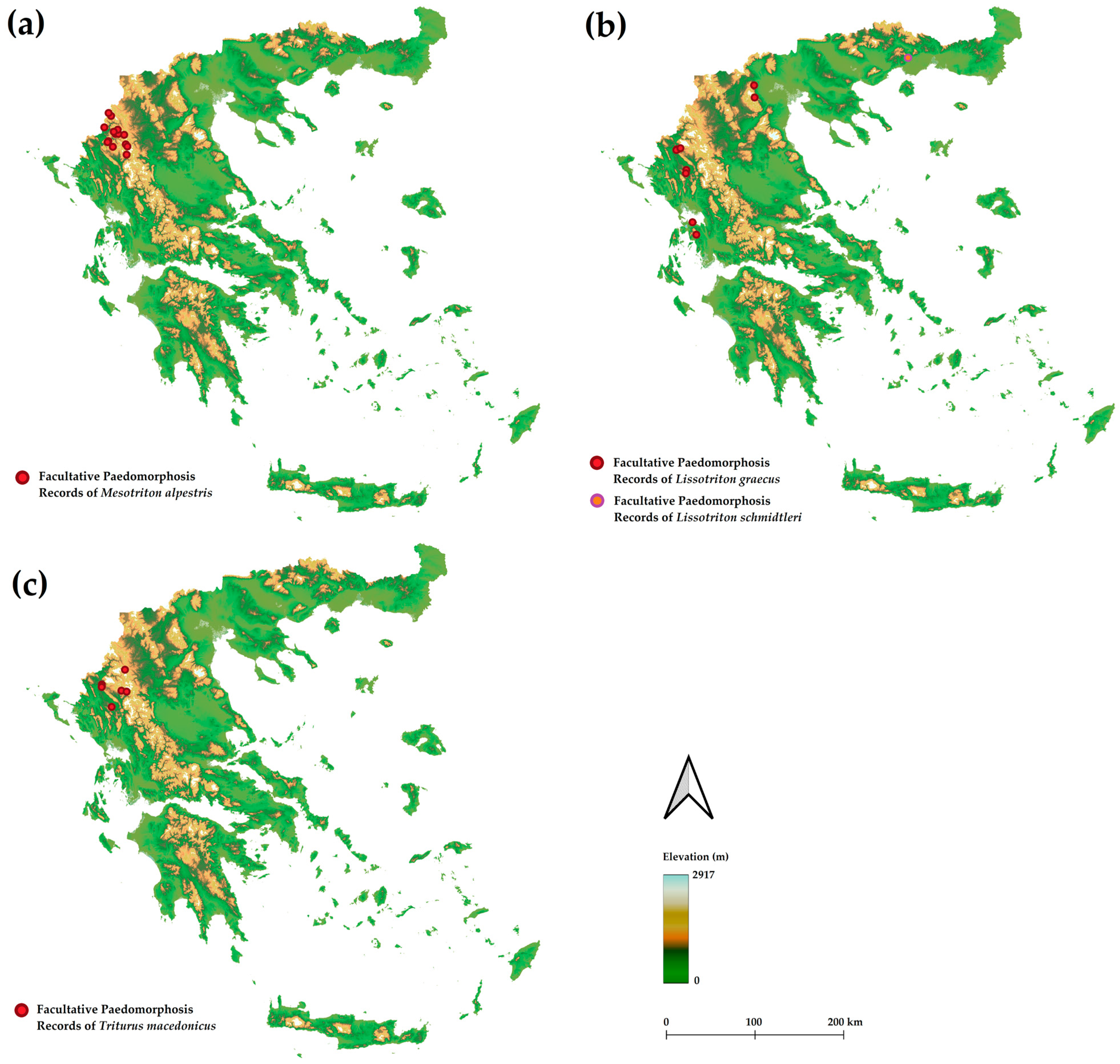
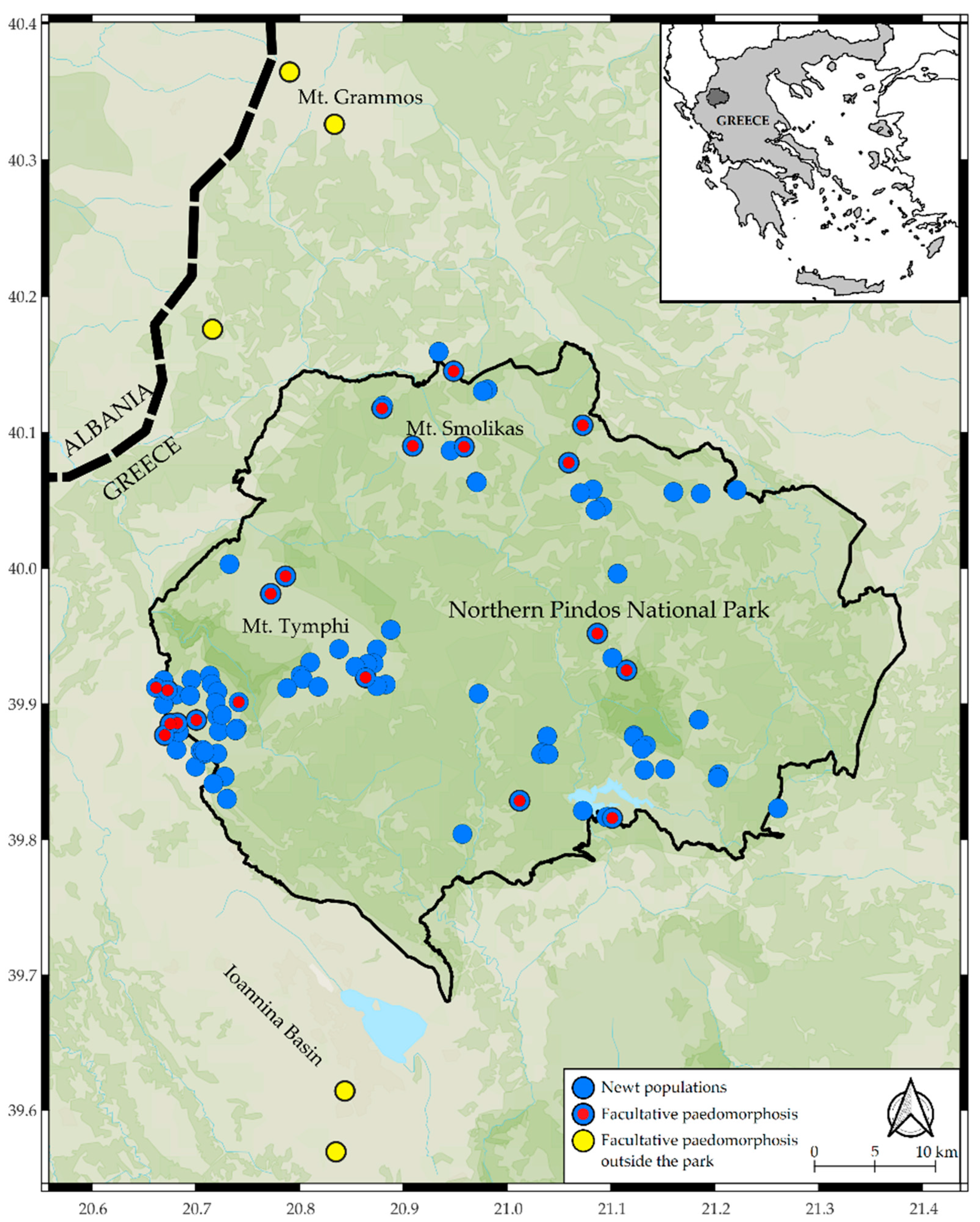
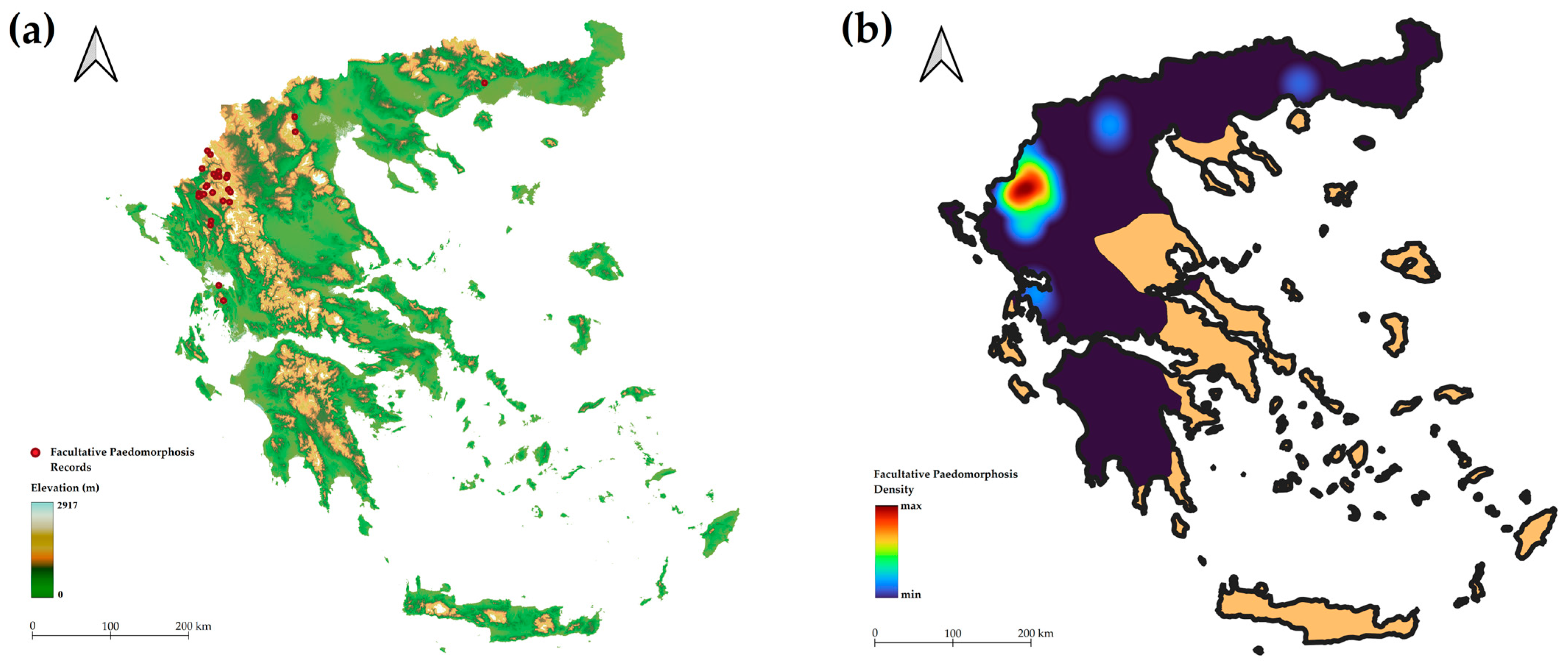
3.1. Frequency of Paedomorphosis
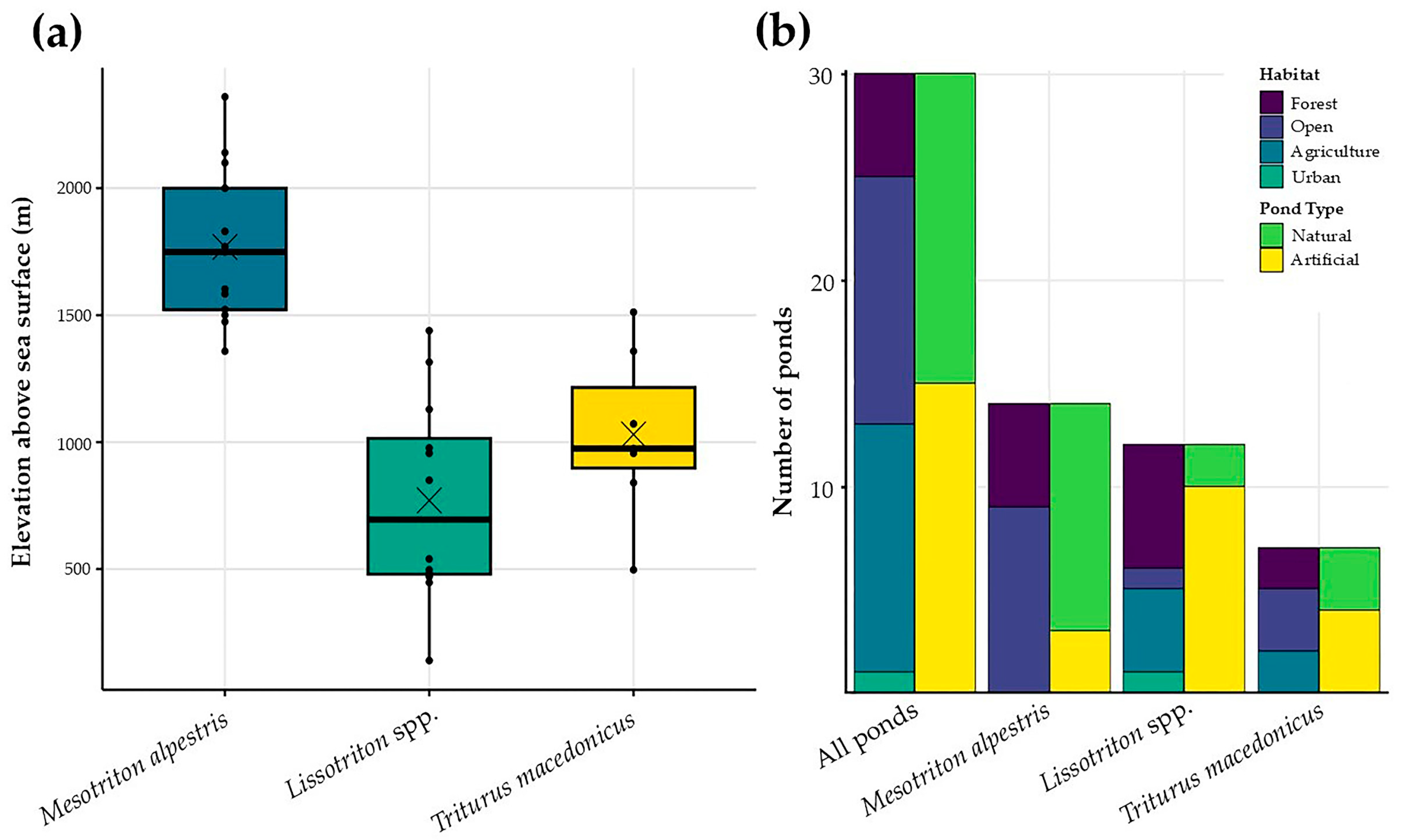
3.2. Characteristics of Paedomorphosis in Greece
3.3. Correlations Among Variables
3.4. Regression Models
4. Discussion
4.1. Frequency of Paedomorphosis
4.2. Environmental Variables
4.3. Species-Specific Patterns of Paedomorphosis Occurrence
4.4. Paedomorphosis in Greek Newts and Conservation Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike’s Information Criterion |
| GLM | Generalized Linear Model |
| HII | Human Influence Index |
| LRT | Likelihood-ratio Test |
| PC1 | First Principal Component |
| PCA | Principal Component Analysis |
| SAI | Shelter Availability Index |
| TWI | Topographic Wetness Index |
| VIF | Variance Inflation Factors |
Appendix A

References
- Gilbert, S.F. Ecological developmental biology: Developmental biology meets the real world. Dev. Biol. 2001, 233, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Keyte, A.L.; Smith, K.K. Heterochrony and developmental timing mechanisms: Changing ontogenies in evolution. Semin. Cell Dev. Biol. 2014, 34, 99–107. [Google Scholar] [CrossRef]
- Kremnev, S.V. Evolutionary and ontogenetic plasticity of conserved signaling pathways in animals’ development. Russ. J. Dev. Biol. 2022, 53, 65–81. [Google Scholar] [CrossRef]
- Ryan, T.J.; Semlitsch, R.D. Intraspecific heterochrony and life history evolution: Decoupling somatic and sexual development in a facultatively paedomorphic salamander. Proc. Natl. Acad. Sci. USA 1998, 95, 5643–5648. [Google Scholar] [CrossRef]
- Bolker, J.A. Modularity in development and why it matters to Evo-Devo. Am. Zool. 2000, 40, 770–776. [Google Scholar] [CrossRef]
- Butuzova, O.; Minarsky, A.; Bessonov, N.; Soulé, C.; Morozova, N. Determinism and variability of the morphogenesis pathways. Dev. Biol. 2021, 479, 1–10. [Google Scholar] [CrossRef]
- Gould, S.J. Ontogeny and Phylogeny; Harvard University Press: Cambridge, MA, USA, 1977. [Google Scholar]
- McKinney, M.L.; McNamara, K.J. Heterochrony. In Heterochrony: The Evolution of Ontogeny; McKinney, M.L., McNamara, K.J., Eds.; Springer: New York, NY, USA, 1991; pp. 1–12. [Google Scholar] [CrossRef]
- Iordansky, N. Paedomorphosis, neoteny, and evolution. Zool. Zh. 2005, 84, 1176–1187. [Google Scholar]
- Whiteman, H.H. Evolution of facultative paedomorphosis in salamanders. Q. Rev. Biol. 1994, 69, 205–221. [Google Scholar] [CrossRef]
- Denoël, M.; Joly, P.; Whiteman, H. Evolutionary ecology of facultative paedomorphosis in newts and salamanders. Biol. Rev. Camb. Philos. Soc. 2005, 80, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.R.; Smith, J.J. Evolution of salamander life cycles. Genetics 2005, 170, 275–281. [Google Scholar] [CrossRef]
- McNamara, K.J. Heterochrony: The Evolution of Development. Evol. Educ. Outreach 2012, 5, 203–218. [Google Scholar] [CrossRef]
- Denoël, M.; Ficetola, G.F. Heterochrony in a complex world: Disentangling environmental processes of facultative paedomorphosis in an amphibian. J. Anim. Ecol. 2014, 83, 606–615. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar] [CrossRef]
- Moran, N.A. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 1994, 25, 573–600. [Google Scholar] [CrossRef]
