Potential of Bacillus halotolerans in Mitigating Biotic and Abiotic Stresses: A Comprehensive Review
Abstract
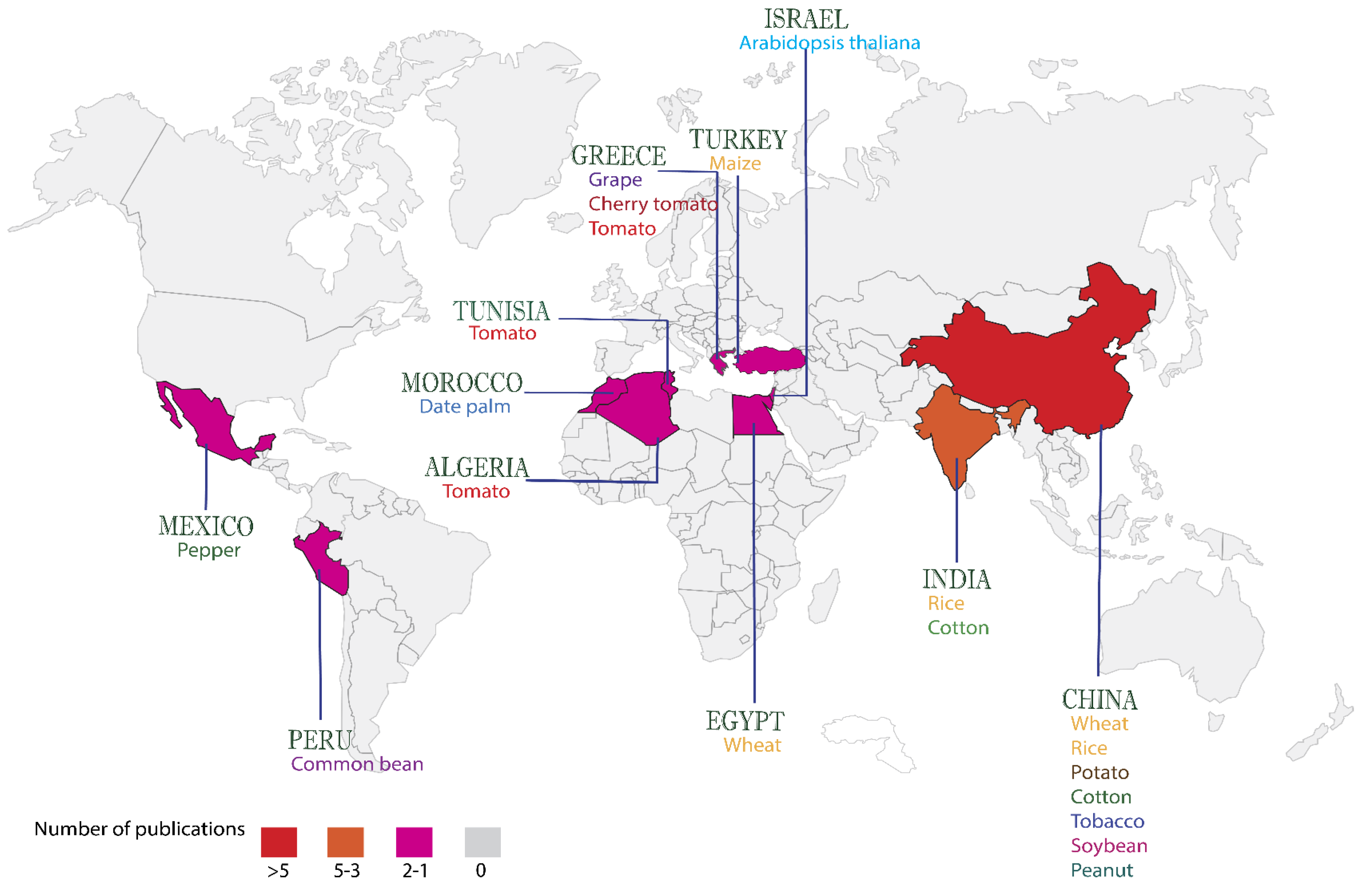
:1. Introduction
2. Morphological, Biochemical and Molecular Characteristics of B. halotolerans
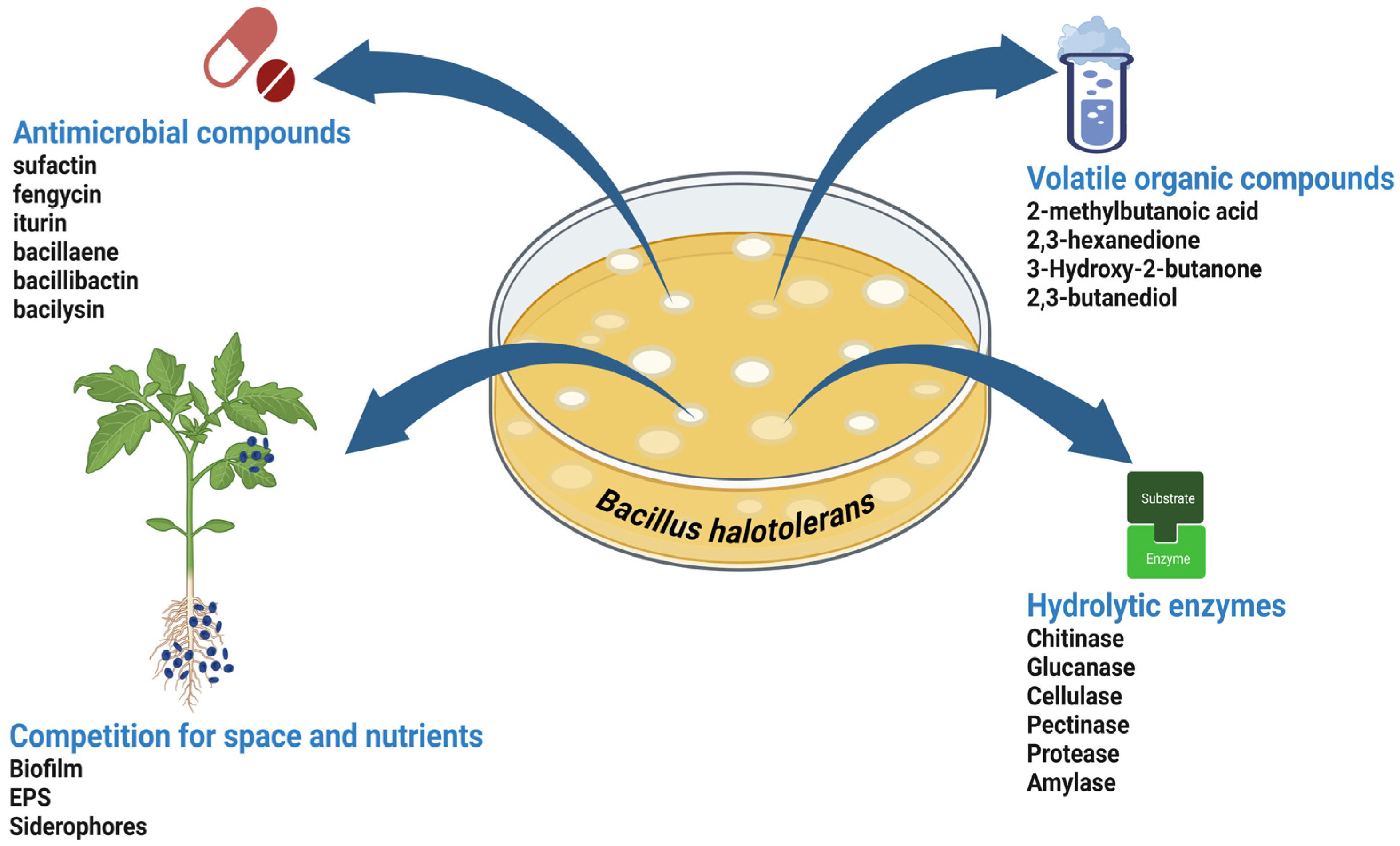
3. Biocontrol Mechanisms of B. halotolerans
3.1. Production of Antimicrobial Compounds
| Crop | B. halotolerans Strains | Targeted Pathogens | Modes of Action | References |
|---|---|---|---|---|
| Tomato | RFP1, RFP10, RFP57, RFP74 | Alternaria spp., Bipolaris spp., F. oxysporum f.sp. lycopersici, Ascochyta sp. | CWDEs, VOC and antimicrobial production | [49,50] |
| LYSX1 | Root-knot nematode (Meloidogyne javanica) | Induced systemic resistance (ISR), nematicidal activity | [51] | |
| Gb67 | - | VOCs (acetoin, 2,3-butanediol), enhanced root/shoot growth, reduction of salinity stress impact | [32] | |
| Cal.l.30, Cal.f.4 | B. cinerea | VOC-mediated suppression, production of surfactin analogs | [42] | |
| Wheat | NYG5 | M. phaseolina, R. solani, P. aphanidermatum, S. sclerotiorum, A. tumefaciens | VOC production (e.g., 2-methylbutanoic acid), nematicidal activity against M. javanica | [52] |
| KKD1 | - | Enhanced soil fertility under saline conditions, phosphate solubilization, soil pH stabilization, nutrient cycling | [23] | |
| MSR-H4 | - | Nitrogen fixation, phosphate solubilization, and improving root-shoot K+/Na+ ratios under saline conditions | [24] | |
| JK-25 | Bipolaris sorokiniana, F. oxysporum, F. graminearum, Rhizoctonia zeae | Surfactin production, CWDEs; reduced antioxidant activity; siderophore | [21] | |
| QTH8 | F. graminearum, B. cinerea, F. pseudograminearum, S. sclerotiorum, Phytophthora nicotianae, | Iturin, surfactin, fengycin; lipopeptides biosynthesis genes; growth promotion (ISR, AMCs) | [22] | |
| Maize | B7, B18, B14 | - | Biofilm and exopolysaccharide production, increased chlorophyll under saline conditions | [53] |
| Potato | SpS5 | Rhizoctonia solani | Biofilm formation, CWDEs | [41] |
| Q2H2 | F. oxysporum, F. graminearum, R.solani, Stemphylium solani | Surfactin, fengycin, bacillaene, subtilosin A; VOCs; phosphate solubilization; nitrogen fixation; IAA and NH3 and biofilm | [54] | |
| F29-3 | R. solani | Fengycin via NRPS genes; antagonistic properties; pathogen suppression in field trials | [55] | |
| Soybean | Ba2-6 | Heterodera glycines (soybean cyst nematode) | Juvenile nematode mortality, antibiosis, ISR, root colonization | [56] |
| Peanut | B28 | - | Benzoic acid breakdown, reduce continuous cropping stress | [57] |
| Lily | LBG-1-13 | Botryosphaeria dothidea, B. cinerea, F. oxysporum | ACC deaminase activity, IAA and siderophore production, ISR and salt/drought tolerance | [58] |
| Cotton | SSVP2 | Soil-borne nematodes | ACC deaminase production, mineral solubilization (P, K), and nematicidal activity | [59] |
| Y6MSR-H4 | Verticillium dahliae | β-glucanase activity; enhanced resistance in cotton in the field | [60] | |
| Date palm | BFOA1–BFOA4 | F. oxysporum f. sp. albedinis, F. solani, F. acuminatum, B. cinerea, A. alternata, Phytophthora infestans, Rhizoctonia bataticola | Antagonism via AMCs (pulegone, 2-undecanone, germacrene D); salt and drought tolerance; auxin and biofilm production; nutrient solubilization, nitrogen fixation | [33] |
| Not specified | HGR5 | F. graminearum, P. infestans, A. alternata | Fengycin, subtilosin, bacilysin; CWDEs (chitinase, cellulase, xylanase); plastic degradation | [43] |
| Wheat, rice, maize | LDFZ001 | R. solani | Antifungal activity via phosphopantetheinyl transferase (SFP) and major facilitator superfamily (MFS) genes; two chitosanases; diverse biosynthetic gene clusters (NRPS, PKS). | [30] |
| Pepper | MS50-18A | Phytophthora capsici, F. solani, R. solani, F. oxysporum | AMCs and auxin production | [29] |
| Tomato, grapes, A. thaliana | Hil4 | B. cinerea | AMCs; ISR elicitors; Mojavensin cluster; secretome extracts; promotes plant growth and mitigates gray mold disease | [35] |
| Common bean | IcBac2.1 | R. solani, F. oxysporum, S. sclerotiorum | Amphiphilic compounds with inhibitory activity; field efficacy against S. sclerotiorum; plant growth promotion | [61] |
| Apple | Pl7 | B. dothidea | CWDEs production, induction of plant secondary metabolite biosynthesis and plant-pathogen interaction | [34] |
| Rice | AD9 | - | High NH3 and phosphate solubilization; salinity reduction via enzymes (SOD, CAT) | [62] |
| Strawberries | KLBC XJ-5 | B. cinerea | Enhancement of disease resistance compounds (phenols, flavonoids), induction of plant defense enzymes (polyphenol oxidase, phenylalanine ammonia lyase) | [26] |
