The Three-Body Problem in Stress Biology: The Balance Between O2, NO, and H2S in the Context of Hans Selye’s Stress Concept
Abstract
1. Introduction
- (1)
- What molecular mechanisms enable a nonspecific response to diverse stressors?
- (2)
- How can the same stressor lead to two opposing outcomes—stress adaptation or stress-induced damage?
2. Stress and Strain in Physics and Engineering: A Foundational Perspective
3. Cannon’s Homeostasis: Introducing the Principle of Balance to Life Sciences
4. Hans Selye’s Original Concept
4.1. Selye’s Concept of Stress
- (1)
- Stress is the nonspecific response of the body to any demand placed upon it.
- (2)
- Stress is inevitable. To be entirely without stress is to be dead!
- (3)
- Stress is not the nonspecific result of damage.
- (4)
- Stress is not something to be avoided.
4.2. Stress as a Biological State: Refinements
- (1)
- The Alarm Stage, characterized by an initial acute response, often exemplified by symptoms like fever during infections.
- (2)
- The Resistance Stage, where adaptation mechanisms stabilize physiological functions.
- (3)
- The Exhaustion Stage, where prolonged or excessive stress exceeds the body’s adaptive capacity, potentially leading to dysfunction or disease.

5. Levitt’s Concepts of Stress and Strain in Plants
5.1. Plant Growth and Environment
5.2. Introduction of Stress and Strain to Plant Science
6. Lichtenthaler’s Application of Chlorophyll Fluorescence as a Marker of Plant Stress
6.1. Four Stages of Plant Stress Responses
6.2. Chlorophyll Fluorescence as a Measure of Stress in Plants
6.3. Broad Applications and Its Limitations
7. Sies’s Concept of Oxidative Stress and Redox Biology
7.1. Oxygen Toxicity
7.2. Oxidative Stress as an Imbalance Between Oxidants and Antioxidants
8. Photooxidative Stress in Plants: The Origin of Oxygen Toxicity
8.1. Oxygenic Photosynthesis as the Origin of Oxygen Toxicity
8.2. Plant Antioxidant Systems and Human Health
8.3. Unification of Plant and Animal Stress Responses by Oxidative Stress
9. The Expanding Universe of Redox Biology
9.1. Updating Oxidative Stress: Integration of Reactive Nitrogen Species (RNS) and Reactive Sulfur Species (RSS)
9.2. Integration of Reactive Nitrogen Species (RNS)
9.2.1. Nitric Oxide (NO) as a Signaling Molecule
9.2.2. Alternative Mechanisms of NO Production
9.3. The Expanding Roles of Reactive Sulfur Species (RSS)
9.3.1. H2S as the Third Gasotransmitter
9.3.2. Endogenous H2S Production in Plants and Animals
9.3.3. Plant-Derived Sulfur Compounds and Human Health
9.4. The Interplay Among ROS, RNS, and RSS
9.4.1. O2-NO-H2S (ONS)
9.4.2. Cysteine Thiol at the Crossroad of Redox Interactions
10. The Three-Body Problem in Stress Biology
10.1. The Dynamic Interplay Among ROS, RNS, and RSS
10.2. Analogy to the Three-Body Problem in Physics
10.3. Broader Implications of the Redox Triad in Stress-Related Diseases
11. Ecological Perspectives: Stress and Neurodegenerative Diseases
11.1. Environmental Stressors and Neurodegeneration
11.2. Natural Neurotoxin BMAA: Linking Disease and Environment
11.3. BMAA and Cyanobacterial Blooms: Global Health Implications
12. The Minimum Machinery for Nonspecific Stress Response
12.1. Multi-Sensitivity of NMDARs
12.2. GLRs in Plants
12.3. TRP Superfamily
12.4. TRP Channels in Response to ONS
12.5. The Minimum Machinery for Selye’s “Filter” Function
13. Updating Selye’s General Adaptation Syndrome (GAS) Model
13.1. O2–NO–H2S (ONS) Homeostasis Model
13.2. Hypoxia: A Distinctive O2–NO–H2S Balance
13.3. Involvement of NO and H2S Productions in Hypoxic Adaptation
14. The Concept of Balance Behind the Opposing Nature of Contributors to the Stress Response: Philosophical Implications
14.1. The Dynamic Harmony of Two Opposites
14.2. The Yin–Yang Principle in Modern Science
14.3. The Balance of Threefold Elements: Evolution of the Yin–Yang Principle
15. Bridging Modern Science and Traditional Medicines While Emphasizing Balance
15.1. Traditional Eastern Medicines
15.2. Acupoints and Meridians
15.3. NO Generation at Acupoints
15.4. Stimulation to a Minimum Machinery
16. Future Perspectives
16.1. O2–NO–H2S (ONS) Dynamics from Physiological, Ecological, and Evolutionary Perspectives

16.2. Special Solutions to the Three-Body Problem in Stress Biology
16.3. The Search for Missing Links
17. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAS | General Adaption Syndrome |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| RSS | Reactive Sulfur Species |
| RONSS | Reactive Oxygen, Nitrogen, and Sulfur Species |
| iGluR | Ionotropic Glutamate Receptors |
| NMDAR | N-methyl-D-aspartate Receptor |
| GLR | Glutamate-Receptor-Like Channel |
| TRP | Transient Receptor Potential |
References
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. A syndrome produced by diverse nocuous agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, aat5314. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Dimsdale, J.E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 2008, 51, 1237–1246. [Google Scholar] [CrossRef]
- Lazarus, R. From psychological stress to the emotions: A history of changing outlooks. Annu. Rev. Psychol. 1993, 44, 1–21. [Google Scholar] [CrossRef]
- Selye, H. What is stress? Metabolism 1955, 5, 525–530. [Google Scholar]
- Selye, H. Stress and disease. Science 1955, 122, 625–631. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.N. Stress hypothesis overload: 131 hypotheses exploring the role of stress in tradeoffs, transitions, and health. Gen. Comp. Endocrinol. 2020, 288, 113355. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Wubshet, N.H.; Cai, G.; Chen, S.J.; Sullivan, M.; Reeves, M.; Mays, D.; Harrison, M.; Varnado, P.; Yang, B.; Arreguin-Martinez, E.; et al. Cellular mechanotransduction of human osteoblasts in microgravity. NPJ Microgravity 2024, 10, 35. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kang, J.H. Mechanical properties of compact bone defined by the stress-strain curve measured using uniaxial tensile test: A concise review and practical guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef]
- Rodrigo-Navarro, A.; Sankaran, S.; Dalby, M.J.; del Campo, A.; Salmeron-Sanchez, M. Engineered living biomaterials. Nat. Rev. Mater. 2021, 6, 1175–1190. [Google Scholar] [CrossRef]
- Yokoyama, M.; Gril, J.; Matsuo, M.; Yano, H.; Sugiyama, J.; Clair, B.; Kubodera, S.; Mistutani, T.; Sakamoto, M.; Ozaki, H.; et al. Mechanical characteristics of aged Hinoki wood from Japanese historical buildings. Comptes Rendus Phys. 2009, 10, 601–611. [Google Scholar] [CrossRef]
- Baloh, R.W. Biological mechanisms of psychosomatic symptoms. In Medically Unexplained Symptoms: A Brain-Centered Approach; Baloh, R.W., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 81–98. [Google Scholar]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Bracha, H.S.; Ralston, T.C.; Matsukawa, J.M.; Williams, A.E.; Bracha, A.S. Does “fight or flight” need updating? Psychosom 2004, 45, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B. The Wisdom of the Body; W. W. Norton and Company, Inc.: New York, NY, USA, 1932. [Google Scholar]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear, and Rage: An Account of Recent Researches into the Function of Emotional Excitement; D. Appleton and Company: New York, NY, USA, 1915. [Google Scholar]
- Tan, S.Y.; Yip, A. Hans Selye (1907–1982): Founder of the stress theory. Singap. Med. J. 2018, 59, 170–171. [Google Scholar] [CrossRef]
- Szabo, S.; Tache, Y.; Somogyi, A. The legacy of Hans Selye and the origins of stress research: A retrospective 75 years after his landmark brief “letter” to the editor# of nature. Stress 2012, 15, 472–478. [Google Scholar]