- Bonett, R.M.; Blair, A.L. Evidence for complex life cycle constraints on salamander body form diversification. Proc. Natl. Acad. Sci. USA 2017, 114, 9936–9941. [Google Scholar] [CrossRef]
- Lejeune, B.; Sturaro, N.; Lepoint, G.; Denoël, M. Facultative paedomorphosis as a mechanism promoting intraspecific niche differentiation. Oikos 2018, 127, 427–439. [Google Scholar] [CrossRef]
- Bonett, R.M.; Ledbetter, N.M.; Hess, A.J.; Herrboldt, M.A.; Denoël, M. Repeated ecological and life cycle transitions make salamanders an ideal model for evolution and development. Dev. Dyn. 2022, 251, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.H.; Cooper, W.S. The evolution of developmental plasticity in reproductive characteristics: An application of the “adaptive coin-flipping” principle. Am. Nat. 1984, 123, 393–410. [Google Scholar] [CrossRef]
- Smith, T.B.; Skúlason, S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 1996, 27, 111–133. [Google Scholar] [CrossRef]
- Denoël, M.; Joly, P. Adaptive significance of facultative paedomorphosis in Triturus alpestris (Amphibia, Caudata): Resource partitioning in an alpine lake. Freshw. Biol. 2001, 46, 1387–1396. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Scott, D.E.; Semlitsch, R.D.; Caldwell, J.P.; Vitt, L.J.; Gibbons, J.W. Declining amphibian populations: The problem of separating human impacts from natural fluctuations. Science 1991, 253, 892–895. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T.; Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- Wilbur, H.; Collins, J.P. Ecological aspects of amphibian metamorphosis. Science 1973, 182, 1305–1314. [Google Scholar] [CrossRef]
- Emel, S.L.; Bonett, R.M. Considering alternative life history modes and genetic divergence in conservation: A case study of the Oklahoma salamander. Conserv. Genet. 2011, 12, 1243–1259. [Google Scholar] [CrossRef]
- Sprules, W.G. The adaptive significance of paedogenesis in North American species of Ambystoma (Amphibia: Caudata): An hypothesis. Can. J. Zool. 1974, 52, 393–400. [Google Scholar] [CrossRef]
- Harris, R.N. Density-dependent paedomorphosis in the salamander Notophthalmus viridescens dorsalis. Ecology 1987, 68, 705–712. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Paedomorphosis in Ambystoma talpoideum: Effects of density, food, and pond drying. Ecology 1987, 68, 994–1002. [Google Scholar] [CrossRef]
- Denoël, M. How do paedomorphic newts cope with lake drying? Ecography 2003, 26, 405–410. [Google Scholar] [CrossRef]
- Denoël, M. Distribution and characteristics of aquatic habitats of newts and yellow-bellied toads in the district of Ioannina (Epirus, Greece). Herpetozoa 2004, 17, 49–64. [Google Scholar]
- Oromi, N.; Michaux, J.; Denoël, M. High gene flow between alternative morphs and the evolutionary persistence of facultative paedomorphosis. Sci. Rep. 2016, 6, 32046. [Google Scholar] [CrossRef]
- Kirk, M.A.; Reider, K.E.; Lackey, A.C.R.; Thomas, S.A.; Whiteman, H.H. The role of environmental variation in mediating fitness trade-offs for an amphibian polyphenism. J. Anim. Ecol. 2023, 92, 1815–1827. [Google Scholar] [CrossRef]
