Salicylic Acid with NaCl Acts as a Stressor and Alters Root Traits and the Estimated Root Surface Area of Rapeseed (Brassica napus L.) Genotypes in Hydroponic Culture
Abstract
1. Introduction
2. Results
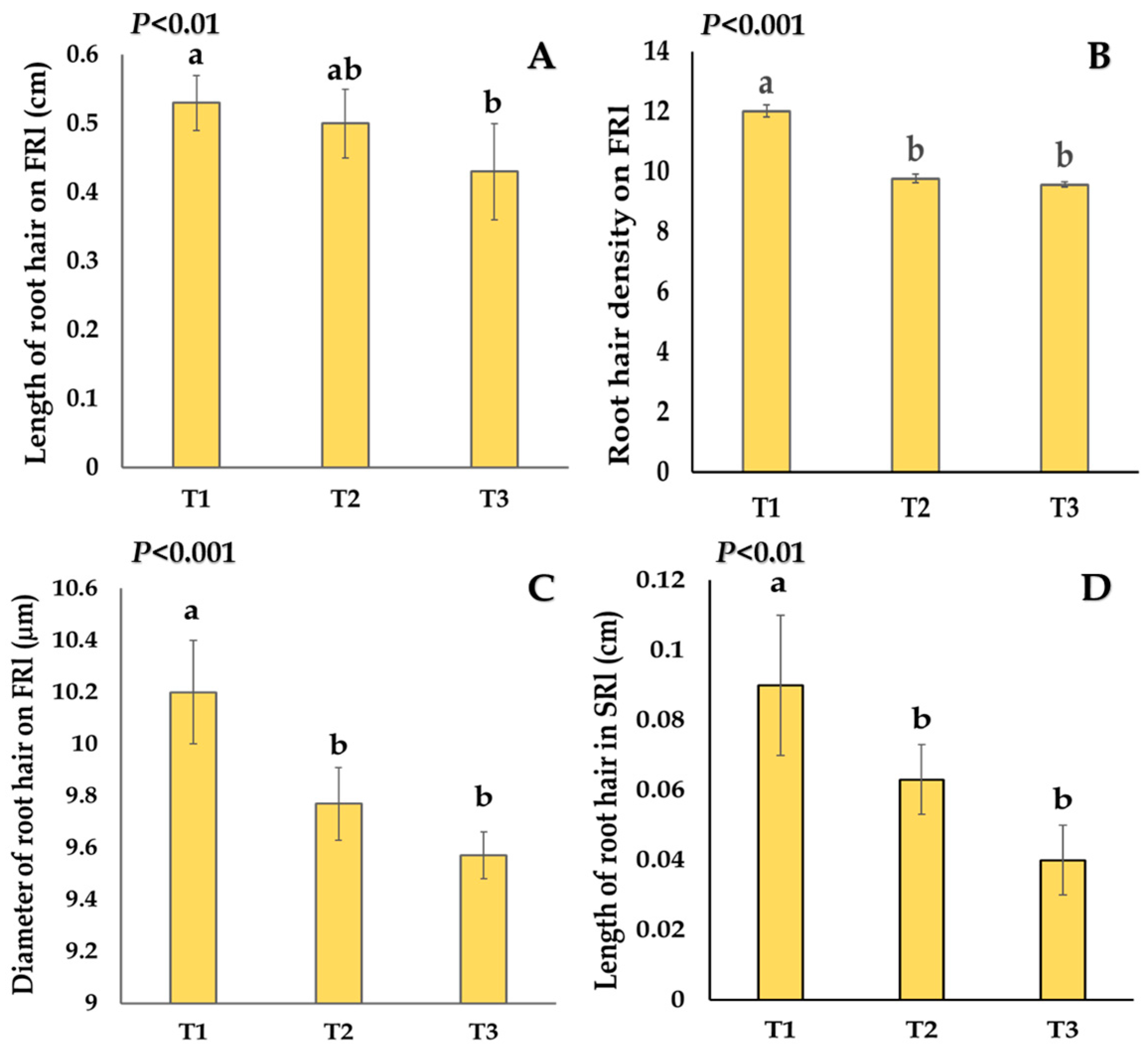
2.1. Effects of Salt Stress and Salicylic Acid on Shoot and Root Traits
2.2. Genotypic Variations
2.3. Trait Associations
3. Discussion
3.1. Salinity Stress Disturbed Shoot Growth and Photosynthetic Capacity
3.2. Salinity Stress Retarded the Development of Roots and New Root Hair Formation
3.3. Salinity Stress Significantly Altered Estimated Root Surface Area (RSA)
3.4. Salicylic Acid (SA) Acted as Stressor in Combination with NaCl upon Vascular Uptake
3.5. Trait Associations Clearly Showed Relationships Among the Traits
4. Materials and Methods
4.1. Culture of Plants and Management of Hydroponic System
4.2. Data Collection
+ a2 n2 πD2 L2 (1 + arh2 nrh2 π Drh2 Lrh2)))
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| PCA | Principal Component Analysis |
| RSA | Estimated Root Surface Area |
| NaCl | Sodium Chloride |
| SA | Salicylic Acid |
References
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Dasgupta, S.; Huq, M.; Khan, Z.H.; Murshed, M.; Ahmed, Z.; Mukherjee, N.; Khan, M.F.; Pandey, K. Cyclones in a changing climate: The case of Bangladesh. Clim. Dev. 2014, 6, 96–110. [Google Scholar] [CrossRef]
- Kabir, M.E.; Sarker, B.C.; Gosh, A.K.; Maiuddin, M.; Bell, R.W. Effect of sowing dates for wheat grown in excess water and salt-affected soils in southwestern coastal Bangladesh. J. Indian Soc. Coast. Agric. Res. 2019, 37, 51–59. [Google Scholar]
- Raihan, S. Economic Reforms and Agriculture in Bangladesh: Assessment of Impacts Using Economy-Wide Simulation Models; MPRA Paper; University Library of Munich: Munich, Germany, 2011; pp. 1–55. [Google Scholar]
- Chakroborty, N.; Kabir, M.A.; Hossen, M.M.; Afrin, J.; Islam, M.M.; Robin, A.H.K. Species-Specific Variation in Oil Content and Fatty Acid Profiles of Short-Duration Rapeseed and Mustard. ACS Food Sci. Technol. 2025, 5, 1082–1090. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Y.; Wang, X.; Bai, J.; Lin, L.; Luo, F.; Zhong, H. A comprehensive review of health-benefiting components in rapeseed oil. Nutrients 2023, 15, 999. [Google Scholar] [CrossRef]
- Helal, M.M.U.; Islam, D.M.N.; Biswas, D.M.; Kadir, D.M.; Miah, D.M.N.H. Performance of rapeseed and mustard (Brassica sp.) Varieties/lines in north-east region (Sylhet) of Bangladesh. Sci. J. Seoul Sci. 2022, 4, 6–13. [Google Scholar]
- Shahzad, B.; Rehman, A.; Tanveer, M.; Wang, L.; Park, S.K.; Ali, A. Salt stress in Brassica: Effects, tolerance mechanisms, and management. J. Plant Growth Regul. 2022, 41, 781–795. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Lv, Y.; Cheng, Y.; Li, C.; Yan, L.; Zou, X. Exogenous serotonin improves salt tolerance in rapeseed (Brassica napus L.) seedlings. Agronomy 2021, 11, 400. [Google Scholar] [CrossRef]