| Not specified | DMC8 | R. solani, P. aphanidermatum, M. phaseolina | CWDEs (protease, chitinase), siderophores, NH3, IAA; nitrogen fixation; phosphate solubilization. | [2] |
3.2. Cell Wall Degrading Enzymes
3.3. Root Colonization and Competition for Space and Nutrients
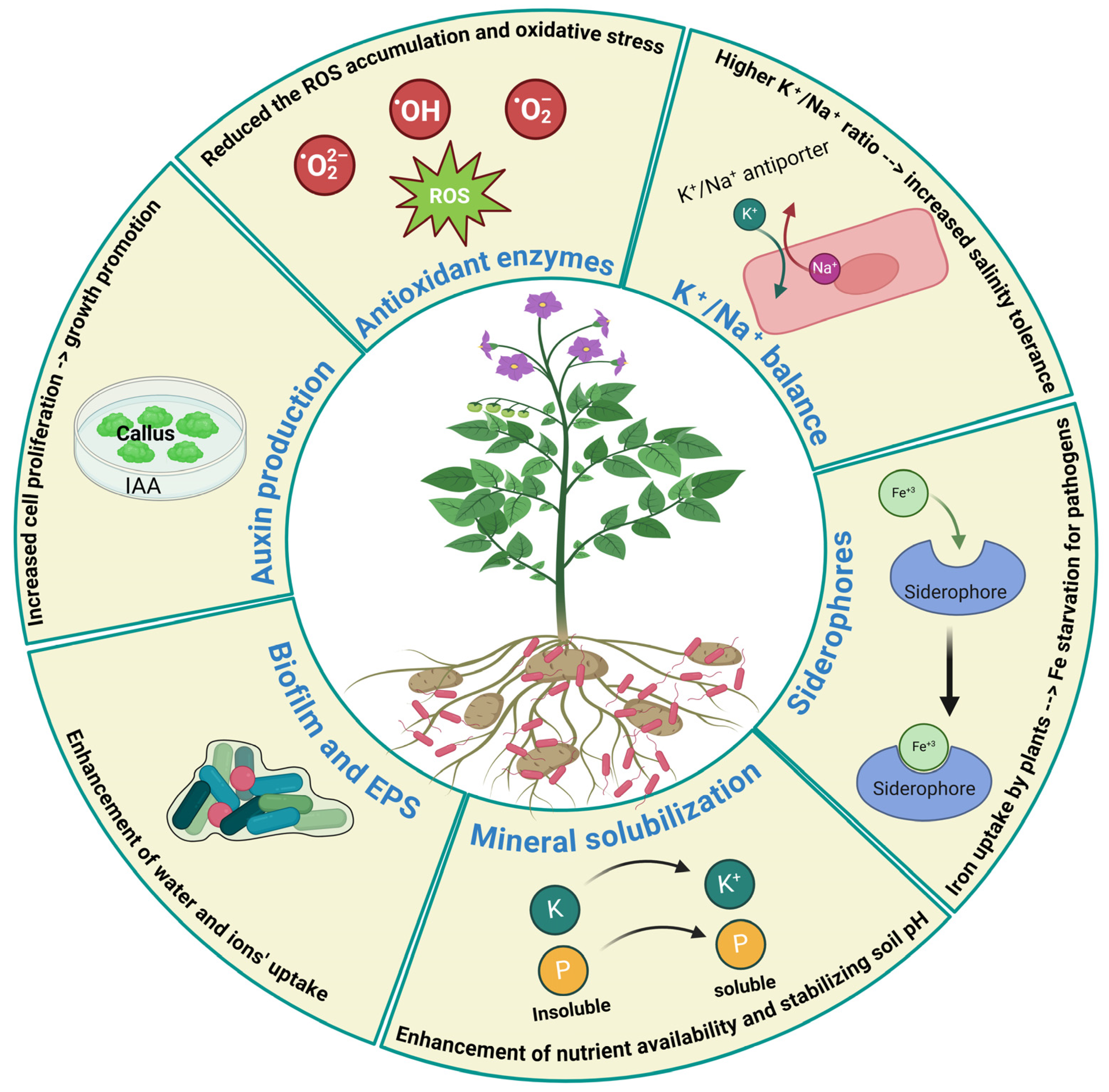
4. Plant Growth-Promoting Effect of Bacillus halotolerans in Alleviating Abiotic and Biotic Stresses
4.1. Production of Indole-3-Acetic Acid
| Mechanism | Example Strains | Impact on Plants | References |
|---|---|---|---|
| IAA production | KKD1, B-4359 | Drought and salinity tolerance, improved nutrient uptake | [23,27] |
| Siderophore production | JK-25, LBG-1-13, BFOA1–BFOA4 | Reduced chlorosis, enhanced biomass, suppression of fungal pathogens | [21,33,58] |
| ACC Deaminase activity | B5, LBG-1-13 | Enhanced growth under salt/drought stress, improved water holding capacity | [58,88] |
| Nitrogen fixation | MSR-H4, SSVP2, KKD1 | Improved soil fertility, supported growth in nutrient-deficient environments | [23,24,59] |
| Mineral solubilization | SSVP2, AD9 | Enhanced nutrient uptake, stabilized soil pH | [59,62] |
| K+/Na+ balance | MSR-H4 | Improved salt tolerance, reduced salinity | [24] |
| Antioxidant enzyme induction | KLBC XJ-5 | Minimized cellular damage, enhanced plant resilience | [26] |
4.2. Siderophore Production
4.3. ACC Deaminase Activity
4.4. Nitrogen Fixation and Mineral Solubilization
4.5. Improving K+/Na+ Balance in Plants
4.6. Induction of Antioxidant Enzymes
5. Additional Applications of B. halotolerans
5.1. Nematode Management
5.2. Bioremediation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1-carboxylate |
| AMCs | Antimicrobial Compounds |
| BCA | Biological Control Agent |
| BGCs | Biosynthetic Gene Clusters |
| CAT | Catalase |
| cPPPs | Chemical Plant Protection Products |
| CWDEs | Cell Wall Degrading Enzymes |
| EPS | Extracellular Polymeric Substances |
| FBCC | Freshwater Bioresources Culture Collection |
| IAA | Indole-3-Acetic Acid |
| IPM | Integrated Pest Management |
| ISR | Induced Systemic Resistance |
| LB | Luria-Bertani Medium |
| MFS | Major Facilitator Superfamily |
| NGS | Next Generation Sequencing |
| NRPS | Non-Ribosomal Peptide Synthetases |
| PAL | Phenylalanine Ammonia-Lyase |
| PGPB | Plant Growth-Promoting Bacteria |
| PKS | Polyketide Synthase |
| PPNs | Plant-Parasitic Nematodes |
| POX | Peroxidase |
| PPO | Polyphenol Oxidase |
| ROS | Reactive Oxygen Species |
| rRNA | Ribosomal Ribonucleic Acid |
| SOD | Superoxide Dismutase |
| tRNA | Tranfer Ribonucleic Acid |
| TSA | Tryptic Soy Agar |
| VOCs | Volatile Organic Compounds |
References
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.N.; Safaie, N.; Shams-Bakhsh, M.; Al-Juboory, H.H. Harnessing Rhizobacteria: Isolation, Identification, and Antifungal Potential against Soil Pathogens. Heliyon 2024, 10, e35430. [Google Scholar] [CrossRef] [PubMed]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Sharma, D.; Kumar, P.; Chandra Dubey, R. Biocontrol Potential of Bacillus spp. for Resilient and Sustainable Agricultural Systems. Physiol. Mol. Plant Pathol. 2023, 128, 102173. [Google Scholar] [CrossRef]
- Keshmirshekan, A.; de Souza Mesquita, L.M.; Ventura, S.P.M. Biocontrol Manufacturing and Agricultural Applications of Bacillus velezensis. Trends Biotechnol. 2024, 42, 986–1001. [Google Scholar] [CrossRef]
- Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 11, 1763. [Google Scholar] [CrossRef]
- Ramírez, V.; Martínez, J.; del Bustillos-Cristales, M.R.; Catañeda-Antonio, D.; Munive, J.-A.; Baez, A. Bacillus cereus MH778713 Elicits Tomato Plant Protection against Fusarium oxysporum. J. Appl. Microbiol. 2022, 132, 470–482. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Pan, Y.; Qi, D.; Zhou, D.; Chen, Y.; Feng, J.; Wei, Y.; Zhao, Y.; Li, K.; et al. Biocontrol Mechanism of Bacillus siamensis sp. QN2MO-1 against Tomato Fusarium Wilt Disease during Fruit Postharvest and Planting. Microbiol. Res. 2024, 283, 127694. [Google Scholar] [CrossRef]
- Espinosa Bernal, M.A.; Mena Navarro, M.P.; Arvizu Gómez, J.L.; Saldaña, C.; Ramos López, M.Á.; Amaro Reyes, A.; Escamilla García, M.; Pacheco Aguilar, J.R.; Moreno, V.P.; Rodríguez Morales, J.A.; et al. Biocontrol Activity of Bacillus altitudinis CH05 and Bacillus tropicus CH13 Isolated from Capsicum annuum L. Seeds against Fungal Strains. Microorganisms 2024, 12, 1943. [Google Scholar] [CrossRef]