- Kirk, M.A.; Lackey, A.C.R.; Reider, K.E.; Thomas, S.A.; Whiteman, H.H. Climate mediates the trade-offs associated with phenotypic plasticity in an amphibian polyphenism. J. Anim. Ecol. 2024, 93, 1747–1757. [Google Scholar] [CrossRef]
- Whiteman, H.H.; Howard, R.D. Conserving alternative amphibian phenotypes: Is there anybody out there? In Status and Conservation of Midwestern Amphibians; Lannoo, M.J., Ed.; University of Iowa Press: Iowa City, AI, USA, 1998; pp. 317–324. [Google Scholar] [CrossRef]
- Denoël, M. Priority areas of intraspecific diversity: Larzac, a global hotspot for facultative paedomorphosis in amphibians. Anim. Conserv. 2007, 10, 110–116. [Google Scholar] [CrossRef]
- Denoël, M.; Ficetola, G.F.; Ćirović, R.; Radović, D.; Džukić, G.; Kalezić, M.L.; Vukov, T.D. A multi-scale approach to facultative paedomorphosis of European newts (Salamandridae) in the Montenegrin karst: Distribution pattern, environmental variables, and conservation. Biol. Conserv. 2009, 142, 509–517. [Google Scholar] [CrossRef]
- Reid, W.V. Biodiversity hotspots. Trends Ecol. Evol. 1998, 13, 275–280. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Pasachnik, S.A.; Echternacht, A.C.; Fitzpatrick, B.M. Gene trees, species and species trees in the Ctenosaura palearis clade. Conserv. Genet. 2010, 11, 1767–1781. [Google Scholar] [CrossRef]
- Fitzpatrick, B.M.; Ryan, M.E.; Johnson, J.R.; Corush, J.; Carter, E.T. Hybridization and the species problem in conservation. Curr. Zool. 2015, 61, 206–216. [Google Scholar] [CrossRef]
- Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef]
- Quilodrán, C.S.; Montoya-Burgos, J.I.; Currat, M. Harmonizing hybridization dissonance in conservation. Commun. Biol. 2020, 3, 391. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Defining ‘Evolutionarily Significant Units’ for conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef]
- Waples, R.S. Evolutionarily significant units and the conservation of biological diversity under the endangered species act. Am. Fish. Soc. Symp. 1995, 17, 8–27. [Google Scholar]
- Crandall, K.A.; Bininda-Emonds, O.R.P.; Mace, G.M.; Wayne, R.K. Considering evolutionary processes in conservation biology. Trends Ecol. Evol. 2000, 15, 290–295. [Google Scholar] [CrossRef]
- Hoelzel, A.R.; Bruford, M.W.; Fleischer, R.C. Conservation of adaptive potential and functional diversity. Conserv. Genet. 2019, 20, 1–5. [Google Scholar] [CrossRef]
- Hoelzel, A.R. Where to now with the evolutionarily significant unit? Trends Ecol. Evol. 2023, 38, 1134–1142. [Google Scholar] [CrossRef]
- Molinari, J. A bare-bones scheme to choose between the species, subspecies, and ‘evolutionarily significant unit’ categories in taxonomy and conservation. J. Nat. Conserv. 2023, 72, 126335. [Google Scholar] [CrossRef]
- Breuil, M.; Parent, G.-H. Essai de caracterisation du Triton alpestre hellénique Triturus alpestris veluchiensis. Historique et présentation de nouvelle Données. Alytes 1987, 6, 131–151. [Google Scholar]
- Denoël, M.; Duguet, R.; Džukić, G.; Kalezić, M.; Mazzotti, S. Biogeography and ecology of paedomorphosis in Triturus alpestris (Amphibia, Caudata). J. Biogeogr. 2001, 28, 1271–1280. [Google Scholar] [CrossRef]
- Denoël, M.; Schabetsberger, R. Resource partitioning in two heterochronic populations of Greek alpine newts, Triturus alpestris veluchiensis. Acta Oecol. 2003, 24, 55–67. [Google Scholar] [CrossRef]