- Chakraborty, K.; Sairam, R.K.; Bhaduri, D. Effects of different levels of soil salinity on yield attributes, accumulation of nitrogen, and micronutrients in Brassica spp. J. Plant Nutr. 2015, 39, 1026–1037. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.; Kumar, S.; Garg, S.; Pandey, R.; Anand, A.; Dikshit, H.K.; Bansal, R. Exploring the genetic variability for root traits in mung bean under salinity stress. Plant Physiol. Rep. 2024, 29, 651–659. [Google Scholar] [CrossRef]
- Arif, M.R.; Islam, M.T.; Robin, A.H.K. Salinity stress alters root morphology and root hair traits in Brassica napus. Plants 2019, 8, 192. [Google Scholar] [CrossRef]
- Hayat, S.; Maheshwari, P.; Wani, A.S.; Irfan, M.; Alyemeni, M.N.; Ahmad, A. Comparative effect of 28-homobrassinolide and salicylic acid in the amelioration of NaCl stress in Brassica juncea L. Plant Physiol. Biochem. 2012, 53, 61–68. [Google Scholar] [CrossRef]
- Khan, M.O.; Farooq, N.; Nawaz, M.A.; Fatima, S.; Islam, E.; Mukhtar, Z.; Ahmad, N. Evaluation of the salt tolerance potential of commercial Brassica cultivars. Commun. Soil Sci. Plant Anal. 2024, 55, 498–516. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Uddin, M.J.; Bayazid, K.N. Polyethylene glycol (PEG)-treated hydroponic culture reduces length and diameter of root hairs of wheat varieties. Agronomy 2015, 5, 506–518. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- El-Hawary, M.M.; Hashem, O.S.; Hasanuzzaman, M. Seed priming and foliar application with ascorbic acid and salicylic acid mitigate salt stress in wheat. Agronomy 2023, 13, 493. [Google Scholar] [CrossRef]
- Chakma, R.; Biswas, A.; Saekong, P.; Ullah, H.; Datta, A. Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci. Hortic. 2021, 280, 109904. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Wahid, A. Exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide improves the productivity of hybrid maize at low temperature stress. Int. J. Agric. Biol. 2014, 16, 825–830. [Google Scholar]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Hasanuzzaman, M.; Khanna, K.; Bhardwaj, R.; Ahmad, P. Salicylic acid-mediated regulation of morpho-physiological and yield attributes of wheat and barley plants in deferring salinity stress. J. Plant Growth Regul. 2022, 41, 1291–1303. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Singh, P.K.; Chaturvedi, V.K.; Bose, B. Effects of salicylic acid on seedling growth and nitrogen metabolism in cucumber (Cucumis sativus L.). J. Stress Physiol. Biochem. 2010, 6, 102–113. [Google Scholar]