- Selye, H. The evolution of the stress concept: The originator of the concept traces its development from the discovery in 1936 of the alarm reaction to modern therapeutic applications of syntoxic and catatoxic hormones. Am. Sci. 1973, 61, 692–699. [Google Scholar] [PubMed]
- Selye, H.; Szent-Györgyi, A. In Vivo: The Case for Supramolecular Biology: Presented in Six Informal, Illustrated Lectures; Liveright Publishing Corporation: New York, NY, USA, 1967. [Google Scholar]
- Mason, J.W. A re-evaluation of the concept of ‘non-specificity’in stress theory. J. Psyiatr Res. 1972, 8, 323–333. [Google Scholar]
- Hariom, S.K.; Ravi, A.; Mohan, G.R.; Pochiraju, H.D.; Chattopadhyay, S.; Nelson, E.J.R. Animal physiology across the gravity continuum. Acta Astronaut. 2021, 178, 522–535. [Google Scholar] [CrossRef]
- Mahdi, S.H.A.; Yamasaki, H.; Otaki, J.M. Heat-shock-induced color-pattern changes of the blue pansy butterfly Junonia orithya: Physiological and evolutionary implications. J. Therm. Biol. 2011, 36, 312–321. [Google Scholar] [CrossRef]
- Mahdi, S.H.; Gima, S.; Tomita, Y.; Yamasaki, H.; Otaki, J.M. Physiological characterization of the cold-shock-induced humoral factor for wing color-pattern changes in butterflies. J. Insect Physiol. 2010, 56, 1022–1031. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Levitt, J. Responses of Plants to Environmental Stresses, 2nd ed.; Academic Press: New York, NY, USA, 1980; Volume 1. [Google Scholar]
- Cohen, M.F.; Mazzola, M.; Yamasaki, H. Nitric oxide research in agriculture; bridging the plant and bacterial realms. In Abiotic Stress Tolerance in Plants; Rai, A.K., Takabe, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 71–90. [Google Scholar]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Fifty-five years of research on photosynthesis, chloroplasts, and stress physiology of plants: 1958–2013. In Progress in Botany; Springer: Cham, Switzerland, 2015; pp. 3–42. [Google Scholar]
- Lichtenthaler, H.K. The stress concept in plants: An introduction. Ann. N. Y. Acad. Sci. 1998, 851, 187–198. [Google Scholar] [CrossRef]
- Schreiber, U.; Lichtenthaler, H. Hans Kautsky’s groundbreaking discovery(ies) in 1931, its scientific environment, and the ensuing developments. Photosynthetica 2025, 63, 20–28. [Google Scholar] [CrossRef]
- Murata, N. Control of excitation transfer in photosynthesis I. Light-induced change of chlorophyll a fluoresence in Porphyridium cruentum. Biochim. Biophys. Acta 1969, 172, 242–251. [Google Scholar] [CrossRef]
- Murata, N. The discovery of state transitions in photosynthesis 40 years ago. Photosynth. Res. 2009, 99, 155–160. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Septiana, A.; Nakamura, S.P.; Naomasa, R.F.; Yamasaki, H. Seawater tolerance of the beach bean Vigna marina (Burm.) Merrill in comparison with mung bean (Vigna radiata) and adzuki bean (Vigna angularis). Agriculture 2025, 15, 228. [Google Scholar] [CrossRef]
- Hossain, K.K.; Nakamura, T.; Yamasaki, H. Effect of nitric oxide on leaf non-photochemical quenching of fluorescence under heat-stress conditions. Russ. J. Plant Physiol. 2011, 58, 629–633. [Google Scholar] [CrossRef]
- Buonasera, K.; Lambreva, M.; Rea, G.; Touloupakis, E.; Giardi, M.T. Technological applications of chlorophyll a fluorescence for the assessment of environmental pollutants. Anal. Bioanal. Chem. 2011, 401, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.; Yoshida, Y.; Vasilkov, A.P.; Yoshida, Y.; Corp, L.A.; Middleton, E.M. First observations of global and seasonal terrestrial chlorophyll fluorescence from space. Biogeosciences 2011, 8, 637–651. [Google Scholar] [CrossRef]
- Guanter, L.; Zhang, Y.; Jung, M.; Joiner, J.; Voigt, M.; Berry, J.A.; Frankenberg, C.; Huete, A.R.; Zarco-Tejada, P.; Lee, J.E.; et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc. Natl. Acad. Sci. USA 2014, 111, E1327–E1333. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakamura, T.; Sakamizu, M.; van Woesik, R.; Yamasaki, H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 2004, 45, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Campo, K.; Baums, I.B. Fitted Fv/Fm temperature response curves: Applying lessons from plant ecophysiology to acute thermal stress experiments in coral holobionts. Coral Reefs 2024, 44, 77–84. [Google Scholar] [CrossRef]
- Hoadley, K.D.; Lockridge, G.; McQuagge, A.; Pahl, K.B.; Lowry, S.; Wong, S.; Craig, Z.; Petrik, C.; Klepac, C.; Muller, E.M. A phenomic modeling approach for using chlorophyll-a fluorescence-based measurements on coral photosymbionts. Front. Mar. Sci. 2023, 10, 1092202. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Alvarez-Noriega, M.; Alvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- van Woesik, R.; Shlesinger, T.; Grottoli, A.G.; Toonen, R.J.; Vega Thurber, R.; Warner, M.E.; Marie Hulver, A.; Chapron, L.; McLachlan, R.H.; Albright, R.; et al. Coral-bleaching responses to climate change across biological scales. Glob. Change Biol. 2022, 28, 4229–4250. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef]
- Sies, H.; Chance, B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970, 11, 172–176. [Google Scholar] [CrossRef]
- Sies, H. Findings in redox biology: From H2O2 to oxidative stress. J. Biol. Chem. 2020, 295, 13458–13473. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and x-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef]
- Sakihama, Y.; Yamasaki, H. Phytochemical antioxidants: Past, present and future. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engle 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Li, Y.R.; Cai, Z.M.; Li, S.H.; Zhu, J.F.; Zhang, F.; Liang, S.S.; Zhang, W.W.; Guan, Y.L.; Shen, D.Q.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Tas, F.; Hansel, H.; Belce, A.; Ilvan, S.; Argon, A.; Camlica, H.; Topuz, E. Oxidative stress in breast cancer. Med. Oncol. 2005, 22, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Siegrist, J.; Sies, H. Disturbed redox homeostasis in oxidative distress: A molecular link from chronic psychosocial work stress to coronary heart disease? Circ. Res. 2017, 121, 103–105. [Google Scholar] [CrossRef]
- Sanchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef]
- Hamilton, T.L. The trouble with oxygen: The ecophysiology of extant phototrophs and implications for the evolution of oxygenic photosynthesis. Free Radic. Biol. Med. 2019, 140, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Yamasaki, H.; Nishimura, M. Non-vectorial light-induced H+ release from CF1-depleted thylakoid membranes. Absence of correlation to stoichiometric H+ production and consumption coupled to electron transfer. Plant Cell Physiol. 1988, 29, 1081–1084. [Google Scholar]
- Mehler, A.H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch. Biochem. Biophys. 1951, 33, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef]
- Takahama, U.; Nishimura, M. Formation of singlet molecular oxygen in illuminated chloroplasts. Effects on photoinactivation and lipid peroxidation. Plant Cell Physiol. 1975, 16, 737–748. [Google Scholar]
- Takahama, U.; Nishimura, M. Effects of electron donor and acceptors, electron transfer mediators, and superoxide dismutase on lipid peroxidation in illuminated chloroplast fragments. Plant Cell Physiol. 1976, 17, 111–118. [Google Scholar] [CrossRef]
- Takahama, U.; Takahashi, K. Suppression of lipid peroxidation by β-carotene in illuminated chloroplast fragments: Evidence for β-carotene as a quencher of singlet molecular oxygen in chloroplasts. Plant Cell Physiol. 1978, 19, 1565–1569. [Google Scholar]