- Serrão, C.P.; Ortega, J.C.G.; Rodrigues, P.C.; de Souza, C.R.B. Bacillus Species as Tools for Biocontrol of Plant Diseases: A Meta-Analysis of Twenty-Two Years of Research, 2000–2021. World J. Microbiol. Biotechnol. 2024, 40, 110. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.J.; Park, A.R.; Vuong, B.S.; Kim, J.-C. Biocontrol of Fusarium Head Blight in Rice Using Bacillus velezensis JCK-7158. Front. Microbiol. 2024, 15, 1358689. [Google Scholar] [CrossRef]
- Balleux, G.; Höfte, M.; Arguelles-Arias, A.; Deleu, M.; Ongena, M. Bacillus Lipopeptides as Key Players in Rhizosphere Chemical Ecology. Trends Microbiol. 2024, 33, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Stanković, S.; Nišavić, M.; Petković, M.; Ristivojević, P.; Fira, D.; Berić, T. The Profile and Antimicrobial Activity of Bacillus Lipopeptide Extracts of Five Potential Biocontrol Strains. Front. Microbiol. 2017, 8, 925. [Google Scholar] [CrossRef]
- Sevugapperumal, N.; Surya, T.; Vinodkumar, S. Antifungal Potential of Plant Growth Promoting Bacillus Species Against Blossom Blight of Rose. J. Plant Growth Regul. 2020, 39, 99–111. [Google Scholar] [CrossRef]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The Surfactin-Like Lipopeptides from Bacillus spp.: Natural Biodiversity and Synthetic Biology for a Broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Dhanabalan, S.; Muthusamy, K.; Iruthayasamy, J.; Kumaresan, P.V.; Ravikumar, C.; Kandasamy, R.; Natesan, S.; Periyannan, S. Unleashing Bacillus Species as Versatile Antagonists: Harnessing the Biocontrol Potentials of the Plant Growth-Promoting Rhizobacteria to Combat Macrophomina phaseolina Infection in Gloriosa superba. Microbiol. Res. 2024, 283, 127678. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Belal, E.E.; Al-Quwaie, D.A.; Ashkan, M.F.; Alqahtani, F.S.; El-Tarabily, K.A.; El-Mageed, T.A.A.; Shami, A.; Nader, M.M.; Hemeda, N.F. Extremophilic Bacterial Strains as Plant Growth Promoters and Biocontrol Agents against Pythium ultimum and Rhizocotnia solani. J. Plant Pathol. 2023, 105, 1347–1369. [Google Scholar] [CrossRef]
- Kang, K.; Niu, Z.; Zhang, W.; Wei, S.; Lv, Y.; Hu, Y. Antagonistic Strain Bacillus halotolerans Jk-25 Mediates the Biocontrol of Wheat Common Root Rot Caused by Bipolaris sorokiniana. Plants 2023, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Fu, L.; Xu, G.; Lin, X.; Qiao, J.; Xia, Y. Biocontrol of Wheat Crown Rot Using Bacillus halotolerans QTH8. Pathogens 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fan, Y.; Wang, R.; Zhao, Q.; Ali, Q.; Wu, H.; Gu, Q.; Borriss, R.; Xie, Y.; Gao, X. Bacillus halotolerans KKD1 Induces Physiological, Metabolic and Molecular Reprogramming in Wheat under Saline Condition. Front. Plant Sci. 2022, 13, 978066. [Google Scholar] [CrossRef]
- El-Akhdar, I.; Elsakhawy, T.; Abo-Koura, H.A. Alleviation of Salt Stress on Wheat (Triticum aestivum L.) by Plant Growth Promoting Bacteria Strains Bacillus halotolerans MSR-H4 and Lelliottia amnigena MSR-M49. J. Adv. Microbiol. 2020, 20, 44–58. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; García-Estévez, I.; Escribano-Bailón, M.T.; García-Fraile, P.; Rivas, R. Bacterial Fertilizers Based on Rhizobium laguerreae and Bacillus halotolerans Enhance Cichorium endivia L. Phenolic Compound and Mineral Contents and Plant Development. Foods 2021, 10, 424. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Li, R.; Liu, L.; Deng, J. Biocontrol Ability and Action Mechanism of Bacillus halotolerans against Botrytis cinerea Causing Grey Mould in Postharvest Strawberry Fruit. Postharvest Biol. Technol. 2021, 174, 111456. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.; Hwang, Y.-J.; Lee, M.-H.; Balaraju, K.; Jeon, Y. Identification and Characterization of Brevibacillus halotolerans B-4359: A Potential Antagonistic Bacterium against Red Pepper Anthracnose in Korea. Front. Microbiol. 2023, 14, 1200023. [Google Scholar] [CrossRef]
- Ben-Gad, D.; Gerchman, Y. Reclassification of Brevibacterium halotolerans DSM8802 as Bacillus halotolerans Comb. Nov. Based on Microbial and Biochemical Characterization and Multiple Gene Sequence. Curr. Microbiol. 2017, 74, 1–5. [Google Scholar] [CrossRef]
- Sagredo-Beltrán, J.; De La Cruz-Rodríguez, Y.; Alvarado-Rodríguez, M.; Vega-Arreguín, J.; Rodríguez-Guerra, R.; Alvarado-Gutiérrez, A.; Fraire-Velázquez, S. Genome Sequence of Bacillus halotolerans Strain MS50-18A with Antifungal Activity against Phytopathogens, Isolated from Saline Soil in San Luís Potosí, Mexico. Genome Announc. 2018, 6, e00135-18. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, M.; Yang, J.; Zhang, R.; Geng, Z.; Mao, T.; Sheng, Y.; Wang, L.; Zhang, J.; Zhang, H. Molecular Characterization of a Novel Strain of Bacillus halotolerans Protecting Wheat from Sheath Blight Disease Caused by Rhizoctonia solani Kühn. Front. Plant Sci. 2022, 13, 1019512. [Google Scholar] [CrossRef]
