Are There Resource Allocation Constraints to Floral Production in the Endangered Barbarea vulgaris subsp. lepuznica (Southern Carpathians, Romania)?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Taxon and Population Sites
2.3. Plant Sampling and Trait Data Collection
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Trait Response to Increasing Elevation
4.2. Trait Response to Grazing
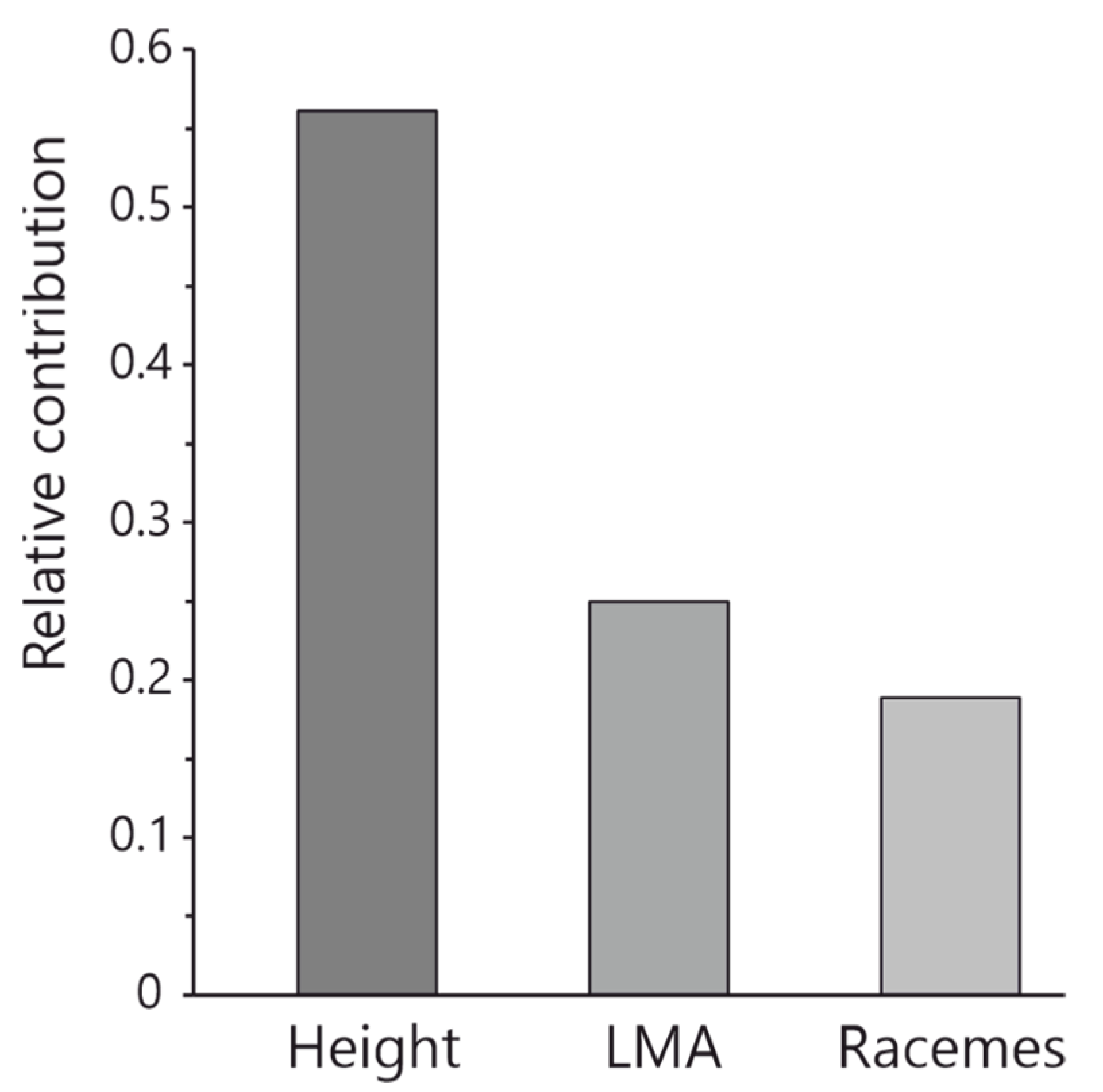
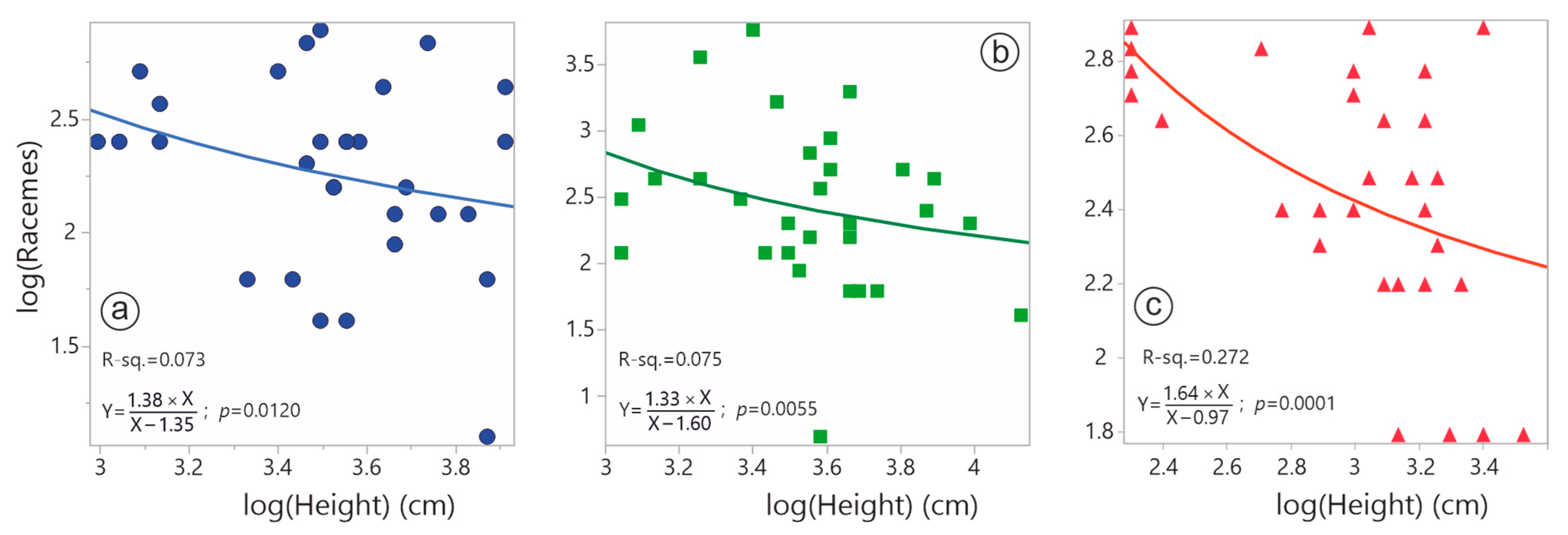
4.3. Allocation Trade-Offs Between Reproductive and Vegetative Traits
4.4. Synthesis, Limitations and Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Bello, F.; Lepš, J.; Sebastià, M.T. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005, 42, 824–833. [Google Scholar] [CrossRef]
- Laliberté, E.; Shipley, B.; Norton, D.A.; Scott, D. Which plant traits determine abundance under long-term shifts in soil resource availability and grazing intensity? J. Ecol. 2012, 100, 662–677. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Grace, J. Plant Resource Allocation; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Ackerly, D.D.; Reekie, E.G. Reproductive Allocation in Plants. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 1–29. [Google Scholar]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Fabbro, T.; Körner, C. Altitudinal differences in flower traits and reproductive allocation. Flora 2004, 199, 70–81. [Google Scholar] [CrossRef]
- Gabel, E.R.; Sattler, J.; Reisch, C. Genetic variation and performance of the alpine plant species Dianthus callizonus differ in two elevational zones of the Carpathians. Alp. Bot. 2017, 127, 65–74. [Google Scholar] [CrossRef]
- He, J.D.; Xue, J.Y.; Gao, J.; Wang, J.; Wu, Y. Adaptations of the floral characteristics and biomass allocation patterns of Gentiana hexaphylla to the altitudinal gradient of the eastern Qinghai-Tibet Plateau. J Mt. Sci. 2017, 14, 1563–1576. [Google Scholar] [CrossRef]
- Takahashi, K.; Matsuki, S. Morphological variations of the Solidago virgaurea L. complex along an elevational gradient on Mt Norikura, central Japan. Plant Species Biol. 2017, 32, 238–246. [Google Scholar] [CrossRef]