- Kaiser, W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim. Biophys. Acta 1976, 440, 476–482. [Google Scholar] [CrossRef]
- Kaiser, W.M. Reversible inhibition of the calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta 1979, 145, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; De Gara, L.; Asard, H.; Horemans, N. Ascorbate and glutathione: Guardians of the cell cycle, partners in crime? Plant Physiol. Biochem. 2002, 40, 537–548. [Google Scholar] [CrossRef]
- Chew, O.; Whelan, J.; Millar, A.H. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003, 278, 46869–46877. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 360203. [Google Scholar] [CrossRef]
- Yamasaki, H.; Uefuji, H.; Sakihama, Y. Bleaching of the red anthocyanin induced by superoxide radical. Arch. Biochem. Biophys. 1996, 332, 183–186. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Yamasaki, H. A function of colour. Trends Plant Sci. 1997, 2, 7–8. [Google Scholar] [CrossRef]
- Takahashi, S.; Tamashiro, A.; Sakihama, Y.; Yamamoto, Y.; Kawamitsu, Y.; Yamasaki, H. High-susceptibility of photosynthesis to photoinhibition in the tropical plant Ficus microcarpa L. f. cv. Golden Leaves. BMC Plant Biol. 2002, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Ferris, B.G., Jr. Health effects of exposure to low levels of regulated air pollutants: A critical review. J. Air Pollut. Control Assoc. 1978, 28, 482–497. [Google Scholar] [CrossRef]
- Yamasaki, H. Nitrite–dependent nitric oxide production pathway: Implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1477–1488. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 2002, 53, 503–514. [Google Scholar]
- Furchgott, R.F. Endothelium-derived relaxing factor: Discovery, early studies, and identifcation as nitric oxide (nobel lecture). Angew. Chem. Int. Ed. 1999, 38, 1870–1880. [Google Scholar] [CrossRef]
- Davis, K.L.; Martin, E.; Turko, I.V.; Murad, F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Konstadt, S. Nitric oxide: Has it progressed from molecule of the year to wonder drug of the decade. J. Cardiothorac. Vasc. Anesth. 1995, 9, 625–626. [Google Scholar] [CrossRef]
- Jackson, G.; Gillies, H.; Osterloh, I. Past, present, and future: A 7-year update of Viagra (sildenafil citrate). Int. J. Clin. Pract. 2005, 59, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.E.; Ohlsson, A.; Shah, P.S. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst. Rev. 2017, 8, CD005494. [Google Scholar] [CrossRef]
- Safaee Fakhr, B.; Wiegand, S.B.; Pinciroli, R.; Gianni, S.; Morais, C.C.A.; Ikeda, T.; Miyazaki, Y.; Marutani, E.; Di Fenza, R.; Larson, G.M.; et al. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 1109–1113. [Google Scholar] [CrossRef]
- Oliynyk, O.V.; Rorat, M.; Strepetova, O.V.; Dubrov, S.O.; Guryanov, V.G.; Oliynyk, Y.V.; Kulivets, O.S.; Slifirczyk, A.; Barg, W. Efficacy of sildenafil in patients with severe COVID-19 and pulmonary arterial hypertension. Viruses 2023, 15, 1157. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef]
- Travis, J. NO-making enzyme no more: Cell, PNAS papers retracted. Science 2004, 306, 960. [Google Scholar] [PubMed]
- Zemojtel, T.; Fröhlich, A.; Palmieri, M.C.; Kolanczyk, M.; Mikula, I.; Wyrwicz, L.S.; Wanker, E.E.; Mundlos, S.; Vingron, M.; Martasek, P. Plant nitric oxide synthase: A never-ending story? Trends Plant Sci. 2006, 11, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.; Galli, M.; Tischner, R.; Heimer, Y.; Okamoto, M.; Mack, A. Response to Zemojtel et al.: Plant nitric oxide synthase: Back to square one. Trends Plant Sci. 2006, 11, 526–527. [Google Scholar] [CrossRef]
- Moreau, M.; Lee, G.I.; Wang, Y.; Crane, B.R.; Klessig, D.F. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 2008, 283, 32957–32967. [Google Scholar] [CrossRef]
- Frohlich, A.; Durner, J. The hunt for plant nitric oxide synthase (NOS): Is one really needed? Plant Sci. 2011, 181, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Cohen, M.F. NO signal at the crossroads: Polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 2006, 11, 522–524. [Google Scholar] [CrossRef]
- Yamasaki, H.; Itoh, R.D.; Bouchard, J.N.; Dghim, A.A.; Hossain, K.K.; Gurung, S.; Cohen, M.F. Nitric oxide synthase-like activities in plants. In Annual Plant Reviews; Foyer, C., Zhang, H., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; Volume 42, pp. 103–125. [Google Scholar]
- Yamasaki, H.; Sakihama, Y.; Takahashi, S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 1999, 4, 128–129. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar]
- Sakihama, Y.; Nakamura, S.; Yamasaki, H. Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 2002, 43, 290–297. [Google Scholar]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Desikan, R.; Griffiths, R.; Hancock, J.; Neill, S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 16314–16318. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Hancock, J.T. Nitric oxide signalling in plants. New Phytol. 2003, 159, 11–35. [Google Scholar] [CrossRef]
- Stoimenova, M.; Libourel, I.G.L.; Ratcliffe, R.G.; Kaiser, W.M. The role of nitrate reduction in the anoxic metabolism of roots II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 2003, 253, 155–167. [Google Scholar] [CrossRef]
- Stöhr, C.; Stremlau, S. Formation and possible roles of nitric oxide in plant roots. J. Exp. Bot. 2006, 57, 463–470. [Google Scholar] [CrossRef]
- Cohen, M.F.; Lamattina, L.; Yamasaki, H. Nitric oxide signaling by plant-associated bacteria. In Nitric Oxide in Plant Physiology; Hayat, M., Mori, J., Pichtel, J., Ahamad, A., Eds.; Wiley-VCH: Weinheim, Germany, 2010; pp. 161–172. [Google Scholar]
- Gladwin, M.T.; Crawford, J.H.; Patel, R.P. The biochemistry of nitric oxide, nitrite, and hemoglobin: Role in blood flow regulation. Free Radic. Biol. Med. 2004, 36, 707–717. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Bender, D.; Schwarz, G. Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett. 2018, 592, 2126–2139. [Google Scholar] [CrossRef]
- Webb, A.J.; Milsom, A.B.; Rathod, K.S.; Chu, W.L.; Qureshi, S.; Lovell, M.J.; Lecomte, F.M.; Perrett, D.; Raimondo, C.; Khoshbin, E.; et al. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: Role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008, 103, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, A.; Jensen, B.S.; Jensen, F.B.; Fago, A. Exploring pathways of NO and H2S signaling in metabolic depression: The case of anoxic turtles. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2021, 253, 110857. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Shimoji, H.; Ohshiro, Y.; Sakihama, Y. Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide 2001, 5, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Metzen, E.; Zhou, J.; Jelkmann, W.; Fandrey, J.; Brüne, B. Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol. Biol. Cell 2003, 14, 3470–3481. [Google Scholar] [CrossRef]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef]
- Modin, A.; Bjorne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Yamasaki, H.; Watanabe, N.S.; Fukuto, J.; Cohen, M.F. Nitrite-dependent nitric oxide production pathway: Diversity of NO production systems. In Studies on Pediatric Disorders; Tsukahara, H., Kaneko, K., Eds.; Springer: New York, NY, USA, 2014; pp. 35–54. [Google Scholar]
- Watanabe, N.S.; Yamasaki, H. Dynamics of nitrite content in fresh spinach leaves: Evidence for nitrite formation caused by microbial nitrate reductase activity. J. Nutr. Food Sci. 2017, 7, 1000572. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef]
