Abstract
Mushrooms play an important role in ecosystem sustainability and are highly valued in medicine and human nutrition. Using AAS and biochemical methods of analysis, the antioxidant status and mineral composition of seven mushroom species (Armillaria mellea, Xeromocus illudens, Leccinum aurantiacum, Leccinum scrabum, Lactarium pubescens, Rusula vesca, and Lycoperpon molle Pers.) gathered near the Pechenganikel smelting plant in the Pasvik Nature Reserve of the Murmansk region were evaluated. The concentrations of Ni and Cu in the fruiting bodies of mushrooms were in the ranges of 0.43–39.7 and 7.9–45.9 mg kg−1 d.w., respectively. An unusually high biological concentration factor (BCF) for Ni, Cu, and Zn levels in mushrooms grown in soils with a low amount of these elements indicates the low suitability of the mentioned parameter for mushroom characteristics in territories with an uneven distribution of elements in soil. On the other hand, selenium (Se) showed high BCF levels, exceeding 1, for all mushrooms tested, with the highest values associated with L. saccatum (5.17) and the lowest values with A. mellea (1.36). A significant excess (3.4) of the Recommended Daily Allowance (RDA) level per 30 g of dry mushrooms was recorded for Ni in Russula vesca gathered 6 km from the Ni/Cu smelting plant, and 1.3 excess of the RDA was recorded in L. scrabum grown in the vicinity of the Shuonyoka waterfall. No RDA excess was revealed for Cu. Positive correlations between Se, polyphenol content, and total antioxidant activity (AOA) (r = 0.915–0.926; p < 0.001) and a negative correlation between Cu–Se and Cu–AOA in Leccinum species indicate the important role of antioxidant defense and Se, particularly in Arctic mushroom growth and survival, providing a specific protection of mushrooms against Cu toxicity.
1. Introduction
Fungi and higher mushrooms, in particular, form an intermediate link between soil and plants to optimize plant nutrition and water availability, protecting against different stresses such as drought, UV radiation, radionuclides, and heavy metals [1,2,3]. In this respect, Pleurotus spp. was shown to increase plant growth in metal-contaminated soils by providing more nutrients and reducing metal toxicity [1]. The large area of hyphae distribution [4] and mycelial age [5] are directly connected with the ability of mushrooms to concentrate metal ions. Furthermore, the accumulation of minerals in mushrooms is known to mainly depend on soil pH, redox potential, the amount of small size soil fraction, mineral and organic matter content, the presence of microorganisms in soils, genetic peculiarities and environmental stresses, growth phases, and the morphological part of the fruiting body [6,7,8,9,10].
The ability of mushrooms to accumulate large amounts of heavy metals also relates to the intensive biosynthesis of metallothionines [11,12,13] and the presence of a natural chelator, i.e., a negatively charged pigment melanin formed via the polymerization of indole and polyphenol derivatives [14].
In conditions of high anthropogenic pollution, the hyperaccumulation ability may cause significant ecological risks for consumers despite the well-known health-promoting properties of most edible mushrooms [15,16].
Indeed, the antioxidant, immunomodulatory, anti-carcinogenic, anti-pathogenic, and anti-inflammatory properties of edible mushrooms, along with their high nutritional value, make their utilization significant in the severe Arctic conditions of the Murmansk region. Most of these properties are directly connected with the ability of mushrooms to synthesize high levels of polyphenols, polysaccharides, phytosterols, and terpenoids [17]. Among polyphenols, phenolic acids predominate in mushrooms, with gallic and cinnamic acids being the most common ones. Other phenolic compounds, such as lignans, stilbene, and pyrogallol, play the role of phytoestrogens along with phytosterols [18].
The accumulation of these health-beneficial compounds may be accompanied by a significant increase in heavy metal content in the case of high environmental pollution [4,19]. Indeed, dangerous soil pollution in the vicinity of Ni/Cu mining and the processing plant remains a significant risk factor in the Pechenga district despite the plant closed in 2020. Since the end of the 20th century, environmental pollution due to Cu/Ni mining and processing at the Kola Peninsula has been intensively monitored [20,21,22,23,24]. Until 2020, the Pechenganikel complex of Ni/Cu mining and smelting was considered one of the most polluted areas of Russia, with intensive emissions of sulfur dioxide (SO2), Ni, and Cu, being released into the atmosphere and water reservoirs, which began in the 1930s [25]. The emissions of large amounts of sulfur oxides and the high soil acidity in the mentioned territory have represented for many decades additional risks connected with the increased bioavailability of heavy metals [20]. Intensive land degradation and an extremely low soil microorganism content [22] are further characteristics of this area.