- Wen, Y.; Qiang, J.; Zhou, G.; Zhang, X.; Wang, L.; Shi, Y. Characterization of Redox and Salinity-Tolerant Alkaline Protease from Bacillus halotolerans Strain DS5. Front. Microbiol. 2022, 13, 935072. [Google Scholar] [CrossRef]
- Abdelkefi, N.; Louati, I.; Mechichi, H.-Z.; Sayahi, N.; El-Sayed, W.S.; Nayal, A.E.; Ismail, W.; Hanin, M.; Mechichi, T. Enhanced Salt Stress Tolerance in Tomato Plants Following Inoculation with Newly Isolated Plant Growth-Promoting Rhizobacteria. Sci. Hortic. 2024, 328, 112921. [Google Scholar] [CrossRef]
- Slama, H.B.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium Antagonistic Bacteria From Contrasting Niches Designated the Endophyte Bacillus halotolerans as Plant Warden Against Fusarium. Front. Microbiol. 2018, 9, 3236. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yuan, M.; Shi, B.; Wang, Z.; Huang, T.; Zhu, J.; Hou, H.; Wang, L.; Tu, H. Biocontrol Activity of Bacillus halotolerans Strain Pl7 against Botryosphaeria dothidea Causing Apple Postharvest Decay and Potential Mechanisms. Front. Microbiol. 2022, 13, 1058167. [Google Scholar] [CrossRef] [PubMed]
- Thomloudi, E.-E.; Tsalgatidou, P.C.; Baira, E.; Papadimitriou, K.; Venieraki, A.; Katinakis, P. Genomic and Metabolomic Insights into Secondary Metabolites of the Novel Bacillus halotolerans Hil4, an Endophyte with Promising Antagonistic Activity against Gray Mold and Plant Growth Promoting Potential. Microorganisms 2021, 9, 2508. [Google Scholar] [CrossRef]
- Tanveer, Y.; Yasmin, H.; Nosheen, A.; Farah, M.A.; Altaf, M.A. Synergizing Bacillus halotolerans, Pseudomonas sihuiensis and Bacillus atrophaeus with Folic Acid for Enhanced Drought Resistance in Wheat by Metabolites and Antioxidants. BMC Plant Biol. 2024, 24, 1003. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, L.; Li, X.; Zhang, C.; Liu, C.; Wu, Z. The Complete Genome Sequence of Bacillus halotolerans ZB201702 Isolated from a Drought- and Salt-Stressed Rhizosphere Soil. Microb. Pathog. 2018, 123, 246–249. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Wang, R.; Wang, Z.; Zhang, Y.; Gu, Q.; Farzand, A.; Yang, X.; Semenov, M.; Borriss, R.; et al. Genomic Features and Molecular Function of a Novel Stress-Tolerant Bacillus halotolerans Strain Isolated from an Extreme Environment. Biology 2021, 10, 1030. [Google Scholar] [CrossRef]
- Chen, K.; Tian, Z.; He, H.; Long, C.; Jiang, F. Bacillus Species as Potential Biocontrol Agents against Citrus Diseases. Biol. Control 2020, 151, 104419. [Google Scholar] [CrossRef]
- Soni, K.; Bagaria, A. GC-MS Based Identification of Anti-Microbial Bioactive Compounds, Isolated from Bacillus halotolerans of Marine Sediment. J. Exp. Mar. Biol. Ecol. 2024, 577, 152026. [Google Scholar] [CrossRef]
- Debez, I.B.S.; Alaya, A.B.; Karkouch, I.; Khiari, B.; Garcia-Caparros, P.; Alyami, N.M.; Debez, A.; Tarhouni, B.; Djébali, N. In Vitro and In Vivo Antifungal Efficacy of Individual and Consortium Bacillus Strains in Controlling Potato Black Scurf and Possible Development of Spore-Based Fungicide. Biol. Control 2024, 193, 105527. [Google Scholar] [CrossRef]
- Tsalgatidou, P.C.; Thomloudi, E.-E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated Genomic and Metabolomic Analysis Illuminates Key Secreted Metabolites Produced by the Novel Endophyte Bacillus halotolerans Cal.l.30 Involved in Diverse Biological Control Activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, B.; Bounabi, H.; Boukerb, A.M.; Gasmi, M. Insights into Genomic Features and Potential Biotechnological Applications of Bacillus halotolerans Strain HGR5. Pol. J. Microbiol. 2023, 72, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Borgio, J.F.; Alhujaily, R.; Alfaraj, A.S.; Alabdullah, M.J.; Alaqeel, R.K.; Kaabi, A.; Alquwaie, R.; Alhur, N.F.; AlJindan, R.; Almofty, S.; et al. Genome-Guided Identification of Surfactin-Producing Bacillus halotolerans AQ11M9 with Anti-Candida Auris Potential. Int. J. Mol. Sci. 2024, 25, 10408. [Google Scholar] [CrossRef]
- Li, P.; Feng, B.; Yao, Z.; Wei, B.; Zhao, Y.; Shi, S. Antifungal Activity of Endophytic Bacillus K1 Against Botrytis cinerea. Front. Microbiol. 2022, 13, 935675. [Google Scholar] [CrossRef]
- Surovy, M.Z.; Rahman, S.; Rostás, M.; Islam, T.; von Tiedemann, A. Suppressive Effects of Volatile Compounds from Bacillus spp. on Magnaporthe oryzae Triticum (MoT) Pathotype, Causal Agent of Wheat Blast. Microorganisms 2023, 11, 1291. [Google Scholar] [CrossRef]