- Yamasaki, H.; Imai, H.; Tanaka, A.; Otaki, J.M. Pleiotropic functions of nitric oxide produced by ascorbate for the prevention and mitigation of COVID-19: A revaluation of Pauling’s vitamin C therapy. Microorganisms 2023, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.D.; Wu, L.Y.; Jiang, B.; Yang, W.; Qi, J.S.; Cao, K.; Meng, Q.H.; Mustafa, A.K.; Mu, W.T.; Zhang, S.M.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Yamasaki, H.; Cohen, M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide 2016, 55–56, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide (H2S)/Polysulfides (H2Sn) signalling and TRPA1 channels modification on sulfur metabolism. Biomolecules 2024, 14, 129. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Wozniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Moraga, J.M.Z.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 2013, 36, 63–71. [Google Scholar] [CrossRef]
- Yamasaki, H.; Itoh, R.D.; Mizumoto, K.B.; Yoshida, Y.S.; Otaki, J.M.; Cohen, M.F. Spatiotemporal characteristics determining the multifaceted nature of reactive oxygen, nitrogen, and sulfur species in relation to proton homeostasis. Antioxid. Redox Signal 2025, 42, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Attia, S.M.; Al-Harbi, N.O.; Alzahrani, K.S.; Bakheet, S.A. Differential regulation of Nrf2 is linked to elevated inflammation and nitrative stress in monocytes of children with autism. Psychoneuroendocrinology 2020, 113, 104554. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gonzalez-Gordo, S.; Munoz-Vargas, M.A.; Rodriguez-Ruiz, M.; Palma, J.M. The Modus Operandi of hydrogen sulfide (H2S)-dependent protein persulfidation in higher plants. Antioxid 2021, 10, 1686. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Merchant, K.; Nudelman, R.; Beyer, W.F.; Keng, T.; DeAngelo, J.; Hausladen, A.; Stamler, J.S. OxyR: A molecular code for redox-related signaling. Cell 2002, 109, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Houk, K.N.; Foote, C.S.; Fukuto, J.M.; Ignarro, L.J.; Squadrito, G.L.; Davies, K.J. Free radical biology and medicine: It’s a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R491–R511. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Butler, A.R.; Woollins, J.D.; Feelisch, M. Inorganic sulfur-nitrogen compounds: From gunpowder chemistry to the forefront of biological signaling. Dalton Trans. 2016, 45, 5908–5919. [Google Scholar] [CrossRef]
- Fra, A.; Yoboue, E.D.; Sitia, R. Cysteines as redox molecular switches and targets of disease. Front. Mol. Neurosci. 2017, 10, 167. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.T.; Gazi, S.K.; Barrow, R.K.; Yang, G.D.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Manta, B.; Botti, H.; Radi, R.; Trujillo, M.; Denicola, A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 2011, 24, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and reactivity of cysteine persulfides in signaling. J. Am. Chem. Soc. 2016, 138, 289–299. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Giuffre, A.; Vicente, J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef]
- Musielak, Z.E.; Quarles, B. The three-body problem. Rep. Prog. Phys. 2014, 77, 065901. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.Y.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Nat. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Schulz, R.; Kelm, M.; Heusch, G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 61, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, D.; Glavnik, N.; Marinsek, M.; Zagozen, P.; Rovan, K.; Goslar, T.; Mars, T.; Podbregar, M. Total plasma sulfide in congestive heart failure. J. Card. Fail. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Edelstein, C.; Gengaro, P.; Meng, X.H.; Lucia, S.; Knotek, M.; Wangsiripaisan, A.; Shi, Y.X.; Schrier, R. Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am. J. Physiol. Ren. Physiol. 1999, 277, F383–F390. [Google Scholar] [CrossRef]
- Ahmad, F.U.D.; Sattar, M.A.; Rathore, H.A.; Abdullah, M.H.; Tan, S.; Abdullah, N.A.; Johns, E.J. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Ren. Fail. 2012, 34, 203–210. [Google Scholar] [CrossRef]
- Ueda, J.; Tsuji, A.; Aoki, T.; Asano, R.; Kiko, T.; Hayashi, H.; Endo, H.; Nishi, N.; Takano, R.; Fujisaki, S.; et al. Phase 2 study to evaluate the efficacy and safety of inhaled nitric oxide therapy in patients with severe right heart failure associated with pulmonary hypertension. Circ. Rep. 2025, 7, 308–312. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, S.; Pan, Z.; Jiang, S.; Fan, J.; Yu, S.; Xue, L.; Yang, J.; Ma, S.; Liu, T.; et al. Hydrogen sulfide alleviates particulate matter-induced emphysema and airway inflammation by suppressing ferroptosis. Free Radic. Biol. Med. 2022, 186, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, K.; Li, M.X.; He, W.; Chang, J.R.; Liao, C.C.; Lin, F.; Qi, Y.F.; Wang, R.; Chen, Y.H. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci. Rep. 2015, 5, 14971. [Google Scholar] [CrossRef]
- Pae, H.O.; Lee, Y.C.; Jo, E.K.; Chung, H.T. Subtle interplay of endogenous bioactive gases (NO, CO and H2S) in inflammation. Arch. Pharm. Res. 2009, 32, 1155–1162. [Google Scholar] [CrossRef]
- Whiteman, M.; Haigh, R.; Tarr, J.M.; Gooding, K.M.; Shore, A.C.; Winyard, P.G. Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: Relation to clinical and laboratory measures of inflammation. Ann. N. Y. Acad. Sci. 2010, 1203, 146–150. [Google Scholar] [CrossRef]
- Kloesch, B.; Liszt, M.; Krehan, D.; Broell, J.; Kiener, H.; Steiner, G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012, 141, 197–203. [Google Scholar] [CrossRef]
- Li, L.; Hsu, A.; Moore, P.K. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation—A tale of three gases! Pharmacol. Ther. 2009, 123, 386–400. [Google Scholar] [CrossRef]
- Kamalian, A.; Asl, M.S.; Dolatshahi, M.; Afshari, K.; Shamshiri, S.; Roudsari, N.M.; Momtaz, S.; Rahimi, R.; Abdollahi, M.; Abdolghaffari, A.H. Interventions of natural and synthetic agents in inflammatory bowel disease, modulation of nitric oxide pathways. World J. Gastroenterol. 2020, 26, 3365–3400. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lu, H.Y.; Zhou, Q.; Chen, D.F.; Zeng, Y.Y.; Shi, J.Y.; Zhang, Y.M.; Wang, X.W.; Shen, X.K.; Cai, X.J. H2S-releasing versatile montmorillonite nanoformulation trilogically renovates the gut microenvironment for inflammatory bowel disease modulation. Adv. Sci. 2024, 11, e2308092. [Google Scholar] [CrossRef]
- Vieira, N.F.; Barbosa, T.P.; Cárnio, E.C. Performance of nitric oxide in sepsis: A scoping review. Acta Paul. Enferm. 2024, 37, eAPE00512. [Google Scholar] [CrossRef]
- Cao, G.D.; Zeng, Y.C.; Zhao, Y.H.; Liang, L.; Luo, X.Q.; Guo, L.C.; Zhang, Y.X.; Cheng, Q.H. H2S regulation of ferroptosis attenuates sepsis-induced cardiomyopathy. Mol. Med. Rep. 2022, 26, 335. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Dawoud, A.; Youness, R.A.; Nafea, H.; Manie, T.; Bourquin, C.; Szabo, C.; Abdel-Kader, R.M.; Gad, M.Z. Pan-inhibition of the three H2S synthesizing enzymes restrains tumor progression and immunosuppression in breast cancer. Cancer Cell Int. 2024, 24, 136. [Google Scholar] [CrossRef]
- Malicka, I.; Martynowicz, H.; Dziegiel, P.; Podhorska-Okolow, M.; Wozniewski, M.; Szuba, A. Asymmetric dimethylarginine in an NMU-induced rat mammary tumor model. Anticancer Res. 2023, 43, 97–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Deng, Y.; Ye, C.; Peng, H. HIF-1alpha signaling: Essential roles in tumorigenesis and implications in targeted therapies. Genes Dis. 2024, 11, 234–251. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Modis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-ß-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide 2017, 70, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Katsouda, A.; Szabo, C.; Papapetropoulos, A. Reduced adipose tissue H2S in obesity. Pharmacol. Res. 2018, 128, 190–199. [Google Scholar] [CrossRef]
