Antioxidant and Histopathological Effects of Paraquat and Fluroxypyr Herbicides on the Apple Snail Pomacea canaliculata (Lamarck, 1822)
Abstract
1. Introduction
2. Results
2.1. Herbicide Toxicity in P. canaliculata
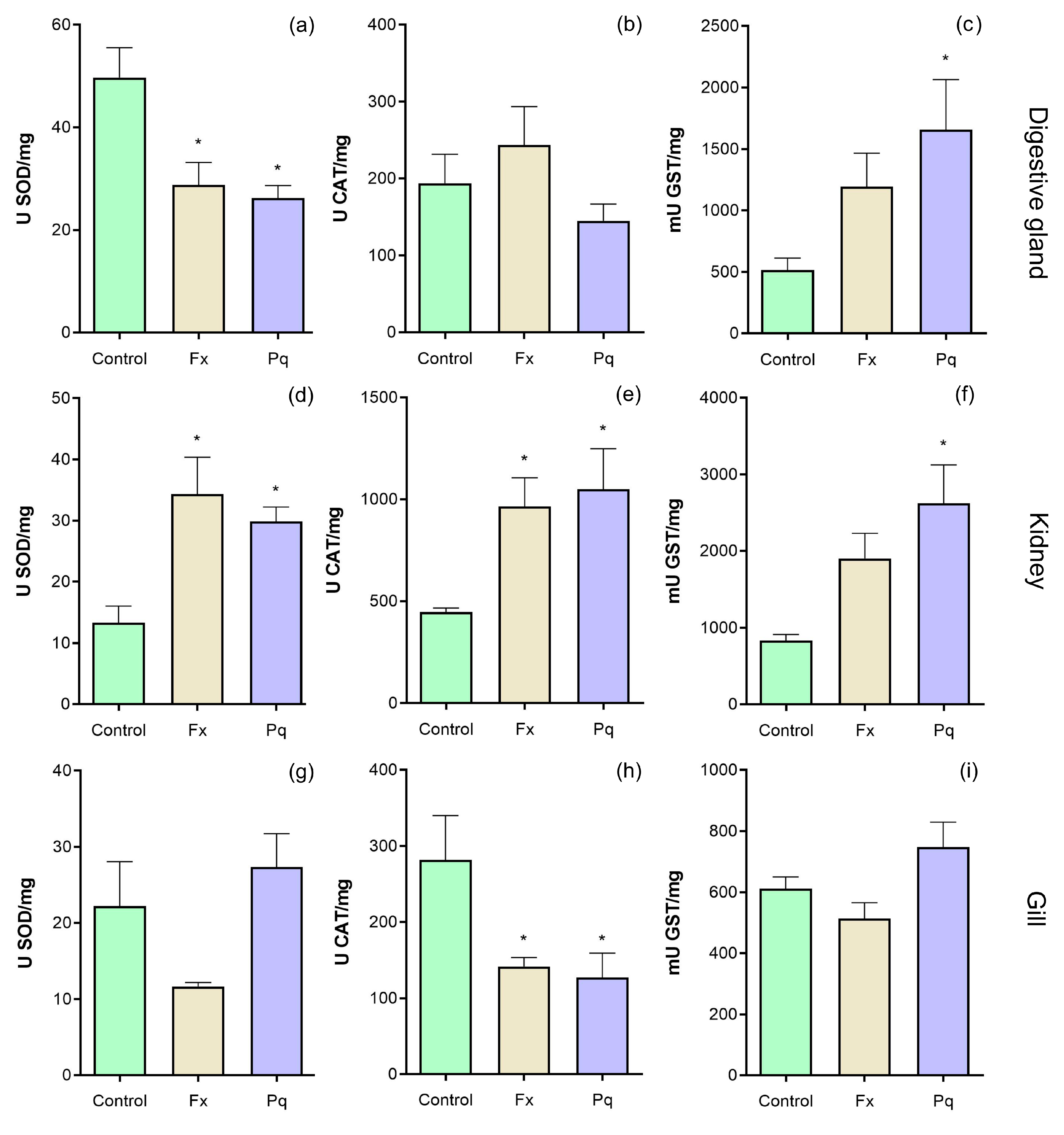
2.2. Antioxidant Enzyme Activities
2.3. Histological Changes in the Digestive Gland Induced by Herbicides
2.4. Symbiotic Occupancy of the Digestive Gland
3. Discussion
3.1. Enzymatic Antioxidant Response to Herbicides
3.2. Effects of Herbicides on Digestive Gland Histology and Its Symbiotic Corpsucles
4. Materials and Methods
4.1. Animals
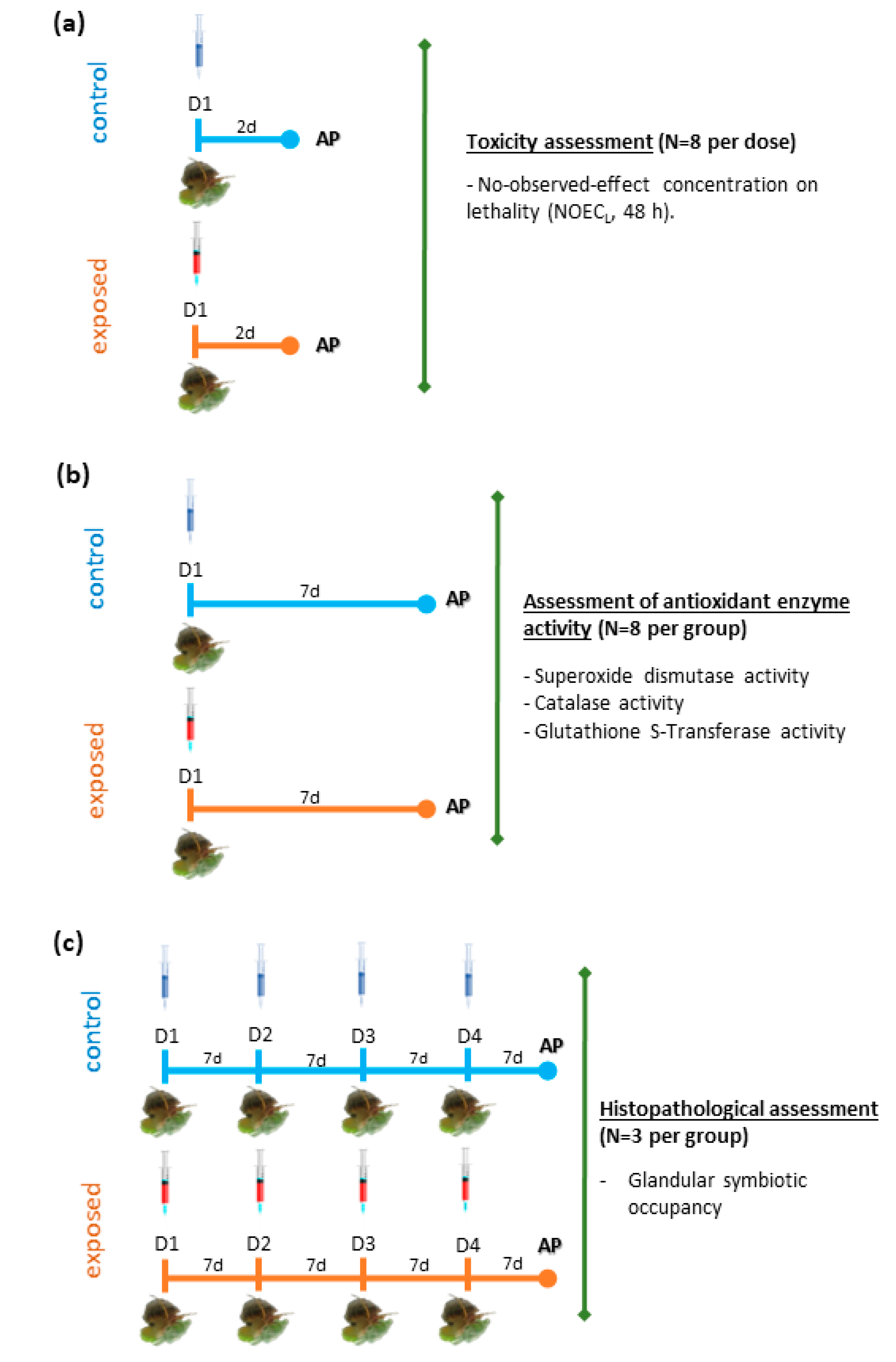
4.2. Agrochemicals and Experimental Design
4.3. Toxicity Assesment
4.3.1. Toxicity Response
4.3.2. Antioxidant Enzymatic Defenses
4.3.3. Glandular Symbiotic Occupancy
4.4. Enzymatic Activities
4.5. Light Microscopy
4.6. Glandular Symbiotic Occupancy
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Z.; Zhang, J.; Yao, F.; Liu, J.; Shi, Z.; Zhao, B.; Guo, J. Niche conservatism and geographical range expansion of Pomacea canaliculata and Pomacea maculata in non-native United States and China. Biol. Invasions 2023, 25, 3391–3405. [Google Scholar] [CrossRef]
- Hayes, K.; Burks, R.; Castro-Vazquez, A.; Darby, P.; Heras, H.; Martín, P.; Qiu, J.-W.; Thiengo, S.; Wada, T.; Yusa, Y.; et al. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 2015, 58, 245–302. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Martín, P.R. Global distribution of the invasive apple snail Pomacea canaliculata: Analyzing possible shifts in climatic niche between native and invaded ranges and future spread. Aquat. Sci. 2024, 86, 17. [Google Scholar] [CrossRef]
- Yao, F.; Chen, Y.; Liu, J.; Qin, Z.; Shi, Z.; Chen, Q.; Zhang, J. A bibliometric analysis of research on apple snails (Ampullariidae). Agronomy 2023, 13, 1671. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species a Selection from the Global Invasive Species Database; ISSG: Auckland, New Zealand, 2000; Volume 12, pp. 1–12. [Google Scholar]
- Rodriguez, C.; Campoy-Diaz, A.D.; Giraud-Billoud, M. Short-term estivation and hibernation induce changes in the blood and circulating hemocytes of the apple snail Pomacea canaliculata. Metabolites 2023, 13, 289. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Campoy-Diaz, A.D.; Dellagnola, F.A.; Rodriguez, C.; Vega, I.A. Antioxidant responses induced by short-term activity–estivation–arousal cycle in Pomacea canaliculata. Front. Physiol. 