Biology and Conservation of Moxostoma spp. Occurring in Canada with Emphasis on the Copper Redhorse (M. hubbsi, Legendre 1952), an Endemic Species on an Extinction Trajectory
Abstract
1. Introduction
2. Methodology
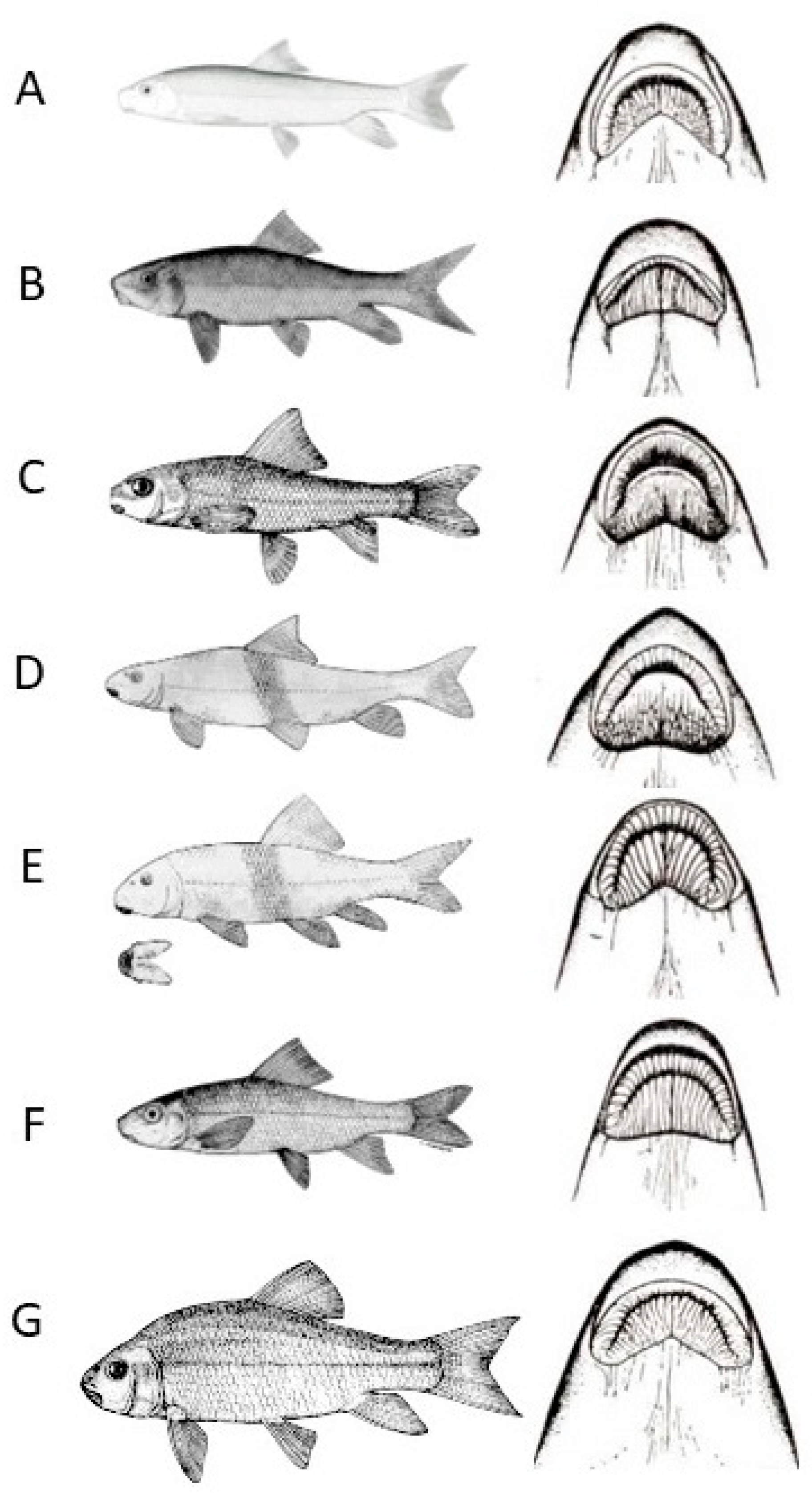
3. Catostomidae (Suckers): Moxostoma spp.
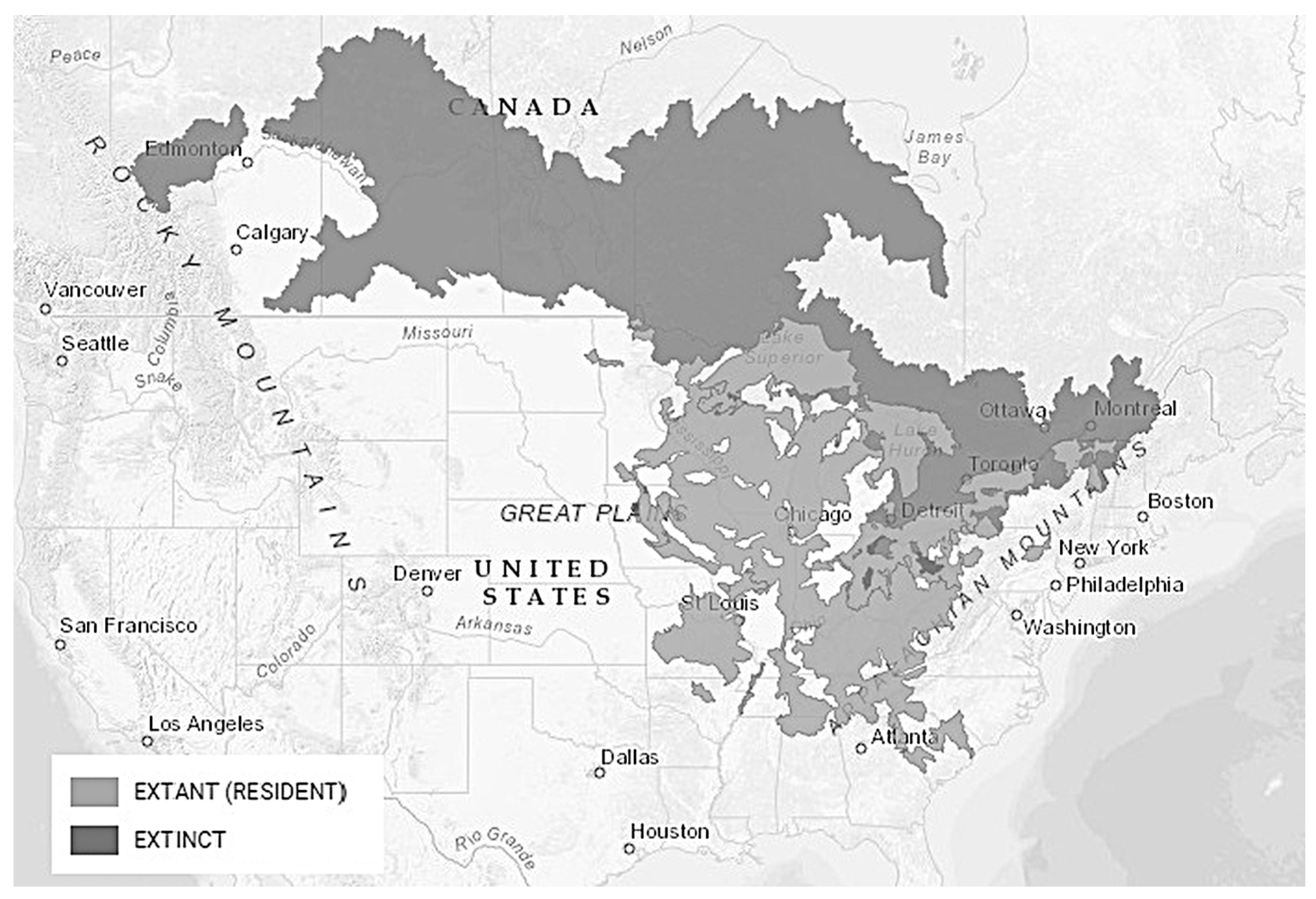
3.1. Silver Redhorse, Moxostoma anisurum (Rafinesque 1820), Chevalier Blanc
3.1.1. Feeding
3.1.2. Reproduction/Spawning
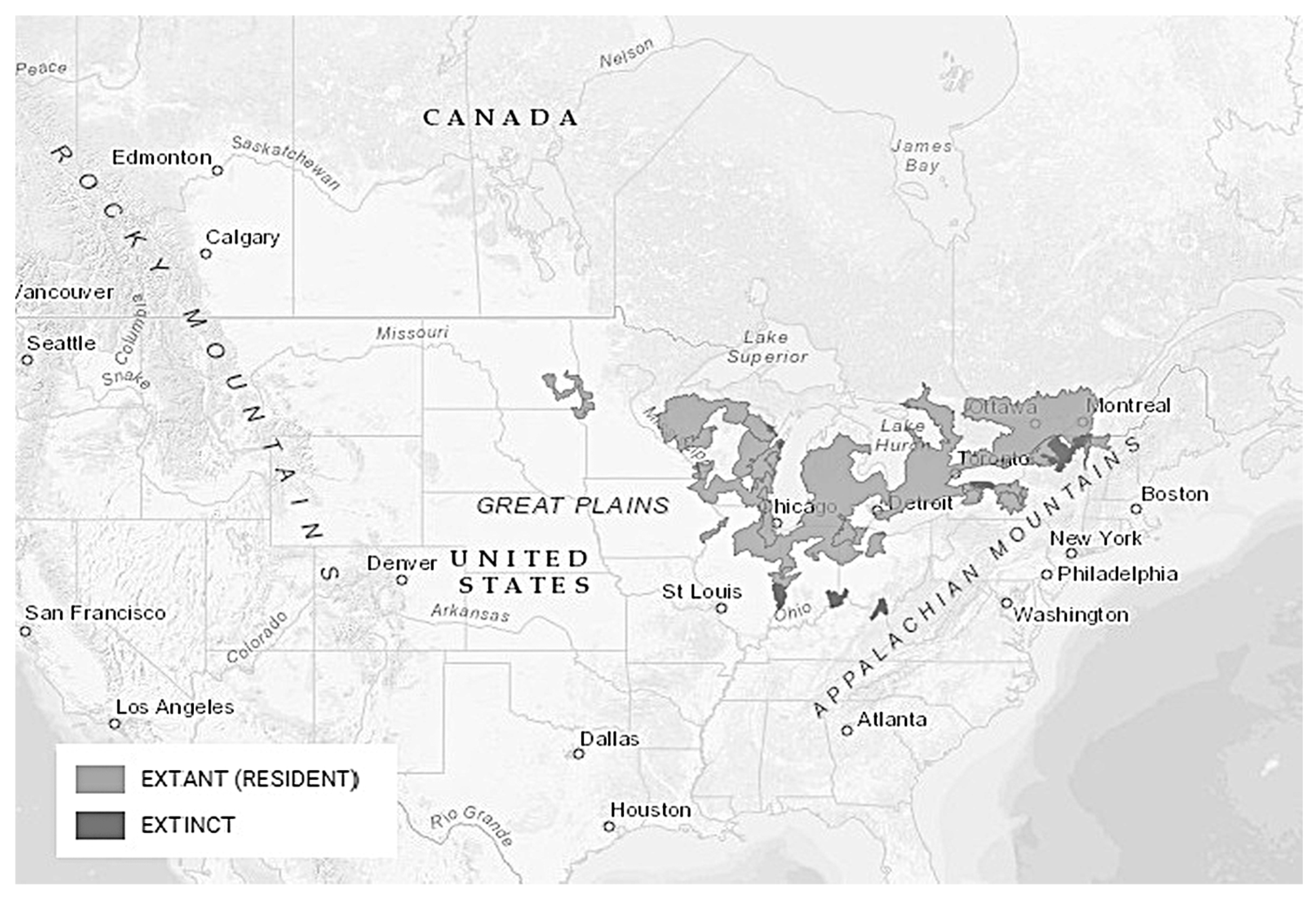
3.1.3. Distribution/Habitat Preferences
3.1.4. Cultivation Activities
3.1.5. Conservation Status and Threats
3.1.6. Other
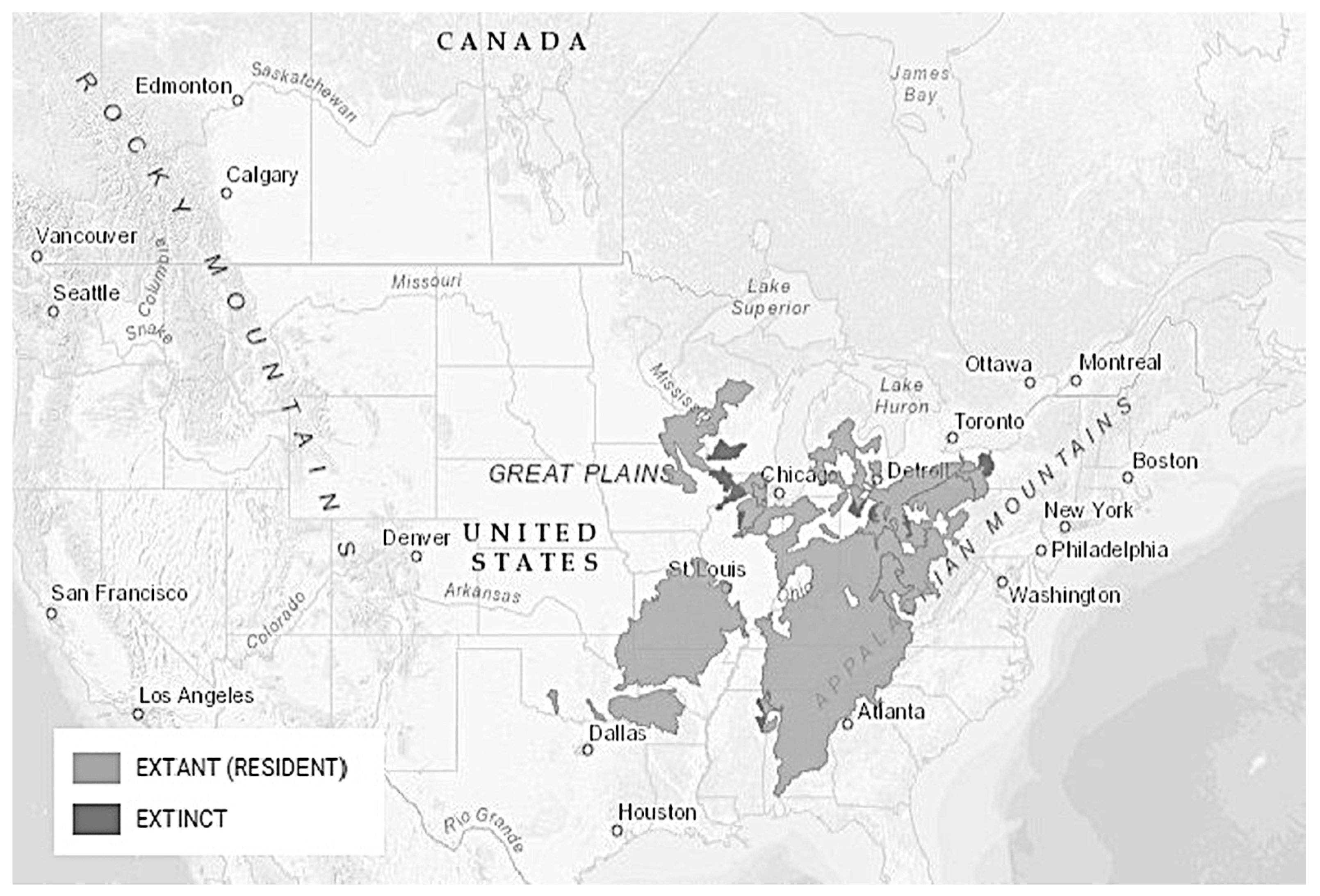
3.2. Black Redhorse, Moxostoma duquesnei (Lesueur 1817), Chevalier Noir
3.2.1. Feeding
3.2.2. Reproduction/Spawning
3.2.3. Distribution and Habitat Preferences
3.2.4. Cultivation Activities
3.2.5. Conservation Status and Threats
3.2.6. Other
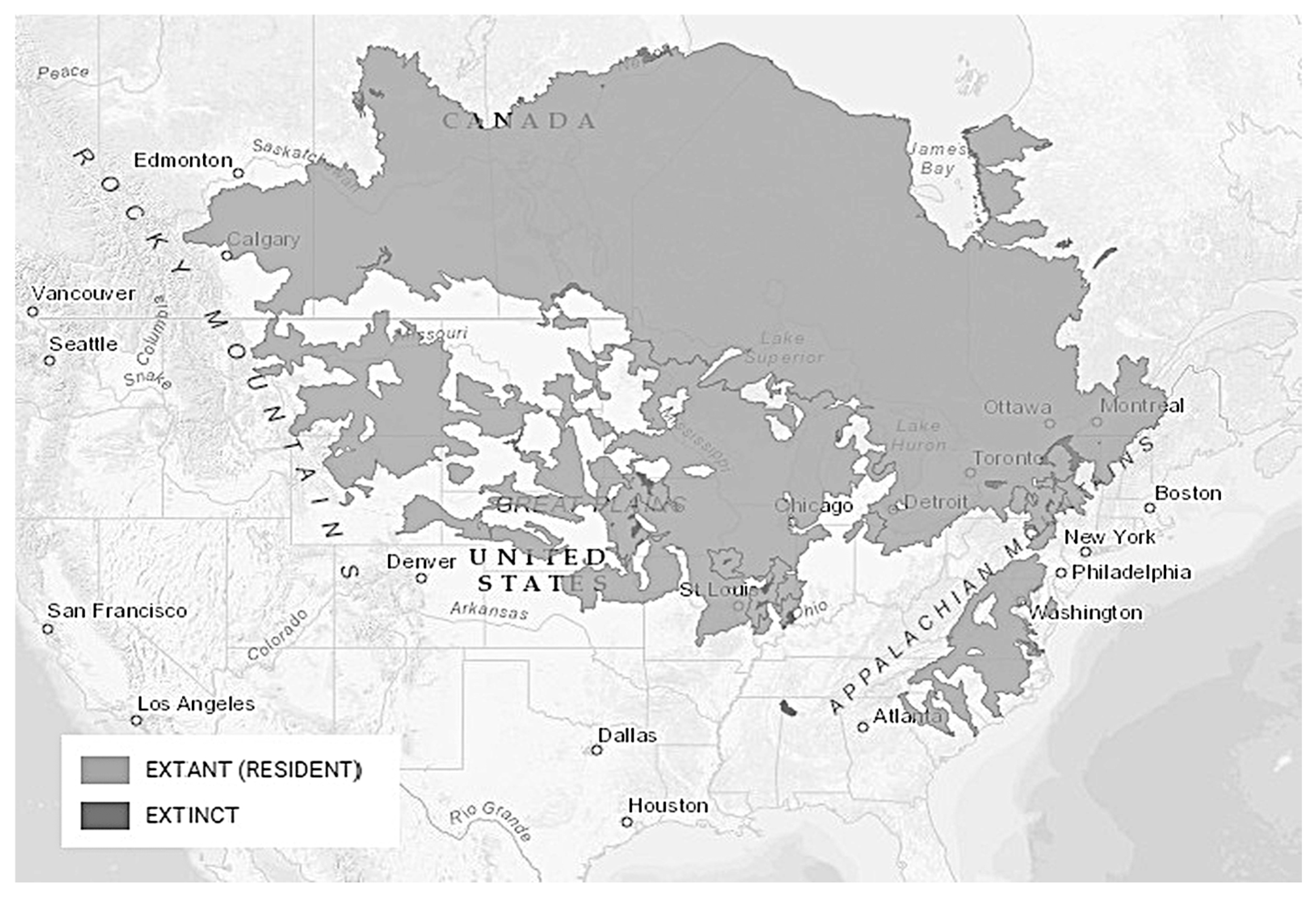
3.3. Golden Redhorse, Moxostoma erythrurum (Rafinesque 1818), Chevalier Doré
3.3.1. Feeding
3.3.2. Reproduction/Spawning
3.3.3. Distribution and Habitat Preferences
3.3.4. Cultivation Activities
3.3.5. Conservation Status and Threats
3.3.6. Other
3.4. Shorthead Redhorse, Moxostoma macrolepidotum (Lesueur 1817), Chevalier Rouge
3.4.1. Feeding
3.4.2. Reproduction/Spawning
3.4.3. Distribution and Habitat Preferences
3.4.4. Cultivation Activities
3.4.5. Conservation Status and Threats
3.5. Greater Redhorse, Moxostoma valenciennesi (Jordan 1885), Chevalier Jaune
3.5.1. Feeding
3.5.2. Reproduction/Spawning
3.5.3. Distribution and Habitat Preferences
3.5.4. Cultivation Activities
3.5.5. Conservation Status and Threats
3.5.6. Other
3.6. River Redhorse, Moxostoma carinatum (Cope 1870), Chevalier de Rivière
3.6.1. Feeding
3.6.2. Reproduction/Spawning
3.6.3. Distribution and Habitat Preferences
3.6.4. Cultivation Activities
3.6.5. Conservation Status and Threats
3.6.6. Other
3.7. Copper Redhorse, Moxostoma hubbsi, (Legendre 1952) Chevalier Cuivré
3.7.1. Feeding
3.7.2. Reproduction/Spawning
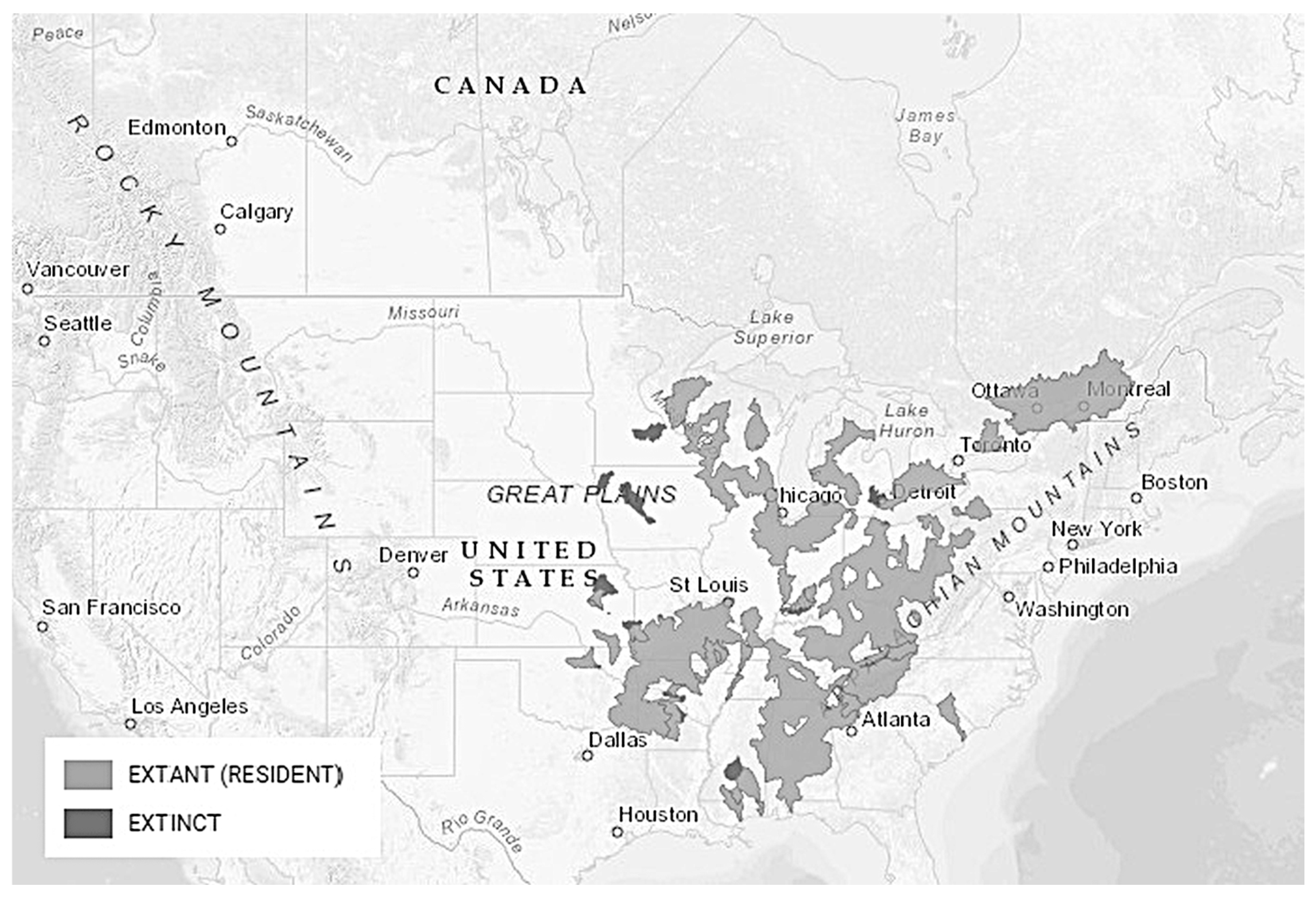
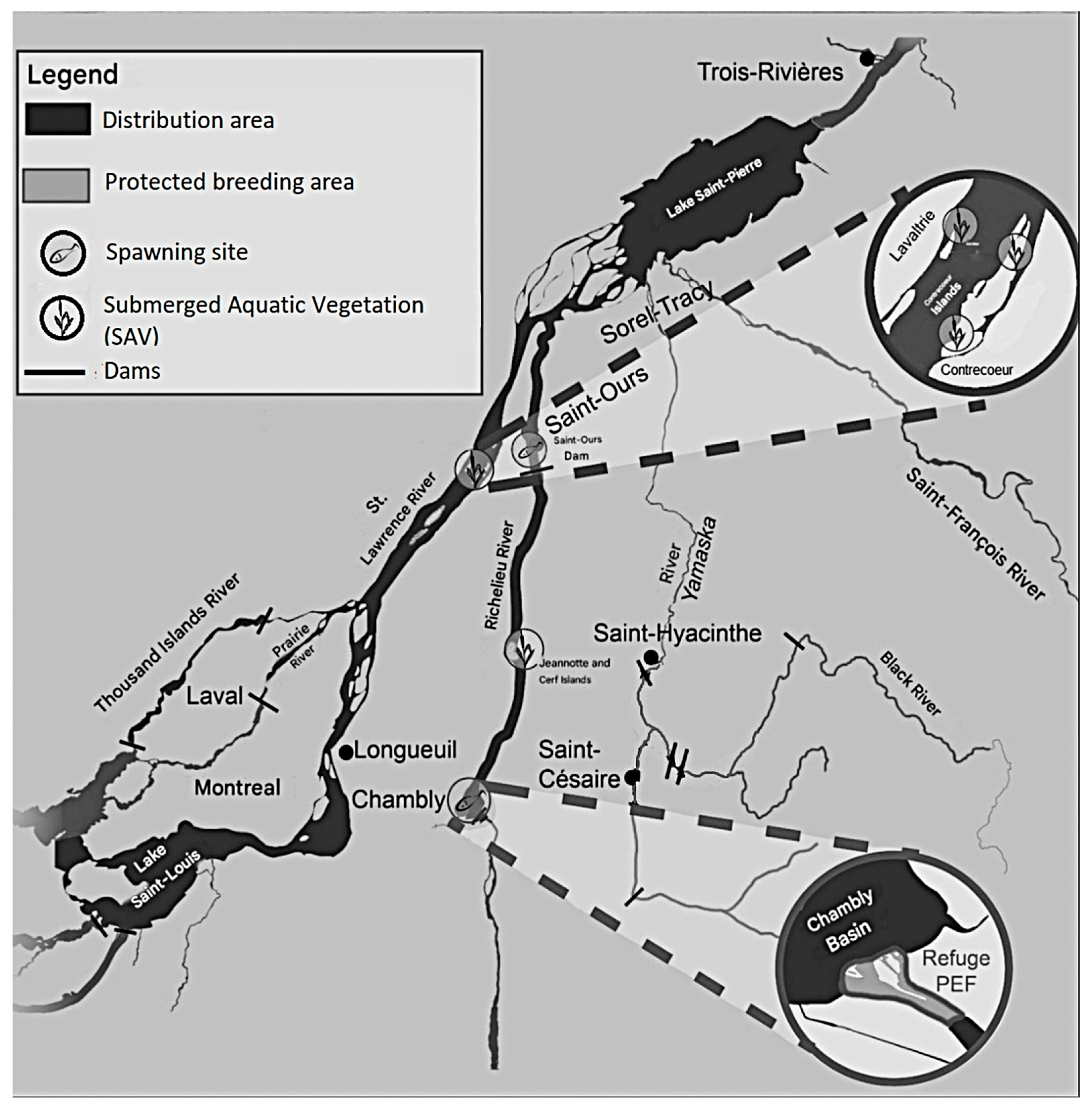
3.7.3. Distribution and Critical Habitat
3.7.4. Cultivation Activities
3.7.5. Conservation Status and Threats