- Paudel, B.R.; Dyer, A.G.; Garcia, J.E.; Shresta, M. The effect of elevational gradient on alpine gingers (Roscoea alpina and R. Purpurea) in the Himalayas. PeerJ 2019, 7, e7503. [Google Scholar] [CrossRef]
- Xu, B.; Wang, J.; Shi, F. Impacts of ontogenetic and altitudinal changes on morphological traits and biomass allocation patterns of Fritillaria unibracteata. J. Mt. Sci. 2020, 17, 83–94. [Google Scholar] [CrossRef]
- Kiełtyk, P. Intraspecific morphological variation of Bellidiastrum michelii (Asteraceae) along a 1,155 m elevation gradient in the Tatra Mountains. PeerJ 2021, 9, e11286. [Google Scholar] [CrossRef]
- Kiełtyk, P. Patterns of floral allocation along an elevation gradient: Variation in Senecio subalpinus growing in the Tatra Mountains. Alp. Bot. 2021, 131, 117–124. [Google Scholar] [CrossRef]
- Milla, R.; Gimenez-Benavides, L.; Escudero, A.; Reich, P.B. Intra- and interspecific performance in growth and reproduction increase with altitude: A case study with two Saxifraga species from northern Spain. Funct. Ecol. 2009, 23, 111–118. [Google Scholar] [CrossRef]
- Ma, W.; Shi, P.; Li, W.; He, Y.; Zhang, X.; Shen, Z.; Chai, S. Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai-Tibetan Plateau. Sci. China Life Sci. 2010, 53, 1142–1151. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Gonzalez-Zurdo, P.; Escudero, A.; Babiano, J.; Garcia-Ciudad, A.; Mediavilla, S. Costs of leaf reinforcement in response to winter cold in evergreen species. Tree Physiol. 2016, 36, 273–286. [Google Scholar] [CrossRef]
- Díaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Tecco, P.A.; Díaz, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Johnston, F.M.; Pickering, C.M. Effect of altitude on resource allocation in the weed Achillea millefolium (yarrow, Asteraceae) in the Australian Alps. Aust. J. Bot. 2004, 52, 639–646. [Google Scholar] [CrossRef]
- Guo, H.; Weiner, J.; Mazer, S.J.; Zhao, Z.G.; Du, G.Z.; Li, B. Reproductive allometry in Pedicularis species changes with elevation. J. Ecol. 2012, 100, 452–458. [Google Scholar] [CrossRef]
- Kelly, C.A. Effects of variable life history and insect herbivores on reproduction in Solidago macrophylla (Asteraceae) on an elevational gradient. Am. Midl. Nat. 1998, 139, 243–254. [Google Scholar] [CrossRef]
