Prediction of Potential Habitat Distributions and Climate Change Impacts on Six Carex L. Species of Conservation Concern in Canada
Abstract
1. Introduction
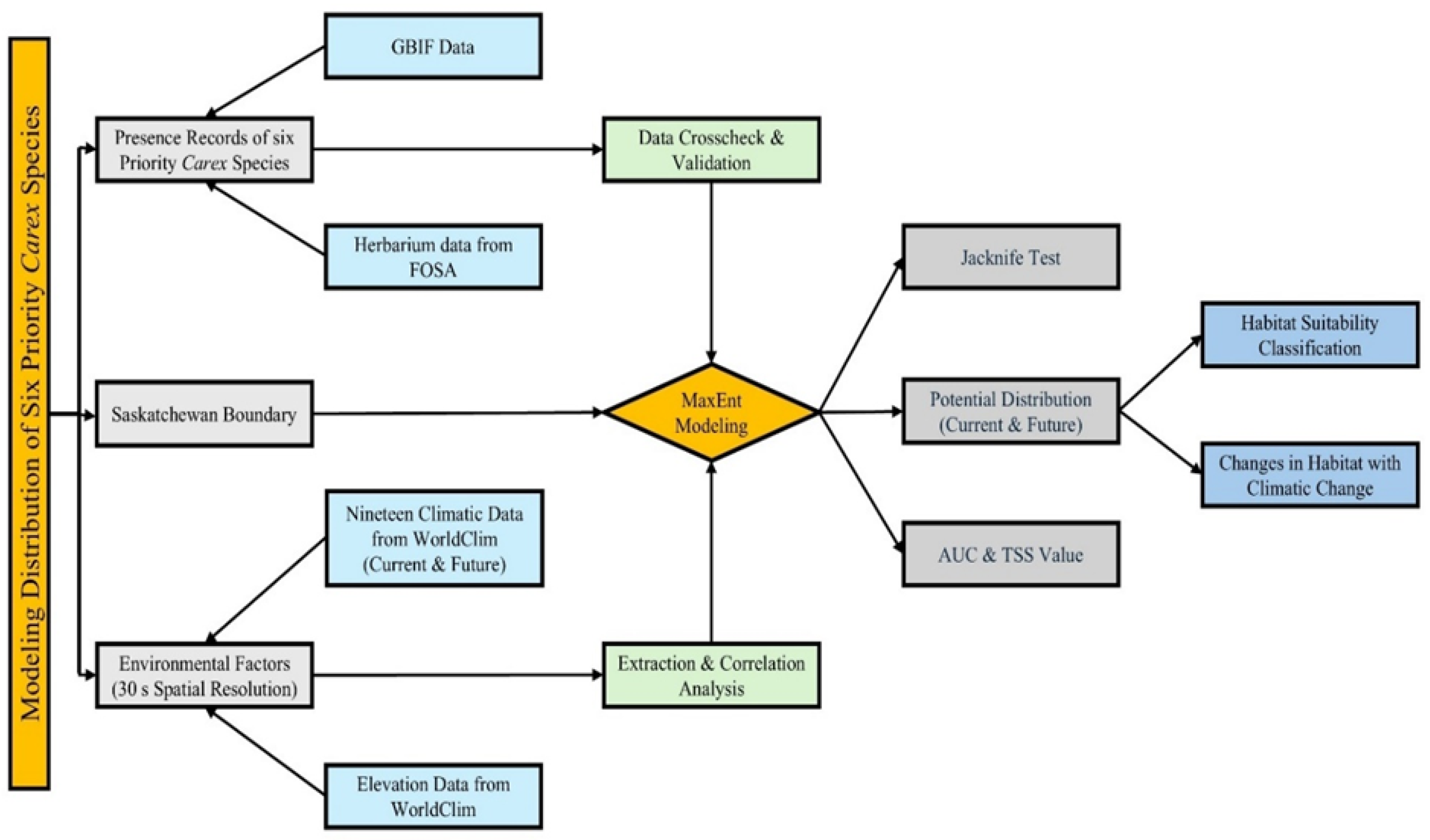
2. Materials and Methods
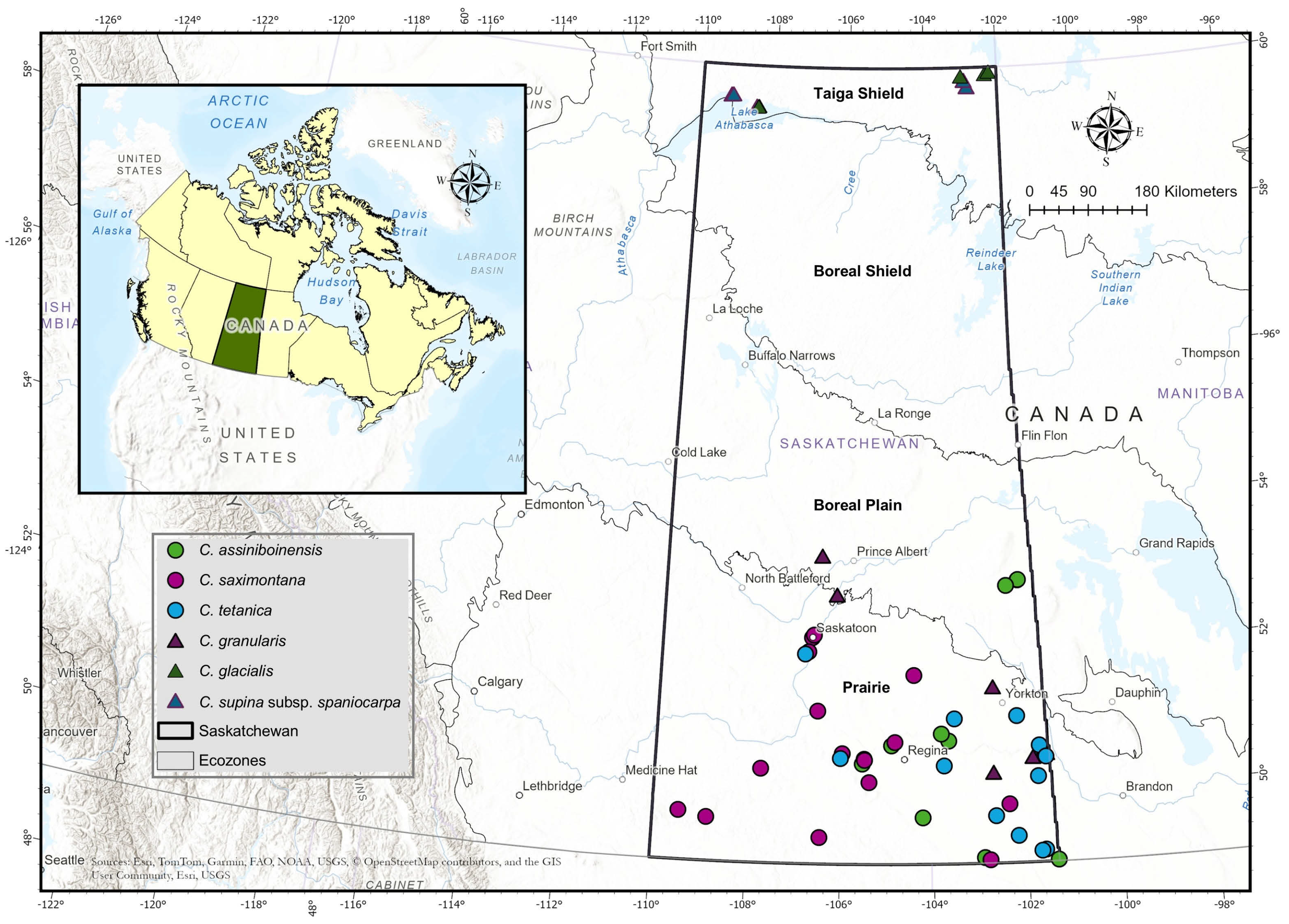
2.1. Study Area
2.2. Species Selection
2.3. Environmental Data Source and Variable Selection
2.4. Model Description
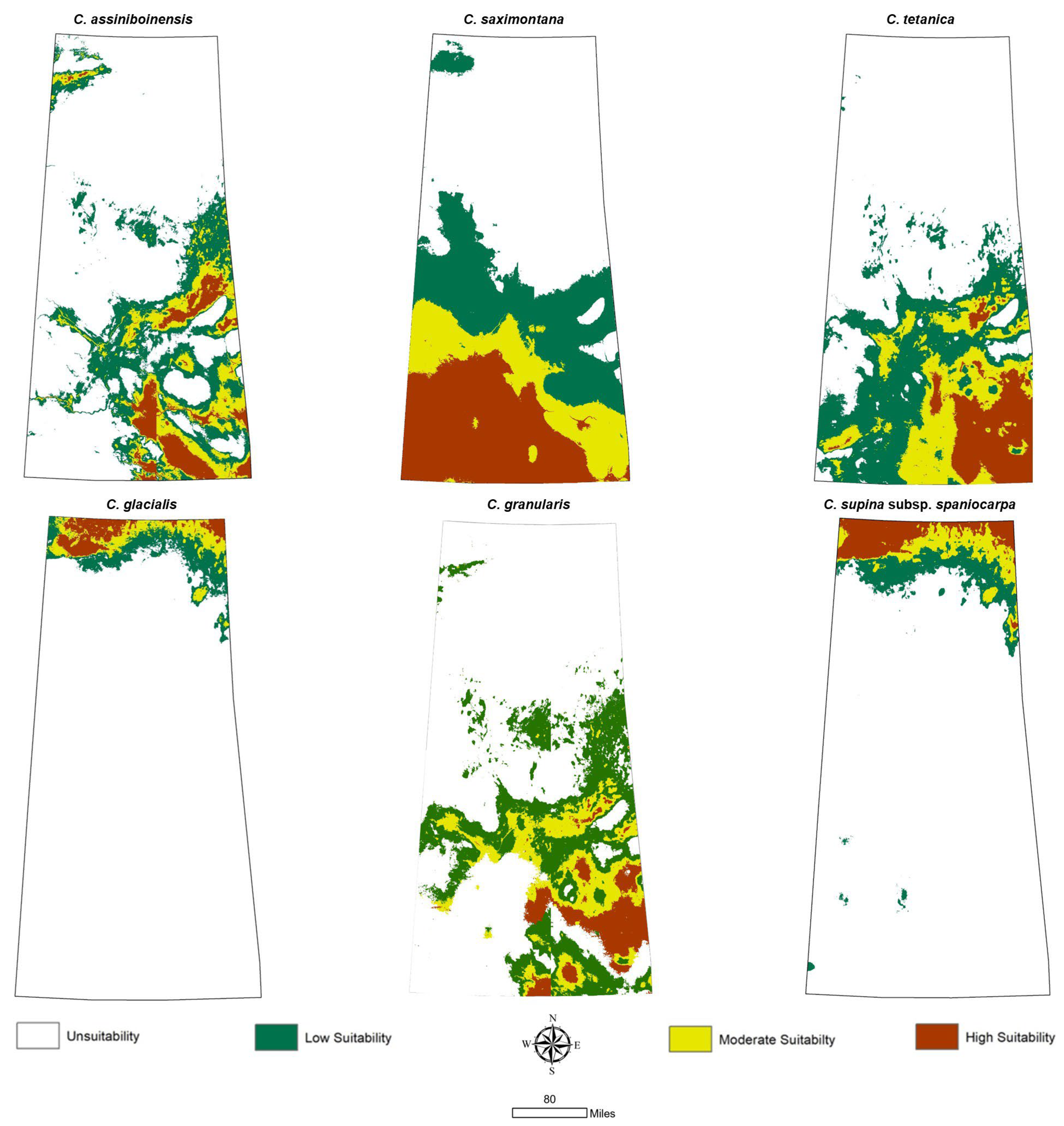
2.5. Suitable Habitat Classification
3. Results
3.1. Model Optimization and Accuracy Evaluation
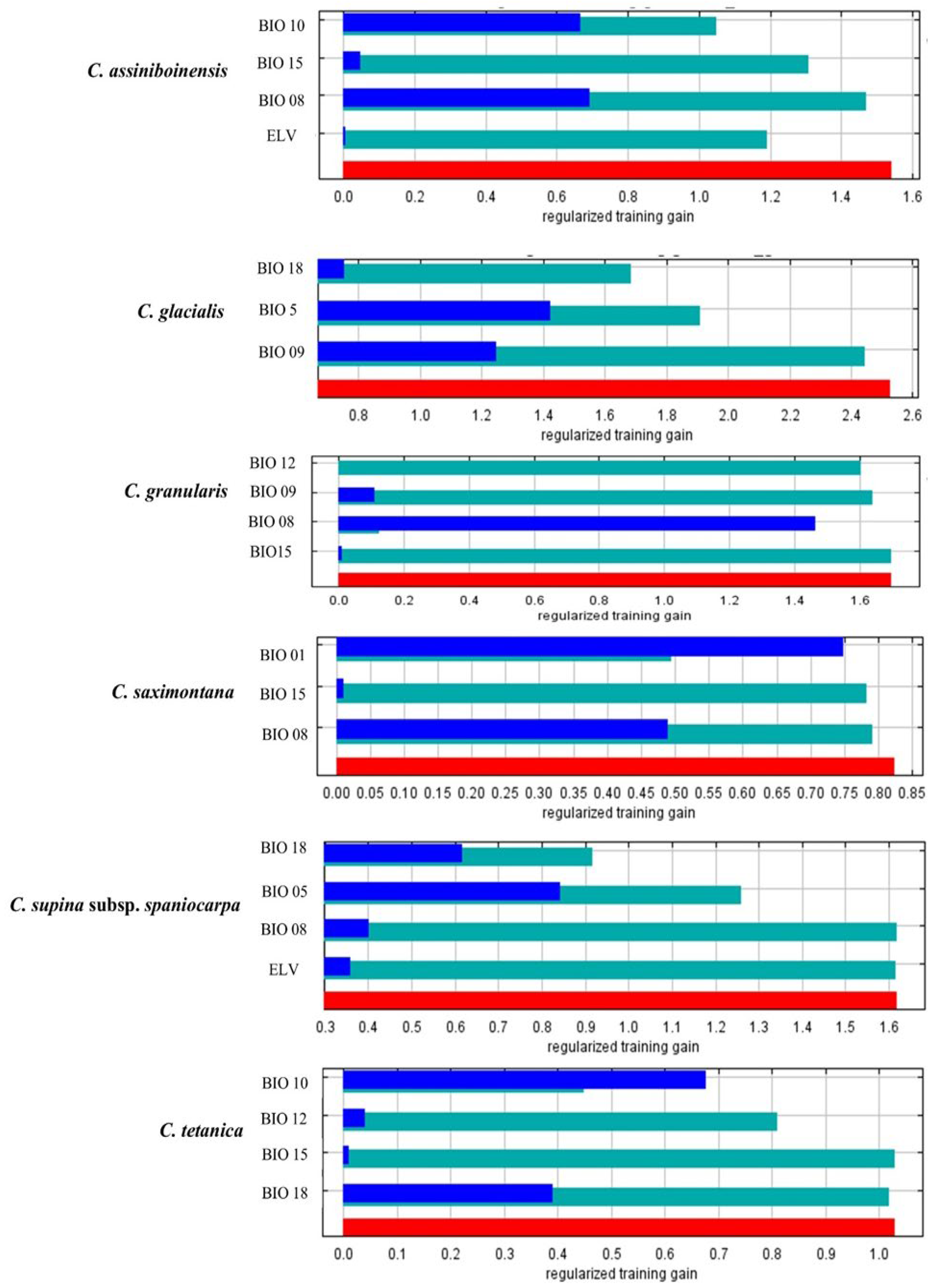
3.2. Climatic Factors Influencing Potential Distribution
3.3. Prediction of Potential Geographic Distribution Under the Current Climatic Period
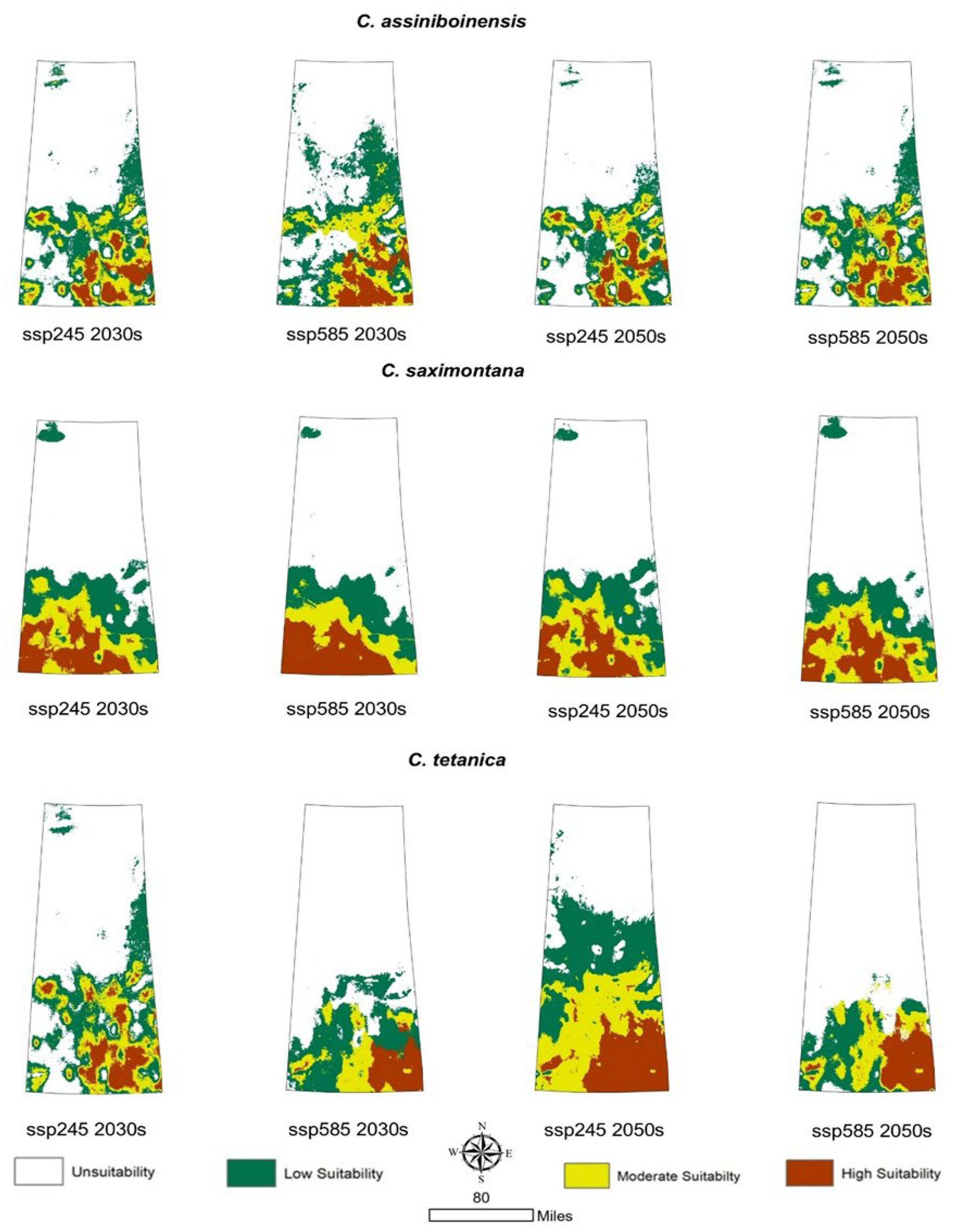
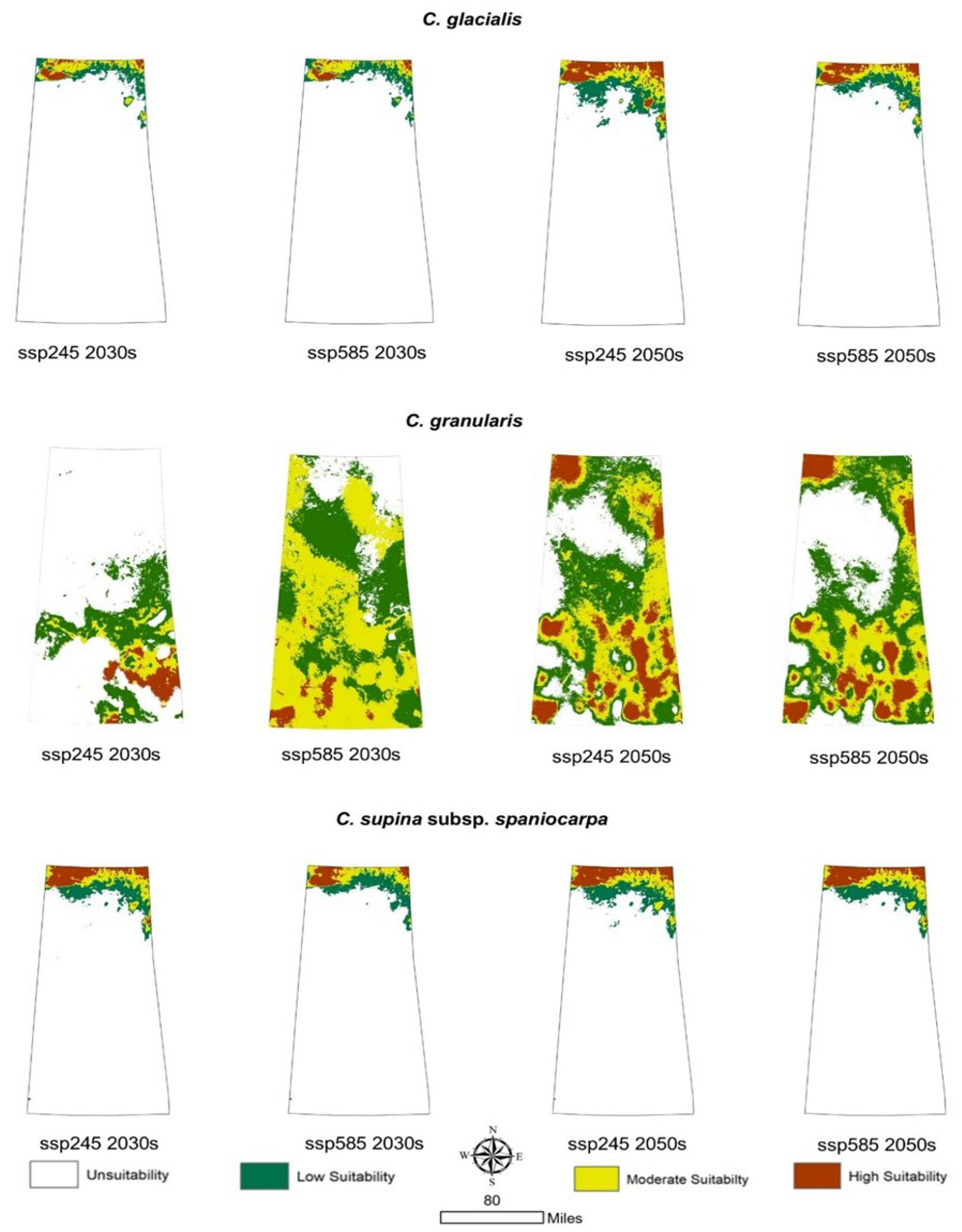
3.4. Prediction of Potential Geographic Distribution Under Future Climatic Scenarios
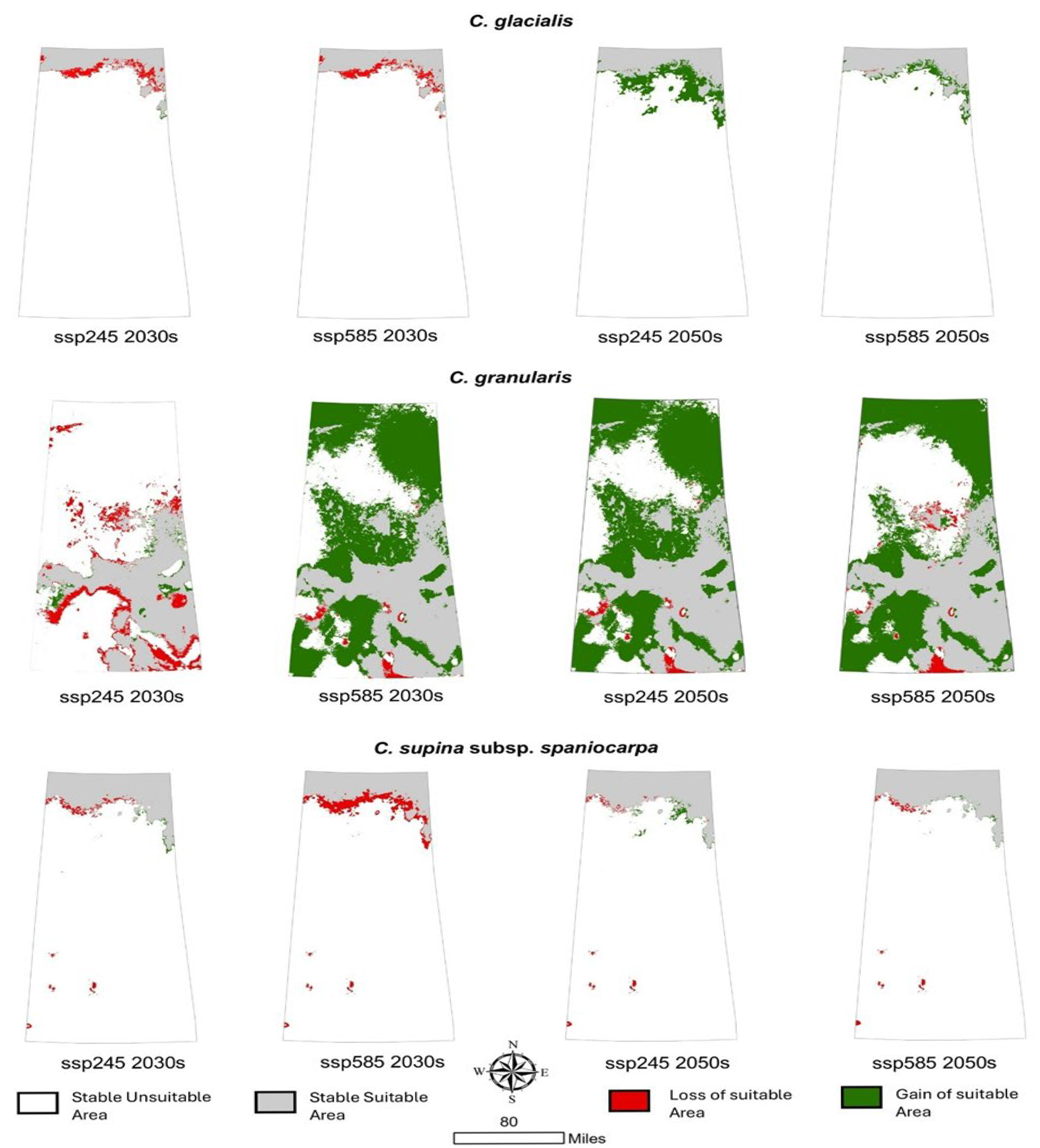
3.5. Dynamic Changes in Suitable Areas Under Future Climatic Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Factor | Description of Factor | Unit |
|---|---|---|
| BIO1 | Annual Mean Temperature | °C |
| BIO2 | Mean Diurnal Range (mean of monthly (max–min temp) | °C |
| BIO3 | Isothermality (BIO2/BIO7) (×100) | Dimensionless |
| BIO4 | Temperature Seasonality (standard deviation × 100) | °C |
| BIO5 | Max Temperature of Warmest Month | °C |
| BIO6 | Min Temperature of Coldest Month | °C |
| BIO7 | Temperature Annual Range (BIO5–BIO6) | °C |
| BIO8 | Mean Temperature of Wettest Quarter | °C |