- Water contaminants: Sixty-four (64) municipalities (total population 300,000) are located along the Richelieu River and 70% of its watershed is dedicated to agriculture [116,136]. Extensive work has evaluated its water quality and biological integrity with regard to industrial, urban, and agricultural pressure, and the impact of wastewater treatment stations installed. Improvements of the global quality of the ecosystem were noted, but pollution from municipalities (wastewater, runoff) and industries increases phosphorus, nitrogen, fecal coliforms, suspended matter, turbidity, and chlorophyll-a, hindering the physicochemical and bacterial quality index of the Richelieu River. Water quality is deemed good from its point of entry and then gradually decreases, becoming severely impacted where it meets the St. Lawrence River. A water management plan addresses habitat degradation (i.e., habitat loss, fragmentation, aquatic vegetation, and invasive species) [137]. Recommendations focus on improving agricultural practices and the enforcing laws for the riparian strip. The main activities directly associated with habitat degradation include urban development, agricultural drainage, riparian strip destruction, erosion, and shoreline artificialization.
- 2.
- Invasive species: The Great Lakes–St Lawrence River basin contains the most non-native species of any freshwater system, with n = 180 established invasive species [146]. The spread of invasive tench (Tinca tinca) in the St Lawrence River has raised concerns in Québec, and was reported by the MELCCFP in 2022, especially regarding the redhorse species, as they share habitats, spawning grounds, and dietary preferences. Two other benthivorous species, the round goby Neogobius melanostomus and the tubenose goby Proterorhinus semilunaris, have also been detected and could exert additional pressure on molluscivorous species such as M. hubbsi and M. carinatum due to their shared diet and possible egg and larvae predation [147]. The presence of the Asian carp species (Ctenopharyngodon idella, Hypophthalmichtys nobilis, H. molitrix, and Mylopharyngodon piceus) has increased in recent years in Canada [148], but only C. idella is confirmed in Québec. Environmental DNA analysis and captures confirmed the presence of grass carp in the M. hubbsi habitat. Grass carp poses threats by (1) occurring in its spawning and feeding grounds; (2) competing for food; (3) being highly performing feeders; and (4) contributing to habitat degradation [149]. Black carp M. piceus, native to East Asia and present in the United States, has not yet been detected in Québec, but as it is an avid consumer of bivalves, its invasion would impact the eight bivalve species on which M. hubbsi relies on for feeding [150].
- 3.