The present research aimed to evaluate the role of antioxidants in the protection of higher mushrooms against oxidative stress caused by environmental pollution in the vicinity of the Ni/Cu smelting plant in the Arctic.
2. Results and Discussion
To evaluate the effect of Ni/Cu processing on mineral accumulation and antioxidant status of edible mushrooms, soil and mushrooms were sampled at seven places situated from 6 to 65.7 km from the plant (Figure 1).
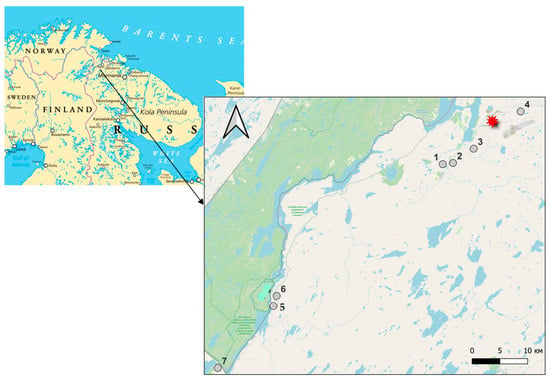
Figure 1.
Sampling places. The red star indicates the Ni/Cu smelting plant. Five and six—the territory of Pasvik Nature Reserve; seven—Rajakoski Settlement; one—Shuonyoka Waterfall.
2.1. Soil Characteristics
The data presented in Table 1 indicate the uneven distribution of Ni and Cu in soils neighboring the Ni/Cu smelting plant. Indeed, while a previous work demonstrated a gradual decrease in the total Ni/Cu content in soils with increasing distance from the Ni/Cu smelting plant [19], the results of the present investigation indicate the existence of a rather chaotic acid soluble Ni/Cu distribution, with the highest levels recorded both in the vicinity of the smelting plant and at the Nature Reserve Pasvik, the Rajakoski Settlement, and the Shuonyoka Waterfall situated far from the Ni/Cu smelting plant (locations 1, 5–7; Table 1). The latter phenomenon may relate to the existence of Ni/Cu deposits at the territory of Pasvik Nature Reserve and neighboring areas [26] and/or with climatic changes since the time of Ni/Cu smelting plant closure, due to leaching processes.

Table 1.
Acid soluble Ni, Cu, Fe, Zn, and Se content in soils of the tested area (mg kg−1 d.w.).
Furthermore, in the present work, soil extracts in 3% HNO3 were used, taking into account that acidic extraction provides information on biologically available elements both for plants and mushrooms, while the total soil content of minerals includes both bioavailable and not available fractions [27]. In this respect, the Athens data indicated a lack of significant correlations between total concentrations of heavy metals in soils and mushrooms, whereas bioavailable fractions of several metals were significantly correlated with their corresponding content in mushrooms [4].
As far as other elements are concerned, Fe and Se content in soils was in a rather narrow concentration range of 166–233 and 0.170–0.259 mg kg−1 d.w., respectively, which is in accordance with the low CV values. The lowest organic matter (OM) content was recorded at the sampling place 4, situated only 6.2 km far away from the smelting plant (OM = 1.6%), with low levels of Zn, Ni, and Cu, and the lowest value of pH, known to decrease soil fertility [23].
2.2. Mushroom Mineral Composition
The smallest distance between the mushroom sampling site and the Ni/Cu smelting plant was 6 km due to the intensive degradation of soils.