| BIO9 | Mean Temperature of Driest Quarter | °C |
| BIO10 | Mean Temperature of Warmest Quarter | °C |
| BIO11 | Mean Temperature of Coldest Quarter | °C |
| BIO12 | Annual Precipitation | mm |
| BIO13 | Precipitation of Wettest Month | mm |
| BIO14 | Precipitation of Driest Month | mm |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) | % |
| BIO16 | Precipitation of Wettest Quarter | mm |
| BIO17 | Precipitation of Driest Quarter | mm |
| BIO18 | Precipitation of Warmest Quarter | mm |
| BIO19 | Precipitation of Coldest Quarter | mm |
| ELV | Elevation | m |
| Species | Environmental Variables | Permutation Importance | Percent Contribution |
|---|---|---|---|
| C. assiniboinensis | BIO10 | 31.2 | 38.1 |
| BIO8 | 28.9 | 36.2 | |
| BIO15 | 10.7 | 17.6 | |
| ELV | 15.2 | 7.4 | |
| C. glacialis | BIO18 | 60.8 | 38.6 |
| BIO5 | 36.9 | 26.7 | |
| BIO9 | 2.3 | 34.6 | |
| C. granularis | BIO8 | 84.9 | 83.6 |
| BIO15 | 10.2 | 8.3 | |
| BIO12 | 3.2 | 7.1 | |
| BIO9 | 1.6 | 0.6 | |
| C. saximontana | BIO1 | 84.4 | 80 |
| BIO18 | 7.3 | 16.4 | |
| BIO15 | 6.3 | 3.6 | |
| C. supina subsp. spaniocarpa | BIO18 | 64.5 | 60.8 |
| BIO5 | 34.6 | 39.2 | |
| C. tetanica | BIO10 | 73.5 | 80 |
| BIO12 | 10.3 | 8.3 | |
| BIO8 | 10.2 | 9.5 | |
| BIO15 | 6.1 | 2.1 |
| Time | Scenario | Unit | Unsuitability | Low Suitability | Moderate Suitability | High Suitability |
|---|---|---|---|---|---|---|
| Present (1970–2000) | km2 | 460,421.65 | 108,235.63 | 51,640.12 | 31,602.60 | |
| % | 70.63 | 16.60 | 7.92 | 4.85 | ||