- Pokorski, M.; Pozdzik, M.; Mazzatenta, A. Antioxidant treatment for impaired hypoxic ventilatory responses in experimental diabetes in the rat. Respir. Physiol. Neurobiol. 2018, 255, 30–38. [Google Scholar] [CrossRef]
- Beltowski, J.; Wojcicka, G.; Jamroz-Wisniewska, A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: Implications for the pathogenesis and treatment of diabetes mellitus. Biochem. Pharmacol. 2018, 149, 60–76. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.S.; Katusic, Z.S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef]
- Vandini, E.; Ottani, A.; Zaffe, D.; Calevro, A.; Canalini, F.; Cavallini, G.M.; Rossi, R.; Guarini, S.; Giuliani, D. Mechanisms of hydrogen sulfide against the progression of severe Alzheimer’s disease in transgenic mice at different ages. Pharmacology 2019, 103, 50–60. [Google Scholar] [CrossRef]
- Kim, J.; Han, J.Y.; Lee, Y.; Kim, K.; Choi, Y.P.; Chae, S.; Hoe, H.S. Genetic deletion of nitric oxide synthase 2 ameliorates Parkinson’s disease pathology and neuroinflammation in a transgenic mouse model of synucleinopathy. Mol. Brain 2023, 16, 7. [Google Scholar] [CrossRef]
- Cao, X.; Cao, L.; Ding, L.; Bian, J.S. A new hope for a devastating disease: Hydrogen sulfide in Parkinson’s disease. Mol. Neurobiol. 2018, 55, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 273283. [Google Scholar] [CrossRef]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2014, 275, 229–250. [Google Scholar] [CrossRef]
- Ince, P.G.; Codd, G.A. Return of the cycad hypothesis—Does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol. Appl. Neurobiol. 2005, 31, 345–353. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Heng, B.; Lane, D.J.R.; Bush, A.I.; Guillemin, G.J.; Tan, V.X. Neuropathological mechanisms of β-N-methylamino-L-alanine (BMAA) with a focus on iron overload and ferroptosis. Neurotox. Res. 2022, 40, 614–635. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, A.R.; Mortimer, J.A.; Schellenberg, G.D.; Galasko, D. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology 2009, 72, 473. [Google Scholar] [CrossRef]
- Borenstein, A.; Mortimer, J.; Schofield, E.; Wu, Y.; Salmon, D.; Gamst, A.; Olichney, J.; Thal, L.; Silbert, L.; Kaye, J. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology 2007, 68, 1764–1771. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Oyanagi, K. The nature of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam and magnesium deficiency. Park. Relat. Disord. 2005, 11 (Suppl. 1), S17–S23. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Pengelly, J.J.; Cuddy, W.S.; Fieker, C.; Forster, P.I.; Neilan, B.A. Host selection of symbiotic cyanobacteria in 31 species of the Australian cycad genus: Macrozamia (Zamiaceae). Mol. Plant Microbe Interact. 2010, 23, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.F.; Sakihama, Y.; Takagi, Y.C.; Ichiba, T.; Yamasaki, H. Synergistic effect of deoxyanthocyanins from symbiotic fern Azolla spp. on hrmA gene induction in the cyanobacterium Nostoc punctiforme. Mol. Plant Microbe Interact. 2002, 15, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.P.; Pereira, A.L.; Azevedo, J.; Bujak, A.A.; Leshyk, V.; Pham Gia, M.; Stadtlander, T.; Vasconcelos, V.; Winstead, D.J. Azolla as a safe food: Suppression of cyanotoxin-related genes and cyanotoxin production in Its symbiont, Nostoc azollae. Plants 2024, 13, 2707. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Peters, S.J.; Mitrovic, S.M.; Rodgers, K.J.; Bishop, D.P. Bioaccumulation of β-methylamino-L-alanine (BMAA) by mussels exposed to the cyanobacteria Microcystis aeruginosa. Environ. Pollut. 2024, 363, 125081. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Elnour, R.O.; Alamri, S.; Hashem, M.; Alshehri, A.M.; Campos, A.; Vasconcelos, V.; Badawye, H. Occurrence of β-N-methylamino-L-alanine (BMAA) toxin in irrigation water and field vegetable plants and assessing its potential risk to human health. Water Air Soil Pollut. 2024, 235, 72. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, W.R.; Liu, Z.Q.; Lin, T.F. Reaction pathways and kinetics of a cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) during chlorination. Environ. Sci. Technol. 2017, 51, 1303–1311. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Popova, A.A.; Rasmussen, U.; Semashko, T.A.; Govorun, V.M.; Koksharova, O.A. Stress effects of cyanotoxin β-methylamino-L-alanine (BMAA) on cyanobacterial heterocyst formation and functionality. Environ. Microbiol. Rep. 2018, 10, 369–377. [Google Scholar] [CrossRef]
- Downing, S.; Banack, S.; Metcalf, J.; Cox, P.; Downing, T. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef]
- Koksharova, O.A.; Butenko, I.O.; Pobeguts, O.V.; Safronova, N.A.; Govorun, V.M. ß-N-methylamino-L-alanine (BMAA) causes severe stress in Nostoc sp. PCC 7120 cells under diazotrophic conditions: A proteomic study. Toxins 2021, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Steele, J.R.; Phillips, C.R.; Violi, J.P.; Rodgers, K.J. The cyanobacterial toxins BMAA and 2,4-DAB perturb the L-serine biosynthesis pathway and induce systemic changes in energy metabolism in human neuroblastoma cells: A proteomic study. Toxicol. Vitr. 2025, 106, 106058. [Google Scholar] [CrossRef] [PubMed]
- Lobner, D.; Piana, P.M.T.; Salous, A.K.; Peoples, R.W. β-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Pierozan, P.; Piras, E.; Brittebo, E.; Karlsson, O. The cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) targets the olfactory bulb region. Arch. Toxicol. 2020, 94, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Kamiya, H.; Tsuzuki, K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 1998, 54, 581–618. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutarnatergic system—Too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Liu, L.D.; Wong, T.P.; Pozza, M.F.; Lingenhoehl, K.; Wang, Y.S.; Sheng, M.; Auberson, Y.P.; Wang, Y.T. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 2004, 304, 1021–1024. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry 2004, 9, 979+984–997. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Hartley, M.; Heinemann, S.F. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 1995, 268, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Kotermanski, S.E.; Johnson, J.W. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J. Neurosci. 2009, 29, 2774–2779. [Google Scholar] [CrossRef]
- Stoll, L.; Hall, J.; Van Buren, N.; Hall, A.; Knight, L.; Morgan, A.; Zuger, S.; Van Deusen, H.; Gentile, L. Differential regulation of ionotropic glutamate receptors. Biophys. J. 2007, 92, 1343–1349. [Google Scholar] [CrossRef]
- Mothet, J.P.; Parent, A.T.; Wolosker, H.; Brady, R.O.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. [Google Scholar] [CrossRef]
- Butler, T.R.; Self, R.L.; Smith, K.J.; Sharrett-Field, L.J.; Berry, J.N.; Littleton, J.M.; Pauly, J.R.; Mulholland, P.J.; Prendergast, M.A. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience 2010, 165, 525–534. [Google Scholar] [CrossRef]
- Chen, H.S.V.; Lipton, S.A. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006, 97, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10. [Google Scholar] [CrossRef]
- Avshalumov, M.V.; Rice, M.E. NMDA receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J. Neurophysiol. 2002, 87, 2896–2903. [Google Scholar] [CrossRef]
