1. Introduction
Silent Spring by Rachel Carson is a collection of observations of wildlife and the failure of the government to act after the spreading of persistent, potent poisons on a grand scale. It appeared in three parts in the
New Yorker magazine in June 1962. The subsequent book drew worldwide attention to the consequences of excessive use of pesticides in the environment [
1]. In the wake of this historic publication, a massive movement to support nature and address some of the wrongs to the natural world began. The world celebrated its first “Earth Day” on 22 April 1970. The United States (U.S.) government reorganized on 2 December 1970 and created the Environmental Protection Agency (EPA). Executive Order 11643, which prohibited the use of predator poisons on federal lands, was signed on 8 February 1972 [
2].
Changes in priorities and perceptions eroded much of the progress that was made as a result of Carson’s seminal book. Invasive species eradication programs on islands are a prime example of an assault against the land and sea using persistent, potent poisons [
1]. Pest eradication requires killing all pests using excessive amounts of persistent and potent pesticides, driven by a series of Executive Orders (E.O. 11987, dated 24 May 1977, E.O. 13112, dated 3 February 1999 [
3], and E.O. 13751, dated 5 December 2016 [
4]).
Silent Spring presented cases where wildlife losses followed the widespread application of persistent insecticides such as dichlorodiphenyltrichloroethane (DDT). Eradication programs on islands are similar to the examples of excessive pesticide applications described in
Silent Spring [
1].
Invasive rodent species on an island are often controlled with the application of palatable baits that contain a proven rodenticide onto the territory of every species, lethal in a single feeding, persistent in the environment long enough for the target species to access the bait and with a low probability of the species preferring other food sources. Currently used anticoagulant rodenticides (ARs) are potent and persistent. Aerial application methods were used in the treatment of 76% of island areas in Australia and New Zealand [
5]. This percentage of the area has likely increased since 2007. To apply bait onto the territory of every target species on islands, the bait goes into the water and the same principles that apply to target species also apply to the non-target marine animals. ARs have been detected in aquatic ecosystems. Fish downstream of a wastewater treatment plant from rat control with ARs [
6] and analyses of fish livers from an environmental specimen bank showed AR residues [
7].
This review calls for attention to consider and possibly establish a reasonable certainty of no harm for ecosystem restoration efforts. Cases relevant to anticoagulants are presented to illustrate the concept and the need of establishing such a certainty. Measures are taken to reduce exposure, but the measures are insufficient, and injury and death occur to marine animals following the use of these persistent, potent poisons. Death of non-target marine animals has followed the application of anticoagulant rodenticides. Proving the cause of death is challenging without collecting samples and adequate analytical tools, for which research effort is needed. This work signifies coincidental cases between AR applications and potential harm to marine animals, which illustrates the research need and proposes the concept of establishing a certainty of no harm for ecosystem restoration efforts.
2. Physicochemical Properties of Anticoagulants
Anticoagulants are substances that interfere with the ability of blood to clot. They were first developed by the University of Wisconsin after a farmer sought help in determining the cause of hemorrhaging that was killing his cattle. The farmer brought a sample of the sweet clover feed to the University of Wisconsin. Karl Link, a biochemist, identified the substance in sweet clover as dicoumarol. It was given the trade name Warfarin [
8]. Warfarin and other anticoagulants developed in the 1940s are considered the first generation of anticoagulant rodenticides. Diphacinone is a first-generation anticoagulant. It requires repeated doses to be effective [
9] (p. 51). More persistent and potent second-generation anticoagulant rodenticides (SGAR’s) were developed in the 1970s. Brodifacoum is a SGAR [
10]. Brodifacoum and diphacinone are currently preferred ARs for eradicating pests on islands. Brodifacoum (C
31H
23BrO
3) and diphacinone (C
23H
16O
3) are large aromatic compounds and have a molecular weight of 523 and 340, respectively. They are practically insoluble in water (
Table 1). The soil organic carbon partition coefficients (K
oc) of brodifacoum and DDT are 9155 and 151,000, respectively, while the K
oc of diphacinone and heptachlor are similar (2555 versus 2400). Such high K
oc and low water solubility indicate that they are likely deposited in the sediment. Brodifacoum has a bioconcentration factor (BCF) of 6000, which is approximately 1.9- and 2.5-fold those of DDT and heptachlor, respectively (
Table 1).
3. Toxicities of Anticoagulants and Coincidences Between the AR Bait Applications and Marine Mammal Strandings
Animals may consume rodenticide baits (i.e., primary poisoning) or prey or carcasses of animals that have consumed the poison (i.e., secondary poisoning).
3.1. Primary Poisoning
The consequences of primary poisoning are death or injury following exposure to the poison. One common method of assessing the toxicity potency is to determine the lethal dose that is fatal to half the population of the test animals. This dose is the median lethal dose (LD
50). This value is reported as a single number and is used to assess acute toxicity (i.e., short-term risks). Some toxicants show shallow dose–response curves (i.e., wide range), while others show sharp dose–response curves. The LD
50 does not reflect the range of lethal doses among the test subjects. For example, diphacinone showed an LD
50 with large variations in mongrel cats. The LD
50 was reported as 14.7 mg/kg of the animal tested, with confidence limits from 0.790 to 273 mg/kg [
12] (
Supplementary File S1), which is a 345-fold difference among the tested individuals. Data from the U.S. EPA review of rodenticides summarizes their acute toxicity. This data shows a single value for cats as 14.7 mg/kg, as do most reference documents on rodenticides [
9,
13]. The LD
50 shows neither the sensitivity nor tolerance of individuals to the poison. The LD
50 of brodifacoum for cats is listed as a single value of approximately 25 mg/kg [
13] (p. 27). A dose of brodifacoum as low as 1 mg in an adult human or 0.014 mg/kg in a child is enough to cause toxicity [
14] (p. 174).