| 2030s (2021–2040) | SSP245 | km2 | 405,177.7 | 134,046.9 | 70,823.69 | 41,851.64 |
| % | 62.15 | 20.56 | 10.86 | 6.41 | ||
| SSP585 | km2 | 350,406.32 | 163,141.22 | 84,000.01 | 54,352.44 | |
| % | 53.75 | 25.02 | 12.88 | 8.33 | ||
| 2050s (2041–2060) | SSP245 | km2 | 435,102 | 114,147.7 | 65,457 | 37,192.94 |
| % | 66.74 | 17.51 | 10.04 | 5.70 | ||
| SSP585 | km2 | 405,506.00 | 127,676.79 | 74,279.63 | 44,437.58 | |
| % | 62.20 | 19.59 | 11.39 | 6.82 |
| Time | Scenario | Unit | Unsuitability | Low Suitability | Moderate Suitability | High Suitability |
|---|---|---|---|---|---|---|
| Present (1970–2000) | km2 | 315,195.04 | 138,229.53 | 74,918.59 | 123,541.79 | |
| % | 48.35 | 21.20 | 11.49 | 18.95 | ||
| 2030s (2021–2040) | SSP245 | km2 | 397,905.04 | 106,058.12 | 78,041.77 | 69,893.05 |
| % | 61.03 | 16.26 | 11.97 | 10.72 | ||
| SSP585 | km2 | 413,945.38 | 94,845.37 | 57,461.99 | 85,646.74 | |
| % | 63.49 | 14.54 | 8.81 | 13.13 | ||
| 2050s (2041–2060) | SSP245 | km2 | 388,359.11 | 110,912.28 | 82,149.70 | 70,477.89 |
| % | 59.57 | 17.01 | 12.60 | 10.81 | ||
| SSP585 | km2 | 410,318.55 | 92,413.78 | 79,130.27 | 70,037.38 | |
| % | 62.94 | 14.17 | 12.13 | 10.74 |
| Time | Scenario | Unit | Unsuitability | Low Suitability | Moderate Suitability | High Suitability |
|---|---|---|---|---|---|---|
| Present (1970–2000) | km2 | 392,988.75 | 126,842.97 | 74,218.49 | 57,849.39 | |
| % | 60.28 | 19.45 | 11.38 | 8.87 | ||
| 2030s (2021–2040) | SSP245 | km2 | 359,657.68 | 138,002.01 | 86,565.85 | 67,673.45 |