- Rauk, A. β-N-Methylamino-L-alanine (BMAA) not involved in Alzheimer’s disease. J. Phys. Chem. B 2018, 122, 4472–7780. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.N.; Omelchenko, I.; Ross, S.M.; Spencer, P. The neurotoxin, ß-N-methylamino-L-alanine (BMAA) interacts with the strychnine-insensitive glycine modulatory site of the N-methyl D-aspartate receptor. Neuropharmacology 1995, 34, 651–658. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Braidy, N.; Guillemin, G.J.; Welch, J.H.; Neilan, B.A. Excitotoxic potential of the cyanotoxin β-methyl-amino-L-alanine (BMAA) in primary human neurons. Toxicon 2012, 60, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gao, Q.F.; Yang, M.; Shao, Q.L.; Xu, X.P.; Zhang, Y.B.; Luan, S. Ca2+/calmodulin-mediated desensitization of glutamate receptors shapes plant systemic wound signalling and anti-herbivore defence. Nat. Plants 2024, 10, 145–160. [Google Scholar] [CrossRef]
- Price, M.B.; Jelesko, J.; Okumoto, S. Glutamate receptor homologs in plants: Functions and evolutionary origins. Front. Plant Sci. 2012, 3, 235. [Google Scholar] [CrossRef]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijó, J.A. Glutamate Receptor-Like genes Form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.L.; Gao, Q.F.; Lhamo, D.; Zhang, H.S.; Luan, S. Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 2020, 13, eaba1453. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 mediate cold acclimation-induced chilling tolerance by regulating apoplastic H2O2 production and redox homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef]
- Gokce, A.; Sekmen Cetinel, A.H.; Turkan, I. Involvement of GLR-mediated nitric oxide effects on ROS metabolism in Arabidopsis plants under salt stress. J. Plant Res. 2024, 137, 485–503. [Google Scholar] [CrossRef]
- Brenner, E.D.; Martinez-Barboza, N.; Clark, A.P.; Liang, Q.S.; Stevenson, D.W.; Coruzzi, G.M. Arabidopsis mutants resistant to S (+)-β-methyl-α, β-diaminopropionic acid, a cycad-derived glutamate receptor agonist. Plant Physiol. 2000, 124, 1615–1624. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B-Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Inoue, R.; Morii, T.; Takahashi, N.; Yamamoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006, 2, 596–607. [Google Scholar] [CrossRef]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S.; Kiyonaka, S.; Takahashi, N.; Wajima, T.; Hara, Y.; Negoro, T.; Hiroi, T.; Kiuchi, Y.; Okada, T.; et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008, 14, 738–747. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Neuberger, A.; Sobolevsky, A.I. Molecular pharmacology of the onco-TRP channel TRPV6. Channels 2023, 17, 2266669. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Nadezhdin, K.D.; Zakharian, E.; Sobolevsky, A.I. Structural mechanism of TRPV3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 2021, 22, e53233. [Google Scholar] [CrossRef]
- Neuberger, A.; Trofimov, Y.A.; Yelshanskaya, M.V.; Khau, J.; Nadezhdin, K.D.; Khosrof, L.S.; Krylov, N.A.; Efremov, R.G.; Sobolevsky, A.I. Molecular pathway and structural mechanism of human oncochannel TRPV6 inhibition by the phytocannabinoid tetrahydrocannabivarin. Nat. Commun. 2023, 14, 4630. [Google Scholar] [CrossRef]
- Neuberger, A.; Trofimov, Y.A.; Yelshanskaya, M.V.; Nadezhdin, K.D.; Krylov, N.A.; Efremov, R.G.; Sobolevsky, A.I. Structural mechanism of human oncochannel TRPV6 inhibition by the natural phytoestrogen genistein. Nat. Commun. 2023, 14, 2659. [Google Scholar] [CrossRef]
- Agam, K.; von Campenhausen, M.; Levy, S.; Ben-Ami, H.C.; Cook, B.; Kirschfeld, K.; Minke, B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J. Neurosci. 2000, 20, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Persson, A.; Cheung, B.; de Bono, M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr. Biol. 2006, 16, 649–659. [Google Scholar] [CrossRef]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef]
- Kozai, D.; Ogawa, N.; Mori, Y. Redox regulation of transient receptor potential channels. Antioxid. Redox Signal 2014, 21, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Wo, Z.G.; Oswald, R.E. Unraveling the modular design of glutamate-gated ion channels. Trends Neurosci. 1995, 18, 161–168. [Google Scholar]
- Sies, H. Dynamics of intracellular and intercellular redox communication. Free Radic. Biol. Med. 2024, 225, 933–939. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Dzhalilova, D.; Makarova, O. Differences in tolerance to hypoxia: Physiological, biochemical, and molecular-biological characteristics. Biomedicines 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Shams, I.; Avivi, A.; Nevo, E. Hypoxic stress tolerance of the blind subterranean mole rat: Expression of erythropoietin and hypoxia-inducible factor 1 alpha. Proc. Natl. Acad. Sci. USA 2004, 101, 9698–9703. [Google Scholar] [CrossRef]
- Horsman, J.W.; Heinis, F.I.; Miller, D.L. A novel mechanism to prevent H2S toxicity in Caenorhabditis elegans. Genetics 2019, 213, 481–490. [Google Scholar] [CrossRef]
- Mustroph, A.; Lee, S.C.; Oosumi, T.; Zanetti, M.E.; Yang, H.; Ma, K.; Yaghoubi-Masihi, A.; Fukao, T.; Bailey-Serres, J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010, 152, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Perata, P. ERFVII transcription factors and their role in the adaptation to hypoxia in Arabidopsis and crops. Front. Genet. 2023, 14, 1213839. [Google Scholar] [CrossRef]
- Yamasaki, H.; Ogura, M.P.; Kingjoe, K.A.; Cohen, M.F. D-Cysteine-induced rapid root abscission in the water fern Azolla pinnata: Implications for the linkage between D-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants 2019, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H. Blood nitrate and nitrite modulating nitric oxide bioavailability: Potential therapeutic functions in COVID-19. Nitric Oxide 2020, 103, 29–30. [Google Scholar] [CrossRef]
- Weitzberg, E.; Hezel, M.; Lundberg, J.O. Nitrate-nitrite-nitric oxide pathway lmplications for anesthesiology and Intensive care. Anesthesiology 2010, 113, 1460–1475. [Google Scholar] [CrossRef]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate-nitrite-ntric oxide pathway: A mechanism of hypoxia and anoxia tolerance in plants. Int. J. Mol. Sci. 2022, 23, 11522. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. The therapeutic potential of hydrogen sulfide: Separating hype from hope. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R297–R312. [Google Scholar] [CrossRef]
- Dombkowski, R.A.; Russell, M.J.; Olson, K.R. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R678–R685. [Google Scholar] [CrossRef]
- Szabo, C.; Ransy, C.; Modis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef]
- Dey, A.; Prabhudesai, S.; Zhang, Y.S.; Rao, G.; Thirugnanam, K.; Hossen, M.N.; Dwivedi, S.K.D.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathione β-synthase regulates HIF-1α stability through persulfidation of PHD2. Sci. Adv. 2020, 6, eaaz8534. [Google Scholar] [CrossRef]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef] [PubMed]
- Capra, F. The Tao of Physics: An Exploration of the Parallels Between Modern Physics and Eastern Mysticism; Shambhala Publications: Boston, MA, USA, 1975. [Google Scholar]
- Edinger, E.F.; Wesley, D.A. The Aion Lectures: Exploring the Self in CG Jung’s Aion Studie; Inner City Books: Toronto, ON, Canada, 1996. [Google Scholar]
- Osmond, C. Quintessential inefficiencies of plant bioenergetics: Tales of two cultures. Funct. Plant Biol. 1995, 22, 123–129. [Google Scholar] [CrossRef]
- Society for Experimental Biology. Journal of Experimental Botany. Available online: https://academic.oup.com/jxb?login=false (accessed on 27 May 2025).