3.2. Field Studies of Primary Poisoning
Anticoagulant rodenticides are non-food-use pesticides [
9]. They are usually confined to bait stations or locations inaccessible to non-target species outside the crop or livestock production area and food handling areas. Their non-food classification means that studies for chronic exposure required for pesticides used on foods are waived. Field studies offer the first opportunity to obtain information including the effects of multiple exposures, subacute effects, and resistance patterns. The next two sections describe field studies that have been conducted on islands in the middle of the Pacific Ocean.
3.2.1. Keauhou Ranch on the Island of Hawaii
An experimental field study to determine the effectiveness and non-target impact of diphacinone baits was made at Keauhou Ranch, on the Island of Hawaii. Keauhou Ranch is owned by the Kamehameha Schools/ Bishop Estate, which is the legacy estate of Queen Liliuokalani, the last Hawaiian sovereign to rule the islands of Hawaii. The study was designed and carried out by the National Wildlife Research Center, Animal and Plant Health Inspection Service, U.S. Department of Agriculture. This center has a field office in Hilo, Hawaii.
The bait application was not effective in eradicating the rats in the treated area [
15]. Feral pigs were monitored for 18 days following the application of diphacinone baits. An amount of 4286 kg of diphacinone bait was applied to 302 hectares (ha) in two treatments, on August 29 and September 4 [
16] (p. 9,
Supplementary File S2). Ten pigs were collared with VHS transmitters. Eleven pigs without collars were also recovered. Of the 21 total pigs, 13 died, 7 were sacrificed, and the collar slipped off 1 of the pigs. A tolerant boar that was sacrificed after 18 days had the highest levels of diphacinone (3.07 ppm in its liver and 0.12 ppm in its muscle tissue). Diphacinone was detected in the liver of 10 of 11 boars and 6 of 7 sows. Diphacinone was detected in the muscle tissue of four boars and one sow [
16] (pp. 17–19,
Supplementary File S2).
3.2.2. Studies on Small Islands
Diphacinone was studied to eradicate rats on Mokapu Island, near Molokai, Lehua Island, near the island of Kauai, and brodifacoum was studied to eradicate rats on Palmyra Atoll (located 1545 km south of Honolulu).
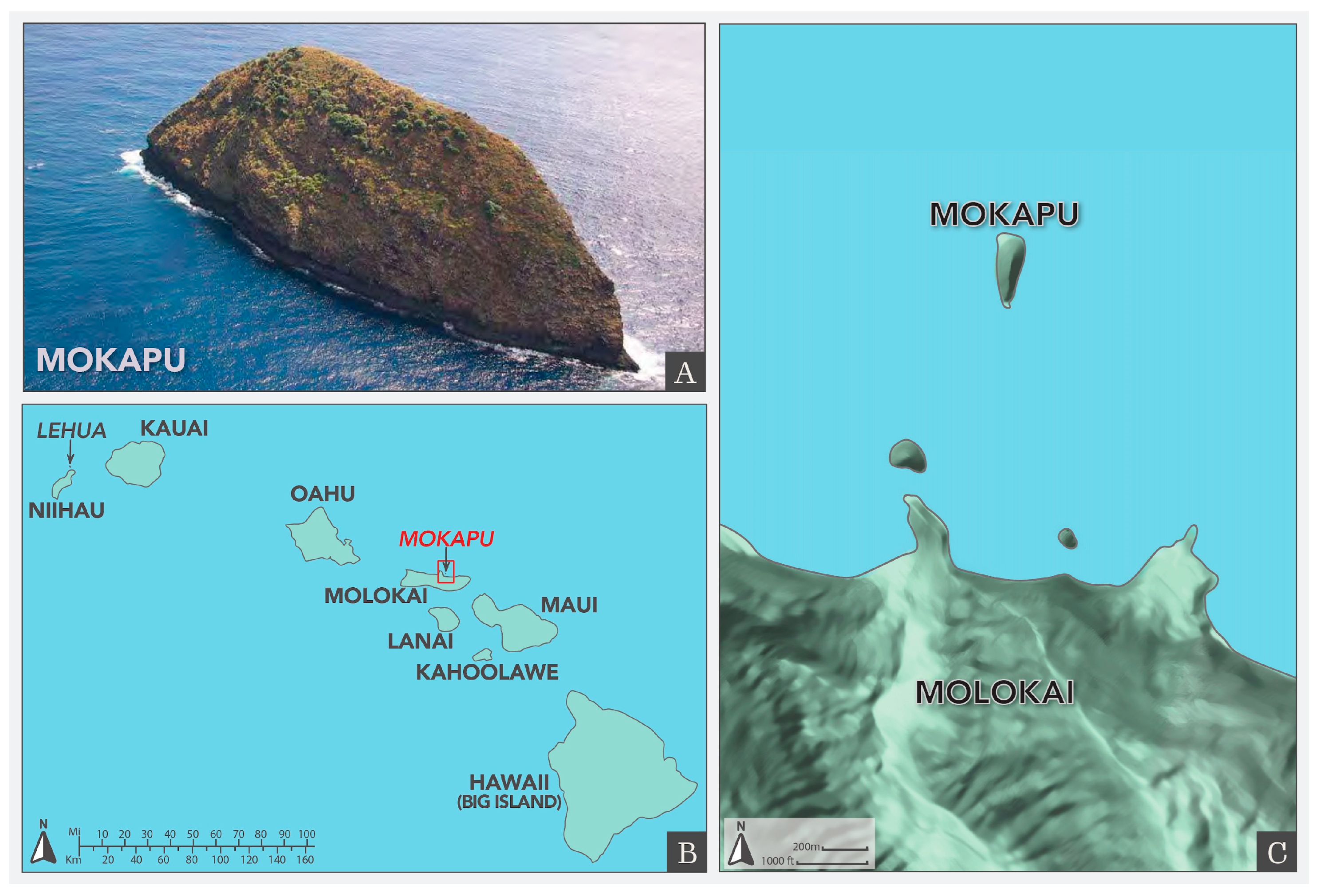
Mokapu Island
Mokapu Island (
Figure 1) is located approximately 1 km off the north coast of Molokai. Its size is approximately 4 ha.