| % | 55.17 | 21.16 | 13.27 | 10.38 | ||
| SSP585 | km2 | 456,205.46 | 107,144.12 | 47,654.97 | 40,894.94 | |
| % | 69.98 | 16.44 | 7.31 | 6.27 | ||
| 2050s (2041–2060) | SSP245 | km2 | 251,735.77 | 162,927.77 | 140,372.45 | 96,863.01 |
| % | 38.62 | 24.99 | 21.53 | 14.9 | ||
| SSP585 | km2 | 472,979.49 | 68,621.13 | 56,963.35 | 53,336.02 | |
| % | 72.55 | 10.53 | 8.738 | 8.18 |
| Time | Scenario | Unit | Unsuitability | Low Suitability | Moderate Suitability | High Suitability |
|---|---|---|---|---|---|---|
| Present (1970–2000) | km2 | 590,469.39 | 27,801.86 | 18,436.35 | 15,192.4 | |
| % | 90.58 | 4.265 | 2.828 | 2.33 | ||
| 2030s (2021–2040) | SSP245 | km2 | 601,839.5 | 24,621.06 | 17,535.78 | 7903.65 |
| % | 92.32 | 3.777 | 2.69 | 1.21 | ||
| SSP585 | km2 | 601,452.11 | 25,948.11 | 17,606.95 | 6892.83 | |
| % | 92.26 | 3.98 | 2.701 | 1.06 | ||
| 2050s (2041–2060) | SSP245 | km2 | 555,675.46 | 39,761.84 | 26,199.68 | 30,262.52 |
| % | 85.24 | 6.099 | 4.019 | 4.64 | ||
| SSP585 | km2 | 579,653.54 | 30,595.78 | 22,535.77 | 19,114.91 | |
| % | 88.92 | 4.693 | 3.457 | 2.93 |
| Time | Scenario | Unit | Unsuitability | Low Suitability | Moderate Suitability | High Suitability |
|---|---|---|---|---|---|---|
| Present (1970–2000) | km2 | 447,937.5 | 119,198.8 | 51,941.82 | 32,821.9 | |
| % | 68.71 | 18.28 | 7.97 | 5.03 | ||
| 2030s (2021–2040) | SSP245 | km2 | 485,550.9 | 106,840.4 | 33,693.4 | 25,815.3 |
| % | 74.48 | 16.39 | 5.17 | 3.96 | ||