- Yamasaki, H. Nitric oxide research in plant biology: Its past and future. In Nitric Oxide SIgnaling in Higher Plants; Magakhaes, J.R., Singh, R.P., Passos, L.P., Eds.; Studium Press: Houston, TX, USA, 2004; pp. 1–23. [Google Scholar]
- Santambrogio, L.; Franco, A. The yin/yang balance of the MHC-self-immunopeptidome. Front. Immunol. 2022, 13, 1035363. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Gross, M.M.; Calleja-Agius, J.; Turner, J.D. The yin and yang of the oxytocin and stress systems: Opposites, yet interdependent and intertwined determinants of lifelong health trajectories. Front. Endocrinol. 2024, 15, 1272270. [Google Scholar] [CrossRef]
- Yamasaki, H. The NO world for plants: Achieving balance in an open system. Plant Cell Environ. 2005, 28, 78–84. [Google Scholar] [CrossRef]
- Reston, J. Now, About My Operation in Peking. The New York Times, 26 July 1971. [Google Scholar]
- Harrison, T.M.; Churgin, S.M. Acupuncture and traditional Chinese veterinary medicine in zoological and exotic animal medicine: A review and introduction of methods. Vet. Sci. 2022, 9, 74. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sato, E.F.; Inoue, M.; Asada, A. Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth. Analg. 2007, 104, 301–307. [Google Scholar] [CrossRef]
- Su, X.T.; Wang, L.; Ma, S.M.; Cao, Y.; Yang, N.N.; Lin, L.L.; Fisher, M.; Yang, J.W.; Liu, C.Z. Mechanisms of acupuncture in the regulation of oxidative stress in treating ischemic stroke. Oxid. Med. Cell Longev. 2020, 2020, 7875396. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.X.; Li, X.Y.; Sakurai, T.; Pandjaitan, M. Evidence of enhanced non-enzymatic generation of nitric oxide on the skin surface of acupuncture points: An innovative approach in humans. Nitric Oxide 2007, 17, 60–68. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y.; Xie, H.; Shu, Q.; Yang, F.; Liu, Y.L.; Liang, F.; Wang, H.; Huang, W.; Zhang, G.J. A sensitive acupuncture needle microsensor for real-time monitoring of nitric oxide in acupoints of rats. Sci. Rep. 2017, 7, 6446. [Google Scholar] [CrossRef]
- Guo, J.; Wei, T.; Huang, Q.; Li, M.; Yang, C.; Mou, J.; Shi, L.; Gao, T.; Li, G. Direct acupuncture of nitric oxide by an electrochemical microsensor with high time-space resolution. Biosens. Bioelectron. 2022, 195, 113667. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K. Cholesterol: Evolution of structure and function. In Biochemistry of Lipids, Lipoproteins and Membranes; Vance, D.E., Vance, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 20, pp. 363–381. [Google Scholar]
- Lefebvre, B.; Furt, F.; Hartmann, M.A.; Michaelson, L.V.; Carde, J.P.; Sargueil-Boiron, F.; Rossignol, M.; Napier, J.A.; Cullimore, J.; Bessoule, J.J.; et al. Characterization of lipid rafts from Medicago truncatula root plasma membranes: A proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007, 144, 402–418. [Google Scholar] [CrossRef]
- Miserocchi, G. Early endothelial signaling transduction in developing lung edema. Life 2023, 13, 1240. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids 2018, 216, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae: Structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef]
- Navarro, G.; Borroto-Escuela, D.O.; Fuxe, K.; Franco, R. Potential of caveolae in the therapy of cardiovascular and neurological diseases. Front. Physiol. 2014, 5, 370. [Google Scholar] [CrossRef]
- Anagnostopoulou, A.; Camargo, L.L.; Rodrigues, D.; Montezano, A.C.; Touyz, R.M. Importance of cholesterol-rich microdomains in the regulation of Nox isoforms and redox signaling in human vascular smooth muscle cells. Sci. Rep. 2020, 10, 17818. [Google Scholar] [CrossRef]
- Noirot, E.; Der, C.; Lherminier, J.; Robert, F.; Moricova, P.; Kieu, K.; Leborgne-Castel, N.; Simon-Plas, F.; Bouhidel, K. Dynamic changes in the subcellular distribution of the tobacco ROS-producing enzyme RBOHD in response to the oomycete elicitor cryptogein. J. Exp. Bot. 2014, 65, 5011–5022. [Google Scholar] [CrossRef]
- Shaul, P.W.; Smart, E.J.; Robinson, L.J.; German, Z.; Yuhanna, I.S.; Ying, Y.; Anderson, R.G.; Michel, T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996, 271, 6518–6522. [Google Scholar] [CrossRef]
- GarciaCardena, G.; Oh, P.; Liu, J.W.; Schnitzer, J.E.; Sessa, W.C. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc. Natl. Acad. Sci. USA 1996, 93, 6448–6453. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.M.; Martens, J.R.; Tamkun, M.M. Localization of ion channels to lipid raft domains within the cardiovascular system. Trends Cardiovasc. Med. 2004, 14, 37–42. [Google Scholar] [CrossRef]
- Angelini, C.; Morellato, A.; Alfieri, A.; Pavinato, L.; Cravero, T.; Bianciotto, O.T.; Salemme, V.; Natalini, D.; Centonze, G.; Raspanti, A.; et al. p140Cap regulates the composition and localization of the NMDAR complex in synaptic lipid rafts. J. Neurosci. 2022, 42, 7183–7200. [Google Scholar] [CrossRef]
- Payrits, M.; Zsidó, B.Z.; Nehr-Majoros, A.K.; Börzsei, R.; Helyes, Z.; Hetényi, C.; Szoke, É. Lipid raft disruption inhibits the activation of transient receptor potential vanilloid 1, but not TRP melastatin 3 and the voltage-gated L-type calcium channels in sensory neurons. Front. Cell Dev. Biol. 2024, 12, 1452306. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Patra, S.K. Lipid raft facilitated receptor organization and signaling: A functional rheostat in embryonic development, stem cell biology and cancer. Stem Cell Rev. Rep. 2023, 19, 2–25. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Brassica napus seed meal soil amendment modifies microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot. Soil. Biol. Biochem. 2005, 37, 1215–1227. [Google Scholar] [CrossRef]