- Grahovac, J.; Pajčin, I.; Vlajkov, V. Bacillus VOCs in the Context of Biological Control. Antibiotics 2023, 12, 581. [Google Scholar] [CrossRef]
- Ling, L.; Jiang, K.; Cheng, W.; Wang, Y.; Pang, M.; Luo, H.; Lu, L.; Gao, K.; Tu, Y. Biocontrol of Volatile Organic Compounds Obtained from Bacillus subtilis CL2 against Aspergillus flavus in Peanuts during Storage. Biol. Control 2022, 176, 105094. [Google Scholar] [CrossRef]
- Rafanomezantsoa, P.; Karkachi, N.; Gharbi, S.; Kihal, M. Antifungal Activity of Bacillus spp. against Fusarium oxysporum f. sp. lycopersici and Ascochyta sp. J. Plant Prot. Res. 2022, 62, 247–257. [Google Scholar]
- Rafanomezantsoa, P.; Gharbi, S.; Karkachi, N.; Kihal, M. Optimization of Amylase Production by the Biological Control Agent Bacillus halotolerans RFP74 Using Response Surface Methodology. J. Genet. Eng. Biotechnol. 2023, 21, 63. [Google Scholar] [CrossRef]
- Xia, Y.; Li, S.; Liu, X.; Zhang, C.; Xu, J.; Chen, Y. Bacillus halotolerans Strain LYSX1-Induced Systemic Resistance against the Root-Knot Nematode Meloidogyne javanica in Tomato. Ann. Microbiol. 2019, 69, 1227–1233. [Google Scholar] [CrossRef]
- Rana, A.; Sudakov, K.; Carmeli, S.; Miyara, S.B.; Bucki, P.; Minz, D. Volatile Organic Compounds of the Soil Bacterium Bacillus halotolerans Suppress Pathogens and Elicit Defense-Responsive Genes in Plants. Microbiol. Res. 2024, 281, 127611. [Google Scholar] [CrossRef] [PubMed]
- Çam, S.; Küçük, Ç.; Almaca, A. Bacillus Strains Exhibit Various Plant Growth Promoting Traits and Their Biofilm-Forming Capability Correlates to Their Salt Stress Alleviation Effect on Maize Seedlings. J. Biotechnol. 2023, 369, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Zhao, Q.; Yang, X.; Li, Y.; Zhou, H.; Zhao, M.; Zheng, H. Whole-Genome Analysis Revealed the Growth-Promoting and Biological Control Mechanism of the Endophytic Bacterial Strain Bacillus halotolerans Q2H2, with Strong Antagonistic Activity in Potato Plants. Front. Microbiol. 2023, 14, 1287921. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Chen, C.-C.; Ku, H.-T.; Wang, C.-L.; Wu, K.-M.; Weng, H.-Y.; Liu, S.-T.; Chen, C.-L.; Chiu, C.-H. Complete Genome Sequence of Bacillus halotolerans F29-3, a Fengycin-Producing Strain. Microbiol. Resour. Announc. 2024, 13, e01246-23. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Feng, Y.; Xiang, P.; Li, J.; Chen, L.; Guo, Y. Biocontrol Potential of Bacillus Strains against Soybean Cyst Nematode (Heterodera glycines) and for Promotion of Soybean Growth. BMC Microbiol. 2024, 24, 371. [Google Scholar] [CrossRef]
- Wang, D.; Sun, L.; Yu, H.; Zhang, C.; Guan, X.; Wang, M.; Cheng, R.; Wang, C.; Xie, Z. Whole-Genome Analysis of the Benzoic Acid-Degrading Bacterium Bacillus halotolerans B28 to Reveal Its Phytoprobiotic Effects. Int. Biodeterior. Biodegrad. 2023, 185, 105668. [Google Scholar] [CrossRef]
- Gao, J.; Khan, M.S.; Sun, Y.; Xue, J.; Du, Y.; Yang, C.; Chebotar, V.K.; Tikunov, V.S.; Rubanov, I.N.; Chen, X.; et al. Characterization of an Endophytic Antagonistic Bacterial Strain Bacillus halotolerans LBG-1-13 with Multiple Plant Growth-Promoting Traits, Stress Tolerance, and Its Effects on Lily Growth. BioMed Res. Int. 2022, 2022, 5960004. [Google Scholar] [CrossRef]
- Poria, V.; Jhilta, P.; Kumar, S.; Kumar, P.; Singh, S.; Rana, A.; Thankappan, S.; Goswami, A.K. Abiotic Stress Tolerance and Antifungal Activities of Rhizobacteria for the Management of Soil-Borne Pathogens. J. Saudi Soc. Agric. Sci. 2024, in press. [CrossRef]
- Zhang, L.; Li, W.; Tao, Y.; Zhao, S.; Yao, L.; Cai, Y.; Niu, Q. Overexpression of the Key Virulence Factor 1,3-1,4-β-d-Glucanase in the Endophytic Bacterium Bacillus halotolerans Y6 To Improve Verticillium Resistance in Cotton. J. Agric. Food Chem. 2019, 67, 6828–6836. [Google Scholar] [CrossRef]
- Memenza-Zegarra, M.; Ormeño-Orrillo, E.; Zúñiga-Dávila, D. Draft Genome Sequence of Bacillus halotolerans IcBac2.1, a Strain with Potential as a Phytopathogen Control Agent. Microbiol. Resour. Announc. 2022, 11, e0085722. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.; Patel, N.; Rana, A.; Vaidya, H.; Alfarraj, S.; Ansari, M.J.; Gafur, A.; Poczai, P.; Sayyed, R.Z. Evaluation of Plant Growth-Promoting and Salinity Ameliorating Potential of Halophilic Bacteria Isolated From Saline Soil. Front. Plant Sci. 2022, 13, 946217. [Google Scholar] [CrossRef] [PubMed]
- Ajuna, H.B.; Lim, H.-I.; Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Yun, J.-Y.; Ahn, Y.S. The Prospect of Hydrolytic Enzymes from Bacillus Species in the Biological Control of Pests and Diseases in Forest and Fruit Tree Production. Int. J. Mol. Sci. 2023, 24, 16889. [Google Scholar] [CrossRef]