| SSP585 | km2 | 99,021.47 | 218,768 | 316,431.4 | 17,679.11 | |
| % | 15.19 | 33.56 | 48.54 | 2.71 | ||
| 2050s (2041–2060) | SSP245 | km2 | 155,927.7 | 217,480.6 | 183,753.1 | 94,738.63 |
| % | 33.56 | 33.36 | 28.19 | 14.53 | ||
| SSP585 | km2 | 213,339.6 | 209,602.5 | 155,726.7 | 73,231.22 | |
| % | 32.73 | 32.15 | 23.80 | 11.23 |
| Time | Scenario | Unit | Unsuitability | Low Suitability | Moderate Suitability | High Suitability |
|---|---|---|---|---|---|---|
| Present (1970–2000) | km2 | 563,283.94 | 35,401.32 | 24,808.99 | 28,405.75 | |
| % | 86.41 | 5.43 | 3.81 | 4.36 | ||
| 2030s (2021–2040) | SSP245 | km2 | 567,456.02 | 33,542.55 | 24,506.29 | 26,394.63 |
| % | 87.05 | 5.15 | 3.76 | 4.05 | ||
| SSP585 | km2 | 584,142.85 | 32,601.39 | 20,868.44 | 14,287.32 | |
| % | 89.61 | 5.01 | 3.201 | 2.19 | ||
| 2050s (2041–2060) | SSP245 | km2 | 562,405.92 | 36,806.05 | 24,850.08 | 27837.45 |
| % | 86.27 | 5.64 | 3.812 | 4.27 | ||
| SSP585 | km2 | 565,538.12 | 33,631.26 | 25,194.37 | 27536.25 | |
| % | 86.75 | 5.159 | 3.865 | 4.22 |
| Time | Scenario | Unit | Unsuitability Stable | Suitability Stable | Gain | Loss |
|---|---|---|---|---|---|---|
| 2030s (2021–2040) | SSP245 | km2 | 360,976.2 | 184,130.5 | 79,562.03 | 27,231.31 |
| % | 55.37 | 28.25 | 12.20 | 4.18 | ||
| SSP585 | km2 | 328,912.6 | 168,255 | 131,509.1 | 23,223.35 | |
| % | 50.45 | 25.81 | 20.17 | 3.56 | ||
| 2050s (2041–2060) | SSP245 | km2 | 375,401 | 2358.29 | 214,409.6 | 59,731.14 |
| % | 57.59 | 0.36 | 32.89 | 9.16 | ||