Applications were completed by aerial broadcast across 100% of the land area of the island at a rate of 11.21 kg/ha in two separate broadcast applications, on 6 February and 12 February, 2008. Coastlines and steep areas were treated with twice the rodenticide for each application. Samples of seawater, limpets, and fish were collected. Samples of fish were filleted and only fillets were tested. No residues of diphacinone were detected in any of the samples [
17].
Lehua Island
Two aerial bait application projects were conducted on Lehua Island (
Figure 2). In total, approximately 3540 kg of diphacinone bait was applied to 115 ha on 6 and 13 January 2009. A coincidental fish kill was reported on Niihau on 17 January 2009 [
18,
19].
In a second project, diphacinone baits were applied on 23 and 30 August and 12 September, 2017. In total, approximately an amount of 8536 kg of diphacinone bait was applied. Coincidental non-target mortality of 45 mullet-type fish and 2 immature boobies in advanced state of decay were reported on social media (
Figure 3 and
Figure 4) and local news 13 days after application [
21,
22].
It is likely that the deaths of these animals coincided with primary poisoning. Diphacinone residues were detected in seabirds and fish. In both cases concerning the bait application to Lehua Island, collecting the carcasses after decaying for days (or weeks) compromised the reliability and representation of the samples.
Palmyra Atoll
Palmyra Atoll had dense foliage, abundant land crabs, and an estimated 20,000 rats. The situation represented a challenge to the eradication principles and planners were authorized to increase the amount of brodifacoum bait 4- to 8-fold over previous eradication projects. Two bait applications were applied by aircraft (80 and 75 kg/ha) in June 2011. The baits contained 25 ppm of brodifacoum. “After the application commenced, carcasses of 84 animals representing 15 species of birds, fish, reptiles, and invertebrates were collected opportunistically as potential non-target mortalities. In addition, fish, reptiles, and invertebrates were systematically collected for residue analysis. Brodifacoum residues were detected in most (84.3%)” [
23]. Twenty-one dead rats were collected, and all had brodifacoum residues. One rat survived. It was found in the proximity of human habitation, so baits were applied by hand at a rate of 71.6 kg/ha over a 10 ha area. The surviving rat had a level of brodifacoum higher than any of the dead rats [
23].
3.3. Secondary Poisoning
Secondary poisoning or relay toxicity is when a predator or scavenger is killed or injured from exposure to the poison by consuming an animal that has been intoxicated or killed by the poison. The EPA’s 2004 document comparing risks of rodenticide describes difficulties with estimating secondary poisonings. “Secondary exposure estimates are considerably more complex and require consideration of residues in tissues of target organisms that are commonly consumed by predators and scavengers, as well as knowledge of what residue level will result in mortality or adverse chronic effects. Moreover, it is important to know how long the residue persists in body tissues, experimental designs and methodologies vary among studies, adding unknown variability to the results and conclusions. Without standard chemical analysis methods for secondary poisoning studies, in vivo toxicity assays were used to determine the mean percent mortality for birds and mammals to estimate the secondary exposure and hazard. Retention time in tissues consumed by scavengers and predators is an important factor in estimating secondary exposure and potential risk, available retention times (half-life, t
1/2, in days) of rodenticide in liver and blood are also factored into secondary exposure and risk estimates” [
13] (p. 9).
Figure 5 illustrates the secondary hazards of rodenticides to mammals [
13] (p. 87).
The t
1/2 of diphacinone in liver tissues of species tested (rats and raptors) ranged from 3 to 29.2 days [
24]. It is noteworthy that the concentration of diphacinone metabolite was twice as abundant as unchanged diphacinone [
25]. The estimated t
1/2 of diphacinone in cattle liver tissues was more than 90 days [
26]. The t
1/2 of brodifacoum in livers of rats, a possum, and a sheep was >80–350 days, >252 days, and >250 days, respectively [
24].
Milk is a route of exposure to mammals because mammals nurse their young. Analyzing cow milk was part of the safety assessment of the use of diphacinone to control vampire bats feeding on cattle. The octanol/water partition coefficient (K
ow) is an important parameter for risk assessments. It is an important factor for estimating the potential for substances to accumulate along the food chain [
7]. This coefficient expresses the affinity of a molecule for lipids. The concentration of diphacinone in the milk, which has a fat content of 3.25%, did not exceed 21 ppb [
24,
27]. The fat content of humpback whales’ milk varies. One factor (months post-partum) shows fat content of milk from a whale estimated to be 5–7 months post-partum as 47%, whereas that of a whale estimated to be 10 months post-partum was shown to be 35% [
28]. The greater fat content is in the milk, the greater the bioaccumulation potential is for lipophilic toxicants.
A study of persistent organic pollutants in the blubber of 16 species of cetaceans (42 individuals) that died in the Pacific Islands from 1997 to 2011 showed that DDT and its metabolites had the highest levels [
29]. The BCF of brodifacoum is approximately two times higher than that of DDT, and the K
ow of diphacinone is similar to that of heptachlor [
11].