- Habitat fragmentation, loss, or degradation: A large proportion of the copper redhorse distribution has been modified by anthropogenic activities. Habitat loss, degradation, and fragmentation exert major restrictions to the distribution range, causing population decline and making recovery more uncertain [127]. Erosion, shoreline hardening, and the construction of dams severely impact M. hubbsi. The Richelieu River is of concern as the quality criterion of 13 mg/L of suspended matter can exceed by more than 20% [137]. Despite policies to improve agricultural practices, the absence of windbreaks, livestock presence, and altered riparian vegetation all contribute to shoreline erosion. Erosion accelerates siltation, increases turbidity, can disrupt the food chain by altering photosynthesis, and accentuate settling of sediments on gravel in the river interstices, possibly covering spawning grounds and mollusk beds adversely.
3.7.6. Ongoing Conservation Efforts and Research
3.7.7. Feeding/Spawning Habitat Creation/Restoration
3.7.8. Genetic Studies (Including Environmental DNA)
3.7.9. Telemetry Surveys
3.7.10. Ecological River Rehabilitation or Restoration
3.7.11. Conservation Aquaculture and Restocking Activities
3.7.12. Cryopreservation and Cryobanking
3.8. Future Conservation and Research Orientation for Moxostoma sp.
3.8.1. Effects of Climate Change and Phenotypic Plasticity
3.8.2. Fish Health
4. Conclusions
- Acquire a better understanding of spawning preferences and develop models to better predict how environmental variables and habitat modification affect reproduction quality, timing, and success.
- Explore novel approaches in vegetation engineering aimed at rehabilitation/creation/restoration and protection of SAV habitats.
- Apply more telemetry monitoring efforts to assess the use of essential habitats.
- Increase regulations for the reduction/control of pesticides and other contaminants in essential habitats and document their effects.
- Implement more efforts aimed at resolving the problems concerning invasive and exotic species.
- Refine/develop eDNA detection techniques for vulnerable Moxostoma species, mollusks (gastropods and bivalves), and plant species found in essential and critical habitats.
- Set forth an extensive research program aimed at copper redhorse conservation physiology aimed at species’ responses to climate change and health status.
- Refine the rearing protocols of the captive copper redhorse population (broodstock, subadults, juveniles on growing, enrichment techniques pre-release, etc.).