- Sotiropoulos, K.; Legakis, A.; Polymeni, R.M. Patterns of morphometric variation in the smooth newt (Lissotriton vulgaris) from Greece: Environmental correlates. J. Nat. Hist. 2008, 42, 435–450. [Google Scholar] [CrossRef]
- Papaioannou, H.; Batistatou, A.; Mavrogiorgou, M.C.; Konidaris, C.; Frillingos, S.; Briasoulis, E.; Vareli, K.; Sainis, I. High incidence of dorsal dark spots in north-western Greek populations of alpine newts Ichthyosaura alpestris (Salamandridae, Caudata). Herpetol. Notes 2015, 8, 589–598. [Google Scholar]
- Sotiropoulos, K.; Moustakas, K.; Konstantinidis, K.; Mantzana-Oikonomaki, V.; Siarabi, S.; Bounas, A. First record of facultative paedomorphosis in the Macedonian crested newt (Triturus macedonicus) and an additional record for the Greek smooth newt (Lissotriton vulgaris) from Greece: Implications on species conservation and preservation of alternative ontogenetic trajectories. Herpetol. Notes 2017, 10, 255–260. [Google Scholar]
- Sotiropoulos, K.; Moustakas, K.; Toli, E.-A. First record of facultative paedomorphosis in the Turkish smooth newt, Lissotriton schmidtleri (Raxworthy, 1988), from Greece. Herpetol. Notes 2020, 13, 1041–1044. [Google Scholar]
- Mutz, T.; Böhme, W. Ichthyosaura as a generic nomen for the Alpine Newt (Caudata: Salamandridae): A doubtful case of literarian archeology. Salamandra 2025, 61, 41–52. [Google Scholar]
- Danelis, T.; Theodoropoulos, A.; Toli, E.-A.; Bounas, A.; Korakis, A.; Sotiropoulos, K. Defining evolutionary conservation units in the Macedonian crested newt, (Amphibia; Salamandridae), in a biodiversity hotspot. Diversity 2023, 15, 671. [Google Scholar] [CrossRef]
- Denoël, M.; Perez, A.; Cornet, Y.; Ficetola, G.F. Similar local and landscape processes affect both a common and a rare newt species. PLoS ONE 2013, 8, e62727. [Google Scholar] [CrossRef]
- Denoël, M. On the identification of paedomorphic and overwintering larval newts based on cloacal shape: Review and guidelines. Curr. Zool. 2016, 63, 165–173. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 24 July 2025).
- Copernicus Land Monitoring Service. Copernicus Land Monitoring Service (CLMS); European Environment Agency (EEA): Copenhagen, Denmark, 2021. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System; QGIS Association: Zurich, Switzerland, 2022; Available online: http://qgis.osgeo.org (accessed on 24 July 2025).
- Google Earth. Google Earth Pro, version 7.3; Google LLC: Mountain View, CA, USA, 2025.
- Beven, K.J.; Kirkby, M.J. A physically based, variable contributing area model of basin hydrology/Un modèle à base physique de zone d’appel variable de l’hydrologie du bassin versant. Hydrol. Sci. J. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Sanderson, E.; Fisher, K.; Robinson, N.; Sampson, D.; Duncan, A.; Royte, L. The March of the Human Footprint. EcoEvorXiv 2022, preprint. [Google Scholar] [CrossRef]
- Bounas, A.; Keroglidou, M.; Toli, E.-A.; Chousidis, I.; Tsaparis, D.; Leonardos, I.; Sotiropoulos, K. Constrained by aliens, shifting landscape, or poor water quality? Factors affecting the persistence of amphibians in an urban pond network. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1037–1049. [Google Scholar] [CrossRef]