| SSP585 | km2 | 378,159.7 | 163,641.4 | 82,261.97 | 27,836.95 | |
| % | 58.01 | 25.10 | 12.62 | 4.27 |
| Time | Scenario | Unit | Unsuitability Stable | Suitability Stable | Gain | Loss |
|---|---|---|---|---|---|---|
| 2030s (2021–2040) | SSP245 | km2 | 313,476.1 | 252,272.1 | 1726.5 | 84,425.31 |
| % | 48.09 | 38.70 | 0.26 | 12.95 | ||
| SSP585 | km2 | 315,202.1 | 237,958.9 | 0.5 | 98,738.51 | |
| % | 48.35 | 36.50 | 0.00 | 15.15 | ||
| 2050s (2041–2060) | SSP245 | km2 | 315,095.3 | 263,438.5 | 107.25 | 73,258.91 |
| % | 48.33 | 40.41 | 0.02 | 11.24 | ||
| SSP585 | km2 | 312,869.7 | 239,253.9 | 2332.91 | 97,443.51 | |
| % | 47.99 | 36.70 | 0.36 | 14.95 |
| Time | Scenario | Unit | Unsuitability Stable | Suitability Stable | Gain | Loss |
|---|---|---|---|---|---|---|
| 2030s (2021–2040) | SSP245 | km2 | 354,273.1 | 253,525.6 | 38,715.97 | 5385.38 |
| % | 54.34 | 38.89 | 5.94 | 0.83 | ||
| SSP585 | km2 | 388,847 | 191,552.2 | 4142.02 | 67,358.78 | |
| % | 59.65 | 29.38 | 0.64 | 10.33 | ||
| 2050s (2041–2060) | SSP245 | km2 | 251,736.8 | 258,910.8 | 141,252.5 | 0 |
| % | 38.62 | 39.72 | 21.67 | 0.00 | ||
| SSP585 | km2 | 384,399.5 | 170,330.8 | 8589.73 | 88,579.98 | |
| % | 58.97 | 26.13 | 1.32 | 13.59 |
| Time | Scenario | Unit | Unsuitability Stable | Suitability Stable | Gain | Loss |
|---|---|---|---|---|---|---|
| 2030s (2021–2040) | SSP245 | km2 | 579,853.4 | 48,698.83 | 11,679.8 | 11,667.98 |
| % | 88.95 | 7.47 | 1.79 | 1.79 | ||
| SSP585 | km2 | 590,121.6 | 50,100.09 | 347.8 | 11,330.53 | |