- Pour, A.P.; Farahbakhsh, H.; Saffari, M.; Keramat, B. Effects of seed priming on germination and seedling growth under salinity stress in fenugreek. Int. J. Agric. Crop Sci. 2012, 4, 779–786. [Google Scholar]
- Bouallègue, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Salicylic acid and hydrogen peroxide pretreatments alleviate salt stress in faba bean (Vicia faba) seeds during germination. Seed Sci. Technol. 2017, 45, 675–690. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.K.N.; Lima, G.D.; Soares, M.D.M.; Soares, L.D.A.; Gheyi, H.R.; Silva, A.D.; Fernandes, P.D. Salicylic acid does not mitigate salt stress on the morphophysiology and production of hydroponic melon. Braz. J. Biol. 2022, 82, 262664. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef] [PubMed]
- Christianson, M.L.; Duffy, S.H. Dose-dependent effect of salicylates in a moss, Funaria hygrometrica. J. Plant Growth Regul. 2002, 21, 200–208. [Google Scholar] [CrossRef]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 164, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ashraf, M.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.; Raza, A.; Fatima, E.M.; Ciarmiello, L.F. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Azari, A.; Sanavi, S.M.; Askari, H.; Ghanati, F.; Naji, A.M.; Alizadeh, B. Effect of salt stress on morphological and physiological traits of two species of rapeseed (Brassica napus and B. rapa). Iran J. Crop Sci. 2012, 14, 121–135. [Google Scholar]
- Jan, S.A.; Shinwari, Z.K.; Rabbani, M.A. Agro-morphological and physiological responses of Brassica rapa ecotypes to salt stress. Pak. J. Bot. 2016, 48, 1379–1384. [Google Scholar]
- Singh, M.P.; Pandey, S.K.; Singh, M.; Ram, P.C.; Singh, B.B. Photosynthesis, transpiration, stomatal conductance and leaf chlorophyll content in mustard genotypes grown under sodic conditions. Photosynthetica 1990, 24, 623–627. [Google Scholar]
- Shah, S.S.; Mohammad, F.; Shafi, M.; Bakht, J.; Zhou, W. Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pak. J. Bot. 2011, 43, 333–340. [Google Scholar]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Tseng, M.J.; Liu, C.W.; Yiu, J.C. Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol. Biochem. 2007, 45, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Molins, A.; Flexas, J.; Conesa, M.À. Coordination between leaf CO2; diffusion and Rubisco properties allows maximizing photosynthetic efficiency in Limonium species. Plant Cell Environ. 2017, 40, 2081–2094. [Google Scholar] [CrossRef]
- Hussain, T.; Huchzermeyer, B.; Koyro, H.W.; Khan, M.A. Linkage between leaf development and photosynthetic response at hyperosmotic salinity in the C4 grass Panicum antidotale. Flora 2019, 256, 52–60. [Google Scholar] [CrossRef]