Figure 6 shows three coincidences between rodenticide baiting and observed marine animal deaths. The first case coincided with aerial applications of diphacinone baits to Mokapu Island in January 2008. A female humpback whale calf died on Paumana beach on Maui 13 days after the final application (a distance of about 40 miles).
Tests of the whale’s liver did not detect diphacinone residues at the limit of quantification (LOQ) of 15 ppb [
30,
31]. Tests were not conducted for a metabolite of concern. The second case coincided with applications of diphacinone baits to Lehua Island (
Figure 7) in January 2009, when two juvenile humpback whales (one on Niihau and another on Kekaha beach on Kauai) died in January–February 2009. The third case coincided with applications of diphacinone baits to the same island in August 2017 when five pilot whales died in October 2017 on Kalapaki beach on Kauai. Whales were observed to be hemorrhaging.
Figure 7 shows the proximity of Lehua Island to Niihau and Kauai.
3.4. Coincidental Kills of Whales and Dolphins
3.4.1. Humpback Whales
A coincidental kill occurred in Maui, Hawaii. Mokapu Island is offshore of the Northern Coast of Molokai, outside of the Humpback Whale Sanctuary. Mokapu Island was baited with diphacinone on 6 and 12 February 2008. The female humpback whale calf died at Paumana Beach near Lahaina, Maui (about 40 miles away), on 25 February 2008 (
Figure 8) [
30,
31]. This calf was one of the three humpback whale calves that died in the Pacific Islands from 1997 to 2011. These calves were tested for persistent organic pollutants [
29].
The order Cetacea is divided into two suborders: Mysticetes and Odontocetes. Mysticetes have baleen plate structures in their mouths which separate food, such as krill and fish, from seawater. Odontocetes have teeth and hunt their prey (usually larger fish, squid, and other marine animals) [
32]. The humpback whale calves showed significantly lower contaminant concentrations than the odontocete cetacean species [
29].
Coincidental killings of juvenile humpback whales also followed the treatment on Lehua Island. Lehua Island was treated on 6 and 13 January 2009 (
Figure 2). Death of juvenile humpback whales was observed on 17 and 29 January 2009, in Niihau and Kauai [
18,
19].
3.4.2. Pilot Whales
Coincident with the eradication project on Lehua Island in August and September 2017, five pilot whales perished on a beach at Kalapaki (south shore of the Island of Kauai) on 13 October 2017. Some were hemorrhaging (
Figure 9).
Whale stranding records were searched from 1997 to 2017. In July 2014, four short-finned pilot whales stranded on Kauai and Oahu at different times. The July 2014 strandings of these pilot whales coincided with the Rim of the Pacific (RIMPAC) naval exercises. RIMPAC is the world’s largest maritime warfare exercise and explosive detonations, sonar, and other practices have an impact on whales. The mass stranding of pilot whales in Hawaii occurred on 13 October 2017.
3.5. Strandings Without Direct Temporal Connection to AR Applications
3.5.1. Pygmy Killer Whales
Other mass strandings occurred well after the eradication drops. On 29 August 2019, a mass stranding of pygmy killer whales occurred on the shores of South Maui, in which 11 pygmy killer whales (a species of dolphin) washed ashore. Out of the eleven, six were successfully re-floated and swam back out to sea, while the remaining five died. Advanced drone technology was used to study the six whales who were successfully re-floated remotely. The study found rapid weight loss resulting from a disruption in feeding and that the whales were outside their typical distribution and habitat. Two individuals died on shore during the 21-day period that remote monitoring was conducted [
33].
3.5.2. Fraser Dolphins
Deaths of three Fraser dolphins in December 2021 occurred in Hawaii approximately four years after diphacinone baits were dropped. All three necropsies reported hemorrhage [
34].
3.5.3. Humpback Whale Calf
A female humpback whale calf died in Honolulu in February 2022. This calf exhibited extensive, fresh hemorrhage through the brain, consistent with severe blunt force trauma. The source of this trauma is unknown. This calf might have been struck by a vessel. However, no tests were conducted for anticoagulant rodenticides [
35].
3.5.4. Uncommon Stranding Events (USE)
The term USE comes from the Navy. The Navy uses sonar and underwater explosives that may impact marine mammals during military training exercises. An uncommon stranding event includes a group of two or more cetaceans of any species exhibiting indicators of distress. A USE occurred in October 2015 when three bottlenose dolphins died on a beach in San Diego, California, USA. Hemorrhage was observed in all three [
36]. Islands in the Gulf of California and Anacapa Islands were treated years earlier with brodifacoum baits [
37].
3.5.5. Unidentified Stranding Events
After the mass stranding of the pilot whales on 13 October 2017, the Hawaii Department of Land and Natural Resources issued a news release. In that release, Gregg Howald, Director of Global and External Affairs for Island Conservation, the organization that led recent rat eradication efforts on Lehua Island, said “As conservationists committed to preserving wildlife, we are deeply saddened by these mortalities. We know, with the highest degree of confidence, that the Lehua Restoration Project and the rodenticides applied in that project have virtually no chance of contributing to the whales’ demise; the likelihood of any impact to pilot whales is so unlikely, it is bordering on the impossible. The good people of Hawaii have had the good fortune to observe natural wildlife in paradise for hundreds of years, and they can tell us that Pilot Whale beachings are quite common” [
38].
Stranding records for whales in the North Central Pacific from 1997 to 2017 were reviewed and summarized (
Figure 10). The graphs below show the total strandings, the strandings where the whale was dead or died at the site or shortly thereafter, and mass strandings.
4. Challenges in Identifying Anticoagulant Involvement in Marine Predator and Scavenger Harm
If marine predators and scavengers are harmed, secondary exposure to anticoagulants may also be involved. Secondary exposure estimates are considerably more complex and require consideration of residues in tissues of target organisms that are commonly consumed by predators and scavengers, as well as knowledge of what residue level will result in mortality or chronic adverse effects.