- Esteban, D.J.; Hysa, B.; Bartow-McKenney, C. Temporal and spatial distribution of the microbial community of Winogradsky columns. PLoS ONE 2015, 10, e0134588. [Google Scholar] [CrossRef]
- Tokuda, G.; Yamada, A.; Nakano, K.; Arita, N.; Yamasaki, H. Occurrence and recent long-distance dispersal of deep-sea hydrothermal vent shrimps. Biol. Lett. 2006, 2, 257–260. [Google Scholar] [CrossRef]
- Tokuda, G.; Yamada, A.; Nakano, K.; Arita, N.O.; Yamasaki, H. Colonization of Sulfurovum sp. on the gill surfaces of Alvinocaris longirostris, a deep-sea hydrothermal vent shrimp. Mar. Ecol. 2007, 29, 106–114. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef] [PubMed]
- Kentgens, A.C.; Pusterla, O.; Bauman, G.; Santini, F.; Wyler, F.; Curdy, M.S.; Willers, C.C.; Bieri, O.; Latzin, P.; Ramsey, K.A. Simultaneous multiple breath washout and oxygen-enhanced magnetic resonance imaging in healthy adults. Respir. Med. Res. 2023, 83, 100993. [Google Scholar] [CrossRef] [PubMed]
- Fago, A. New insights into survival strategies to oxygen deprivation in anoxia-tolerant vertebrates. Acta Physiol. 2022, 235, e13841. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Gomez, C.R.; Richards, J.G.; Milsom, W.K. Mitochondrial responses to prolonged anoxia in brain of red-eared slider turtles. Biol. Lett. 2016, 12, 20150797. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Hogg, D.W.; Buck, L.T. Endogenous reductions in N-methyl-D-aspartate receptor activity inhibit nitric oxide production in the anoxic freshwater turtle cortex. FEBS Lett. 2008, 582, 1738–1742. [Google Scholar] [CrossRef]
- Chiu, J.; DeSalle, R.; Lam, H.M.; Meisel, L.; Coruzzi, G. Molecular evolution of glutamate receptors: A primitive signaling mechanism that existed before plants and animals diverged. Mol. Biol. Evol. 1999, 16, 826–838. [Google Scholar] [CrossRef]
- Burnstock, G.; Verkhratsky, A. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009, 195, 415–447. [Google Scholar] [CrossRef]
- Bright, M.; Gollner, S.; de Oliveira, A.L.; Espada-Hinojosa, S.; Fulford, A.; Hughes, I.V.; Hourdez, S.; Karthauser, C.; Kolar, I.; Krause, N.; et al. Animal life in the shallow subseafloor crust at deep-sea hydrothermal vents. Nat. Commun. 2024, 15, 8466. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Damer, B. Can life begin on Enceladus? A perspective from hydrothermal chemistry. Astrobiology 2017, 17, 834–839. [Google Scholar] [CrossRef] [PubMed]



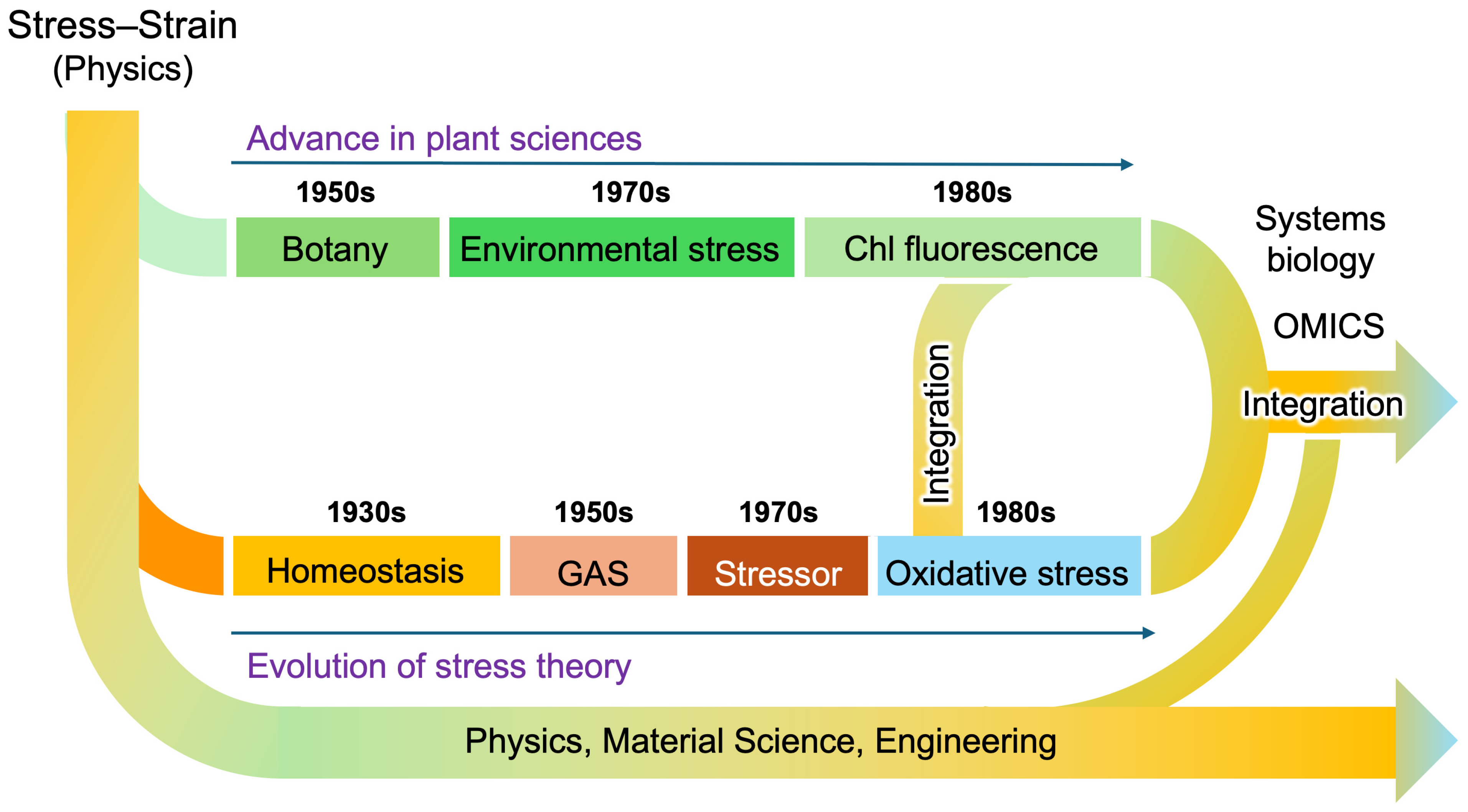





| Category | Chemical Formula | Common Name |
|---|---|---|
| ROS | O2•− | Superoxide |
| H2O2 | Hydrogen Peroxide | |
| •OH | Hydroxyl Radical | |
| 1O2 | Singlet Oxygen | |
| RNS | NO• | Nitric Oxide |
| NO2• | Nitrogen Dioxide | |
| ONOO− | Peroxynitrite | |
| HNO | Nitroxyl | |
| RSS | H2S | Hydrogen Sulfide |
| HS− | Hydrosulfide Ion | |
| RSSH | Persulfide | |
| RSSn− | Polysulfide |
| Category | Reactive Species | Modification | Product |
|---|---|---|---|
| ROS | H2O2 | S-Sulfenylation | Cys–SOH |
| HOCl | S-Glutathionylation | Cys–SSG | |
| H2O2 (excess) | S-Sulfinylation | Cys–SO2H | |
| H2O2 (excess) | S-Sulfonylation | Cys–SO3H | |
| RNS | NO• | S-Nitrosylation | Cys–SNO |
| ONOO− | S-Nitrosylation | Cys–SNO | |
| RSS | H2S | S-Persulfidation | Cys–SSH |
| RSSH | S-Persulfidation | Cys–SSH | |
| RSS2− | S-Polysulfidation | Cys–SnH | |
| CysSSH | Protein S-Polysulfidation | Protein–S–SnH |
| Category | Pathology | O2 | NO | H2S | References |
|---|---|---|---|---|---|
| Cardiovascular Diseases | Myocardial ischemia/infarction | ↓ | ↓ | ↓ | [190,191,192] |
| Heart failure | ↓ | ↓ | ↓ | [154,193,194] | |
| Pulmonary Disorders | Pulmonary hypertension | ↓ | ↓ | ↓ | [155,195,196] |
| COPD/Emphysema | ↓ | ↓ | ↓ | [197,198] | |
| Inflammatory Conditions | Rheumatoid arthritis | ↓ | ↑ | ↑ | [199,200,201] |
| Inflammatory bowel disease | ↓ | ↑ | ↑ | [202,203,204] | |
| Sepsis | ↓ | ↑ | ↑ | [202,205,206] | |
| Cancer | Solid tumors | ↓ | ↑ | ↑ | [207,208,209] |
| Hematological malignancies | ↓ | ↑ | ↑ | [210,211,212] | |
| Metabolic Disorders | Obesity | ↓ | ↓ | ↓ | [213,214,215] |
| Diabetes | ↓ | ↓ | ↓ | [214,216,217] | |
| Neurodegenerative Disorders | Alzheimer’s disease | ↓ | ↓ | ↓ | [218,219,220] |
| Parkinson’s disease | ↓ | ↓ | ↓ | [218,221,222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, H.; Naomasa, R.F.; Mizumoto, K.B.; Cohen, M.F. The Three-Body Problem in Stress Biology: The Balance Between O2, NO, and H2S in the Context of Hans Selye’s Stress Concept. Stresses 2025, 5, 37. https://doi.org/10.3390/stresses5020037
Yamasaki H, Naomasa RF, Mizumoto KB, Cohen MF. The Three-Body Problem in Stress Biology: The Balance Between O2, NO, and H2S in the Context of Hans Selye’s Stress Concept. Stresses. 2025; 5(2):37. https://doi.org/10.3390/stresses5020037
Chicago/Turabian StyleYamasaki, Hideo, Riko F. Naomasa, Kakeru B. Mizumoto, and Michael F. Cohen. 2025. "The Three-Body Problem in Stress Biology: The Balance Between O2, NO, and H2S in the Context of Hans Selye’s Stress Concept" Stresses 5, no. 2: 37. https://doi.org/10.3390/stresses5020037
APA StyleYamasaki, H., Naomasa, R. F., Mizumoto, K. B., & Cohen, M. F. (2025). The Three-Body Problem in Stress Biology: The Balance Between O2, NO, and H2S in the Context of Hans Selye’s Stress Concept. Stresses, 5(2), 37. https://doi.org/10.3390/stresses5020037