- Kudo, G.; Molau, U. Variations in reproductive traits at inflorescence and flower levels of an arctic legume, Astragalus alpinus L.: Comparisons between a subalpine and an alpine population. Plant Species Biol. 1999, 14, 181–191. [Google Scholar] [CrossRef]
- Hautier, Y.; Randin, C.F.; Stöcklin, J.; Guisan, A. Changes in reproductive investment with altitude in an alpine plant. J. Plant Ecol. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Maad, J.; Armbruster, W.S.; Fenster, C.B. Floral size variation in Campanula rotundifolia (Campanulaceae) along altitudinal gradients: Patterns and possible selective mechanisms. Nor. J. Bot. 2013, 31, 361–371. [Google Scholar] [CrossRef]
- Bliss, L.C. Arctic and alpine plant life cycles. Annu. Rev. Ecol. Syst. 1971, 2, 405–438. [Google Scholar] [CrossRef]
- Weiner, J.; Campbell, L.G.; Pino, J.; Echarte, L. The allometry of reproduction within plant populations. J. Ecol. 2009, 97, 1220–1233. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Posse, G.; Collantes, M.B. Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. J. Appl. Ecol. 2005, 42, 50–59. [Google Scholar] [CrossRef]
- Whitworth-Hulse, J.I.; Cingolani, A.M.; Zeballos, S.R.; Poca, M.; Gurvich, D.E. Does grazing induce intraspecific trait variation in plants from a sub-humid mountain ecosystem? Austral Ecol. 2016, 41, 745–755. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Li, Z.; Liu, H.; Wang, L.; Wang, W.; Wang, Y.; Liang, C. Single grazing is more detrimental to grasslands than mixed grazing: Evidence from the response of functional traits of dominant plants to grazing systems. Front. Ecol. Evol. 2021, 9, 682289. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, J.; Tang, Y.; Li, Z.; Liu, H.; Wang, L.; Wu, Y.; Liang, C. Plant functional traits and biodiversity can reveal the response of ecosystem functions to grazing. Sci. Total Environ. 2023, 899, 165636. [Google Scholar] [CrossRef] [PubMed]
- Briske, D.D. Strategies of plant survival in grazed systems: A functional interpretation. In The Ecology and Management of Grazing Systems; Hodgson, J., Illius, A.W., Eds.; CABI International: Wallingford, UK, 1996; pp. 37–67. [Google Scholar]