4.1. Exposure of Humpback Whale to Anticoagulants via Opportunistic Feeding and Lactating
The bait applications to Mokapu and to Lehua in 2009 were in the winter, when humpback whales were present. Opportunistic feeding by a juvenile humpback whale on fish identified as Pacific chub mackerel (
Scomber japonicus) was first reported in 1989 [
39] (
Supplementary File S10). Therefore, exposure may occur when they opportunistically feed.
Three female humpback whale calves died from 1997 to 2011 [
28]. Their only source of possible exposure was their mothers’ milk. It is noteworthy that humpback whale milk generally contains 35–45% fat [
28]. The blubber of the three calves showed very low levels of all persistent organic pollutants [
29], and no dead adults were found during the period from 1997 to 2011. No parent diphacinone was detected at a LOQ of 15 ppb in the liver of the calf that died on Maui, and the method was not suitable for the detection of diphacinone metabolites [
25].
The 1976 study of cattle showed that diphacinone persisted at the original dose in the livers for over 90 days [
26]. It is not known how long diphacinone would persist in the liver of a humpback whale. Diphacinone and brodifacoum are lipophilic. It is not known how much diphacinone and brodifacoum in the milk would be lethal to a calf. It is known that diphacinone can be metabolized. However, little is known about the toxicity and persistence of the metabolites. There are very few opportunities to collect samples of whale’s milk.
4.2. Secondary Exposure of Toothed Whales Through Prey
Pilot whales are toothed whales. The size of their prey is larger than the baleen whales (e.g., humpback whales) [
40]. The yellowfin tuna (
Thunnus albacores) is a predatory fish. Fishermen routinely discard the entrails, including the liver and kidneys, at sea, where they are consumed by scavengers (sharks and sea birds). This may have added to exposure to diphacinone and resulted in the stranding event on 13 October 2017, which followed the application of diphacinone to Lehua in August and September 2017. Brodifacoum has been found in fish three years after its use on Wake Island [
41]. AR residues may be in a marine predator or scavenger’s next meal.
5. Research Challenges and Opportunities
5.1. Development and Application of New Analytical Methods
The analytical methods that were used for the parent diphacinone were not sensitive and required specialized equipment and a large sample size to detect trace levels of the toxicant.
Figure 11 shows the detection limit of the analytical methods used to measure diphacinone in the liver of the humpback whale calf that died in 2008 in comparison with a sensitive method that was published in 2009 [
42]. There are many advanced analytical methods and equipment available nowadays. However, analysis of ARs remains challenging. Few methods have been available in the literature for the analysis of ARs metabolites [
43].
One of the challenges in determining secondary risk of ARs to mammals is testing for metabolites.
Figure 12 shows the relative concentrations of unchanged diphacinone and one of its metabolites. The structure of metabolite E was not elucidated, although C
14-labeled diphacinone was used in the study [
25]. The metabolite accounted for about twice the concentration of diphacinone, and there is no method to detect it. Methods must be developed to analyze ARs and their metabolites quickly and accurately.
5.2. Other Research Challenges and Opportunities
The EPA document entitled Potential Risks of Nine Rodenticides to Birds and Nontarget Mammals: a Comparative Approach [
13], p. iv, lists many factors that require data to support the use of rodenticides. There are at least eight factors that may contribute to the most uncertainty of potential risks. The first is missing data, including acute, chronic, and secondary toxicity as well as retention of some active ingredients (and their metabolites) in the liver, blood, and other body tissues. The second is the variable quality and quantity of existing data on metabolism and retention times in rodents and nontarget species. The third is related to specific use information by formulation and label, which includes typical amounts applied by use site, seasonally, and annually; distances applied from buildings; amounts used in rural versus urban areas; use by professional applicators versus homeowners and other applicators; and other such relevant information. The fourth is about the information on the number and species of birds and nontarget mammals frequenting baited areas and the likelihood of their finding and consuming bait or poisoned primary consumers in the various use areas. The fifth is the methods that are used to determine organ (e.g., liver) concentration(s) and total body burdens of rodenticide that would corroborate death, or if such a cause–effect relationship is even appropriate (e.g., the “threshold of toxicity” concentration). The sixth is sublethal effects on reproduction and nontarget mortality (e.g., clotting abnormalities, hemorrhaging, stress factors including environmental stressors, such as adverse weather conditions, food shortages, and predation). The seventh is bioaccumulation of repeated sublethal exposures to bait or poisoned rodents utilized as food by predators and scavengers. Finally, lack of incident reporting contributes largely to the uncertainty of potential risks. Much of these data have been waived because the use of rodenticides is considered a non-food use. Prohibitions against contaminating water and collecting and disposing of animal carcasses that have perished have been waived to facilitate use on islands [
13].
Silent Spring describes how stakeholders with special interests fail to identify harm they cause. Rachel Carson used the Biblical parable of the Good Samaritan to show how observers familiar with the ways of wildlife identify harm that is not recognized by stakeholders with special interests [
1] (p. 86). Harm to wildlife was not recognized by stakeholders on Niihau; but was recognized by observers familiar with wildlife [
18].
6. Conclusions and Outlook
This review is intended to serve as an advocate for invasive species management, island ecosystem restoration, AR research, and adequate management of anticoagulant rodenticides. One may consider that the coincidences described above are apparent or casual or hypothetical associations. However, they warrant a need for investigating potential causes of anticoagulant applications contributing to marine mammal deaths. The analysis methods for the parent substances such as diphacinone and brodifacoum are already good (limit of quantitation ≤ 0.1 ng/g liver) [
43]. There is a need for further research into metabolites, which can serve as markers of exposure. In addition, other research opportunities include development and application of portable and precise analytical methods, long-term monitoring (decades), incident reporting, and toxicity studies of metabolites.