The present results (Table 2) indicate high variability between Ni, Cu, and Fe contents in mushrooms, reflecting both genetic variations in the ability to accumulate Ni, Cu, Fe, Zn, and Se, and the valuable effects of environmental factors. In this respect, Russila vesca showed the highest Ni concentration in the fruiting body at location 4 (6 km from the smelting plant), while L. aurantiacum demonstrated a high Ni content, both at location 4 and in the vicinity of a well-known waterfall situated 10.8 km from the plant (location 1). Copper contamination was recorded in the fruiting body of Russula vesca, 6 km from the smelting plant. On the contrary, puffballs displayed a high Cu content in the Pasvik Nature Reserve (location 6). Interestingly, all species gathered at various distances from the smelting plant demonstrated high levels of Se regardless of the low levels of the elements in soils. The results only partly confirm the large-scale research of Hansen et al. [20] about the peculiarities of Ni/Cu accumulation in local wild food from the border regions of Norway, Finland, and Russia where a significant decrease in Ni content in mushrooms with the increase in the distance from the smelting Pechenganikel plant was recorded, while the latter correlation was not detected for copper.

Table 2.
Mineral composition of mushrooms (mg kg−1 d.w.).
The comparison of Ni, Fe, Cu, and Zn content in mushrooms and plants gathered in the same area, 65.7 km far away from Ni/Cu smelting plant (sampling place 7), confirms the extremely high accumulation abilities of mushrooms, exceeding that of plants by approximately 10.5, 15.4, 91.9, and 29.6 times, respectively (Table 2 and Table 3).

Table 3.
The accumulation of microelements in plants at the sampling place 7 (mg kg−1 d.w.).
While the high coefficients of variation regarding mineral content in mushrooms reflect both species variability and differences in soil mineral content, the corresponding CV values relevant to the mineral composition of plants gathered in the vicinity of Rajakoski settlement relate to differences in plant ability to tolerate high levels of minerals, highlighting Salix caprea and Callúna vulgáris as the most powerful accumulators of Ni, Fe, and Cu. Nevertheless, Cu and Fe concentrations in the mentioned species were much lower than the corresponding values detected in Russia vesca sampled at the same place (Table 2 and Table 3).
2.3. Biological Concentration Factor (BCF)
In plants, the intensity of metal accumulation is clearly reflected by the value of the Biological Concentration Factor (BCF), though the application of this parameter to mushrooms may be debatable due to the enormous area of mycelia distribution. The calculated BCF levels for mushrooms revealed 106–150 values for Cu in L. aurantiacum and R. vesca 6 km far from the smelting plant, contrary to low levels of Ni and Cu in soil (Table 4).

Table 4.
Biologically concentration factor (BCF) of mushrooms sampled in the Pechenga region of Murmansk region.
Similar phenomena of high BCF values for Ni and Zn (1.14–6.00 and 138–184, respectively) at the location with low soil Ni/Zn and organic matter content (location 4) were recorded. Such a discrepancy may relate to the enormous area of hyphae distribution [4], fostering high Cu, Ni, and Zn accumulation in the mushroom fruiting body, and reflecting low suitability of the BCF for mushroom characteristics in conditions of significant variations in soil mineral composition.
However, it is worth highlighting that all tested mushrooms demonstrated increased levels of BCF for Zn and Se, in the range of 1.92–184 and 1.36–5.17, respectively (Table 4).
Most of the mushroom species examined are good Se accumulators [19], with the exception of Russula species known to accumulate high levels of Se only in Se-contaminated areas [19] and Armillaria mellea. In the inspected areas, Se BCF in Russula mushrooms exceeded 1, being in the range of 1.7–2.31. The high ability to accumulate Se has been previously discussed for Leccinum aurantiacum, L. scrabum, Xerocomus elludens, and Lycoperdon species [28]. In addition, high Se levels were also reported in L. pubescens and Russula vesca in the vicinity of the phosphorous fertilizer production plant [19]. Supposedly, the detected phenomenon may relate to the highly acidic soils of the territory tested and the adaptation of mushrooms, though further investigations are necessary to clarify the factors affecting Se accumulation in mushrooms.
The zinc hyperaccumulation ability of Russula species has been previously reported by Leonhardt et al. [29] who indicated the ability of peptides to effectively bind Zn. The present results suggest the existence of the mentioned ability in conditions of low soil Zn content in Arctic conditions.
2.4. Antioxidant Status of Mushrooms
The antioxidant status of Arctic mushrooms has been poorly investigated. The only exception is the work of Singh et al. [30], who demonstrated the high antioxidant activity of Lycoperdon molle Pers., which was positively correlated with the polysaccharide level. Furthermore, Burulianu et al. [31] found a positive correlation between Mn and flavonoid content in mushrooms in Romania.