- Díaz, S.; Noy-Meir, I.; Cabido, M. Can grazing response of herbaceous plants be predicted from simple vegetative traits? J. Appl. Ecol. 2001, 38, 497–508. [Google Scholar] [CrossRef]
- Cayssials, V.; Rodríguez, C. The adaptive value of grass traits in response to grazing. J. Plant Ecol. 2018, 11, 248–255. [Google Scholar] [CrossRef]
- Hendrickson, J.; Olson, B. Understanding plant response to grazing. In Targeted Grazing: A Natural Approach to Vegetation Management and Landscape Enhancement; Launchbaugh, K., Walker, J.W., Eds.; American Sheep Industry Association: Englewood, CO, USA, 2006; pp. 32–39. [Google Scholar]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Diaz, S.; Vendramini, F.; Cornelissen, J.H.C.; Gurvich, D.E.; Cabido, M. Leaf traits and herbivore selection in the field and in cafeteria experiments. Austral Ecol. 2003, 28, 642–650. [Google Scholar] [CrossRef]
- Adler, P.; Raff, D.; Lauenroth, W. The effect of grazing on the spatial heterogeneity of vegetation. Oecologia 2001, 128, 465–479. [Google Scholar] [CrossRef]
- Louault, F.; Pillar, V.D.; Aufrère, J.; Garnier, E.; Soussana, J.F. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Veg. Sci. 2005, 16, 151–160. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; McIntyre, S.U.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant trait responses to grazing—A global synthesis. Glob. Change Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 157–178. [Google Scholar] [CrossRef]
- Kostrakiewicz-Gierałt, K.; Kozak, M.; Kozłowska-Kozak, K. The impact of extensive sheep grazing on the population and individual traits of Trollius altissimus Crantz. Pol. J. Ecol. 2015, 63, 523–533. [Google Scholar] [CrossRef]
- Wentao, M.; Shiming, T.; Le, Q.; Weibo, R.; Fry, E.L.; De Long, J.R.; Margerison, R.C.; Yuan, C.; Xiaomin, L. Grazing reduces plant sexual reproduction but increases asexual reproduction: A global meta-analysis. Sci. Total Environ. 2023, 879, 162850. [Google Scholar] [CrossRef]
- Menges, E.S. Applications of population viability analyses in plant conservation. Ecol. Bull. 2000, 48, 73–84. [Google Scholar]