2022, 13, 805168. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Castro-Vazquez, A.; Campoy-Diaz, A.D.; Giuffrida, P.M.; Vega, I.A. Tolerance to hypometabolism and arousal induced by hibernation in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 129–137. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Vega, I.A.; Rinaldi Tosi, M.E.; Abud, M.A.; Calderón, M.L.; Castro-Vazquez, A. Antioxidant and molecular chaperone defenses during estivation and arousal in the South American apple-snail Pomacea canaliculata. J. Exp. Biol. 2013, 216, 614–622. [Google Scholar] [CrossRef]
- Andrews, E.B. The functional anatomy of the gut of the prosobranch gastropod Pomacea canaliculata and of some other pilids. Proc. Zool. Soc. Lond. 1965, 145, 19–36. [Google Scholar] [CrossRef]
- Godoy, M.S.; Castro-Vasquez, A.; Vega, I.A. Endosymbiotic and host proteases in the digestive tract of the invasive snail Pomacea canaliculata: Diversity, origin and characterization. PLoS ONE 2013, 8, e66689. [Google Scholar] [CrossRef]
- Vega, I.A.; Arribére, M.A.; Almonacid, A.V.; Ribeiro Guevara, S.; Castro-Vazquez, A. Apple snails and their endosymbionts bioconcentrate heavy metals and uranium from contaminated drinking water. Environ. Sci. Pollut. Res. 2012, 19, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Campoy-Diaz, A.D.; Malanga, G.; Giraud-Billoud, M.; Vega, I.A. Changes in the oxidative status and damage by non-essential elements in the digestive gland of the gastropod Pomacea canaliculata. Front. Physiol. 2023, 14, 467. [Google Scholar] [CrossRef] [PubMed]
- Campoy-Diaz, A.D.; Escobar-Correas, S.; Canizo, B.V.; Wuilloud, R.G.; Vega, I.A. A freshwater symbiosis as sensitive bioindicator of cadmium. Environ. Sci. Pollut. Res. 2020, 27, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Campoy-Diaz, A.D.; Arribére, M.A.; Guevara, S.R.; Vega, I.A. Bioindication of mercury, arsenic and uranium in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Bioconcentration and depuration in tissues and symbiotic corpuscles. Chemosphere 2018, 196, 196–205. [Google Scholar] [CrossRef]
- Arrighetti, F.; Landro, S.M.; Lavarías, S.M. Sensitivity of histopathological and histochemical parameters in the digestive gland of the apple snail Pomacea canaliculata exposed to cypermethrin. Aquat. Toxicol. 2022, 252, 106292. [Google Scholar] [CrossRef]