In the present work, the redox titration method was used for the determination of the total antioxidant activity of mushrooms [32]. In this respect, the utilization of one and the same ethanolic extract for the antioxidant activity and polyphenol content determination, using the same standard (gallic acid) and the same units (mg GAE g−1 d.w.), allows to evaluate the intensity of polyphenol participation in the total antioxidant activity of mushroom extracts, compared to other possible antioxidants extracted with 70% EtOH. The present results show wide variations in antioxidant activity (AOA) and polyphenol content (TP) between different mushroom species, with the highest AOA and TP values recorded in Lycoperdon fruiting body (Table 5). The data presented in Table 5 reveal that polyphenols provided from 31.7 to 57.8% out of the total antioxidant activity of mushrooms, with the highest values in Leccinum auranticum (44.7–57.8%) and the lowest in Lycoperdon molle Pers. (31.7%).

Table 5.
The antioxidant status of mushrooms.
Furthermore, a positive correlation between the total antioxidant activity and polyphenol content arose, similarly to most of the terrestrial plants, which confirms the ubiquitous character of this phenomenon [33] (Figure 2).
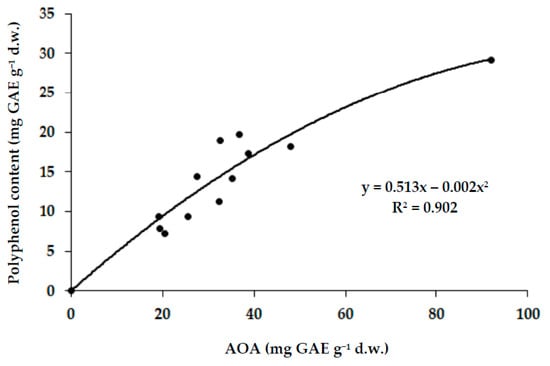
Figure 2.
Correlation between polyphenol content and antioxidant activity of Arctic mushrooms (r = 0.950; p < 0.001).
2.5. Mushroom Antioxidant/Se Defense Against Heavy Metal Uptake
A high antioxidant content in plants provides a powerful defense in conditions of environmental stresses, including heavy metal pollution [34]. The presented data about the Arctic mushroom antioxidant activity indicate a significant role of both polyphenols and Se in the antioxidant status, which is confirmed by significant coefficients of correlation between AOA, TP, and Se (Figure 3A,B).
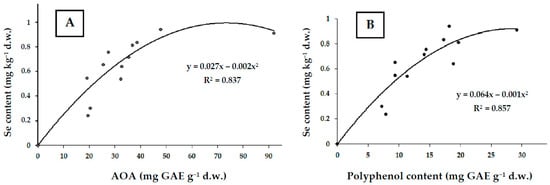
Figure 3.
Correlations between Se and AOA (A) and Se and TP (B) in Arctic mushrooms (r = 0.915 and r = 0.926, respectively; p < 0.001).
In this respect, AOA of mushrooms is also positively correlated to the BCF Se factor (Figure 4), suggesting the existence of a unique system of antioxidant defense composed of polyphenols, Se and, possibly, polysaccharides, based on Singh et al.’s [19] investigation.
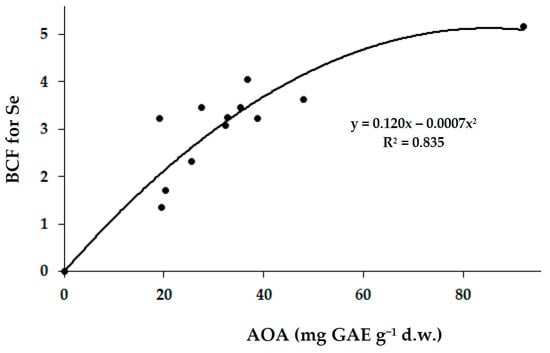
Figure 4.
Correlation between total antioxidant activity of Arctic mushrooms and Se biological concentration factor (r = 0.916; p < 0.001).
Selenium is a well-known antagonist of heavy metals, including Cu and Ni, providing remarkable protection to plants via improvement of the antioxidant defense, activation of phytohormones, and the regulation of genes involved in heavy metal resistance [35]. The protection effect of Se against the toxic effects of Cd and Pb [36] and against Hg in Pleurotus mushrooms has been previously confirmed [37,38]. In this respect, it would be tempting to reveal the relationship between Ni, Cu, and Se in Arctic mushrooms.