- Develop more adapted anesthesia techniques for the genus Moxostoma.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAV | Submerged aquatic vegetation |
| COVABAR | Comité de concertation et de valorisation du bassin de la rivière Richelieu |
| SARA | Species at Risk Act (Canada) |
| DFO | Department of Fisheries and Oceans (Canada) |
| IUCN | International Union for Conservation of Nature |
| COSEWIC | Committee on the Status of Endangered Wildlife in Canada |
| MELCCFP | Ministère de l’Environnement, de la Lutte contre les Changements Climatiques, de la Faune et des Parcs |
References
- Desforges, J.E.; Clarke, J.; Harmsen, E.J.; Jardine, A.M.; Robichaud, J.A.; Serré, S.; Chakrabarty, P.; Bennett, J.R.; Hanna, D.E.L.; Smol, J.P.; et al. The Alarming State of Freshwater Biodiversity in Canada. Can. J. Fish. Aquat. Sci. 2022, 79, 352–365. [Google Scholar] [CrossRef]
- Moyle, P.B.; Leidy, R.A. Loss of Biodiversity in Aquatic Ecosystems: Evidence from Fish Faunas. In Conservation Biology; Fiedler, P.L., Jain, S.K., Eds.; Springer: Boston, MA, USA, 1992; pp. 127–169. [Google Scholar] [CrossRef]
- Cambray, J.A.; Bianco, P.G. Freshwater Fish in Crisis, a Blue Planet Perspective. Ital. J. Zool. 1998, 65 (Suppl. 1), 345–356. [Google Scholar] [CrossRef]
- Ricciardi, A.; Neves, R.J.; Rasmussen, J.B. Impending Extinctions of North American Freshwater Mussels (Unionoida) following the Zebra Mussel (Dreissena polymorpha) Invasion. J. Anim. Ecol. 1998, 67, 613–619. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. Bioscience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ Warning to Humanity on the Freshwater Biodiversity Crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef]
- Sayer, C.A.; Fernando, E.; Jimenez, R.R.; Macfarlane, N.B.W.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Contreras-MacBeath, T.; Cox, N.A.; Harrisson, I.; et al. One-Quarter of Freshwater Fauna Threatened with Extinction. Nature 2025, 638, 138–145. [Google Scholar] [CrossRef]
- Quist, M.C.; Spiegel, J.R. Population Demographics of Catostomids in Large River Ecosystems: Effects of Discharge and Temperature on Recruitment Dynamics and Growth. Riv. Res. Appl. 2012, 28, 1567–1586. [Google Scholar] [CrossRef]
- Rypel, A.L.; Saffarinia, P.; Vaughn, C.C.; Nesper, L.; O’Reilly, K.; Parisek, C.A.; Miller, M.L.; Moyle, P.B.; Fangue, N.A.; Bell-Tilcock, M.; et al. Goodbye to “Rough Fish”: Paradigm Shift in the Conservation of Native Fishes. Fisheries 2021, 46, 605–616. [Google Scholar] [CrossRef]
- Childress, E.S.; McIntyre, P.B. Life History Traits and Spawning Behavior Modulate Ecosystem-Level Effects of Nutrient Subsidies from Fish Migrations. Ecosphere 2016, 7, e01301. [Google Scholar] [CrossRef]
- Booth, M.T.; Hairston, N.G., Jr.; Flecker, A.S. Consumer Movement Dynamics as Hidden Drivers of Stream Habitat Structure: Suckers as Ecosystem Engineers on the Night Shift. Oikois 2020, 129, 194–208. [Google Scholar] [CrossRef]
- Cooke, S.J.; Bunt, C.M.; Hamilton, S.J.; Jennings, C.A.; Pearson, M.P.; Cooperman, M.S.; Markle, D.F. Threats, Conservation Strategies, and Prognosis for Suckers (Catostomidae) in North America: Insights from Regional Case Studies of a Diverse Family of Non-Game Fishes. Biol. Conserv. 2005, 121, 317–331. [Google Scholar] [CrossRef]
- Harris, P.M.; Hubbard, G.; Sandel, M. Catostomidae: Suckers. In Freshwater Fishes of North America: Volume 1: Petromyzontidae to Catostomidae; Warren, M.L., Burr, B.M., Tomerelli, J.R., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2014; pp. 451–502. ISBN 978-1421412016. [Google Scholar]
- Ricciardi, A.; Rasmussen, J.B. Extinction rates of North American freshwater fauna. Conserv. Biol. 1999, 13, 1220–1222. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Moxostoma#:~:text=Teretulus%20Rafinesque%2C%201820-,Species,%2C%201820)%20(Silver%20redhorse) (accessed on 7 March 2025).
- Scott, W.E.; Crossman, E.J. Suckers-Catostomidae. In Freshwater Fishes of Canada; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1973; pp. 563–567. [Google Scholar]
- Mongeau, J.R.; Dumont, P.; Cloutier, L. La Biologie du Suceur Cuivré (Moxostoma hubbsi) Comparée à Celle de Quatre Autres Espèces de Moxostoma (M. anisurum, M. carinatum, M. macrolepidotum et M. valenciennesi). Can. J. Zool. 1992, 70, 1354–1363. [Google Scholar] [CrossRef]
- Hatry, C.; Thiem, J.D.; Hatin, D.; Dumont, P.; Smokorowski, K.E.; Cooke, S.J. Fishway Approach Behaviour and Passage of Three Redhorse Species (Moxostoma anisurum, M. carinatum, and M. macrolepidotum) in the Richelieu River, Quebec. Environ. Biol. Fishes 2016, 99, 249–263. [Google Scholar] [CrossRef]
- Jelks, H.L.; Walsh, S.J.; Burkhead, N.M.; Contreras-Balderas, S.; Diaz-Pardo, E.; Hendrickson, D.A.; Lyons, J.; Mandrak, N.E.; McCormick, F.; Nelson, J.S.; et al. Conservation Status of Imperiled North American Freshwater and Diadromous Fishes. Fisheries 2008, 33, 372–407. [Google Scholar] [CrossRef]
- IUCN Red List. Moxostoma valenciennesi. Available online: https://www.iucnredlist.org/species/202259/18228653 (accessed on 7 March 2025).
- IUCN Red list. Moxostoma duquesnii. Available online: https://www.iucnredlist.org/species/18227213/18234003 (accessed on 7 March 2025).
- UICN Red List. Moxostoma erythrurum. Available online: https://www.iucnredlist.org/species/202165/76571620 (accessed on 7 March 2025).
- UICN Red List. Moxostoma anisurum. Available online: https://www.iucnredlist.org/species/202159/18230929 (accessed on 7 March 2025).
- IUCN Red List. Moxostoma macrolepidotum. Available online: https://www.iucnredlist.org/species/202253/18228990 (accessed on 7 March 2025).
- IUCN Red List. Moxostoma carinatum. Available online: https://www.iucnredlist.org/species/202161/18234655 (accessed on 7 March 2025).
- Lamothe, K.A.; Drake, D.A.R.; Pitcher, T.E.; Broome, J.E.; Dextrase, A.J.; Gillespie, A.; Mandrak, N.E.; Poesch, M.S.; Reid, S.M.; Vachon, N. Reintroduction of Fishes in Canada: A Review of Research Progress for SARA-Listed Species. Environ. Rev. 2019, 27, 575–599. [Google Scholar] [CrossRef]
- IUCN Red List. Moxostoma robustustum. Available online: https://www.iucnredlist.org/species/202257/19033267 (accessed on 7 March 2025).
- Department of Fisheries and Oceans. Management Plan for the River Redhorse (Moxostoma carinatum) in Canada; Species at Risk Act Management Plan Series; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2018; 48p.
- Boyko, A. (Department of Fisheries and Oceans Canada). Personal communication, 2020.
- DFO. Recovery Strategy and Action Plan for the Black Redhorse (Moxostoma duquesnei) in Canada; Species at Risk Act Recovery Strategy Series; Fisheries and Oceans: Ottawa, ON, Canada, 2022; Available online: https://publications.gc.ca/site/eng/9.906661/publication.html (accessed on 7 March 2025).
- IUCN Red List. Moxostoma hubbsi. Available online: https://www.iucnredlist.org/species/13917/58135857 (accessed on 7 March 2025).
- Tognelli, M.F.; Máiz-Tomé, L.; Kraus, D.; Lepitzki, D.; Mackie, G.; Morris, T.; Carney, J.; Alfonso, N.; Tonn, B.; Cox, N.A.; et al. Freshwater Key Biodiversity Areas in Canada; Informing Species Conservation and Development Planning in Freshwater Ecosystems; IUCN: Arlington, TX, USA, 2017; 42p. [Google Scholar]
- Port de Montréal. Available online: https://www.port-montreal.com/en/the-port-of-montreal/projects/terminal-in-contrecoeur (accessed on 7 March 2025).
- Day, J.L.; Jacobs, J.L.; Rasmussen, J. Considerations for the Propagation and Conservation of Endangered Lake Suckers of the Western Lake Suckers of the Western United States. J. Wildl. Manag. 2017, 8, 301–312. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Rytwinski, T.; Taylor, J.J.; Bennett, J.R.; Drake, D.A.R.; Martel, A.; Cooke, S.J. Can Conservation Targets for Imperilled Freshwater Fishes and Mussels be Achieved by Captive Breeding and Release Programs? A Systematic Map Protocol to Determine Available Evidence. Environ. Evid. 2019, 8, 16. [Google Scholar] [CrossRef]
- Taylor, J.J.; Rytwinski, T.; Bennett, J.R.; Smokorowski, K.E.; Lapointe, N.W.R.; Janusz, R.; Clarke, K.; Tonn, B.; Walsh, J.C.; Cooke, S.J. The Effectiveness of Spawning Habitat Creation or Enhancement for Substrate-Spawning Temperate Fish: A Systematic Review. Environ. Evid. 2019, 8, 19. [Google Scholar] [CrossRef]
- Schmucker, C.M.; Blümle, A.; Schell, L.K.; Schwarzer, G.; Cabrera, L.; von Elm, E.; Briel, M.; Meerpöhl, J.J. Systematic Review Finds that Study Data Not Published in Full Text Article Have Unclear Impact on Meta-Analysis Results in Medical Research. PLoS ONE 2017, 12, e0176210. [Google Scholar] [CrossRef]
- Buxton, R.T.; Bennett, J.R.; Reid, A.J.; Shulman, C.; Cooke, S.J.; Francis, C.M.; Nyboer, E.A.; Pritchard, G.; Binley, A.D.; Avery-Gomm, S.; et al. Key Information Needs to Move from Knowledge to Action for Biodiversity Conservation in Canada. Biol. Conserv. 2021, 256, 108983. [Google Scholar] [CrossRef]
- Eberhard, E.K.; Wilcove, D.S.; Dobson, A.P. Too Few, Too Late: U.S. Endangered Species Act Undermined by Inaction and Inadequate Funding. PLoS ONE 2022, 17, e0275322. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, H.E.; Gentry, R.R.; Halpern, B.S. Conservation Aquaculture: Shifting the Narrative and Paradigm of Aquaculture’s Role in Resource Management. Biol. Conserv. 2017, 215, 162–168. [Google Scholar] [CrossRef]
- Ridlon, A.D.; Wasson, K.; Waters, T.; Adams, J.; Donatuto, J.; Fleener, G.; Froehlich, H.; Govender, R.; Kornbluth, A.; Lorda, J.; et al. Conservation Aquaculture as a Tool for Imperiled Marine Species: Evaluation of Opportunities and Risks for Olympia Oysters, Ostrea lurida. PLoS ONE 2021, 16, e0252810. [Google Scholar] [CrossRef]
- Robitaille, J.M.; Bérubé, M.; Gosselin, A.; Baril, M.; Beauchamp, J.; Boucher, J.; Dionne, S.; Legault, M.; Mailhot, Y.; Ouellet, B.; et al. Recovery Strategy for the Striped bass (Morone saxatilis), St-Lawrence Estuary Population, Canada; Species at risk act recovery strategy series; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2011; 51p. [Google Scholar]
- Valiquette, E.; Harvey, V.; Pelletier, A.-M. Mise à Jour des Connaissances sur l’Identification, la Description et l’Utilisation Spatio-Temporelle des Habitats du Bar Rayé (Morone saxatilis) de la Population du Fleuve Saint-Laurent, Québec. Secrétariat Canadien de Consultation Scientifique (SCCS). 2017. Doc. Rech. 2292-4272; 2017/005, Région du Québec. Available online: https://publications.gc.ca/site/eng/9.833505/publication.html (accessed on 7 March 2025).
- Gaudreau, C.M. (Ministère de l’Environnement, de la Lutte contre les Changements Climatique, Faune et Parc MELCCFP, QC, Canada). Personal communication, 2024.