- Jehle, R.; Arntzen, J.W. Post-breeding migrations of newts (Triturus cristatus and T. marmoratus) with contrasting ecological requirements. J. Zool. 2000, 251, 297–306. [Google Scholar] [CrossRef]
- Langton, T.E.S.; Beckett, C.L.; Foster, J.P. Great Crested Newt Conservation Handbook; Froglife: Halesworth, UK, 2001. [Google Scholar]
- Malmgren, J.; Andersson, P.-Å.; Ekdahl, S. Modelling terrestrial interactions and shelter use in Great crested newts (Triturus cristatus). Amphib. Reptil. 2007, 28, 205–215. [Google Scholar] [CrossRef]
- Gustafson, D.H.; Malmgren, J.C.; Mikusiński, G. Terrestrial habitat predicts use of aquatic habitat for breeding purposes—A study on the Great crested newt (Triturus cristatus). Ann. Zool. Fenn. 2011, 48, 295–307. [Google Scholar] [CrossRef]
- Denoël, M.; Ficetola, G.F.; Sillero, N.; Džukić, G.; Kalezić, M.L.; Vukov, T.; Muhovic, I.; Ikovic, V.; Lejeune, B. Traditionally managed landscapes do not prevent amphibian decline and the extinction of paedomorphosis. Ecol. Monogr. 2019, 89, e01347. [Google Scholar] [CrossRef]
- Albero, L.; Martínez-Solano, Í.; Hermida, M.; Vera, M.; Tarroso, P.; Bécares, E. Open areas associated with traditional agriculture promote functional connectivity among amphibian demes in Mediterranean agrosystems. Landsc. Ecol. 2023, 38, 3045–3059. [Google Scholar] [CrossRef]
- Walters, P.J.; Greenwald, L. Physiological adaptations of aquatic newts (Notophthalmus viridescens) to a terrestrial environment. Physiol. Zool. 1977, 50, 88–98. [Google Scholar] [CrossRef]
- Roe, A.W.; Grayson, K.L. Terrestrial movements and habitat use of juvenile and emigrating adult Eastern red-spotted newts, Notophthalmus viridescens. J. Herpetol. 2008, 42, 22–30. [Google Scholar] [CrossRef]
- Jarvis, L.E. Terrestrial ecology of juvenile great crested newts (Triturus cristatus) in a woodland area. Herpetol. J. 2016, 26, 287–296. [Google Scholar]
- González-del-Pliego, P.; Scheffers, B.R.; Freckleton, R.P.; Basham, E.W.; Araújo, M.B.; Acosta-Galvis, A.R.; Medina Uribe, C.A.; Haugaasen, T.; Edwards, D.P. Thermal tolerance and the importance of microhabitats for Andean frogs in the context of land use and climate change. J. Anim. Ecol. 2020, 89, 2451–2460. [Google Scholar] [CrossRef]
- Shaffer, H.B.; Austin, C.C.; Huey, R.B. The consequences of metamorphosis on salamander (Ambystoma) locomotor performance. Physiol. Zool. 1991, 64, 212–231. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Zumbach, S. Amphibian Road Mortality and How to Prevent It: A Review. In Urban Herpetology; Mitchell, J.C., Jung Brown, R.E., Bartholomew, B., Eds.; Society for the Study of Amphibians & Reptiles: Salt Lake City, UT, USA, 2008; pp. 157–167. [Google Scholar]
- Hijmans, R.J. Terra: Spatial Data Analysis, version 1.8-70; The R Project Community: Vienna, Austria, 2020. [CrossRef]
- Bowerman, B.L.; O’Connell, R.T. Linear Statistical Models: An Applied Approach, 2nd ed.; PWS-Kent Publishing Company: Boston, MA, USA, 1990. [Google Scholar]
- Alkharusi, H. Categorical Variables in Regression Analysis: A Comparison of Dummy and Effect Coding. Int. J. Educ. 2012, 4, 202–210. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference, version 1.47.5; The R Project Community: Vienna, Austria, 2024. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 16 October 2025).