The present results indicate that there were no correlations between Ni and Se in the tested mushrooms, which may be attributed both to the detected uneven distribution of the mentioned elements in soils and species differences in Ni tolerance.
Taking into account the insignificant correlation between Se and Cu in the whole bulk of mushroom collection, we have supposed the existence of a powerful genetic influence on Se/Cu interaction. Irrespective of the sampling place, two species of Leccinum genus (L. aurantiacum and L. scrabum) demonstrated a negative correlation between Se and Cu levels (Figure 5A,B), suggesting the existence of Se protection against Cu toxicity.
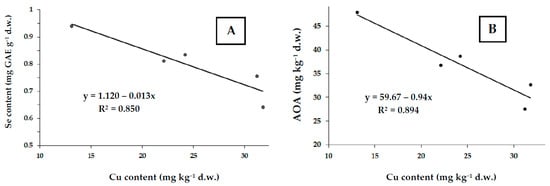
Figure 5.
Correlations between Cu and Se (A) and Cu and AOA (B) levels in (r = 0.922; p < 0.002 and r = 0.946; p < 0.001).
A similar correlation between total antioxidant activity and Cu content in Leccinum species (Figure 5) is in accordance with the confirmed protective effect of flavonoids against Cu toxicity in mushrooms [39]. This suggests the importance of antioxidant defense in mushrooms grown in Arctic conditions with high soil Cu content, known to inhibit their growth and development [40]. However, further investigations are needed to confirm the hypothesis mentioned.
2.6. Risks of Mushroom Consumption
The official regulations in force in the European Union countries do not report the maximum safe content of elements in dried wild-grown mushrooms. To assess the ecological risks of mushroom consumption, a daily dose of 30 g of dry mushrooms has been chosen (Table 6).

Table 6.
The daily consumption levels of Ni, Fe, Cu, Zn, and Se with 30 g of dry mushrooms (% of the Recommended Daily Allowance, RDA).
The results of the present investigation suggest that the consumption issues of mushrooms gathered in the vicinity of the smelting plant and near the Suonijoka Waterfall (location 1) may increase the risk of Ni-neurotoxicity [41]. Notably, relatively high concentrations of Se corresponding to 10–40.3% of the RDA of this microelement should be considered beneficial for human health due to the known antagonistic relationship between Se and heavy metals, decreasing the probability of cancer occurrence [42].
3. Material and Methods
3.1. Study Area and Sample Preparation
Research was carried out in 2023 and 2024 at a territory situated in the sub-Arctic region in the north-western part of Russia, in the near vicinity of Finland and Norway, in tundra and taiga soil with dissected mountainous terrain (Figure 1). The flat part is dominated by tundra, podzolic, bog-podzolic, swamp, and soddy soils. The territory includes: the close vicinity of the Ni/Cu smelting plant (sampling places 3,4 on the map); the neighborhood of the Pasvik River: the Pasvik Nature Reserve (sampling places 5,6), and the Rajakoski Settlement, 65 km far from the plant (number 7 on the map); and the neighborhood of the Shuonyoka Waterfall at the Shuonyoka River (number 1 on the map). The lack of forest was recorded only at sampling places 3 and 4, which were characterized by the existence of small shrubs and birches not higher than 2.0 m.
Five to fifteen fruit bodies of seven mushroom species (Armilaria mellea, Xeromocus illudens, Leccinum aurantiacum, Leccinum scrabum, Lactarium pubescens, Rusula vesca, and Lycoperpon saccatum) were sampled in late August 2023–2024, at seven sampling places indicated in Figure 1. The geographical coordinates of sampling places were determined using the GPS navigator (Table 7).

Table 7.
Geographical coordinates of mushroom and soil sampling places.