- Comitetul de Stat al Geologiei. Harta Geologică 1:200000, Foaia L-34-XXIV; Institutul Geologic: București, Romania, 1968. [Google Scholar]
- Fărcaș, I.; Scorocovschi, V. Clima Munților Retezat. In Parcul Național Retezat—Studii Ecologice; Popovici, I., Ed.; West Side Computers: Brașov, Romania, 1992; pp. 13–20. [Google Scholar]
- Diklić, N.; Lakušić, D. Barbarea vulgaris R. Br subsp. lepuznica (E.I. Nyárády) Soó. In The Red Data Book of Flora of Serbia I: Extinct and Critically Endangered Taxa; Stevanović, V., Ed.; Institute for Protection of Nature of the Republic of Serbia: Belgrade, Serbia, 1999; pp. 221–222. [Google Scholar]
- Dihoru, G.; Negrean, G. Cartea Roșie a Plantelor Vasculare din România; Editura Academiei Române: București, Romania, 2009. [Google Scholar]
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Nyárády, E.I. Adnotațiuni la Flora României, vol. IX. Bul. Grad. Bot. Muz. Bot. Cluj 1934, 14, 3–4. [Google Scholar]
- Ciocârlan, V. Flora Ilustrată a României: Pteridophyta et Spermatophyta; Editura Ceres: București, Romania, 2009. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A. Plante vasculare din Romania: Determinator Ilustrat de Teren; Editura Victor B Victor: București, Romania, 2013. [Google Scholar]
- Kliment, J.; Turis, P.; Janišová, M. Taxa of vascular plants endemic to the Carpathian Mts. Preslia 2016, 88, 19–76. [Google Scholar]
- Euro+Med PlantBase. 2025. Available online: https://europlusmed.org (accessed on 2 April 2025).
- EUNIS. 2025. Available online: https://eunis.eea.europa.eu/species/163785 (accessed on 2 April 2025).
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Hikosaka, K.; Kurokawa, H.; Arai, T.; Takayanagi, S.; Tanaka, H.O.; Nagano, S.; Nakashizuka, T. Intraspecific variations in leaf traits, productivity and resource use efficiencies in the dominant species of subalpine evergreen coniferous and deciduous broadleaved forests along the altitudinal gradient. J. Ecol. 2021, 109, 1804–1818. [Google Scholar] [CrossRef]
- Martínková, J.; Hájek, T.; Adamec, L.; Klimešová, J. Growth, root respiration and photosynthesis of a root-sprouting short-lived herb after severe biomass removal. Flora 2021, 284, 151915. [Google Scholar] [CrossRef]