- Muttray, A.F.; Muir, D.C.G.; Tetreault, G.R.; McMaster, M.E.; Sherry, J.P. Spatial Trends and Temporal Declines in Tissue Metals/Metalloids in the Context of Wild Fish Health and the St.Clair River Area of Concern. J. Great Lakes Res. 2021, 47, 900–915. [Google Scholar] [CrossRef]
- Page, L.M.; Johnston, C.E. Spawning in the Creek Chubsucker, Erimyzon oblongus, With a Review of Spawning Behavior in Suckers (Catostomidae). Environ. Biol. Fish. 1990, 27, 265–272. [Google Scholar] [CrossRef]
- Kwak, T.J.; Skelly, T.M. Spawning Habitat, Behavior, and Morphology as Isolating Mechanisms of the Golden Redhorse, Moxostoma erythrurum, and the Black Redhorse, M. duquesnei, Two Syntopic Fishes. Environ. Biol. Fish. 1992, 34, 127–137. [Google Scholar] [CrossRef]
- Cooke, S.J.; Bunt, C.M. Spawning and Reproductive Biology of the Greater Redhorse, Moxostoma valenciennesi, in the Grand River, Ontario. Can. Field-Nat. 1999, 113, 497–502. [Google Scholar] [CrossRef]
- Jenkins, R.E.; Jenkins, D.J. Reproductive Behavior of the Greater Redhorse, Moxostoma valenciennesi, in the Thousand Islands region. Can. Field-Nat. 1980, 94, 426–430. [Google Scholar] [CrossRef]
- Grabowski, T.B.; Isely, J.J. Seasonal and Diel Movements and Habitat Use of Robust Redhorse in the Lower Savannah River, Georgia and South Carolina. Trans. Am. Fish. Soc. 2006, 135, 1145–1155. [Google Scholar] [CrossRef]
- Catalano, M.J.; Bozek, M.A. Influence of Environmental Variables on Catostomid Spawning Phenology in a Warmwater River. Am. Mid. Nat. 2015, 173, 1–16. Available online: https://www.jstor.org/stable/43822778 (accessed on 7 March 2025). [CrossRef]
- Reid, S.M.; Wilson, C.C.; Carl, L.M.; Zorn, T.G. Species Traits Influence the Genetic Consequences of River Fragmentation of Two Co-Occurring Redhorse (Moxostoma) Species. Can. J. Fish. Aquat. Sci. 2008, 65, 1892–1904. [Google Scholar] [CrossRef]
- Reid, S.M.; Wilson, C.C.; Mandrak, N.E.; Carl, L.M. Population Structure and Genetic Diversity of Black Redhorse (Moxostoma duquesnei) in a Highly Fragmented Watershed. Conserv. Genet. 2008, 9, 531–546. [Google Scholar] [CrossRef]
- Jenkins, R.E.; Burkhead, N.M. Freshwater Fishes of Virginia; American Fisheries Society: Bethesda, MD, USA, 1994; 1084p. [Google Scholar]
- Reid, S.M. Comparison of Scales, Pectoral Fin Rays and Opercles for Age Estimation of Ontario Redhorse, Moxostoma, Species. Can. Field-Nat. 2007, 121, 29–34. [Google Scholar]
- Lippé, C.; Dumont, P.; Bernatchez, L. Isolation and Identification of 21 Microsatellite Loci in the Copper Redhorse (Moxostoma hubbsi; Catostomidae) and their Variability in Other Catostomids. Mol. Ecol. Notes 2004, 4, 638–641. [Google Scholar] [CrossRef]
- Lippé, C.; Dumont, P.; Bernatchez, L. High Genetic Diversity and No Inbreeding in the Endangered Copper Redhorse, Moxostoma hubbsi (Catostomidae, Pisces): The Positive Sides of a Long Generation Time. Mol. Ecol. 2006, 15, 1769–1780. [Google Scholar] [CrossRef]
- Reid, S.M.; Wilson, C.C. PCR-RFLP Based Diagnostic Tests for Moxostoma Species in Ontario. Conserv. Genet. 2006, 7, 997–1000. [Google Scholar] [CrossRef]
- Lacoursière-Roussel, A.; Rosabal, M.; Bernatchez, L. Estimating Fish Abundance and Biomass from eDNA Concentrations: Variability Among Capture Methods and Environmental Conditions. Mol. Ecol. Res. 2016, 16, 1401–1414. [Google Scholar] [CrossRef]
- Rider, H.J.; Janosik, A.M. Using Environmental DNA Metabarcoding to Assess the Spatiotemporal Occurrence of the Imperiled River Redhorse (Moxostoma carinatum) in the Escambia-Conecuh River System of Florida and Alabama. USA Environ. Biol. Fish 2024, 107, 1593–1608. [Google Scholar] [CrossRef]
- MELCCFP. Available online: https://mffp.gouv.qc.ca/documents/faune/GM_catostomides_MFFP.pdf (accessed on 7 March 2025).
- Moxostoma.com. Available online: https://moxostoma.com/redhorse-id-downloads/ (accessed on 7 March 2025).
- Page, L.M.; Burr, B.M. A Field Guide to Freshwater Fishes of North America North of Mexico; Houghton Mifflin Company: Boston, MA, USA, 1991; 432p. [Google Scholar]
- Hatry, C.; Thiem, J.D.; Binder, T.R.; Hatin, D.; Dumont, P.; Stamplecoskie, K.M.; Molina, J.M.; Smokorowski, K.E.; Cooke, S.J. Comparative Physiology and Relative Swimming Performance of Three Redhorse (Moxostoma spp.) Species: Associations with Fishway Passage Success. Physiol. Biochem. Zool. 2014, 87, 148–159. [Google Scholar] [CrossRef]
- Spiegel, J.R.; Quist, M.C.; Morris, J.E. Trophic Ecology and Gill Raker Morphology of Seven Catostomid Species in Iowa Rivers. J. Appl. Ichthyol. 2011, 27, 1159–1164. [Google Scholar] [CrossRef]
- Vachon, N. Écologie des Juvéniles 0+ et 1+ de Chevalier Cuivré (Moxostoma hubbsi), une Espèce Menacée, Comparée à Celle des Quatre Autres Espèces de Moxostoma (M. anisurum, M. carinatum, M. macrolepidotum, M. valenciennesi) dans le Système de la Rivière Richelieu. Master’s Thesis, Université du Québec à Montréal, Montréal, QC, Canada, 1999. [Google Scholar]
- Reid, S.M. Timing and Demographic Characteristics of Redhorse Spawning Runs in Three Great Lakes Basin Rivers. J. Freshw. Ecol. 2006, 21, 249–258. [Google Scholar] [CrossRef]
- Uhrovič, D.; Oros, M.; Kudlai, O.; Choudhury, A.; Scholz, T. Molecular Evidence of Three Closely Related Species of Biacetabulum Hunter, 1927 (Cestoda: Caryophyllidea): A Case of Recent Speciation in Different Fish Hosts (Catostomidae)? Parasitology 2021, 148, 1040–1056. [Google Scholar] [CrossRef] [PubMed]
- Fraker, M.E.; Keitzer, S.C.; Sinclair, J.S.; Aloysius, N.R.; Dippold, D.A.; Yen, H.; Arnold, J.G.; Daggupati, P.; Johnson, M.-V.V.; Martin, J.F.; et al. Projecting the Effects of Agricultural Conservation Practices on Stream Fish Communities in a Changing Climate. Sci. Total Environ. 2020, 747, 141112. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.M. Age, Growth and Mortality of Black Redhorse (Moxostoma duquesnei) and Shorthead Redhorse (M. macrolepidotum) in the Grand River, Ontario. J. Appl. Ichthyol. 2009, 25, 178–183. [Google Scholar] [CrossRef]
- Howlett, D.T. Age, Growth and Population Structure of Black Redhorse (Moxostoma duquesnei) and Golden Redhorse (Moxostoma erythrurum) in Southwest Missouri. Master’s Thesis, Southwest Missouri State University, Springfield, MO, USA, 1999. [Google Scholar]
- Bunt, C.M.; Heiman, T.; Mandrak, N.E. Ontogeny of Larval and Juvenile Black Redhorse (Moxostoma duquesnei). Copeia 2013, 2013, 121–126. [Google Scholar] [CrossRef]
- Bunt, C.M.; Mandrak, N.E.; Eddy, D.C.; Choo-Wing, S.A.; Heiman, T.; Taylor, E. Habitat Utilization, Movement and Use of Groundwater Seepages by Larval and Juvenile Black Redhorse Moxostoma duquesnei. Environ. Biol. Fish. 2013, 96, 1281–1287. [Google Scholar] [CrossRef]
- Curry, K.D.; Spacie, A. Differential Use of Stream Habitat by Spawning Catastomids. Am. Mid. Nat. 1984, 111, 267–279. [Google Scholar] [CrossRef]
- Reid, S.M.; Mandrak, N.E.; Carl, L.M.; Wilson, C.C. Influence of Dams and Habitat Conditions on the Distribution of Redhorse (Moxostoma) Species in the Grand River Watershed, Ontario. Environ. Biol. Fish. 2008, 81, 111–125. [Google Scholar] [CrossRef]
- Young, J.A.M.; Koops, M.A. Population Modelling of Black Redhorse (Moxostoma duquesni) in Canada. DFO Can. Sci. Advis. Sec. Res. Doc. 2014. 14p. Available online: https://publications.gc.ca/collections/collection_2014/mpo-dfo/Fs70-5-2014-020-eng.pdf (accessed on 12 March 2025).
- Brown, B.A. Comparative Life-Histories of Some Species of Redhorse, Subgenus Moxostoma, Genus Moxostoma. Ph.D. Thesis, Indiana State University, Terre Haute, ID, USA, 1984. Available online: https://scholars.indianastate.edu/etds/2349/ (accessed on 7 March 2025).
- Bowman, M.L. Life History of the Black Redhorse, Moxostoma duquesnei (Lesueur), in Missouri. Trans. Am. Fish. Soc. 1970, 99, 546–559. [Google Scholar] [CrossRef]
- Page, L.M.; Burr, B.M. Peterson Field Guide to Freshwater Fishes; Houghton Mifflin Company: Boston, MA, USA, 2011; 663p. [Google Scholar]
- Holm, E.; Mandrak, N.E.; Burridge, M.E. The ROM Field Guide to Freshwater Fishes of Ontario; Royal Ontario Museum: Toronto, ON, Canada, 2009; 462p. [Google Scholar]
- Bouvier, L.D.; Burridge, M.E.; Glass, W.R.; Caskenette, A. Information in Support of a Recovery Potential Assessment of Black Redhorse (Moxostoma duquesnei) in Canada. DFO Can. Sci. Advis. Sec. Res. Doc. 2021. 38p. Available online: https://www.dfo-mpo.gc.ca/csas-sccs/Publications/ResDocs-DocRech/2021/2021_021-eng.html (accessed on 12 March 2025).
- Beckman, D.W.; Howlett, D.T. Otolith Annulus Formation and Growth of Two Redhorse Suckers (Moxostoma: Catastomidae). Copeia 2013, 3, 390–395. [Google Scholar] [CrossRef]
- Reid, S.M.; Glass, W.R. Precision and Comparability of Black Redhorse (Moxostoma duquesnei) Age Estimates Using Scales, Pectoral Fin Rays, and Opercle Bones. Can. Manuscr. Rep. Fish. Aquat. Sci. 2014. 9p. Available online: https://publications.gc.ca/collections/collection_2014/mpo-dfo/Fs97-4-3034-eng.pdf (accessed on 12 March 2025).
- Bunt, C.M.; van Poorten, B.T.; Wong, L. Denil Fishway Utilization Patterns and Passage of Several Warmwater Species Relative to Seasonal, Thermal and Hydraulic Dynamics. Ecol. Freshw. Fish 2001, 10, 212–219. [Google Scholar]
- Department of Fisheries and Oceans. Black Redhorse (Moxostoma duquesnei): Recovery Strategy and Action Plan 2022. Available online: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/recovery-strategies/black-redhorse-2022.html#toc0 (accessed on 12 March 2025).