The identification of mushrooms was carried out according to Courtecuisse and Duhem [43], and Gorlenko et al. [44]. Fruit bodies were collected by a plastic knife and were not rinsed prior to chemical analysis since local people normally do not rinse them before consumption. In this way, we aimed at better estimating the true element concentrations the inhabitants are exposed to through ingestion. The mentioned materials were cut into thin slices, dried to constant weight at 70 °C, and homogenized to form a fine powder suitable for biochemical and elemental analysis. From the sites where mushrooms were harvested, the respective soil substrates (n = 13) were also obtained as follows: for each fruiting body collected, three soil samples were taken from the upper horizon (3–20 cm); the top organic layer including leaves, twigs, and other debris was discarded. The three soil samples were pooled together, air-dried, and sieved through a 2 mm sieve. To evaluate the mineral accumulation ability of mushrooms, additional comparisons were performed with the leaves of several plants gathered in the vicinity of the Rajakoski settlement (site 7, Figure 1): Vaccinium myrtillus, Vaccinium vitis-idaea, Calluna vulgaris, Salix caprea, Lycopodium, Urtica, and Equisetum arvense.
3.2. Determination of pH
For pH measurements, 1:1 w/v soil-to-water ratio slurries were used, and the determination was performed by an ionomer Expert-001 (Econix co, St-Petersburg, Russia).
3.3. Mineral Content
The mineral contents in mushrooms, plant leaves, and soil extracts (Ni, Cu, Zn, and Fe) were determined by atomic absorption spectrophotometry using a Shimadzu GFA-7000 spectrophotometer (Shimadzu, Kyoto, Japan). For AAS determinations, the relative standard deviation of three measurements was lower than 3%. Analysis was performed on 3% of HNO3 soil extracts (1:5) and on 3% of HNO3 mushroom/plant extracts after sample digestion at 425 °C [45]. The results reflected the means of three determinations and were expressed in mg per kg d.w.
3.4. Selenium Content
The concentration of Se in dried homogenized mushrooms was determined using the microfluorimetric method [46]. The soil content of this microelement was analyzed analogically using some modifications [27]. About 100 mg of dry, homogenized soil samples were digested with a 7:3 (v/v) mixture of nitric and perchloric acids, under the increasing temperature sequence of 120, 150, and 180 °C, each of them for 1 h, in 15 mL tubes. Then, the samples were cooled down to 150 °C and kept at this temperature for 10 min after supplying 1–2 drops of concentrated H2O2 to remove traces of nitric acid. Next, they were taken out of the digestion block, cooled, added with 1 mL of 6 N HCl, and kept at 120 °C for 10 min to reduce the selenate to Se+4. After the addition of 1 mL of distilled water and the delamination of solid and liquid phases, the upper layer was decanted, and 1 mL of 10% EDTA was added and mixed, and after phase separation, the upper layer was decanted again. The combined liquid extract was mixed with 1 mL of 10% EDTA and 1 mL of freshly produced 2,3-diaminonaphtalene solution in 1% HCl (0.1 g in 10 mL). Tubes were kept at 53 °C for half an hour, cooled, and the piasoselenol complex between selenite and 2,3-diaminonaphjtalene was extracted with 3 mL of hexane. Selenium content in hexane extracts was measured via the determination of fluorescence using 376 nm excitation and 519 nm emission on fluorimeter Fluorate 4M; (Lumex, Sanct-Petersburg, Russia). To evaluate the precision of the determination reference standards, a known Se content was used: soil from the experimental fields of the Federal Scientific Vegetable Centre and lyophilized mitsuba stem powder with Se content of 240 µg kg−1 and 1865 µg kg−1, respectively. All determinations were performed in triplicate.
3.5. Organic Matter (OM)
Organic matter content (OM) was determined by the Walkley–Black procedure [47].
3.6. Bio Concentration Factor (BCF)
The bio concentration factor (BCF) was calculated by dividing the element concentrations in mushrooms by their respective available concentrations in the underlying soil [48]:
BCF = Nimushroom/Nisoil
3.7. Antioxidant Activity (AOA)
The antioxidant activity of dried mushroom powder was assessed in ethanolic extracts after sample heating in 70% ethanol at 80 °C for 1 h using a redox titration method [32]. The values were expressed in mg gallic acid equivalents per g of dry weight (mg GAE g−1 d.w.).