- Arrighetti, F.; Ambrosio, E.; Astiz, M.; Capítulo, A.R.; Lavarías, S. Differential response between histological and biochemical biomarkers in the apple snail Pomacea canaliculata (Gasteropoda: Amullariidae) exposed to cypermethrin. Aquat. Toxicol. 2018, 194, 140–151. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, P.; Soto, I.; Haubrock, P.J.; Chen, J.; Ji, L. Global economic costs and knowledge gaps of invasive gastropods. Ecol. Indic. 2022, 145, 109614. [Google Scholar] [CrossRef]
- Panda, F.; Pati, S.G.; Bal, A.; Das, K.; Samanta, L.; Paital, B. Control of invasive apple snails and their use as pollutant ecotoxic indicators: A review. Environ. Chem. Lett. 2021, 19, 4627–4653. [Google Scholar] [CrossRef]
- Panda, F.; Pati, S.G.; Anwar, T.N.; Samanta, L.; Paital, B. Seasonal variation of water quality modulated redox regulatory system in the apple snail Pila globosa and its use as a bioindicator species in freshwater ecosystems across India. Water 2022, 14, 3275. [Google Scholar] [CrossRef]
- Juarez, A.; Vega, I.A.; Mayorga, L.S.; Guevara, S.R.; Arribére, M.A. An Arsenic-76 radiotracer to study the routes of assimilation, hemolymph distribution, and tissue inventories in the bioindicator organism Pomacea canaliculata. Sci. Total Environ. 2022, 815, 152760. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Castro-Vazquez, A. Aging and retinoid X receptor agonists on masculinization of female Pomacea canaliculata, with a critical appraisal of imposex evaluation in the Ampullariidae. Ecotoxicol. Environ. Saf. 2019, 169, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Vega, I.A.; Wuilloud, R.G.; Clément, M.E.; Castro-Vazquez, A. Imposex and novel mechanisms of reproductive failure induced by tributyltin (TBT) in the freshwater snail Pomacea canaliculata. Environ. Toxicol. Chem. 2013, 32, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.L.; Piol, M.N.; Nudelman, N.S.; Guerrero, N.R.V. Tributyltin bioaccumulation and toxic effects in freshwater gastropods Pomacea canaliculata after a chronic exposure: Field and laboratory studies. Ecotoxicology 2017, 26, 691–701. [Google Scholar] [CrossRef]
- Zou, X.-H.; Xie, H.Q.-H.; Zha, G.-C.; Chen, V.P.; Sun, Y.-J.; Zheng, Y.-Z.; Tsim, K.W.-K.; Dong, T.T.-X.; Choi, R.C.-Y.; Luk, W.K.-W. Characterizations of cholinesterases in golden apple snail (Pomacea canaliculata). J. Mol. Neurosci. 2014, 53, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Bhagat, J.; Ingole, B. Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as a biomarkers of oxidative stress in snails: A review. Invertebr. Surviv. J. 2016, 13, 336–349. [Google Scholar] [CrossRef]