- Lynch, D.R.; Foroud, N.; Kozub, G.C.; Fames, B.C. The effect of moisture stress at three growth stages on the yield, components of yield and processing quality of eight potato varieties. Am. Potato J. 1995, 72, 375–385. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Bell, D.L.; Sultan, S.E. Dynamic phenotypic plasticity for root growth in Polygonum: A comparative study. Am. J. Bot. 1999, 86, 807–819. [Google Scholar] [CrossRef]
- Kano, M.; Inukai, Y.; Kitano, H.; Yamauchi, A. Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 2011, 342, 117–128. [Google Scholar] [CrossRef]
- Neumann, P.M.; Azaizeh, H.; Leon, D. Hardening of root cell-walls—A growth-inhibitory response to salinity stress. Plant Cell Environ. 1994, 17, 303–309. [Google Scholar] [CrossRef]
- Neves, G.Y.S.; Marchiosi, R.; Ferrarese, M.L.L.; Siqueira-Soares, R.C.; Ferrarese, O. Root growth inhibition and lignification induced by salt stress in soybean. J. Agron. Crop Sci. 2010, 196, 467–473. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2022, 45, 695–704. [Google Scholar] [CrossRef]
- Sun, J.; Xia, J.; Shao, P.; Ma, J.; Gao, F.; Lang, Y.; Li, C. Response of the fine root morphological and chemical traits of Tamarix chinensis to water and salt changes in coastal wetlands of the Yellow River Delta. Front. Plant Sci. 2022, 13, 952830. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Matthew, C.; Uddin, M.J.; Bayazid, K.N. Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat. J. Exp. Bot. 2016, 67, 3719–3729. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Li, K.; Sun, F.; Han, C.; Wang, Y.; Li, X. Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J. Plant Res. 2008, 121, 87–96. [Google Scholar] [CrossRef]
- Jin, D.; Li, S.; Li, Z.; Yang, L.; Han, X.; Hu, Y.; Jiang, Y. Arabidopsis ABRE-binding factors modulate salinity-induced inhibition of root hair growth by interacting with and suppressing RHD6. Plant Sci. 2023, 332, 111728. [Google Scholar] [CrossRef]
- Srinivasarao, C.H.; Benzioni, A.; Eshel, A.; Waisel, Y. Effects of salinity on root morphology and nutrient acquisition by faba beans (Vicia faba L.). J. Indian Soc. Soil Sci. 2004, 52, 184–191. [Google Scholar]
- Waisel, Y.; Breckle, S.W. Differences in responses of various radish roots to salinity. Plant Soil 1987, 104, 191–194. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Bačkor, M.; Strnad, M.; Repčák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- Luo, J.P.; Jiang, S.T.; Pan, L.J. Enhanced somatic embryogenesis by salicylic acid of Astragalus adsurgens Pall.: Relationship with H2O2 production and H2O2 metabolizing enzyme activities. Plant Sci. 2001, 161, 125–132. [Google Scholar] [CrossRef]
- Kawano, T.; Muto, S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J. Exp. Bot. 2000, 51, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Klessig, D.F. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. USA 1995, 92, 11312–11316. [Google Scholar] [CrossRef]