3.8. Total Polyphenols (TPs)
Total polyphenols were determined in 70% ethanolic extracts of dried mushroom powder using the Folin–Ciocâlteu colorimetric method as previously described [32]. One gram of dry homogenate was extracted with 20 mL of 70% ethanol/water at 80 °C for 1 h. The mixture was cooled down and quantitatively transferred to a volumetric flask, and the volume was adjusted to 25 mL. The mixture was filtered through a filter paper, and 1 mL of the resulting solution was transferred to a 25 mL volumetric flask, to which 2.5 mL of saturated Na2CO3 solution and 0.25 mL of diluted (1:1) Folin–Ciocâlteu reagent were added. The volume was brought to 25 mL with distilled water. One hour later, the solutions were analyzed through a spectrophotometer (Unico 2804 UV, Suite E Dayton, NJ, USA) and the concentration of polyphenols was calculated according to the absorption of the reaction mixture at 730 nm. As an external standard, 0.02% gallic acid was used. The results were expressed as mg of gallic acid equivalent per g of dry weight (mg GAE g−1 d.w.).
3.9. Statistical Analysis
The mean values of three replicates were presented after the data were processed by analysis of variance and mean separations were performed through the Duncan’s multiple range test, with reference to 0.05 probability level, using SPSS software version 29 (Armonk, NY, USA).
4. Conclusions
The results of the present research confirm the existence of active Se participation in Arctic mushroom antioxidant defense, with a positive correlation between mushroom antioxidant status, polyphenol content, and Se, and negative correlations between Se—Cu and AOA—Cu in the fruiting body of the Leccinum species. The obtained outcomes suggest the low suitability of the BCF values for the mushroom Cu, Ni, and Zn accumulation ability at the areas of uneven soil mineral distribution. Health risks connected with wild mushroom consumption may relate to both the vicinity of the Ni/Cu smelting plant and the remote territories with naturally high levels of heavy metals situated in the southern part of Pasvik Nature Reserve, the Rajakoski Settlement and the Shuonyoka Waterfall. Further annual monitoring is desirable to confirm the general validity of the patterns found.
Author Contributions
Conceptualization: N.G. and G.C.; investigation: U.P., A.K., and E.S.; methodology: N.G., A.V.T., and O.C.M.; formal analysis: O.H. and N.P.; data curation: N.P. and G.C.; software: O.H. and A.V.T.; validation: N.G., E.S., O.C.M., and G.C.; supervision: N.G.; writing original draft: N.G. and U.P.; writing, review and editing: N.G., O.C.M., and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
Agreement No. 200-2023, 13 February 2023 ‘Comprehensive monitoring of ecosystems and their component biodiversity at the area neighboring Zapolyarny and Nikel of ‘Nornikel’ corporation and Pasvik Nature Reserve’. Sponsor ‘Kola Mining and Metallurgical Company’.
Institutional Review Board Statement
This manuscript does not contain any studies with human participants or animals performed by any author.
Data Availability Statement
The data that support the findings of this study are available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohamadhasani, F.; Rahimi, M. Growth response and mycoremediation of heavy metals by fungus Pleurotus sp. Sci. Rep. 2022, 12, 19947. [Google Scholar]
- Adriaensen, K.; Vealstad, T.; Nobel, J.-P.; Vangrobaveld, J.; Colpaert, J.V. Copper-adapted Suillus luteus, a symbolic solution for lines colonizing Xu mine soils. Appl. Environ. Microbiol. 2005, 71, 7279–7284. [Google Scholar]
- Colpaert, J.V.; Vandenkoornhuyse, P.; Adriaensen, K.; Vangronsveld, G. Genetic variation and heavy metal tolerance in the ectomycorrhizal Basidimycete Suillus luteus. New Phytol. 2000, 147, 367–379. [Google Scholar]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [PubMed]