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Bivand, R.; Wong, D.W.S. Comparing implementations of global and local indicators of spatial association. TEST 2018, 27, 716–748. [Google Scholar] [CrossRef]
- Maggini, R.; Lehmann, A.; Zimmermann, N.E.; Guisan, A. Improving Generalized Regression Analysis for the Spatial Prediction of Forest Communities. J. Biogeogr. 2006, 33, 1729–1749. [Google Scholar] [CrossRef]
- Chongsuvivatwong, V. epiDisplay: Epidemiological Data Display Package, version 3.5.0; The R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://cran.r-project.org/web/packages/epiDisplay/index.html (accessed on 15 October 2025).
- Nagelkerke, N.J.D. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Mangiafico, S. Rcompanion: Functions to Support Extension Education Program Evaluation, version 2.5.0; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2016. [CrossRef]
- Mathiron, A.G.E.; Lena, J.-P.; Baouch, S.; Denoël, M. The ‘male escape hypothesis’: Sex-biased metamorphosis in response to climatic drivers in a facultatively paedomorphic amphibian. Proc. R. Soc. B 2017, 284, 20170176. [Google Scholar] [CrossRef]
- Toli, E.-A.; Moustakas, K.; Siarabi, S.; Bounas, A.; Sotiropoulos, K. Contrasting sex ratios between coexisting alternative morphs of the smooth newt, Lissotriton vulgaris: An example of fitness advantage? In Program & Abstracts, Proceedings of the 19th European Congress of Herpetology, Salzburg, Austria, 18–23 September 2017; The Societas Europaea Herpetologica (SEH): Bonn, Germany, 2017. [Google Scholar] [CrossRef]
- Bruni, G.; Tessa, G.; Angelini, C. Body size, age and population structure of Triturus carnifex (Urodela: Salamandridae) in the context of facultative paedomorphosis. Acta Herpetol. 2018, 13, 177–183. [Google Scholar] [CrossRef]
- Meng, F.; Yan, S.; Tian, G.; Wang, Y. Surface urban heat island effect and its spatiotemporal dynamics in metropolitan area: A case study in the Zhengzhou metropolitan area, China. Front. Environ. Sci. 2023, 11, 1247046. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Edwards, D.P.; Diesmos, A.; Williams, S.E.; Evans, T.A. Microhabitats reduce animal’s exposure to climate extremes. Glob. Change Biol. 2014, 20, 495–503. [Google Scholar] [CrossRef]
- Bell, G. The life of the Smooth newt (Triturus vulgaris) after metamorphosis. Ecol. Monogr. 1977, 47, 279–299. [Google Scholar] [CrossRef]
- Litvinchuk, S.N.; Rudyk, A.M.; Borkin, L.J. Observations on paedomorphic newts (Triturus vulgaris) from the former Soviet Union. Russ. J. Herpetol. 1996, 3, 39–48. [Google Scholar]
- Gvoždík, V.; Javůrková, V.; Kopecký, O. First evidence of a paedomorphic population of the smooth newt (Lissotriton vulgaris) in the Czech Republic. Acta Herpetol. 2013, 8, 53–57. [Google Scholar] [CrossRef]
- Covaciu-Marcov, S.-D.; Cicort-Lucaciu, A. Notes on the presence of facultative paedomorphosis in the Smooth newt Lissotriton vulgaris (Linnaeus, 1758) in Western Romania. North-West. J. Zool. 2007, 3, 47–52. [Google Scholar]
- Covaciu-Marcov, S.-D.; Cicort-Lucaciu, A.-Ș. Big and nonmetamorphic Triturus cristatus larvae from North-Western Romania. Biharean Biol. 2009, 3, 73–74. [Google Scholar]
- Covaciu-Marcov, S.D.; Sas, I.; Cicort-Lucaciu, A.Ş.; Bogdan, H.V. Lissotriton vulgaris paedomorphs in South-Western Romania: A consequence of a human modified habitat? Acta Herpetol. 2011, 6, 15–18. [Google Scholar]
- Gherghel, I.; Strugariu, A.; Ghira, I. On the presence of paedomorphosis in Lissotriton vulgaris (Amphibia: Salamandridae) from Danube Delta. Herpetol. Rom. 2010, 4, 62–64. [Google Scholar]
- Haggerty, C.J.E.; Crisman, T.L.; Rohr, J.R. Effects of forestry-driven changes to groundcover and soil moisture on amphibian desiccation, dispersal, and survival. Ecol. Appl. 2019, 29, e01870. [Google Scholar] [CrossRef]
- Denoël, M.; Hervant, F.; Schabetsberger, R.; Joly, P. Short- and long-term advantages of an alternative ontogenetic pathway: Benefits of paedomorphosis in newts. Biol. J. Linn. Soc. 2002, 77, 105–112. [Google Scholar] [CrossRef]
- Lukanov, S.; Atanasova, I. Correlation between individual body condition and seasonal activity in Buresch’s crested newt, Triturus ivanbureschi. Diversity 2025, 17, 350. [Google Scholar] [CrossRef]
- Covaciu-Marcov, S.-D.; Rosioru, C.; Cicort-Lucaciu, A.-Ș.; Sas-Kovacs, I. Lissotriton vulgaris (Amphibia) paedomorphs in Carei Plain natural protected area, North-Western Romania. North-West. J. Zool. 2013, 9, 217–220. [Google Scholar]
- Ceacero, F.; Donaire-Barroso, D.; García-Muñoz, E.; Beltrán, J.F.; Tejedo, M. On the occurrence of facultative paedomorphosis in the three newt species of Southern Iberian Peninsula (Amphibia, Salamandridae). Amphib. Reptil. 2010, 31, 571–575. [Google Scholar] [CrossRef]