- Ashraf, M. Effect of salicylic acid applied through rooting medium on drought tolerance of wheat. Pak. J. Bot. 2006, 38, 1127–1136. [Google Scholar]
- Moharekar, S.T.; Lokhande, S.D.; Hara, T.; Tanaka, R.; Tanaka, A.; Chavan, P.D. Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica 2003, 41, 315–317. [Google Scholar] [CrossRef]
- Anandhi, S.; Ramanujam, M.P. Effect of salicylic acid on black gram (Vigna mungo) cultivars. Indian J. Plant Physiol. 1997, 2, 138–141. [Google Scholar]
- Michniewicz, M.; Zago, M.K.; Abas, L.; Weijers, D.; Schweighofer, A.; Meskiene, I.; Heisler, M.G.; Ohno, C.; Zhang, J.; Huang, F. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.C.; Lin, K.H.; Huang, Y.J.; Wu, C.W.; Chang, Y.S. Evaluation of vegetation indices and plant growth regulator use on the rooting of azalea cuttings. Hortic. Bras. 2020, 38, 153–159. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Prasad, P.V. Variability of root traits in spring wheat germplasm. PLoS ONE 2014, 9, e100317. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.H.K.; Ghosh, S.; Shahed, M.A. PEG-induced osmotic stress alters root morphology and root hair traits in wheat genotypes. Plants 2021, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]



| Source of Variation | df | FS | NL | NDL | MAL | MAd | FRl | NFR | FRd | RHDf | RHlf | RHdf |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | 14 | 0.64 *** | 33.85 *** | 2.49 NS | 135.63 ** | 0.34 *** | 41.67 NS | 4.36 *** | 0.045 *** | 30.18 *** | 0.092 *** | 4.55 *** |
| Treatment (T) | 2 | 0.02 NS | 100.02 *** | 214.03 *** | 515.54 *** | 1.23 *** | 203.92 ** | 2.69 NS | 0.033 NS | 21.3 *** | 0.12 ** | 4.54 *** |
| G × T | 28 | 0.19 NS | 5.25 ** | 1.81 * | 109.23 *** | 0.75 *** | 63.34 *** | 2.16 *** | 0.014 *** | 7.58 *** | 0.033 ** | 0.38 *** |
| Error | 90 | 0.19 | 2.81 | 1.08 | 29.34 | 0.012 | 21.70 | 0.78 | 0.006 | 1.86 | 0.016 | 0.12 |
| SRl | NSR | SRd | RHDs | RHls | RHds | DWS | DWR | Cc | RSA | |||
| Genotype (T) | 14 | 23.16 *** | 2.39 * | 0.008 *** | 43.72 *** | 0.101 *** | 3.082 *** | 0.53 *** | 0.008 *** | 142.74 *** | 132,827 *** | |
| Treatment (T) | 2 | 84.7 *** | 2.29 NS | 0.021 *** | 1.92 NS | 0.1 ** | 0.95 NS | 1.48 *** | 0.031 *** | 3476.62 *** | 811,345 *** | |
| G × T | 28 | 24.1 *** | 1.54 NS | 0.003 *** | 3.25 ** | 0.035 ** | 1.12 NS | 0.145 *** | 0.0042 *** | 47.50 *** | 98,839 *** | |
| Error | 90 | 5.13 | 1.04 | 0.0007 | 1.348 | 0.018 | 0.96 | 0.037 | 0.0012 | 11.63 | 23,613 |
| Traits | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | G11 | G12 | G13 | G14 | G15 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FS | 0.44 ab | 0.0 b | 0.55 ab | 0.33 ab | 0.55 ab | 0.67 ab | 0.44 ab | 0.89 a | 0.56 ab | 0.56 ab | 0.67 ab | 0.89 a | 1.0 a | 0.78 a | 1.0 a | 0.62 |