It is imperative that a balanced and holistic approach to the conservation of species biodiversity be prioritized, with due consideration of ethical practices, human cultural heritage values, and the well-being and dignity of living organisms. Moreover, extreme solutions and radical concepts should be avoided in conservation initiatives, and instead, a focus on balanced and pragmatic approaches should be emphasized. It is crucial to give precedence to the expansion of environmental awareness and education, while simultaneously challenging the legitimacy of existing policies and practices that may not align with the principles of sustainability and ethical conduct [
45]. In order to achieve successful outcomes in nature conservation, it is essential to demonstrate a profound dedication to scientific integrity, a comprehensive approach to environmental conservation, and an unwavering respect for the natural world [
46,
47].
Ecosystem restoration needs to be science-based, ecologically balanced, and holistic. Different anticoagulants have different toxicities. Some anticoagulants have a wide toxicity range. The LD
50 of diphacinone in mongrel cats demonstrates that the range of toxicity is not factored into risk assessments [
12]. The tolerance of the boar to diphacinone at Keauhou Ranch [
16] and the tolerance of the rat to brodifacoum at Palmyra [
23] illustrate that not all individuals perished. Tolerant individuals that survived will hypothetically pass on their genetic tolerance to anticoagulants to their offspring. Marine predators and scavengers that have long life spans and low fecundity may suffer greater mortality.
Anticoagulant rodenticides are designed to kill pest rodents. Therefore, harm is associated with anticoagulant use. Harm (injury) may not be recognized because of the delay in toxicity and the absence of long-term monitoring and incident reporting. The abovementioned incidents, as well as documented residues of anticoagulants in land animals, are a harbinger and call for attention. Having sensitive methods is a first step to studying the effects of rodenticides on marine mammals. Timely sample collection and analysis are also essential. Portable sensitive diagnostic tools offer quick in situ analysis of the samples. Suitable techniques are also essential to manage invasive rodent pests without harming marine mammals. However, development of effective and safe rodent control techniques requires adequate resources and long-term commitment. It is suggested that anticoagulant rodenticides be listed under the Stockholm Convention on Persistent Organic Pollutants.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/conservation4040045/s1, S1: Hazleton Laboratories, Inc. Project Report of Diphacinone (2-Diphenylacetyl-1,3-indandione); 1957. S2: Pitt, W.C., et.al.,
Diphacinone Residues in Free-Ranging Wild Pigs Following Aerial Broadcast of a Rodenticide Bait in a Hawaiian Forest; Laboratory Project No. QA-1077; Unpublished Report QA-1077; 2005. S3: Letter from Niihau Ranch to Robert Boesch, dated 7 April 2011. S4: Lehua Island Complaint-entire KA-17-08_redacted, p. 167. S5: Marine Mammal Stranding Report, 25 February 2008, Field NO. KW2008003 NMFS Registration NO. NMFS-MN-08-04-SD. S6: Gale, R.W. et. al., No Diphacinone Residues Detected in a Beached Juvenile Humpback Whale (
Megaptera novaeangliae); 2008. S7: Necropsy Summary of 3 Fraser Dolphins on 5 and 6 December 2021. S8: Necropsy of Humpback Calf Stranding on 6 February 2022. S9: Supporting information for
Figure 10. Total of strandings and number of dead whales at the stranding sites found between 1997 and 2017 (A) and number of whales perishing in mass stranding events between 1997 and 2021 in the central Pacific (B). S10: Salden, D.R., An Observation of Apparent Feeding by a Sub-adult Humpback Whale off Maui, Hawaii, 1989. S11: Letter from Patrick Leonard (U.S. Fish and Wildlife Services) to Chris Yates (National Marine Fisheries Service) dated 22 August 2008.
Funding
This work was supported in part by the USDA Hatch multistate project HAW05044R.
Acknowledgments
Qing X. Li provided inspiration, resources, office space, guidance, and a forum to present concepts to colleagues. Lyle Wong, Hawaii Department of Agriculture, provided supervision, encouragement, and support. The Chemical Analysis Laboratory at the Hawaii Department of Agriculture tested samples and gave advice about the limits of detection of analytical capabilities. The author thanks Amy Holmes for graphical support and the three anonymous reviewers for their positive comments and suggestions.
Conflicts of Interest
The author declares no conflicts of interest.
References and Notes
- Carson, R. Silent Spring; Mariner Books: Boston, MA, USA, 1962. [Google Scholar]
- Nixon, R. Executive Order 11643—Environmental Safeguards on Activities for Animal Damage Control on Federal Lands. Available online: https://www.presidency.ucsb.edu/documents/executive-order-11643-environmental-safeguards-activities-for-animal-damage-control (accessed on 5 August 2024).
- Clinton, W.J. Executive Order 13112—Invasive Species. Available online: https://www.invasivespeciesinfo.gov/executive-order-13112 (accessed on 5 August 2024).
- Obama, B. Executive Order 13751—Safeguarding the Nation from the Impacts of Invasive Species. Available online: https://www.doi.gov/invasivespecies/executive-order-13751 (accessed on 5 August 2024).