- Blazer, V.S.; Iwanowicz, D.D.; Sperry, A.J.; Iwanowicz, L.R.; Alvarez, D.A.; Brightbill, R.A.; Smith, G.; Foreman, W.T.; Manning, R. Reproductive Health Indicators of Fishes from Pennsylvania Watersheds: Association with Chemicals of Emerging Concern. Environ. Monit. Assess. 2014, 186, 6471–6491. [Google Scholar] [CrossRef] [PubMed]
- Rosencranz, J.; Cuddington, K.; Brook, M.; Koops, M.A.; Drake, D.A. Data-Limited Models to Predict River Temperatures for Aquatic Species at Risk. Can. J. Fish. Aquat. Sci. 2020, 78, 1268–1277. [Google Scholar] [CrossRef]
- iNaturalist.org. Available online: https://www.inaturalist.org/taxa/106212-Moxostoma-erythrurum (accessed on 12 March 2025).
- Meyer, W.H. Life History of Three Species of Redhorse (Moxostoma) in the Des Moines River, Iowa. Trans. Am. Fish. Soc. 1962, 91, 412–419. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Witman, D.C. Descriptions and Comparisons of Catostomid Fish Larvae: Catastomus catastomus and Moxostoma erythrurum. Trans. Am. Fish. Soc. 1979, 108, 604–619. [Google Scholar] [CrossRef]
- Moyer, G.R.; Shaw, R.; Wertz, T. Mitochondrial DNA Barcoding Reveals First Documented Evidence of Golden Redhorse (Moxostoma erythrurum) in the Susquehanna River, Pennsylvania. Northeast. Nat. 2021, 28, 149–155. [Google Scholar] [CrossRef]
- Reash, R.J.; Seegert, G.L.; Goodfellow, W.L. Experimentally-Derived Upper Thermal Tolerances for Redhorse Suckers: Revised 316(A) Variance Conditions at Two Generating Facilities in Ohio. Environ. Sci. Policy 2000, 3 (Suppl. 1), 191–196. [Google Scholar] [CrossRef]
- Keplinger, B.; Hedrick, J.; Blazer, V.S. Temporal Trends in Macroscopic Indicators of Fish Health in the South Branch Potamac River. N. Am. J. Fish. Manag. 2022, 42, 277–294. [Google Scholar] [CrossRef]
- Al-Ansari, A.M.; Saleem, A.; Kimpe, L.E.; Sherry, J.P.; McMaster, M.E.; Trudeau, V.L.; Blais, J.M. Bioaccumulation of the Pharmaceutical 17α-Ethinylestradiol in Shorthead Redhorse Suckers (Moxostoma macrolepidotum) from the St. Clair River, Canada. Environ. Pollut. 2010, 158, 2566–2571. [Google Scholar] [CrossRef]
- Choudhury, A.; Nelson, P.A. Lissorchi s macropharynx n. sp. (Digenea: Lissorchiidae) From the Shorthead Redhorse, Moxostoma macrolepidotum (Lesueur) (Osteichthyes: Catostomidae). J. Parasitol. 1998, 84, 1196–1202. Available online: https://www.jstor.org/stable/3284652 (accessed on 7 March 2025). [PubMed]
- DeBofsky, A.; Xie, Y.; Jardine, T.D.; Hill, J.E.; Jones, P.D.; Giepsy, J.P. Effects of the Husky Oil Spill on Gut Microbiota of Native Fishes in the North Saskatchewan River, Canada. Aquat. Toxicol. 2020, 229, 105658. [Google Scholar] [CrossRef]
- Moravec, F.; Levron, C.; de Buron, I. Morphology and Taxonomic Status of Two Little-Known Nematode Species Parasitizing North American Fishes. J. Parasitol. 2011, 97, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.R.; Al-Ansari, A.M.; Gendron, R.L.; White, P.A.; Blais, J.M. A method to estimate sediment ingestion by fish. Aquat. Toxicol. 2011, 103, 121–127. [Google Scholar] [CrossRef]
- Bister, T.J.; Willis, D.W.; Brown, M.L.; Jordan, S.M.; Newmann, R.M.; Quist, M.C.; Guy, C.S. Proposed Standard Weight (Ws) Equations and Length Categories for 18 Warmwater Nongame and Riverine Fish Species. N. Am. Fish. Manag. 2000, 20, 570–574. [Google Scholar] [CrossRef]
- Buynak, G.L.; Mohr, H.W. Larval Development of the Shorthead Redhorse (Moxostoma macrolepidotum) from the Susquehanna River. Trans. Am. Fish. Soc. 1979, 108, 161–165. [Google Scholar] [CrossRef]
- den Haas, T.C.; Bouvier, L.D.; Mandrak, N.E. Identifying and Comparing Annuli across Calcified Structures in Shorthead Redhorse (Moxostoma macrolepidotum). Can. Manuscr. Rep. Fish. Aquat. Sci. 2013, 3023, 10. Available online: https://publications.gc.ca/site/eng/475020/publication.html (accessed on 12 March 2025).
- Coughlan, D.J.; Baker, B.K.; Barwick, D.H.; Garner, A.B.; Doby, W.H. Catastomi Fishes of the Wateree River, South Carolina. Southeast. Nat. 2007, 6, 305–320. [Google Scholar] [CrossRef]
- Edge, E.N.; Paukert, C.P.; Lobb III, M.D.; Landwer, B.H.P.; Bonnot, T.W. Seasonal Selection Habitat by Spotted Bass and Shorthead Redhorse in a Regulated River in the Midwest, USA. River Res. Appl. 2000, 36, 1087–1096. [Google Scholar] [CrossRef]
- Healy, B.D. Conservation Assessment for the Greater Redhorse (Moxostoma valenciennesi); USDA Forest Service: Eastern Region, Ghana, 2002.
- Harris, P.M.; Mayden, R.L.; Espinosa Pérez, H.S.; Garcia de Leon, F. Phylogenetic Relationships of Moxostoma and Scartomyzon (Catostomidae) Based on Mitochondrial Cytochrome b Sequence Data. J. Fish Biol. 2005, 61, 1433–1452. [Google Scholar] [CrossRef]
- Clements, M.D.; Bart, H.L., Jr.; Hurley, D.L. A Different Perspective on the Phylogenetic Relationships of the Moxostomatini (Cypriniformes: Catostomidae) Based on Cytochrome-b and Growth Hormone Intronsequences. Mol. Phylogenet. Evol. 2012, 63, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bunt, C.M.; Cooke, S.J. Ontogeny of Larval Greater Redhorse (Moxostoma valenciennesi). Am. Mid. Nat. 2004, 151, 93–100. Available online: https://www.jstor.org/stable/3566791 (accessed on 7 March 2025). [CrossRef]
- Bunt, C.M.; Cooke, S.J. Post-Spawn and Habitat Use by Greater Redhorse, Moxostoma valenciennesi. Ecol. Freshw. Fish 2001, 10, 57–60. [Google Scholar] [CrossRef]
- Tomsic, C.A.; Granata, T.C.; Murphy, R.P.; Livchak, C.J. Using a Coupled Eco-Hydrodynamic Model to Predict Habitat for Target Species Following Dam Removal. Ecol. Eng. 2007, 30, 215–230. [Google Scholar] [CrossRef]
- Cheng, F.; Granata, T. Sediment Transport and Channel Adjustments Associated with Dam Removal: Field Observations. Water Resour. Res. 2007, 43, W03444. [Google Scholar] [CrossRef]
- Parker, B.J. Updated Status of the River Redhorse, Moxostoma carinatum, in Canada. Can. Field-Nat. 1988, 102, 140–146. [Google Scholar] [CrossRef]
- French, J.R.P. How Well Can Fishes Prey on Zebra Mussels in Eastern North America. Fisheries 1993, 18, 13–19. [Google Scholar] [CrossRef]
- Butler, S.E.; Wahl, D.H. Movements and Habitat Use of River Redhorse (Moxostoma carinatum) in the Kankakee River, Illinois. Copeia 2017, 105, 734–742. [Google Scholar] [CrossRef]
- Straight, C.A.; Jakson, C.R.; Freeman, B.J.; Freeman, M.C. Diel Patterns and Temporal Trends in Spawning Activities of Robust Redhorse and River Redhorse in Georgia, Assessed Using Passive Acoustic Monitoring. Trans. Am. Fish. Soc. 2015, 144, 563–576. [Google Scholar] [CrossRef]
- Preville, N.M.; Snyder, E.B.; O’Keefe, D.; Hanshue, S.; Russel, A.; Radecki, J. Habitat Use of the Threatened River Redhorse (Moxostoma carinatum) in the Grand River, MI, USA. Aquat. Sci. 2022, 84, 43. [Google Scholar] [CrossRef]
- Marchand, H.; Barst, B.D.; Boulanger, E.; Vachon, N.; Houde, M.; Xia, J.; Liu, P.; Ewald, J.D.; Bayen, S.; Liu, L.; et al. Exposure to contaminated river water is associated with early hatching and dysregulation of gene expression in early life stages of the endangered copper redhorse (Moxostoma hubbsi). Environ. Toxicol. Chem. 2022, 41, 1950–1966. [Google Scholar] [CrossRef] [PubMed]
- Gagnon-Poiré, R. (Ministère de l’Environnement, de la Lutte contre les Changements Climatique, Faune et Parc MELCCFP, QC, Canada). Personal communication, 2024.
- Cooke, S.J. (University of Waterloo, Waterloo, ON, Canada). Personal communication, 2024.
- Vachon, N. Guide et Clé d’Identification des Juvéniles de Chevaliers (Genre Moxostoma) du Québec. Société de la Faune et des Parcs du Québec, Direction de L’aménagement de la Faune de Montréal, de Laval et de la Montérégie, Longueuil, Rapport Technique 16-14F. 2003. 26p. Available online: https://mffp.gouv.qc.ca/documents/faune/chevalier_guide_cle.pdf (accessed on 12 March 2025).
- McAllister, D.E. The Copper Redhorse, Moxostoma hubbsi A disappearing Canadian endemic. Biodiversity 2011, 2, 27–28. [Google Scholar] [CrossRef]
- Eastman, J.T. The Pharyngeal Bones and Teeth of Catostomid Fishes. Am. Mid. Nat. 1977, 97, 68–88. [Google Scholar] [CrossRef]
- Doosey, M.H.; Bart, H.L. Morphological Variation of the Palatal Organ and Chewing Pad of Catostomidae (Teleostei: Cypriniformes). J. Morphol. 2011, 272, 1092–1108. [Google Scholar] [CrossRef]
- DFO Recovery Strategy for the Copper Redhorse (Moxostoma hubbsi) in Canada; Species at Risk Act Recovery Strategy Series; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2012; 60p, Available online: www.registrelep-sararegistry.gc.ca/virtual_sara/files/plans/rs_chevalier_cuivre_copper_redhorse_0312_eng.pdf (accessed on 12 March 2025).
- Vachon, N.; Chagnon, Y. Caractérisation de la Population de Chevalier Cuivré (Moxostoma hubbsi) du Fleuve Saint-Laurent (Secteur Lavaltrie-Contrecoeur) à Partir des Captures Fortuites d’un Pêcheur Commercial en 1999, 2000 et 2001. Report Prepared for Direction de L’aménagement de la Faune de Montréal, de Laval et de la Montérégie, Ministère des Ressources Naturelles et de la Faune et des Parcs. 2004. Available online: https://doi.org/10.13140/RG.2.2.24794.64965 (accessed on 14 March 2025).