| NL | 7.78 b–d | 6.44 d | 7.78 b–d | 6.56 cd | 7.67 b–d | 8.78 b–d | 9.22 bc | 8.11 b–d | 8.00 b–d | 8.67 b–d | 8.67 b–d | 9.89 b | 9.56 b | 8.78 b–d | 14.78 a | 8.711 |
| MAL (cm) | 26.78 ab | 25.33 a–d | 21.24 b–d | 22.18 a–d | 20.06 b–d | 30.32 a | 27.64 ab | 21.12 b–d | 26.42 a–c | 17.17 d | 26.29 a–c | 23.73 a–d | 22.07 a–d | 17.56 cd | 27.43 ab | 23.690 |
| MAd (mm) | 1.05 c–f | 0.93 e–h | 0.82 h | 0.85 gh | 1.02 d–g | 1.42 a | 1.21 bc | 0.917 f–h | 1.38 ab | 0.974 e–h | 0.997 e–h | 1.22 bc | 1.2 b–d | 1.12 c–e | 1.38 ab | 1.10 |
| NFR | 3.78 b | 5.00 ab | 4.22 b | 3.78 b | 4.89 ab | 4.44 b | 4.00 a–c | 4.22 b | 5.11 ab | 4.22 b | 3.89 b | 3.89 b | 4.56 b | 5.11 ab | 6.33 a | 4.496 |
| FRd (mm) | 0.48 a–d | 0.34 e | 0.39 c–e | 0.42 b–e | 0.42 b–e | 0.40 b–e | 0.49 a–c | 0.38 c–e | 0.59 a | 0.43 b–e | 0.34 e | 0.53 ab | 0.42 b–e | 0.35 de | 0.38 c–e | 0.424 |
| RHDf | 8.67 a | 8.67 a | 9.33 de | 9.78 c–e | 11.00 b–d | 11.56 a–d | 9.33 de | 12.44 ab | 12.11 ab | 12.67 ab | 13.44 a | 13.33 a | 11.78 a–c | 13.78 a | 13.33 a | 11.415 |
| RHlf (cm) | 0.63 a | 0.35 f | 0.37 d–f | 0.60 ab | 0.56 a–e | 0.57 a–d | 0.46 a–f | 0.41 b–f | 0.44 a–f | 0.39 c–f | 0.36 ef | 0.62 a | 0.60 a–c | 0.51 a–f | 0.49 a–f | 0.491 |
| RHdf | 11.11 a | 9.89 b | 10.44 ab | 8.33 c | 8.33 c | 10.44 ab | 9.78 b | 9.78 b | 10 b | 10 b | 10 b | 10 b | 10 b | 9.67 b | 10 b | 9.852 |
| SRl (cm) | 10.61 a | 5.89 b | 6.64 b | 5.86 b | 7.23 ab | 6.58 b | 5.17 b | 4.43 b | 4.30 b | 4.57 b | 5.73 b | 6.12 b | 7.03 ab | 4.43 b | 6.97 ab | 6.105 |
| SRd (mm) | 0.22 a–d | 0.16 d | 0.25 a–c | 0.25 a–c | 0.24 a–c | 0.19 b–d | 0.20 a–d | 0.16 d | 0.22 a–d | 0.19 b–d | 0.19 cd | 0.21 a–d | 0.20 a–d | 0.21 a–d | 0.26 a | 0.209 |
| RHDs | 9.11 d | 9.11 d | 10.67 cd | 9.78 d | 12.22 bc | 10.67 cd | 13.67 ab | 13.11 ab | 15.00 a | 14.67 a | 14.00 ab | 14.11 ab | 15.00 a | 14.00 ab | 14.78 a | 12.659 |
| RHls (cm) | 0.463 a–c | 0.321 c | 0.370 bc | 0.549 a–c | 0.618 ab | 0.464 a–c | 0.432 a–c | 0.323 c | 0.379 bc | 0.422 a–c | 0.324 c | 0.60 ab | 0.632 a | 0.392 a–c | 0.468 a–c | 0.451 |
| RHds (µm) | 10.11 a | 8.78 a–c | 9.78 ab | 8.00 c | 8.00 c | 9.22 a–c | 9.44 a–c | 8.33 bc | 9.00 a–c | 8.67 a–c | 9.33 a–c | 9.00 a–c | 9.00 a–c | 9.00 a–c | 9.00 a–c | 8.978 |
| DWS (mg) | 0.384 cd | 0.267 cd | 0.180 d | 0.160 d | 0.277 cd | 0.573 bc | 0.544 bc | 0.308 cd | 0.571 bc | 0.271 cd | 0.787 ab | 0.736 ab | 0.539 bc | 0.294 cd | 0.985 a | 0.458 |
| DWR (mg) | 0.059 b–e | 0.076 b–e | 0.041 c–e | 0.03 de | 0.053 c–e | 0.057 b–e | 0.074 b–e | 0.041 c–e | 0.085 a–d | 0.025 e | 0.089 a–c | 0.137 a | 0.062 b–e | 0.036 c–e | 0.110 ab | 0.065 |
| Cc (SPAD unit) | 33.13 a–c | 34.74 a | 24.71 f | 25.72 ef | 24.12 f | 27.02 d–f | 29.09 b–f | 26.26 ef | 31.07 a–e | 32.33 a–d | 32.86 a–c | 27.50 c–f | 35.20 a | 33.61 ab | 35.00 a | 30.158 |