- Suntres, Z.E. Role of antioxidants in paraquat toxicity. Toxicology 2002, 180, 65–77. [Google Scholar] [CrossRef]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef]
- Schininà, M.E.; Barra, D.; Bossa, F.; Calabrese, L.; Montesano, L.; Carrì, M.T.; Mariottini, P.; Amaldi, F.; Rotilio, G. Primary structure from amino acid and cDNA sequences of two Cu, Zn superoxide dismutase variants from Xenopus laevis. Arch. Biochem. Biophys. 1989, 272, 507–515. [Google Scholar] [CrossRef]
- Orbea, A.; Dariush Fahimi, H.; Cajaraville, M.P. Immunolocalization of four antioxidant enzymes in digestive glands of mollusks and crustaceans and fish liver. Histochem. Cell Biol. 2000, 114, 393–404. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Sun, J.; Mu, H.; Ip, J.C.H.; Li, R.; Xu, T.; Accorsi, A.; Sánchez Alvarado, A.; Ross, E.; Lan, Y.; Sun, Y.; et al. Signatures of divergence, invasiveness, and terrestrialization revealed by four apple snail genomes. Mol. Biol. Evol. 2019, 36, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Seulalae, A. Antioxidant activity of biopigment fractions from golden apple snail eggs (Pomacea canaliculata). Proc. IOP Conf. Ser. Earth Environ. Sci. 2020, 404, 012003. [Google Scholar] [CrossRef]
- Andrews, E.B. Osmoregulation and excretion in prosobranch gastropods Part 2: Structure in relation to function. J. Molluscan Stud. 1981, 47, 248. [Google Scholar] [CrossRef]
- Tsushima, M.; Katsuyama, M.; Matsuno, T. Metabolism of carotenoids in the apple snail, Pomacea canaliculata. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 118, 431–436. [Google Scholar] [CrossRef]
- Vega, I.A.; Dellagnola, F.A.; Hurst, J.A.; Godoy, M.S.; Castro-Vazquez, A. A study of chlorophyll-like and phycobilin pigments in the C endosymbiont of the apple-snail Pomacea canaliculata. Biocell 2012, 36, 47–55. [Google Scholar] [CrossRef]
- Cochón, A.; Della Penna, A.; Kristoff, G.; Piol, M.; De Viale, L.S.M.; Guerrero, N.V. Differential effects of paraquat on oxidative stress parameters and polyamine levels in two freshwater invertebrates. Ecotoxicol. Environ. Saf. 2007, 68, 286–292. [Google Scholar] [CrossRef]