- Howald, G.; Donlan, C.J.; Galv’an, J.P.; Russell, J.; Parkes, J.; Samaniego, A.; Wang, Y.; Veitch, D.; Genovesi, P.; Pascal, M.; et al. Invasive Rodent Eradication on Islands. Conserv. Biol. 2007, 21, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Regnery, J.; Schulz, R.S.; Parrhysius, P.; Bachtin, J.; Brinke, M.; Schäfer, S.; Reifferscheid, G.; Friesen, A. Heavy Rainfall Provokes Anticoagulant Rodenticides’ Release from Baited Sewer Systems and Outdoor Surfaces into Receiving Streams. Sci. Total Environ. 2020, 740, 139905. [Google Scholar] [CrossRef] [PubMed]
- Kotthoff, M.; Rüdel, H.; Jürling, H.; Severin, K.; Hennecke, S.; Friesen, A.; Koschorreck, J. First Evidence of Anticoagulant Rodenticides in Fish and Suspended Particulate Matter: Spatial and Temporal Distribution in German Freshwater Aquatic Systems. Environ. Sci. Pollut. Res. Int. 2019, 26, 7315–7325. [Google Scholar] [CrossRef] [PubMed]
- Link, K.P. The Discovery of Dicumarol and its Sequels. Circulation 1959, 19, 97–107. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency, Prevention, Pesticides and Toxic Substances (7508W). Reregistration Eligibility Decision (RED): Rodenticide Cluster. EPA738-R-98-007. July 1998. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/2100red.pdf (accessed on 24 September 2024).
- US EPA. Restrictions on Rodenticide Products. Available online: https://www.epa.gov/rodenticides/restrictions-rodenticide-products (accessed on 5 August 2024).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Hazleton Laboratories, Inc. Project Report of Diphacinone (2-Diphenylacetyl-1,3-indandione); Hazleton Laboratories, Inc.: Vienna, VA, USA, 1957. [Google Scholar]
- Erickson, W.; Urban, D. Potential Risks of Nine Rodenticides to Birds and Nontarget Mammals: A Comparative Approach; Office of Pesticides Programs, Environmental Fate and Effects Division, United States Environmental Protection Agency: Washington, DC, USA, 2004; Available online: http://pesticideresearch.com/site/docs/bulletins/EPAComparisonRodenticideRisks.pdf (accessed on 5 August 2024).
- US EPA. Recognition and Management of Pesticide Poisonings: Sixth Edition: 2013. EPA 735K13001. Available online: https://www.epa.gov/pesticide-worker-safety/recognition-and-management-pesticide-poisonings (accessed on 5 August 2024).
- Tummons, P.; Pig Deaths Quench Hopes to Employ Helicopters to Spread Rat Bait in Forests. Environment Hawaii 11 May 2004; Volume 14. Available online: https://www.environment-hawaii.org/?p=2984 (accessed on 5 August 2024).
- Pitt, W.C.; Eisemann, J.D.; Swift, C.E.; Sugihara, R.T.; Dengler-Germain, B.; Driscoll, L. Diphacinone Residues in Free-Ranging Wild Pigs Following Aerial Broadcast of a Rodenticide Bait in a Hawaiian Forest; Laboratory Project No. QA-1077; Unpublished Report QA-1077; National Wildlife Research Center: Fort Collins, CO, USA, 2005. [Google Scholar]
- Gale, R.W.; Tanner, M.; Orazio, C.E. Determination of Diphacinone in Seawater, Vertebrates, Invertebrates, and Bait Pellet Formulations Following Aerial Broadcast on Mokapu Island, Molokai, Hawai’i: U.S. Geological Survey Open-File Report 2008–1285; USGS: Columbia, MO, USA, 2008; 16p. Available online: https://www.usgs.gov/publications/determination-diphacinone-sea-water-vertebrates-invertebrates-and-bait-pellet (accessed on 5 August 2024).
- Letter from Niihau Ranch to Robert Boesch, dated 7 April 2011. See Supplementary File S3.
- Parkes, J.; Fisher, P. Review of the Lehua Island Rat Eradication Project 2009; Pacific Cooperative Studies Unit Technical Report 195; University of Hawai‘i at Mānoa: Honolulu, HI, USA, 2017; 48p, Available online: https://manoa.hawaii.edu/hpicesu/techr/195/v195.pdf (accessed on 5 August 2024).
- Lehua Island Complaint-entire KA-17-08_redacted, p. 167. See Supplementary File S4.
- Lehua Island Document Archive. Lehua Aerial Poison Drop: A Document Archive for Researchers and Policy Makers. Available online: https://www.lehua-island-hawaii-conservation.org (accessed on 5 August 2024).
- Siers, S.R.; Foster, D.K.; Niebuhr, C.N.; Leinbach, I.; Shiels, A.B.; Volker, S.F. Monitoring Diphacinone Residues after an Eradication of Polynesian Rats from Lehua Island, Hawaii; Final Report QA-2802; USDA, APHIS, WS, NWRC: Hilo, HI, USA, 2018; p. 14 + appendices. Available online: https://dlnr.hawaii.gov/wildlife/files/2018/07/QA-2802-FINAL-REPORT-Lehua-diphacinone-residues-REV1.pdf (accessed on 5 August 2024).
- Pitt, W.C.; Berentsen, A.R.; Shiels, A.B.; Volker, S.F.; Eisemann, J.D.; Wegmann, A.S.; Howald, G.R. Non-target Species Mortality and the Measurement of Brodifacoum Rodenticide Residues after a Rat (Rattus rattus) Eradication on Palmyra Atoll, Tropical Pacific. Biol. Conserv. 2015, 185, 36–46. [Google Scholar] [CrossRef]
- Horak, K.; Fisher, P.M.; Hopkins, B.M. Pharmacokinetics of Anticoagulant Rodenticides in Target and Nontarget Organisms; DigitalCommons; University of Nebraska-Lincoln: Lincoln, NE, USA, 2018; Available online: https://digitalcommons.unl.edu/icwdm_usdanwrc/2091 (accessed on 5 August 2024).