| RSA (cm2) | 421.05 a | 143.6 bc | 86.97 c | 80.95 c | 152.46 bc | 268.08 a–c | 146.93 bc | 100.97 bc | 344.64 ab | 55.85 c | 110.9 bc | 207.96 a–c | 222.26 a–c | 92.29 bc | 418.47 a | 177.27 |
| Sl. No. | Genotypes | Generation |
|---|---|---|
| 1 | M-205 | Parents |
| 2 | M-206 | |
| 3 | M-223 | |
| 4 | M-232 | |
| 5 | M-245 | |
| 6 | M-232×M-223 | F3 segregants |
| 7 | M-205×M-232 | |
| 8 | M-205×M-223 | |
| 9 | M-223×M-206 | |
| 10 | M-223×M-205 | |
| 11 | M-205×M-245 | |
| 12 | M-206×M-223 | |
| 13 | M-232×M-245 | |
| 14 | M-206×M-232 | |
| 15 | M-245×M-206 |
| Level of Injury | Visual Status of Leaves | Visual Status of Flowers | Visual Status of Siliquae |
|---|---|---|---|
| 1 | Normal pigmentation and growth | Healthy and normal color, blossoms properly | Normal color and growth |
| 3 | Almost normal, but the tip becomes pale and wilting initiates | Bud does not blossom properly; blossomed bud starts shrinking | Nearly normal, but slight discoloration is observed |
| 5 | Leaves become rolled, a large, discolored proportion start drying | Compacted or twisted petals; young bud starts to die instead of blossoming | No further growth or very slow growth, almost discolored |
| 7 | Mostly dry, pigmentation can hardly be observed | Unopened flower bud dies; fertilization is totally stunted | Siliqua dries and growth is totally stunted |
| 9 | On the verge of death | Most of the buds die | Siliqua dead or about to die |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, J.; Chakroborty, N.; Sultana, R.; Naher, J.; Robin, A.H.K. Salicylic Acid with NaCl Acts as a Stressor and Alters Root Traits and the Estimated Root Surface Area of Rapeseed (Brassica napus L.) Genotypes in Hydroponic Culture. Stresses 2025, 5, 48. https://doi.org/10.3390/stresses5030048
Afrin J, Chakroborty N, Sultana R, Naher J, Robin AHK. Salicylic Acid with NaCl Acts as a Stressor and Alters Root Traits and the Estimated Root Surface Area of Rapeseed (Brassica napus L.) Genotypes in Hydroponic Culture. Stresses. 2025; 5(3):48. https://doi.org/10.3390/stresses5030048
Chicago/Turabian StyleAfrin, Jannatul, Nikunjo Chakroborty, Rebeka Sultana, Jobadatun Naher, and Arif Hasan Khan Robin. 2025. "Salicylic Acid with NaCl Acts as a Stressor and Alters Root Traits and the Estimated Root Surface Area of Rapeseed (Brassica napus L.) Genotypes in Hydroponic Culture" Stresses 5, no. 3: 48. https://doi.org/10.3390/stresses5030048
APA StyleAfrin, J., Chakroborty, N., Sultana, R., Naher, J., & Robin, A. H. K. (2025). Salicylic Acid with NaCl Acts as a Stressor and Alters Root Traits and the Estimated Root Surface Area of Rapeseed (Brassica napus L.) Genotypes in Hydroponic Culture. Stresses, 5(3), 48. https://doi.org/10.3390/stresses5030048