- Little, C. Osmoregulation and excretion in prosobranch gastropods Part I: Physiology and biochemistry. J. Molluscan Stud. 1981, 47, 221–247. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Koch, E.; Vega, I.; Gamarra-Luques, C.; Castro-Vazquez, A. Urate cells and tissues in the South American apple snail Pomacea canaliculata. J. Molluscan Stud. 2008, 74, 259–266. [Google Scholar] [CrossRef]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Assessment of the kidney and lung as immune barriers and hematopoietic sites in the invasive apple snail Pomacea canaliculata. PeerJ 2018, 6, e5789. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Functional and evolutionary perspectives on gill structures of an obligate air-breathing, aquatic snail. PeerJ 2019, 7, e7342. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. A J. Virtual Libr. 2008, 13, 7210. [Google Scholar] [CrossRef]
- Banan, A.; Zhang, Y.; Losurdo, J.; Keshavarzian, A. Carbonylation and disassembly of the F-actin cytoskeleton in oxidant induced barrier dysfunction and its prevention by epidermal growth factor and transforming growth factor α in a human colonic cell line. Gut 2000, 46, 830–837. [Google Scholar] [CrossRef]
- Bignell, J.; Stentiford, G.; Taylor, N.; Lyons, B. Histopathology of mussels (Mytilus sp.) from the Tamar estuary, UK. Mar. Environ. Res. 2011, 72, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vazquez, A.; Albrecht, E.; Vega, I.; Koch, E.; Gamarra-Luques, C. Pigmented corpuscles in the midgut gland of Pomacea canaliculata and other Neotropical apple-snails (Prosobranchia, Ampullariidae): A possible symbiotic association. Biocell 2002, 26, 101–109. [Google Scholar]
- Koch, E.; Vega, I.A.; Albrecht, E.A.; Ortega, H.H.; Castro-Vazquez, A. A light and electron microscopic study of pigmented corpuscles in the midgut gland and feces of Pomacea canaliculata (Caenogastropoda: Ampullariidae). Veliger 2006, 48, 17–25. [Google Scholar]
- Zhang, S.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Tansai, S.; Issakul, K.; Ngearnpat, N. Toxicity of paraquat on growth of cyanobacteria (Nostoc sp. N1 and Anabaena sp. A1) and germination of rice seed (san-pah-twang 1). In Proceedings of the The 6th International Conference on Biochemistry and Molecular Biology, Rayong, Thailand, 20–22 June 2018. [Google Scholar]
- Bai, F.; Jia, Y.; Li, J.; Wu, Z.; Li, L.; Song, L. Paraquat induces different programmed cell death patterns in Microcystis aeruginosa and Chlorella luteoviridis. Ecotoxicol. Environ. Saf. 2023, 249, 114429. [Google Scholar] [CrossRef]