- Martínková, J.; Šmilauer, P.; Mihulka, S.; Latzel, V.; Klimešová, J. The effect of injury on whole-plant senescence: An experiment with two root-sprouting Barbarea species. Ann. Bot. 2016, 117, 667–679. [Google Scholar] [CrossRef]
- Samson, D.A.; Werk, K.S. Size-dependent effects in the analysis of reproductive effort in plants. Am. Nat. 1986, 127, 667–680. [Google Scholar] [CrossRef]
- Stoecklin, J.; Favre, P. Effects of plant size and morphological constraints on variation in reproductive components in two related species of Epilobium. J. Ecol. 1994, 82, 735–746. [Google Scholar] [CrossRef]
- Pluess, A.; Stoecklin, J. The importance of population origin and environment on clonal and sexual reproduction in the alpine plant Geum reptans. Funct. Ecol. 2005, 19, 228–237. [Google Scholar] [CrossRef]
- Aarssen, L.W.; Taylor, D.R. Fecundity allocation in herbaceous plants. Oikos 1992, 65, 225–232. [Google Scholar] [CrossRef]
- Laiolo, P.; Obeso, J.R. Life-history responses to the altitudinal gradient. In High Mountain Conservation in a Changing World; Catalan, J., Ninot, J.M., Aniz, M.M., Eds.; Springer: Cham, Switzerland, 2017; Volume 62, pp. 253–283. [Google Scholar]
- Dai, W.; Gao, R.; He, M.; Yang, Y.; Li, F.; Mu, J. Linkages among the functional traits, insect visitation rate and seed set of Gentiana taxa on the Tibetan Plateau. J. Mt. Sci. 2022, 19, 2312–2321. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
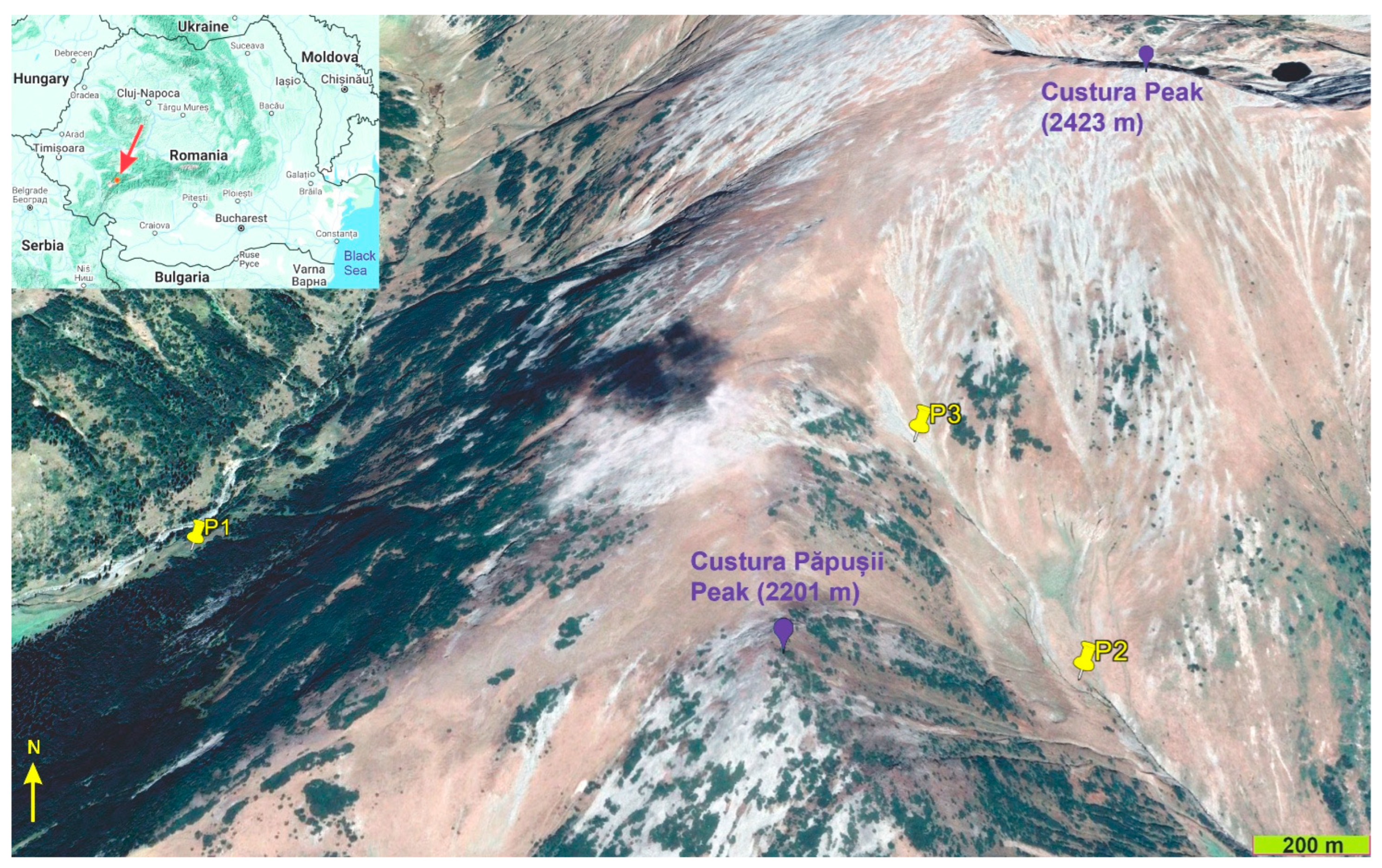

| Feature | P1 Site | P2 Site | P3 Site |
|---|---|---|---|
| Latitude north (degrees) | 45.344717 | 45.340710 | 45.343711 |
| Longitude east (degrees) | 22.906873 | 22.927006 | 22.923895 |
| Mean elevation (m) | 1700 | 1900 | 2100 |
| Mean slope (degrees) | 4 | 19 | 25 |
| Slope aspect | SW | SE | SE |
| Soil pH (at 5 cm depth) | 6.6 | 6.4 | 7.1 |
| Sheep grazing intensity | Moderate | Very low | Absent |
| Fixed Effect | F Ratio | Prob > F |
| Population | 4.151 | 0.0190 |
| Random Effects | Variance Component | Wald p-Value |
| Plant [within Population] | 0.0298 | <0.0001 |
| Leaf [within Population and Plant] | 0.0271 | <0.0001 |