- Silva, A.T.; Hatry, C.; Thiem, J.D.; Gutowsky, L.F.G.; Hatin, D.; Zhu, D.Z.; Dawson, J.W.; Katopodis, C.; Cooke, S.J. Behaviour and Locomotor Activity of a Migratory Catostomid during Fishway Passage. PLoS ONE 2015, 10, e0123051. [Google Scholar] [CrossRef] [PubMed]
- Gariépy, S. Déplacements, Domaines Vitaux, Sélection et Caractérisation des Habitats des Chevaliers Cuivrés Adultes dans le Système du Fleuve Saint-Laurent, Québec, Canada. Master’s Thesis, Université du Québec à Rimouski, Rimouski, QC, Canada, 2008. Available online: https://semaphore.uqar.ca/id/eprint/58/1/Simone_Gariepy_juin2008.pdf (accessed on 7 March 2025).
- Équipe de Rétablissement du Chevalier Cuivré. Plan de Rétablissement du Chevalier Cuivré (Moxostoma hubbsi) au Québec—2012–2017; Ministère des Ressources Naturelles et de la Faune du Québec, Faune, QC, Canada; 2012; 55p, Available online: https://mffp.gouv.qc.ca/documents/faune/plan-retablissement-chevalier.pdf (accessed on 14 March 2025).
- DFO. Report on the Progress of Recovery Strategy Implementation for the Copper Redhorse (Moxostoma hubbsi) in Canada for the Period 2012 to 2018; Species at Risk Act Recovery Strategy Report Series; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2022; 47p, Available online: https://www.canada.ca/content/dam/eccc/documents/pdf/lep-sara/2022/20220526-CopperRedhorseProgressReport-eng.pdf (accessed on 14 March 2025).
- Vachon, N.; Velasquez-Medina, S.; Grondin, P. Motilité des Spermatozoïdes du Chevalier cuivré dans les Différents Traitements de Cryopréservation en 2013. Ministère des Forêts, de la Faune et des Parcs (MFFP), Direction de la Gestion de la Faune de l’Estrie, de Montréal, de la Montérégie et de Laval, Secteur de la Faune, Rapport Technique. 2019. 24p. Available online: https://mffp.gouv.qc.ca/documents/faune/Motilite_spermatozoide_chevalier_cuivre_cryopreservation.pdf (accessed on 14 March 2025).
- Branchaud, A.; Gendron, A.D. Artificial Spawning and Rearing of the Copper Redhorse, Moxosoma hubbsi (Teleostei: Catostomidae). Can. Field-Nat. 1996, 107, 279–282. Available online: https://www.biodiversitylibrary.org/item/108204#page/295/mode/1up (accessed on 17 March 2025). [CrossRef]
- Couillard, M.-A.; Poiré-Gagnon, R. (MELCCFP, QC, Canada). Personal communications, 2024.
- Vachon, N. Reproduction Artificielle, Ensemencements et Suivi de la Population du Chevalier Cuivré (Moxostoma hubbsi) en 2018. Direction de la Gestion de la Faune de l’Estrie, de Montréal, de la Montérégie et de Laval, Secteur des Opérations Régionales, Ministère des Forêts, de la Faune et des Parcs. Rapport Technique. 2021, pp. 16–57, 43p. Available online: https://mffp.gouv.qc.ca/nos-publications/reproduction-artificielle-ensemencements-suivi-population-chevalier-cuivre/ (accessed on 17 March 2025).
- Lair, S.; Vergneau-Grosset, C.; Coulombe, C.; Vachon, N.; Couillard, M.-A. Conservation of the Endangered Copper Redhorse (Moxostoma hubbsi): Implication of the Aquarium du Québec. In Proceedings of the International Association for Aquatic Animal Medecine (IAAAM) Annual Conference, Salt Lake City, UT, USA, 21–24 May 2025; 2023. Available online: https://www.vin.com/doc/?id=11463515&meta=Generic (accessed on 17 March 2025).
- Bernatchez, L. Considérations Génétiques et Protocole de Reproduction Relatifs au Plan de Rétablissement du Chevalier Cuivré (Moxostoma hubbsi). Ministère des Ressources Naturelles, de la Faune et des Parcs, Direction de l’Aménagement de la Faune de Montréal, de Laval et de la Montérégie, Longueuil et Pêches et Océans Canada, Région du Québec. 2004. Rapport Technique. pp. 16–22, 38p. Available online: https://mffp.gouv.qc.ca/documents/faune/chevalier_cuivre_reproduction.pdf (accessed on 17 March 2025).
- Woolnough, D.A.; Bellamy, A.; Longstaff Hummel, S.; Annis, M. Environmental Exposure of Freshwater Mussels to Contaminants of Emerging Concern: Implications for Species Conservation. J. Great Lakes Res. 2020, 46, 1625–1638. [Google Scholar] [CrossRef]
- Giroux, I.; Simoneau, M. État de l’Écosystème Aquatique du Bassin Versant de la Rivière Nicolet: Faits Saillants 2004–2006; Ministère du Développement Durable, de l’Environnement et des Parcs, Direction du Suivi de l’état de l’environnement: Longueuil, QC, Canada, 2008; 21p, ISBN 978-2-550-53174-6. Available online: https://belsp.uqtr.ca/id/eprint/1372/1/Giroux%20et%20al._2008_Écosystème_aquatique_Nicolet.pdf (accessed on 17 March 2025).
- COVABAR. Plan Directeur de l’Eau—Plan d’Action 2015–2020 du Bassin Versant de la Rivière Richelieu et de la Zone Saint-Laurent, Beloeil. 2015. Available online: www.covabar.qc.ca/documents/PDE/Portrait/Planaction2015-2020.pdf (accessed on 17 March 2025).
- Montiel-León, J.M.; Munoz, G.; Duy, S.V.; Do, D.T.; Vaudreuil, M.A.; Goeury, K.; Guillemette, F.; Amyot, M.; Sauvé, S. Widespread Occurrence and Spatial Distribution of Glyphosate, Atrazine, and Neonicotinoids Pesticides in the St. Lawrence and Tributary Rivers. Environ. Pollut. 2019, 250, 29–39. [Google Scholar] [CrossRef]
- Patoine, M.; Hébert, S.; D’Auteuil-Potvin, F. Water Quality Trends in the Last Decade for Ten Watersheds Dominated by Diffuse Pollution in Québec, Canada. Water Sci. Technol. 2012, 65, 1095–1101. [Google Scholar] [CrossRef]
- De Lafontaine, Y.; Gilbert, N.; Dumouchel, F.; Brochu, C.; Moore, S.; Pelletier, E.; Dumont, P.; Branchaud, A. Is Chemical Contamination Responsible for the Decline of the Copper Redhorse (Moxostoma hubbsi), an Endangered Fish Species, in Canada? Sci. Total Environ. 2002, 298, 25–44. [Google Scholar] [CrossRef]
- Giroux, I.; Hébert, S.; Berryman, D. Qualité de l’Eau du Saint-Laurent de 2000 à 2014: Paramètres Classiques, Pesticides et Contaminants Émergents. Nat. Can. 2016, 140, 26–34. [Google Scholar] [CrossRef][Green Version]
- Giroux, I. Pesticides dans les Sédiments de Cours d’Eau au Québec-Échantillonnages Exploratoires de 2018–2021. MELCCFP, Direction Générale du Suivi de l’état de L’environnement. 2023. 38p. Available online: https://www.environnement.gouv.qc.ca/eau/flrivlac/pesticides.htm (accessed on 17 March 2025).
- Maltais, D.; Roy, L.R. Purification and Partial Characterization of Vitellogenin from Shorthead Redhorse (Moxostoma macrolepidotum) and Copper Redhorse (Moxostoma hubbsi) and Detection in Plasma and Mucus with a Heterologous Antibody. Fish Physiol. Biochem. 2009, 35, 241–254. [Google Scholar] [CrossRef]
- Maltais, D.; Roy, R.L.; Couillard, C.M. Hybrid ELISAs for Vitellogenins of the Endangered Copper Redhorse Moxostoma hubbsi and the Shorthead Redhorse Moxostoma macrolepidotum (Cypriniformes, Catostomidae). Ecotoxicol. Environ. Saf. 2010, 73, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Maltais, D.; Roy, R.L. Effects of Nonylphenol and Ethinylestradiol on Copper Redhorse (Moxostoma hubbsi), an Endangered Species. Ecotoxicol. Environ. Saf. 2014, 108, 168–178. [Google Scholar] [CrossRef]
- Pagnucco, K.S.; Maynard, G.A.; Fera, S.A.; Yan, N.D.; Nalepa, T.F.; Ricciardi, A. The Future of Species Invasions in the Great Lakes-St. Lawrence River Basin. J. Great Lakes Res. 2015, 4, 96–107. [Google Scholar] [CrossRef]
- Kipp, R.; Ricciardi, A. Impacts of the Eurasian Round Goby (Neogobius melanostomus) on Benthic Communities in the Upper St. Lawrence River Can. J. Fish. Aquat. Sci. 2012, 69, 469–486. [Google Scholar] [CrossRef]
- Hernandez, C.; Bougas, B.; Perreault-Payette, A.; Simard, A.; Côté, G.; Bernatchez, L. 60 specific eDNA qPCR assays to detect invasive, threatened, and exploited freshwater vertebrates and invertebrates in Eastern Canada. Environ. DNA 2020, 2, 373–386. [Google Scholar] [CrossRef]
- Pouliot, R.; Morissette, O. Risques de Dispersion des Carpes Asiatiques dans les Tributaires du Fleuve Saint-Laurent, Aspects Théoriques, Évaluation Préliminaire de la Franchissabilité des Obstacles pour les Carpes Asiatiques et Actions Pouvant être Mises en Oeuvre pour Contribuer à la Protection des Eaux Intérieures, Ministère des Forêts, de la Faune et des Parcs, Direction de L’expertise sur la Faune Aquatique, 2019; 163p. Available online: https://belsp.uqtr.ca/id/eprint/1469/1/Pouliot%20%26%20Morissette_risques_dispersion_carpes_asiatiques_St-Laurent.pdf (accessed on 17 March 2025).
- Morrissette, O.; Paradis, Y.; Pouliot, R.; Lecomte, F. Spatio-Temporal Changes in Littoral Fish Community Structure Along the St. Lawrence River (Québec, Canada) Following Round Goby (Neogobius melanostomus) invasion. Aquat. Inv. 2018, 13, 501–512. [Google Scholar] [CrossRef]
- Avlijaš, S.; Mandrak, N.E.; Ricciardi, A. Effects of Substrate and Elevated Temperature on the Growth and Feeding Efficiency of an Invasive Cyprinid Fish, Tench (Tinca tinca). Biol. Invasions 2022, 24, 2383–2397. [Google Scholar] [CrossRef]
- Marcogliese, D.J.; Dumont, P.; Gendron, A.D. Parasites of Illegally Introduced Tench (Tinca tinca) in the Richelieu River, Québec: Potential Risks for Native Fishes. Comp. Parasitol. 2009, 76, 222–228. [Google Scholar] [CrossRef]
- Uhrovič, D.; Oros, M.; Reyda, F.; Kudlai, O.; Scholz, T. Redescription of Biacetabulum giganteum Hunter, 1929 (Cestoda: Caryophyllidea), Description of Two New, Closely Related Species from Suckers (Catostomidae) in North America, and a Critical Review of Host Specificity of Species of Biacetabulum Hunter, 1927. Syst. Biodivers. 2021, 19, 1062–1079. [Google Scholar] [CrossRef]
- Marcogliese, D.J.; Gendron, A.D.; Forest, J.J.; Li, W.; Boyce, K.; El-Shehabi, F.; McLaughlin, J.D. Range Expansion and Molecular Confirmation of the Asian Fish Tapeworm in the Lower Great Lakes and St. Lawrence River with Notes on Infections in Baitfish. J. Great Lakes Res. 2016, 42, 819–828. [Google Scholar] [CrossRef]
- Miller, J.W.; Kocovsky, P.M.; Wiegmann, D.; Miner, J.G. Fish Community Responses to Submerged Aquatic Vegetation in Maumee Bay, Western Lake Erie. N. Am. J. Fish. Manag. 2018, 38, 623–629. [Google Scholar] [CrossRef]
- IAAC-AEIC Canada. Available online: https://iaac-aeic.gc.ca/050/documents/p83969/145788F.pdf (accessed on 20 March 2025).