- Bai, F.; Gao, G.; Li, T.; Liu, J.; Li, L.; Jia, Y.; Song, L. Integrated physiological and metabolomic analysis reveals new insights into toxicity pathways of paraquat to Microcystis aeruginosa. Aquat. Toxicol. 2023, 259, 106521. [Google Scholar] [CrossRef]
- Bai, F.; Jia, Y.; Yang, C.; Li, T.; Wu, Z.; Liu, J.; Song, L. Multiple physiological response analyses aid the understanding of sensitivity variation between Microcystis aeruginosa and Chlorella sp. under paraquat exposures. Environ. Sci. Eur. 2019, 31, 83. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Zhang, P.; Yu, F.; Lu, S.; Li, P.; Zhou, J. Effects of paraquat on photosynthetic pigments, antioxidant enzymes, and gene expression in Chlorella pyrenoidosa under mixotrophic compared with autotrophic conditions. Arch. Environ. Contam. Toxicol. 2014, 67, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Lester, P., Ed.; Academic Press: Cambridge, MA USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130. [Google Scholar] [CrossRef]
- Dellagnola, F.A.; Rodriguez, C.; Castro-Vazquez, A.; Vega, I.A. A multiple comparative study of putative endosymbionts in three coexisting apple snail species. PeerJ 2019, 7, e8125. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campoy-Diaz, A.D.; Vega, I.A.; Giraud-Billoud, M. Antioxidant and Histopathological Effects of Paraquat and Fluroxypyr Herbicides on the Apple Snail Pomacea canaliculata (Lamarck, 1822). Stresses 2025, 5, 33. https://doi.org/10.3390/stresses5020033
Campoy-Diaz AD, Vega IA, Giraud-Billoud M. Antioxidant and Histopathological Effects of Paraquat and Fluroxypyr Herbicides on the Apple Snail Pomacea canaliculata (Lamarck, 1822). Stresses. 2025; 5(2):33. https://doi.org/10.3390/stresses5020033
Chicago/Turabian StyleCampoy-Diaz, Alejandra D., Israel A. Vega, and Maximiliano Giraud-Billoud. 2025. "Antioxidant and Histopathological Effects of Paraquat and Fluroxypyr Herbicides on the Apple Snail Pomacea canaliculata (Lamarck, 1822)" Stresses 5, no. 2: 33. https://doi.org/10.3390/stresses5020033
APA StyleCampoy-Diaz, A. D., Vega, I. A., & Giraud-Billoud, M. (2025). Antioxidant and Histopathological Effects of Paraquat and Fluroxypyr Herbicides on the Apple Snail Pomacea canaliculata (Lamarck, 1822). Stresses, 5(2), 33. https://doi.org/10.3390/stresses5020033