| Fixed Effect | F Ratio | Prob > F |
| Population | 32.964 | <0.0001 |
| Random Effect | Variance Component | Wald p-Value |
| Plant [within Population] | 0.0878 | <0.0001 |
| Fixed Effect | F Ratio | Prob > F |
| Population | 1.530 | 0.2223 |
| Random Effect | Variance Component | Wald p-Value |
| Plant [within Population] | 0.2267 | <0.0001 |
| Term | Raw Estimate | Standardised Estimate | t Ratio | Prob > |t| | R-Square |
|---|---|---|---|---|---|
| Population 1 | |||||
| Intercept | 3.239 | 0 | 1.79 | 0.0851 | |
| LMA | 0.141 | 0.076 | 0.41 | 0.6839 | 0.081 |
| Height | −0.435 | −0.266 | −1.44 | 0.1626 | |
| Population 2 | |||||
| Intercept | 3.099 | 0 | 0.74 | 0.4682 | |
| LMA | 0.373 | 0.089 | 0.45 | 0.6542 | 0.095 |
| Height | −0.611 | −0.263 | −1.33 | 0.1943 | |
| Population 3 | |||||
| Intercept | 6.719 | 0 | 5.69 | <0.0001 | |
| LMA | −0.668 | −0.352 | −2.42 | 0.0226 | 0.428 |
| Height | −0.519 | −0.534 | −3.66 | 0.0011 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafta, D.; Aczel, E.; Carpa, R.; Dănău, C.; Goia, I. Are There Resource Allocation Constraints to Floral Production in the Endangered Barbarea vulgaris subsp. lepuznica (Southern Carpathians, Romania)? Conservation 2025, 5, 56. https://doi.org/10.3390/conservation5040056
Gafta D, Aczel E, Carpa R, Dănău C, Goia I. Are There Resource Allocation Constraints to Floral Production in the Endangered Barbarea vulgaris subsp. lepuznica (Southern Carpathians, Romania)? Conservation. 2025; 5(4):56. https://doi.org/10.3390/conservation5040056
Chicago/Turabian StyleGafta, Dan, Emilia Aczel, Rahela Carpa, Claudia Dănău, and Irina Goia. 2025. "Are There Resource Allocation Constraints to Floral Production in the Endangered Barbarea vulgaris subsp. lepuznica (Southern Carpathians, Romania)?" Conservation 5, no. 4: 56. https://doi.org/10.3390/conservation5040056
APA StyleGafta, D., Aczel, E., Carpa, R., Dănău, C., & Goia, I. (2025). Are There Resource Allocation Constraints to Floral Production in the Endangered Barbarea vulgaris subsp. lepuznica (Southern Carpathians, Romania)? Conservation, 5(4), 56. https://doi.org/10.3390/conservation5040056