| % | 90.52 | 7.69 | 0.05 | 1.74 | ||
| 2050s (2041–2060) | SSP245 | km2 | 555,672.5 | 61,427.11 | 34,796.93 | 3.51 |
| % | 85.24 | 9.42 | 5.34 | 0.00 | ||
| SSP585 | km2 | 579,469.1 | 61,246.19 | 11,000.27 | 184.42 | |
| % | 88.89 | 9.40 | 1.69 | 0.03 |
| Time | Scenario | Unit | Unsuitability Stable | Suitability Stable | Gain | Loss |
|---|---|---|---|---|---|---|
| 2030s (2021–2040) | SSP245 | km2 | 437,966.6 | 156,378.2 | 9970.9 | 47,584.3 |
| % | 67.18 | 23.99 | 1.53 | 7.30 | ||
| SSP585 | km2 | 91,177.44 | 196,118.5 | 356,761 | 7844.01 | |
| % | 13.99 | 30.08 | 54.73 | 1.20 | ||
| 2050s (2041–2060) | SSP245 | km2 | 148,753.2 | 196,788 | 299,184.3 | 7174.48 |
| % | 22.82 | 30.19 | 45.89 | 1.10 | ||
| SSP585 | km2 | 201,612.1 | 192,235.1 | 11,727.44 | 246,325.3 | |
| % | 30.93 | 29.49 | 1.80 | 37.79 |
| Time | Scenario | Unit | Unsuitability Stable | Suitability Stable | Gain | Loss |
|---|---|---|---|---|---|---|
| 2030s (2021–2040) | SSP245 | km2 | 561,754.4 | 82,913.96 | 1529.51 | 5702.1 |
| % | 86.17 | 12.72 | 0.23 | 0.87 | ||
| SSP585 | km2 | 563,283.9 | 67,757.15 | 0 | 20,858.92 | |
| % | 86.41 | 10.39 | 0.00 | 3.20 | ||
| 2050s (2041–2060) | SSP245 | km2 | 559,481.2 | 85,690.84 | 3802.74 | 2925.22 |
| % | 85.82 | 13.14 | 0.58 | 0.45 | ||
| SSP585 | km2 | 561,919.8 | 84,997.75 | 1364.13 | 3618.31 | |
| % | 86.20 | 13.04 | 0.21 | 0.56 |
References
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2022; Available online: https://www.ipcc.ch/assessment-report/ar6 (accessed on 1 July 2025).