- Yu, C.C.; Atallah, Y.H.; Whitacre, D.M. Metabolism and Disposition of Diphacinone in Rats and Mice. Drug Metab. Dispos. 1982, 10, 645–648. [Google Scholar] [PubMed]
- Bullard, R.W.; Thompson, R.D.; Holguin, G. Diphenadione Residues in Tissues of Cattle. J. Agric. Food Chem. 1976, 24, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Bullard, R.W.; Thompson, R.D.; Kilburn, S.R. Diphenadione Residues in Milk of Cattle. J. Agric. Food Chem. 1977, 25, 79–81. [Google Scholar] [CrossRef] [PubMed]
- West, K.L.; Oftedal, O.T.; Carpenter, J.R.; Krames, B.J.; Campbell, M.; Sweeney, J.C. Effect of Lactation Stage and Concurrent Pregnancy on Milk Composition in the Bottlenose Dolphin. J. Zool. 2007, 273, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Bachman, M.J.; Keller, J.M.; West, K.L.; Jensen, B.A. Persistent Organic Pollutant Concentrations in Blubber of 16 Species of Cetaceans Stranded in the Pacific Islands from 1997 to 2011. Sci. Total Environ. 2014, 488–489, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Marine Mammal Stranding Report, February 25, 2008, Field NO. KW2008003 NMFS Registration NO. NMFS-MN-08-04-SD. See Supplementary File S5.
- Gale, R.W.; Tanner, M.; Orazio, C.E. No Diphacinone Residues Detected in a Beached Juvenile Humpback Whale (Megaptera novaeangliae); U.S. Geological Survey Administrative Report; 2008; 18p. See Supplementary File S6.
- International Whaling Commission. Available online: https://iwc.int/about-whales/cetacea (accessed on 6 August 2023).
- Currie, J.J.; van Aswegen, M.; Stack, S.H.; West, K.L.; Vivier, F.; Bejder, L. Rapid Weight Loss in Free Ranging Pygmy Killer Whales (Feresa attenuata) and the Implications for Anthropogenic Disturbance of Odontocetes. Sci. Rep. 2021, 11, 8181. [Google Scholar] [CrossRef] [PubMed]
- Necropsy Summary of 3 Fraser Dolphins on 5 and 6 December 2021. See Supplementary File S7.
- Necropsy of Humpback Calf Stranding on 6 February 2022. See Supplementary File S8.
- Danil, K.; Beaulieu-McCoy, N.; Dennison, S.; Rotstein, D.; Rowles, T.; Wilkin, S. Uncommon Stranding Event of Bottlenose Dolphins (Tursiops truncatus) in San Diego, California (October 2015); NOAA Tech. Memo. NMFS-SWFSC-641; NOAA: La Jolla, CA, USA, 2021; 26p. Available online: https://swfsc-publications.fisheries.noaa.gov/publications/CR/2021/2021Danil.pdf (accessed on 6 August 2023).
- DIISE. The Database of Island Invasive Species Eradications, Developed by Island Conservation, Coastal Conservation Action Laboratory UCSC, IUCN SSC Invasive Species Specialist Group, University of Auckland and Landcare Research New Zealand. 2018. Available online: http://diise.islandconservation.org (accessed on 6 August 2024).
- Hawaii Department of Land and Natural Resources. 10/13/17—Multi-Agency Community Response to Pilot Whales Stranding on Kaua‘i. Available online: https://dlnr.hawaii.gov/blog/2017/10/13/nr17_0161/ (accessed on 6 August 2024).
- Salden, D.R. An Observation of Apparent Feeding by a Sub-adult Humpback Whale off Maui, Hawaii. In Proceedings of the Eighth Biennial Conference on the Biology of Marine Mammals, Pacific Grove, CA, USA, 7–11 December 1989; p. 58. [Google Scholar]
- Baird, R.W. The Lives of Hawai’i’s Dolphins and Whales: Natural History and Conservation; University of Hawaii Press: Honolulu, HI, USA, 2016; p. 56. [Google Scholar]
- Siers, S.R.; Shiels, A.B.; Volker, S.F.; Rex, K.; Pitt, W.C. Brodifacoum Residues in Fish Three Years after an Island-wide Rat Eradication Attempt in the Tropical Pacific. Manag. Biol. Invasions 2020, 11, 105–121. [Google Scholar] [CrossRef]
- Jin, M.C.; Chen, X.H.; Ye, M.L.; Zhu, Y. Analysis of Indandione Anticoagulant Rodenticides in Animal Liver by Eluent Generator Reagent Free Ion Chromatography Coupled with Electrospray Mass Spectrometry. J. Chromatogr. A 2008, 1213, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shafi, M.; Wattoo, S.A.; Chaudhary, M.T.; Usman, H.F. Analytical Methods for Determination of Anticoagulant Rodenticides in Biological Samples. Forensic Sci. Int. 2015, 253, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Letter from Patrick Leonard (U.S. Fish and Wildlife Services) to Chris Yates (National Marine Fisheries Service) dated 22 August 2008. See Supplementary File S11.
- Cianfaglione, K. Editorial from the new editor in chief, open questions and outlooks for the future. J. Zool. Bot. Gard. 2022, 3, 714–724. [Google Scholar] [CrossRef]
- Conservation Editorial Office. A retrospective and interview with Dr. Kevin Cianfaglione—editorial board member of Conservation. Conservation 2023, 3, 319–333. [Google Scholar] [CrossRef]
- Littin, K.E.; Mellor, D.J. Strategic animal welfare issues: Ethical and animal welfare issues arising from the killing of wildlife for disease control and environmental reasons. Rev. Sci. Et Tech. -Off. Int. Des Epizoot. 2005, 24, 767–782. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).