- Bach, E.; dos Seger, G.D.S.; de Fernandes, G.C.; Lisboa, B.B.; Passaglia, L.M.P. Evaluation of Biological Control and Rhizosphere Competence of Plant Growth Promoting Bacteria. Appl. Soil Ecol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Sritongon, N.; Boonlue, S.; Mongkolthanaruk, W.; Jogloy, S.; Riddech, N. The Combination of Multiple Plant Growth Promotion and Hydrolytic Enzyme Producing Rhizobacteria and Their Effect on Jerusalem Artichoke Growth Improvement. Sci. Rep. 2023, 13, 5917. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Hombalimath, V.S.; Shaikh, I.A.; Muddapur, U.M.; Desai, S.V.; Achappa, S.; El-Sherbiny, M.; Ghoneim, M.M.; Jefri, O.A.; Alshahrani, M.M.; et al. Production of Extracellular Lipase by Bacillus halotolerans from Oil-Contaminated Soil in a Pilot-Scale Submerged Bioreactor. Processes 2022, 10, 1548. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Zhang, Z.; Luo, J.; Shen, B.; Zhang, R.; Shen, Q. Antagonist Bacillus subtilis HJ5 Controls Verticillium Wilt of Cotton by Root Colonization and Biofilm Formation. Biol. Fertil. Soils 2013, 49, 295–303. [Google Scholar] [CrossRef]
- Tian, T.; Sun, B.; Shi, H.; Gao, T.; He, Y.; Li, Y.; Liu, Y.; Li, X.; Zhang, L.; Li, S.; et al. Sucrose Triggers a Novel Signaling Cascade Promoting Bacillus subtilis Rhizosphere Colonization. ISME J. 2021, 15, 2723–2737. [Google Scholar] [CrossRef]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Pomerleau, M.; Charron-Lamoureux, V.; Léonard, L.; Grenier, F.; Rodrigue, S.; Beauregard, P.B. Adaptive Laboratory Evolution Reveals Regulators Involved in Repressing Biofilm Development as Key Players in Bacillus subtilis Root Colonization. mSystems 2024, 9, e00843-23. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Wees, S.C.M.V.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Fessia, A.; Sartori, M.; García, D.; Fernández, L.; Ponzio, R.; Barros, G.; Nesci, A. In Vitro Studies of Biofilm-Forming Bacillus Strains, Biocontrol Agents Isolated from the Maize Phyllosphere. Biofilm 2022, 4, 100097. [Google Scholar] [CrossRef]
- Elumalai, P.; Gao, X.; Cui, J.; Kumar, A.S.; Dhandapani, P.; Parthipan, P.; Karthikeyan, O.P.; Theerthagiri, J.; Kheawhom, S.; Choi, M.Y. Biofilm Formation, Occurrence, Microbial Communication, Impact and Characterization Methods in Natural and Anthropic Systems: A Review. Environ. Chem. Lett. 2024, 22, 1297–1326. [Google Scholar] [CrossRef]
- Santoyo, G.; del Orozco-Mosqueda, M.C.; Afridi, M.S.; Mitra, D.; Valencia-Cantero, E.; Macías-Rodríguez, L. Trichoderma and Bacillus Multifunctional Allies for Plant Growth and Health in Saline Soils: Recent Advances and Future Challenges. Front. Microbiol. 2024, 15, 1423980. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; Elrys, A.S.; El-Mageed, T.A.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.A.; Al Kafaas, S.S.; et al. Halotolerant Plant Growth-Promoting Rhizobacteria Improve Soil Fertility and Plant Salinity Tolerance for Sustainable Agriculture—A Review. Plant Stress 2024, 12, 100482. [Google Scholar] [CrossRef]
- Ravindran, A.; Manivannan, A.C.; Bharathi, G.S.J.; Balasubramanian, V.; Velmurugan, P.; Sivasubramanian, K.; Muruganandham, M.; Arumugam, N.; Almansour, A.I.; Kumar, R.S.; et al. Production and Characterization of Exopolysaccharide (EPS) from Marine Bacillus halotolerans and Its Antibacterial Activity against Clinical Pathogens. Biologia 2024, 79, 605–619. [Google Scholar] [CrossRef]
- Boulahouat, S.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Biocontrol Efficiency of Rhizospheric Bacillus against the Plant Pathogen Fusarium oxysporum: A Promising Approach for Sustainable Agriculture. Microbiol. Res. 2023, 14, 892–908. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Canette, A.; Aymerich, S.; Briandet, R. Should the Biofilm Mode of Life Be Taken into Consideration for Microbial Biocontrol Agents? Microb. Biotechnol. 2017, 10, 719–734. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Yang, J.; Zhang, Y.; Yu, P.; Cao, Y.; Fang, J.; Chen, S.; Li, L.; Sun, L.; et al. Auxin-Producing Bacteria Promote Barley Rhizosheath Formation. Nat. Commun. 2023, 14, 5800. [Google Scholar] [CrossRef]
- Lata, D.L.; Abdie, O.; Rezene, Y. IAA-Producing Bacteria from the Rhizosphere of Chickpea (Cicer arietinum L.): Isolation, Characterization, and Their Effects on Plant Growth Performance. Heliyon 2024, 10, e39702. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; Hasan, S.F.; Elhateir, M.M. Optimization of Indole-3-Acetic Acid Production by Bacillus velezensis Isolated from Pyrus Rhizosphere and Its Effect on Plant Growth. Biocatal. Agric. Biotechnol. 2023, 50, 102714. [Google Scholar] [CrossRef]