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, D.-S.; Kwon, T.-S.; Athar, M.; Park, Y.-S. Predicting the global distribution of Solenopsis geminata (Hymenoptera: Formicidae) under climate change using the MaxEnt model. Insects 2021, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, J.; Huang, Z. The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol. Evol. 2022, 12, e9165. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.; Ghorbanpour, M.; Mostafavi, H. Habitat potential modelling and the effect of climate change on the current and future distribution of three Thymus species in Iran using MaxEnt. Sci. Rep. 2024, 14, 3641. [Google Scholar] [CrossRef]
- Pettorelli, N. Climate change as a main driver of ecological research. J. Appl. Ecol. 2012, 49, 542–545. [Google Scholar] [CrossRef]
- Smallwood, P.A.; Trapnell, D.W. Species distribution modeling reveals recent shifts in suitable habitat for six North American Cypripedium spp. (Orchidaceae). Divers 2022, 14, 694. [Google Scholar] [CrossRef]
- Mkala, E.M.; Muthoni, F.K.; Ngumbau, V.M.; Ndang’ang’a, K.P.; Wambua, L.; Musili, P.M.; Mwachala, G.; Hu, G.-W.; Yan, H.-F. Predicting the potential impacts of climate change on the endangered endemic Annonaceae species in East Africa. Heliyon 2023, 9, e14613. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H. Study on rare and endangered plants under climate: MaxEnt modeling for identifying hot spots in Northwest China. Cerne 2021, 27, e102667. [Google Scholar] [CrossRef]
- Ouyang, X.; Bai, S.; Strachan, G.B.; Chen, A. Simulation of the potential distribution of rare and endangered Satyrium species in China under climate change. Ecol. Evol. 2022, 12, e9054. [Google Scholar] [CrossRef]
- Lian, Y.; Bai, Y.; Huang, Z.; Ali, M.; Wang, J.; Chen, H. Spatio-temporal changes and habitats of rare and endangered species in Yunnan Province based on MaxEnt model. Land 2024, 13, 240. [Google Scholar] [CrossRef]
- Yao, W.; Luo, Z.; Zhang, X.; Zhu, Y.; Li, H.; Wang, B.; Xu, L. Prediction of potential habitat distributions and climate change impacts on the rare species Woonyoungia septentrionalis (Magnoliaceae) in China based on MaxEnt. Plants 2024, 14, 86. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning (ICML 2004), Banff, AB, Canada, 4–8 July 2004; ACM Press: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Sánchez-Flores, E. GARP modeling of natural and human factors affecting the potential distribution of the invasives Schismus arabicus and Brassica tournefortii in ‘El Pinacate y Gran Desierto de Altar’ Biosphere Reserve. Ecol. Model. 2007, 204, 457–474. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Danylyk, I.M.; Kricsfalusy, V.V. Phytogeographical differentiation of the genus Carex (Cyperaceae) in Saskatchewan, Canada. Thaiszia J. Bot. 2020, 30, 001021. [Google Scholar] [CrossRef]
- Kricsfalusy, V.V.; Godfrey, A.; Chakma, K.; Stewart, A.; Danylyk, I.M. Evaluating the diversity, distribution patterns and habitat preferences of Carex species (Cyperaceae) in western Canada using geospatial analysis. Biodivers. Data J. 2025, 13, e144840. [Google Scholar] [CrossRef]
- Kricsfalusy, V.; Chakma, K.; Godfrey, A. Integrating herbarium data with spatial biodiversity assessment into conservation plans: A case study of the genus Carex L. (Cyperaceae) in Saskatchewan, Canada. Nat. Conserv. 2025, 59, 65–88. [Google Scholar] [CrossRef]
- Benítez-Benítez, C.; Sanz-Arnal, M.; Urbani, M.; Jiménez-Mejías, P.; Martín-Bravo, S. Dramatic impact of future climate change on the genetic diversity and distribution of ecologically relevant Western Mediterranean Carex (Cyperaceae). PeerJ 2022, 10, e13464. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, B.; Zhang, Y.; Wang, H.; Jin, Y.; Liu, G.; Guo, M. Evolution of potential spatial distribution patterns of Carex tussock wetlands under climate change scenarios, Northeast China. Chin. Geogr. Sci. 2022, 32, 142–154. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Zhang, Y.; Liu, X.; He, Y.; Xu, W. The current distribution of Carex alatauensis in the Qinghai—Tibet Plateau estimated by MaxEnt. Agronomy 2023, 13, 564. [Google Scholar]
- FOSA. Flora of Saskatchewan Association Carex Occurrence Dataset; Nature Saskatchewan: Regina, SK, Canada, 2021. [Google Scholar]
- GBIF. The Global Biodiversity Information Facility, GBIF.org GBIF Occurrence Download; GBIF: Copenhagen, Denmark, 2024; Available online: https://doi.org/10.15468/dl.ez4xa9 (accessed on 10 September 2025).
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef] [PubMed]
- Songer, M.; Delion, M.; Biggs, A.; Huang, Q. Modeling impacts of climate change on giant panda habitat. Int. J. Ecol. 2012, 2012, 108752. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Pica, A.; Vela, D.; Magrini, S. Forest orchids under future climate scenarios: Habitat suitability modelling to inform conservation strategies. Plants 2024, 13, 1810. [Google Scholar] [CrossRef]
- Wei, B.O.; Wang, R.; Hou, K.; Wang, X.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Rs. Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2015; Available online: https://cir.nii.ac.jp/crid/1370004240607707919 (accessed on 9 July 2025).
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2021, 35, 213–226. [Google Scholar] [CrossRef]
- Shrestha, N. Detecting multicollinearity in regression analysis. Am. J. Appl. Math. Stat. 2020, 8, 39–42. [Google Scholar] [CrossRef]
- Deng, X.; Xu, D.; Liao, W.; Wang, R.; Zhuo, Z. Predicting the distributions of Scleroderma guani (Hymenoptera: Bethylidae) under climate change in China. Ecol. Evol. 2022, 12, e9410. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P.; McPherson, J. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for MAXENT ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, X.; Sun, Y.; Liu, Y. Species distribution modeling based on MaxEnt to inform biodiversity conservation in the Central Urban Area of Chongqing Municipality. Ecol. Indic. 2024, 158, 111491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kricsfalusy, V.; Chakma, K. Prediction of Potential Habitat Distributions and Climate Change Impacts on Six Carex L. Species of Conservation Concern in Canada. Conservation 2025, 5, 55. https://doi.org/10.3390/conservation5040055
Kricsfalusy V, Chakma K. Prediction of Potential Habitat Distributions and Climate Change Impacts on Six Carex L. Species of Conservation Concern in Canada. Conservation. 2025; 5(4):55. https://doi.org/10.3390/conservation5040055
Chicago/Turabian StyleKricsfalusy, Vladimir, and Kakon Chakma. 2025. "Prediction of Potential Habitat Distributions and Climate Change Impacts on Six Carex L. Species of Conservation Concern in Canada" Conservation 5, no. 4: 55. https://doi.org/10.3390/conservation5040055
APA StyleKricsfalusy, V., & Chakma, K. (2025). Prediction of Potential Habitat Distributions and Climate Change Impacts on Six Carex L. Species of Conservation Concern in Canada. Conservation, 5(4), 55. https://doi.org/10.3390/conservation5040055