- Pizzuti Piccoli, A. First record of paedomorphosis for the Smooth Newt Lissotriton vulgaris meridionalis (Boulenger, 1882), (Amphibia, Urodela) in the “Bosco di Palo” Natural Park (Northern Latium, Italy). Herpetol. Rom. 2013, 7, 23–27. [Google Scholar]
- Çiçek, K.; Ayaz, D. New data on facultative paedomorphism of the smooth newt, Lissotriton vulgaris, in Western Anatolia, Turkey. J. Freshw. Ecol. 2011, 26, 99–103. [Google Scholar] [CrossRef]
- Scillitani, G.; Scalera, R.; Carafa, M.; Tripepi, S. Conservation and biology of Triturus italicus in Italy (Amphibia, Salamandridae). Ital. J. Zool. 2004, 71, 45–54. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.; Garcia-Vazquez, E. The value of traditional troughs as freshwater shelters for amphibian diversity. Aquat. Conserv. 2011, 21, 74–81. [Google Scholar] [CrossRef]
- Caballero–Diaz, C.; Sanchez–Montes, G.; Butler, H.; Vrendenburg, V.; Martinez-Solano, I. The Role of artificial breeding sites in amphibian conservation: A case study in rural areas in central Spain. Herpetol. Conserv. Biol. 2020, 15, 87–104. [Google Scholar]
- Smith, R.K.; Meredith, H.; Sutherland, W.J. Amphibian Conservation. In What Works in Conservation; Sutherland, W.J., Dicks, L.V., Petrovan, S.O., Smith, R.K., Eds.; Open Book Publishers: Cambridge, UK, 2020; pp. 9–66. [Google Scholar] [CrossRef]
- Stellati, L.; Mirabasso, J.; Luiselli, L.; Bologna, M.A.; Vignoli, L.; Bissattini, A.M. Can we share? feeding strategy in three syntopic newts in artificial habitats. Diversity 2021, 13, 32. [Google Scholar] [CrossRef]
- Arillo, A.; Canessa, S.; Costa, A.; Oneto, F.; Ottonello, D.; Rosa, G.; Salvidio, S. Artificial tanks for amphibian conservation in Mediterranean rural landscapes. Bull. Environ. Life Sci. 2022, 4, 4–11. [Google Scholar] [CrossRef]
- Bissattini, A.M.; D’Ambrosio, D.; Buono, V.; Bologna, M.A.; Vignoli, L. Home is where the newts are: Suitability of artificial aquatic sites for the Italian crested newt in a traditional farming landscape in Central Italy. Anim. Conserv. 2025; in press. [Google Scholar] [CrossRef]
- Costa, A.; Bernabò, I.; Rosa, G.; Salvidio, S.; Romano, A. Artificial water sites increase amphibian resilience in a changing Mediterranean landscape. Agric. Ecosyst. Environ. 2025, 394, 109912. [Google Scholar] [CrossRef]
- Bernabò, I.; Iannella, M.; Cittadino, V.; Corapi, A.; Romano, A.; Andreone, F.; Biondi, M.; Gallo Splendore, M.; Tripepi, S. Survived the glaciations, will they survive the fish? Allochthonous ichthyofauna and alpine endemic newts: A road map for a conservation strategy. Animals 2023, 13, 871. [Google Scholar] [CrossRef] [PubMed]
- Denoël, M.; Džukić, G.; Kalezić, M.L. Effects of widespread fish introductions on paedomorphic newts in Europe. Conserv. Biol. 2005, 19, 162–170. [Google Scholar] [CrossRef]
- Seglie, D.; Evangelista, M. On the discovering and paedomorphosis of Ichthyosaura alpestris apuanus in a lowland river floodplain (Scrivia, NW Italy). In Proceedings of the Atti VIII Congresso Nazionale Societas Herpetologica Italica, Chieti, Italy, 22–26 September 2010; Di Tizio, L., Di Cerbo, A.R., Di Francesco, N., Cameli, A., Eds.; Ianieri Edizioni: Pescara, Italy, 2010; pp. 119–125. [Google Scholar]
- Toli, E.A.; Chavas, C.; Denoël, M.; Bounas, A.; Sotiropoulos, K. A Subtle threat: Behavioral and phenotypic consequences of invasive mosquitofish on a native paedomorphic newt. Biol. Invasions 2020, 22, 1299–1308. [Google Scholar] [CrossRef]
- Denoël, M.; Winandy, L. The importance of phenotypic diversity in conservation: Resilience of palmate newt morphotypes after fish removal in Larzac ponds (France). Biol. Conserv. 2015, 192, 402–408. [Google Scholar] [CrossRef][Green Version]
- De Troyer, N.; Eurie Forio, M.A.; Roels, K.; De Meester, L.; Lemmens, P.; Declerck, S.A.J.; Martens, K.; Goethals, P. Key management rules for agricultural Alpine newt breeding ponds based on habitat suitability models. Glob. Ecol. Conserv. 2020, 23, e01086. [Google Scholar] [CrossRef]
- Pupins, M.; Nekrasova, O.; Marushchak, O.; Tytar, V.; Theissinger, K.; Čeirāns, A.; Skute, A.; Georges, J.-Y. Potential threat of an invasive fish species for two native newts inhabiting wetlands of Europe vulnerable to climate change. Diversity 2023, 15, 201. [Google Scholar] [CrossRef]
- Denoël, M. Insights from paedomorphic newts in introduced populations. NeoBiota 2025, 99, 249–263. [Google Scholar] [CrossRef]