- Figueredo, E.F.; da Cruz, T.A.; de Almeida, J.R.; Batista, B.D.; Marcon, J.; de Andrade, P.A.M.; de Hayashibara, C.A.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The Key Role of Indole-3-Acetic Acid Biosynthesis by Bacillus thuringiensis RZ2MS9 in Promoting Maize Growth Revealed by the IpdC Gene Knockout Mediated by the CRISPR-Cas9 System. Microbiol. Res. 2023, 266, 127218. [Google Scholar] [CrossRef]
- Goud, M.S.; Sharma, S.K.; Kharbikar, L.L.; Prasanna, R.; Sangwan, S.; Dahuja, A.; Dixit, A. Bacillus Species Consortium with Tryptophan-Dependent and -Independent Pathways Mediated Production of IAA and Its Derivatives Modulates Soil Biological Properties, Growth and Yield of Wheat. Plant Soil 2024. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant Plant Growth–Promoting Bacteria: Prospects for Alleviating Salinity Stress in Plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Reang, L.; Bhatt, S.; Tomar, R.S.; Joshi, K.; Padhiyar, S.; Vyas, U.M.; Kheni, J.K. Plant Growth Promoting Characteristics of Halophilic and Halotolerant Bacteria Isolated from Coastal Regions of Saurashtra Gujarat. Sci. Rep. 2022, 12, 4699. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; Gui, Y.; Xu, F.; Liu, J.; Zhang, J.; Xu, W. Moderate Water Stress in Rice Induces Rhizosheath Formation Associated with Abscisic Acid and Auxin Responses. J. Exp. Bot. 2020, 71, 2740–2751. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, H.; Xu, F.; Ding, Y.; Gui, Y.; Zhang, J.; Xu, W. Root-Bacteria Associations Boost Rhizosheath Formation in Moderately Dry Soil through Ethylene Responses. Plant Physiol. 2020, 183, 780–792. [Google Scholar] [CrossRef]
- Orhan, F. Potential of Halophilic/Halotolerant Bacteria in Enhancing Plant Growth Under Salt Stress. Curr. Microbiol. 2021, 78, 3708–3719. [Google Scholar] [CrossRef]
- Khan, A.; Doshi, H.V.; Thakur, M.C. Bacillus spp.: A Prolific Siderophore Producer. In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 309–323. ISBN 978-3-319-44409-3. [Google Scholar]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Ghazanfar, S.; Hussain, A.; Dar, A.; Ahmad, M.; Anwar, H.; Al Farraj, D.A.; Rizwan, M.; Iqbal, R. Prospects of Iron Solubilizing Bacillus Species for Improving Growth and Iron in Maize (Zea mays L.) under Axenic Conditions. Sci. Rep. 2024, 14, 26342. [Google Scholar] [CrossRef]
- Santoyo, G.; Equihua, A.; Flores, A.; Sepulveda, E.; Valencia-Cantero, E.; Sanchez-Yañez, J.M.; Morales, L.R.; Govindappa, M.; de los Santos-Villalobos, S. Plant Growth Promotion by ACC Deaminase-Producing Bacilli Under Salt Stress Conditions. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Islam, M.T., Rahman, M.M., Pandey, P., Boehme, M.H., Haesaert, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 81–95. ISBN 978-3-030-15175-1. [Google Scholar]
- Misra, S.; Chauhan, P.S. ACC Deaminase-Producing Rhizosphere Competent Bacillus spp. Mitigate Salt Stress and Promote Zea mays Growth by Modulating Ethylene Metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Jain, S.; Varma, A.; Choudhary, D.K. Perspectives on Nitrogen-Fixing Bacillus Species. In Soil Nitrogen Ecology; Cruz, C., Vishwakarma, K., Choudhary, D.K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 359–369. ISBN 978-3-030-71206-8. [Google Scholar]
- Singh, R.K.; Singh, P.; Li, H.-B.; Song, Q.-Q.; Guo, D.-J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; et al. Diversity of Nitrogen-Fixing Rhizobacteria Associated with Sugarcane: A Comprehensive Study of Plant-Microbe Interactions for Growth Enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef]
- Jalal-Ud-Din, S.; Elahi, N.N.; Mubeen, F. Significance of Zinc-Solubilizing Plant Growth-Promoting Rhizobacterial Strains in Nutrient Acquisition, Enhancement of Growth, Yield, and Oil Content of Canola (Brassica napus L.). Front. Microbiol. 2024, 15, 1446064. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of Potassium Solubilizing Bacteria in Improved Potassium Assimilation and Cytosolic K+/Na+ Ratio in Rice (Oryza sativa L.) under Saline-Sodic Conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Srithaworn, M.; Jaroenthanyakorn, J.; Tangjitjaroenkun, J.; Suriyachadkun, C.; Chunhachart, O. Zinc Solubilizing Bacteria and Their Potential as Bioinoculant for Growth Promotion of Green Soybean (Glycine max L. Merr.). PeerJ 2023, 11, e15128. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. K+/Na+ Balance and Transport Regulatory Mechanisms in Weedy and Cultivated Rice (Oryza sativa L.) Under Salt Stress. BMC Plant Biol. 2018, 18, 375. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, M.S.; Mosharaf, M.K.; Haque, M.A.; Islam, M.S.; Nahar, K.; Islam, M.M.; Shozib, H.B.; Islam, M.M. Ferdous-E-Elahi Halotolerant Biofilm-Producing Rhizobacteria Mitigate Seawater-Induced Salt Stress and Promote Growth of Tomato. Sci. Rep. 2022, 12, 5599. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.; Yang, X.; Wang, X. Quantitative Changes of Plant Defense Enzymes and Phytohormone in Biocontrol of Cucumber Fusarium Wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a Bio-Control Agent Enhances the Activity of Antioxidant Defense Enzymes in Rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef]
- Díaz-Manzano, F.E.; Amora, D.X.; Martínez-Gómez, Á.; Moelbak, L.; Escobar, C. Biocontrol of Meloidogyne spp. in Solanum lycopersicum Using a Dual Combination of Bacillus Strains. Front. Plant Sci. 2023, 13, 1077062. [Google Scholar] [CrossRef]
- Migunova, V.D.; Sasanelli, N. Bacteria as Biocontrol Tool against Phytoparasitic Nematodes. Plants 2021, 10, 389. [Google Scholar] [CrossRef]
- Liu, G.; Lin, X.; Xu, S.; Liu, G.; Liu, F.; Mu, W. Screening, Identification and Application of Soil Bacteria with Nematicidal Activity against Root-Knot Nematode (Meloidogyne incognita) on Tomato. Pest Manag. Sci. 2020, 76, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Tileubayeva, Z.; Avdeenko, A.; Avdeenko, S.; Stroiteleva, N.; Kondrashev, S. Plant-Parasitic Nematodes Affecting Vegetable Crops in Greenhouses. Saudi J. Biol. Sci. 2021, 28, 5428–5433. [Google Scholar] [CrossRef] [PubMed]
- Harun, F.A.; Yakasai, H.M.; Jagaba, A.H.; Usman, S.; Umar, H.A.; Shukor, M.Y. Bioremediation Potential of Bacillus sp. and Paenebacillus sp. Novel Lead-Resistant Isolates: Identification, Characterization, and Optimization Studies. Microbe 2024, 3, 100087. [Google Scholar] [CrossRef]
- Khan, M.; Kamran, M.; Kadi, R.H.; Hassan, M.M.; Elhakem, A.; Sakit ALHaithloul, H.A.; Soliman, M.H.; Mumtaz, M.Z.; Ashraf, M.; Shamim, S. Harnessing the Potential of Bacillus altitudinis MT422188 for Copper Bioremediation. Front. Microbiol. 2022, 13, 878000. [Google Scholar] [CrossRef]
- Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of Heavy Metals by the Genus Bacillus. Int. J. Environ. Res. Public Health 2023, 20, 4964. [Google Scholar] [CrossRef]
- Deng, Z.; Jiang, Y.; Chen, K.; Gao, F.; Liu, X. Petroleum Depletion Property and Microbial Community Shift After Bioremediation Using Bacillus Halotolerans T-04 and Bacillus Cereus 1-1. Front Microbiol 2020, 11, 353. [Google Scholar] [CrossRef]
- Karaoğlan, B.; Alkassab, A.T.; Borges, S.; Fisher, T.; Link-Vrabie, C.; McVey, E.; Ortego, L.; Nuti, M. Microbial Pesticides: Challenges and Future Perspectives for Non-Target Organism Testing. Environ. Sci. Eur. 2024, 36, 205. [Google Scholar] [CrossRef]
- Lankinen, Å.; Witzell, J.; Aleklett, K.; Furenhed, S.; Karlsson Green, K.; Latz, M.; Liljeroth, E.; Larsson, R.; Löfkvist, K.; Meijer, J.; et al. Challenges and Opportunities for Increasing the Use of Low-Risk Plant Protection Products in Sustainable Production. A Review. Agron. Sustain. Dev. 2024, 44, 21. [Google Scholar] [CrossRef]
- Wend, K.; Zorrilla, L.; Freimoser, F.M.; Gallet, A. Microbial Pesticides—Challenges and Future Perspectives for Testing and Safety Assessment with Respect to Human Health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Kaleh, A.M.; Singh, P.; Mazumdar, P.; Chua, K.O.; Harikrishna, J.A. Halotolerant Rhizobacteria Isolated from a Mangrove Forest Alleviate Saline Stress in Musa acuminata Cv. Berangan. Microbiol. Res. 2022, 265, 127176. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafanomezantsoa, P.; El-Hasan, A.; Voegele, R.T. Potential of Bacillus halotolerans in Mitigating Biotic and Abiotic Stresses: A Comprehensive Review. Stresses 2025, 5, 24. https://doi.org/10.3390/stresses5020024
Rafanomezantsoa P, El-Hasan A, Voegele RT. Potential of Bacillus halotolerans in Mitigating Biotic and Abiotic Stresses: A Comprehensive Review. Stresses. 2025; 5(2):24. https://doi.org/10.3390/stresses5020024
Chicago/Turabian StyleRafanomezantsoa, Pelias, Abbas El-Hasan, and Ralf Thomas Voegele. 2025. "Potential of Bacillus halotolerans in Mitigating Biotic and Abiotic Stresses: A Comprehensive Review" Stresses 5, no. 2: 24. https://doi.org/10.3390/stresses5020024
APA StyleRafanomezantsoa, P., El-Hasan, A., & Voegele, R. T. (2025). Potential of Bacillus halotolerans in Mitigating Biotic and Abiotic Stresses: A Comprehensive Review. Stresses, 5(2), 24. https://doi.org/10.3390/stresses5020024