- SNAP Québec.org. Available online: https://snapquebec.org/wp-content/uploads/2021/04/Science-advice-copper-redhorse-2021.pdf (accessed on 20 March 2025).
- Stamp, T.; West, E.; Colclough, S.; Plenty, S.; Ciotti, B.; Robbins, T.; Sheehan, E. Suitability of Compensatory Saltmarsh Habitat for Feeding and Diet of Multiple Estuarine Fish Species. Fish. Manag. Ecol. 2022, 30, 44–55. [Google Scholar] [CrossRef]
- Bannester-Marchand, N.; Vachon, N.; Mandrak, N.E.; Blanchet, F.G. Population Viability Analysis of the Endangered Copper Redhorse Moxostoma hubbsi. Endanger. Species Res. 2025, 57, 21–31. [Google Scholar] [CrossRef]
- Botrel, M.; Maranger, R. Global Historical Trends and Drivers of Submerged Aquatic Vegetation Quantities in Lakes. Glob. Change Biol. 2023, 29, 2493–2509. [Google Scholar] [CrossRef]
- Looby, A.; Reynolds, L.K.; Reinhardt, C.; Martin, C.W. Submerged Aquatic Vegetation Patch Size Affects Fish Communities in a Turbid-Algal Lake. Front. Conserv. Sci. 2021, 2, 657691. [Google Scholar] [CrossRef]
- Angradi, T.R.; Pearson, M.S.; Bolgrien, D.W.; Bellinger, B.J.; Starry, M.A.; Reschke, C. Predicting Submerged Aquatic Vegetation Cover and Occurrence in a Lake Superior Study. J. Great Lakes Res. 2013, 39, 536–546. [Google Scholar] [CrossRef]
- Plan St-Laurent. Available online: https://www.planstlaurent.qc.ca/en/water-quality/improving-water-quality-2011-2016-projects/nonpoint-source-pollution-2011-2016/brochure-richelieus-corridor-vert-et-bleu (accessed on 7 March 2025).
- Gagnon, K.; Rinde, E.; Bengil, E.G.T.; Carugati, L.; Christianen, M.J.A.; Danovaro, R.; Gambi, C.; Govers, L.L.; Kipson, S.; Meysick, L.; et al. Facilitating Foundation Species: The Potential for Plant-Bivalve Interactions to Improve Habitat Restoration. J. Appl. Ecol. 2020, 57, 1161–1179. [Google Scholar] [CrossRef]
- Couillard, M.-A.; Côté, G.; Garant, D.; Houle, C. État Génétique du Chevalier Cuivré: Constats et Recommandations pour le Plan de Production. Ministère de l’Environnement, de la Lutte Contre les Changements Climatiques, de la Faune et des Parcs. Gouvernement du Québec. 2024. Available online: https://bibliotheque.cecile-rouleau.gouv.qc.ca/documents/archives/pgq/E5A1_C683_2024.pdf (accessed on 7 March 2025).
- Evans, N.T.; Shirey, P.D.; Wieringa, J.G.; Mahon, A.R.; Lamberti, G.A. Comparative Cost and Effort of Fish Distribution Detection via Environmental DNA Analysis and Electrofishing. Fisheries 2017, 42, 90–99. [Google Scholar] [CrossRef]
- Lacoursière-Roussel, A.; Deiner, K. Environmental DNA is Not the Tool by Itself. Fish Biol. 2019, 98, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.S.; Hernandez, C.; Laporte, M.; Côté, G.; Paradis, Y.; Kameni, T.D.W.; Normandeau, E.; Bernatchez, L. Fine-Scale Environmental Heterogeneity Shapes Fluvial Fish Communities as Revealed by eDNA Metabarcoding. Environ. DNA 2020, 2, 647–666. [Google Scholar] [CrossRef]
- Langlois, V.S.; Allison, M.J.; Bergman, L.C.; To, T.A.; Helbing, C.C. The Need for Robust qPCR-based eDNA Detection Assays in Environmental Monitoring and Species Inventory. Environ. DNA 2020, 3, 519–527. [Google Scholar] [CrossRef]
- McCauley, M.; Koda, S.A.; Loesgen, S.; Duffy, D.J. Multicellular Species Environmental DNA (eDNA) Research Constrained by Overfocus on Mitochondrial DNA. Sci. Tot. Environ. 2024, 912, 169550. [Google Scholar] [CrossRef]
- Koda, S.A.; McCauley, M.; Farrel, J.A.; Duffy, I.J.; Duffy, F.G.; Loesgen, S.; Whilde, J.; Duffy, D.J. A Novel eDNA Approach for Rare Species Monitoring: Application of Long-Read Shotgun Sequencing to Lyns rufus Soil Pawprints. Biol. Conserv. 2023, 287, 110315. [Google Scholar] [CrossRef]
- Portik, D.M.; Brown, C.T.; Pierce-Ward, N.T. Evaluation of Taxonomic Classification and Profiling Methods for Long-Read Shotgun Metagenomic Sequencing Datasets. BM Bioinform. 2022, 23, 541. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Griffin, L.P.; Brooks, J.L.; Danylchuk, A.J.; Cooke, S.J.; Midwood, J.D. Applications of Telemetry to Fish Habitat Science and Management. Can. J. Fish. Aquat. Sci. 2022, 79, 1347–1359. [Google Scholar] [CrossRef]
- Wohl, E.; Lane, S.N.; Wilcox, A.C. The Science and Practice of River Restoration. Water Resour. Res. 2015, 51, 5974–5997. [Google Scholar] [CrossRef]
- Li, P.; Li, D.; Sun, X.; Chu, Z.; Xia, T.; Zheng, B. Application of Ecological Restoration Technologies for the Improvement of Biodiversity and Ecosystem in the River. Water 2022, 14, 1402. [Google Scholar] [CrossRef]
- Cochran-Biederman, J.L.; Wyman, K.E.; French, W.E.; Loppnow, G.L. Identifying Correlates of Success and Failure of Native Freshwater Fish Reintroductions: Native Freshwater Fish Reintroduction. Conserv. Biol. 2015, 29, 175–186. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, W.; Li, Y.; Yuan, X.; He, X.; Zhao, H.; Mo, J.; Lin, J.; Yang, L.; Liang, B.; et al. Physical Enrichment for Improving Welfare in Fish Aquaculture and Fitness of Stocking Fish: A Review of Fundamentals, Mechanisms and Applications. Aquaculture 2023, 574, 739651. [Google Scholar] [CrossRef]
- Mayer, I.; Psenicka, M. Conservation of Teleost Fishes: Application of Reproductive Technologies. Theriogenol. Wild 2024, 4, 100078. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Available online: https://www.fws.gov/project/cryopreservation-lab (accessed on 20 March 2025).
- Cooke, S.J.; Sack, L.; Franklin, C.E.; Farrell, A.P.; Beardall, J.; Wikelski, M.; Chown, S.L. What is Conservation Physiology? Perspectives on an Increasingly Integrated and Essential Science. Conserv. Physiol. 2013, 1, cot001. [Google Scholar] [CrossRef]
- Whitney, J.A.; Al-Chokhachy, R.; Bunnell, D.B.; Caldwell, C.A.; Cooke, S.J.; Elliason, E.J.; Rogers, M.; Lynch, A.J.; Paukert, C.P. Physiological Basis of Climate Change Impacts on North American inland Fishes. Fisheries 2016, 41, 332–345. [Google Scholar] [CrossRef]
- Carosi, A.; Padula, R.; Ghetti, L.; Lorenzoni, M. Endemic Freshwater Fish Range Shifts Related to Global Climate Changes: A Long-Term Study Provides Some Observational Evidence for the Mediterranean Area. Water 2019, 11, 2349. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pysek, P.; Winter, M.; Arianoutsou, M.; et al. No Saturation in the Accumulation of Alien Species Worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Mallet, M.C.; Thiem, J.D.; Butler, G.L.; Kennard, M.J. A Systematic Review of Approaches to Assess Fish Health Responses to Anthropogenic Threats in Freshwater Ecosystems. Conserv. Physiol. 2024, 12, coae022. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, P.; Walker, P.; Johnston, H.; Johnston, C.; Symonds, J.E. Comparative Assessment of Blood Biochemistry and Haematology Normal Ranges Between Chinook Salmon (Oncorhynchus tshawytscha) From Seawater and Freshwater Farms. Aquaculture 2021, 537, 736464. [Google Scholar] [CrossRef]
- Jeong, H.; Kang, M.; Cha, S.-Y.; Byun, J.; Kim, J.; Baek, J.W.; Park, J.J.; Shin, S.R.; Kim, H.J.; Lee, J.S.; et al. Usefulness of Clustering Blood Biochemical Markers to Assess Thermal Stress and Acclimation in Red Seabream, Pagrus major. Aquaculture 2021, 545, 737197. [Google Scholar] [CrossRef]
- Seibel, H.; Baabmann, B.; Rebl, A. Blood will tell: What hematological analyses can reveal about fish. Front. Vet. Sci. 2021, 8, 616955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Q.; Cai, J.; Yang, J.; Shen, Q.; Xu, S. Chlorpyrifos Exposure in Common Carp (Cyprinus carpio L.) Leads to Oxidative Stress and Immune Responses. Fish Shellfish Immunol. 2017, 67, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.U. Fish Health: An Oxidative Stress Perspective. Fish. Aquacult. J. 2014, 5, e105. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A Comparative and Evolutionary Approach to Oxidative Stress in Fish: A Review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le François, N.R.; Drouin-Johnson, C.; Marchand, H.C.; Lemire, S.; Blier, P.U. Biology and Conservation of Moxostoma spp. Occurring in Canada with Emphasis on the Copper Redhorse (M. hubbsi, Legendre 1952), an Endemic Species on an Extinction Trajectory. Conservation 2025, 5, 27. https://doi.org/10.3390/conservation5020027
Le François NR, Drouin-Johnson C, Marchand HC, Lemire S, Blier PU. Biology and Conservation of Moxostoma spp. Occurring in Canada with Emphasis on the Copper Redhorse (M. hubbsi, Legendre 1952), an Endemic Species on an Extinction Trajectory. Conservation. 2025; 5(2):27. https://doi.org/10.3390/conservation5020027
Chicago/Turabian StyleLe François, Nathalie R., Charles Drouin-Johnson, Hugo C. Marchand, Sophie Lemire, and Pierre U. Blier. 2025. "Biology and Conservation of Moxostoma spp. Occurring in Canada with Emphasis on the Copper Redhorse (M. hubbsi, Legendre 1952), an Endemic Species on an Extinction Trajectory" Conservation 5, no. 2: 27. https://doi.org/10.3390/conservation5020027
APA StyleLe François, N. R., Drouin-Johnson, C., Marchand, H. C., Lemire, S., & Blier, P. U. (2025). Biology and Conservation of Moxostoma spp. Occurring in Canada with Emphasis on the Copper Redhorse (M. hubbsi, Legendre 1952), an Endemic Species on an Extinction Trajectory. Conservation, 5(2), 27. https://doi.org/10.3390/conservation5020027