- Alarcos, G. Ejemplares pedomórficos de tritón jaspeado (Triturus marmoratus) y larvas de gallipato (Pleurodeles waltl) con longitudes más grandes de lo habitual en Zamora y Salamanca. Bol. Asoc. Herpetol. Esp. 2020, 31, 50–53. [Google Scholar]
- Daftsios, T.; Sagonas, K.; Strachinis, I. Extending the known vertical distribution for the highly adaptive Triturus macedonicus (Karaman, 1922). Herpetozoa 2024, 37, 107–110. [Google Scholar] [CrossRef]

| Regression Analysis Variables | Type of Data | Numeric Encoding for Regression Models |
|---|---|---|
| Pond characteristics (biotic/hydrological) | ||
| Fish presence | categorical | Absence = 0; Presence = 1 |
| Pond type | categorical | Natural = 0; Artificial = 1 |
| Hydroperiod | categorical | Temporary = 0; Permanent = 1 |
| Climatic variables | ||
| Maximum Temperature of July (Tmax) | continuous | - |
| Minimum Temperature of January (Tmin) | continuous | - |
| Mean annually Temperature (Tmean) | continuous | - |
| Mean annually Precipitation | continuous | - |
| Spatial/Remote sensing data | ||
| Altitude | continuous | - |
| Topographic Wetness Index (TWI) | continuous | - |
| Human Influence Index (HII) | continuous | - |
| Shelter Availability Index (SAI) | continuous | - |
| Rank | K | Δ-AIC | Variables in Selected Models | R2N |
|---|---|---|---|---|
| Mesotriton alpestris | ||||
| 1 | 1 | PC1 (-) | 0.819 | |
| 2 | 2 | 0.12 | PC1 (-), Hydroperiod (+) | 0.779 |
| 3 | 3 | 0.56 | PC1 (-), Hydroperiod (+), SAI (+) | 0.744 |
| 4 | 2 | 0.70 | PC1 (-), SAI (+) | 0.790 |
| 5 | 2 | 1.11 | PC1 (-), HII (-) | 0.798 |
| 6 | 3 | 1.58 | PC1 (-), Hydroperiod (+), HII (-) | 0.764 |
| 7 | 2 | 1.99 | PC1 (-), TWI (-) | 0.815 |
| Lissotriton graecus | ||||
| 1 | 3 | PC1 (+), Precipitation (-), Pond type (+) | 0.482 | |
| 2 | 4 | 0.07 | Fish (-), PC1 (+), Precipitation (-), Pond type (+) | 0.423 |
| 3 | 4 | 0.58 | PC1 (+), Precipitation (-), Pond type (+), TWI (-) | 0.435 |
| 4 | 2 | 0.96 | PC1 (+), Precipitation (-) | 0.567 |
| 5 | 2 | 1.01 | Precipitation (-), Pond type (+) | 0.569 |
| 6 | 4 | 1.05 | PC1 (+), Precipitation (-), Pond type (+), SAI (+) | 0.445 |
| 7 | 3 | 1.44 | PC1 (+), Precipitation (-), Hydroperiod (+) | 0.517 |
| 8 | 3 | 1.46 | Fish (-), PC1 (+), Precipitation (-) | 0.517 |
| 9 | 4 | 1.64 | Fish (-), PC1 (+), Precipitation (-), Hydroperiod (+) | 0.459 |
| 10 | 3 | 1.71 | Fish (-), Precipitation (-), Pond type (+) | 0.523 |
| 11 | 5 | 1.80 | Fish (-), PC1 (+), Precipitation (-), Pond type (+), SAI (+) | 0.400 |
| 12 | 5 | 1.82 | Fish (-), PC1 (+), Precipitation (-), Pond type (+), Hydroperiod (+) | 0.400 |
| 13 | 4 | 1.88 | PC1 (+), Precipitation (-), Pond type (+), Hydroperiod (+) | 0.464 |
| 14 | 3 | 1.91 | PC1 (+), Precipitation (-), TWI (-) | 0.528 |
| Triturus macedonicus | ||||
| 1 | 2 | PC1 (+), Precipitation (-) | 0.693 | |
| 2 | 3 | 1.57 | PC1 (+), Precipitation (-), Hydroperiod (+) | 0.667 |
| 3 | 3 | 1.90 | Fish (-), PC1 (+), Precipitation (-) | 0.677 |
| All species | ||||
| 1 | 2 | Precipitation (-), Hydroperiod (+) | 0.880 | |
| 2 | 3 | 0.31 | Fish (-), Precipitation (-), Hydroperiod (+) | 0.857 |
| 3 | 2 | 1.13 | Fish (-), Hydroperiod (+) | 0.893 |
| 4 | 2 | 1.24 | Hydroperiod (+) | 0.921 |
| 5 | 3 | 2.00 | PC1 (+), Precipitation (-), Hydroperiod (+) | 0.878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danelis, T.; Theodoropoulos, A.; Bounas, A.; Toli, E.-A.; Paraskevopoulou, A.; Korakis, A.; Sotiropoulos, K. Environmental Correlates of Facultative Paedomorphosis in Newts from a Greek Biodiversity Hotspot: Is Staying Young Enough to Stay Alive? Conservation 2025, 5, 79. https://doi.org/10.3390/conservation5040079
Danelis T, Theodoropoulos A, Bounas A, Toli E-A, Paraskevopoulou A, Korakis A, Sotiropoulos K. Environmental Correlates of Facultative Paedomorphosis in Newts from a Greek Biodiversity Hotspot: Is Staying Young Enough to Stay Alive? Conservation. 2025; 5(4):79. https://doi.org/10.3390/conservation5040079
Chicago/Turabian StyleDanelis, Taxiarchis, Anagnostis Theodoropoulos, Anastasios Bounas, Elisavet-Aspasia Toli, Aristea Paraskevopoulou, Athanasios Korakis, and Konstantinos Sotiropoulos. 2025. "Environmental Correlates of Facultative Paedomorphosis in Newts from a Greek Biodiversity Hotspot: Is Staying Young Enough to Stay Alive?" Conservation 5, no. 4: 79. https://doi.org/10.3390/conservation5040079
APA StyleDanelis, T., Theodoropoulos, A., Bounas, A., Toli, E.-A., Paraskevopoulou, A., Korakis, A., & Sotiropoulos, K. (2025). Environmental Correlates of Facultative Paedomorphosis in Newts from a Greek Biodiversity Hotspot: Is Staying Young Enough to Stay Alive? Conservation, 5(4), 79. https://doi.org/10.3390/conservation5040079