- Lalotra, P.; Gupta, D.; Yangdol, R.; Sharma, Y.; Gupta, S. Bioaccumulation of heavy metals in the sporocarps of some wild mushrooms. Curr. Res. Environ. Appl. Mycol. 2016, 6, 159–165. [Google Scholar]
- Dowlati, M.; Sobhi, H.R.; Esrafili, A.; FarzadKia, M.; Yeganeh, M. Heavy metals content in edible mushrooms: A systematic review, meta-analysis and health risk assessment. Trends Food Sci. Technol. 2021, 109, 527–535. [Google Scholar]
- Qin, G.; Liu, J.; Zou, K.; He, F.; Li, Y.; Liu, R.; Zhang, P.; Zhao, G.; Wang, T.; Chen, B. Analysis of heavy metal characteristics and health risks of edible mushrooms in the mid-western region of China. Sci. Rep. 2024, 14, 26960. [Google Scholar]
- Širic, I.; Rukavina, K.; Miŏc, B.; Držaic, V.; Kumar, P.; Taher, M.A.; Eid, E.M. Bioaccumulation and health risk assessment of nickel uptake by five wild edible saprotrophic mushroom species collected from Croatia. Forests 2023, 14, 879. [Google Scholar] [CrossRef]
- Bucurica, I.A.; Dulama, I.D.; Radulescu, C.; Banica, A.L.; Stanescu, S.G. Heavy metals and associated risks of wild edible mushrooms consumption: Transfer factor, carcinogenic risk, and health risk index. J. Fungi 2024, 10, 844. [Google Scholar] [CrossRef]
- Radulescu, C.; Stihi, C.; Busuioc, G.; Gheboianu, A.I.; Popescu, I.V. Studies concerning heavy metals bioaccumulation of wild edible mushrooms from industrial area by using spectrometric techniques. Bull. Environ. Contam. Toxicol. 2010, 84, 641–646. [Google Scholar]
- Damodaran, D.; Balakrishnan, R.M.; Shetty, V.K. The uptake mechanism of Cd(II), Cr(VI), Cu(II), Pb(II), and Zn(II) by mycelia and fruiting bodies of Galerina vittiformis. BioMed Res. Int. 2013, 2013, 149120. [Google Scholar]
- Collin-Hansen, C.; Yttri, K.E.; Andersen, R.A.; Berthelsen, B.O.; Steinnes, E. Mushrooms from two metal-contaminated areas in Norway: Occurrence of metals and metallothionein-like proteins. Geochem. Explor. Environ. Anal. 2002, 2, 121–130. [Google Scholar]
- Kuziemska, B.; Wysokiński, A.; Jaremko, D.; Pakuła, K.; Popek, M.; Kożuchowska, M. The content of copper, zinc, and nickel in the selected species of edible mushrooms. Envrion. Prot. Nat. Res. 2019, 30, 7–10. [Google Scholar]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar]
- Phillips, J.M.; Ooi, S.L.; Pak, S.C. Health-promoting properties of medicinal mushrooms and their bioactive compounds for the COVID-19 era-an appraisal: Do the pro-health claims measure up? Molecules 2022, 27, 2302. [Google Scholar] [CrossRef]
- Árvay, J.; Tomáš, J.; Hauptvog, M.; Kopernická, M.; Kováčik, A.; Bajčan, D.; Massányi, P. Contamination of wild-grown edible mushrooms by heavy metals in a former mercury-mining area. J. Environ. Sci. Health B 2014, 4, 815–827. [Google Scholar]
- Zhou, Y.; Chu, M.; Ahmadi, F.; Agaa, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleri, H.A.R. A comprehensive review on phytochemical profiling in mushrooms: Occurrence, biological activities, applications and future prospective. Food Rev. Int. 2024, 40, 924–951. [Google Scholar]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules. 2019, 24, 917. [Google Scholar]
- Golubkina, N.; Mironov, J. Element composition of mushrooms in contrasting anthropogenic loading. Geochem. Int. 2018, 56, 1263–1275. [Google Scholar]
- Hansen, M.D.; Nøst, T.H.; Heimstad, E.S.; Evenset, A.; Dudarev, A.A.; Rautio, A.; Myllynen, P.; Dushkina, E.V.; Jagodic, M.; Christensen, G.N.; et al. The impact of a nickel-copper smelter on concentrations of toxic elements in local wild food from the Norwegian, Finnish, and Russian border regions. Int. J. Environ. Res. Public Health 2017, 14, 694. [Google Scholar]
- Gregurek, D.; Reimann, C.; Stumpf, E.F. Trace elements and precious metals in snow samples from the immediate vicinity of nickel processing plants, Kola Peninsula, northwest Russia. Environ. Pollut. 1998, 102, 221–232. [Google Scholar]
- Redkina, V.V.; Shalygina, R.R. Algae and cyanobacteria in soils polluted with heavy metals (Northwest Russia, Murmansk region). Czech Polar Rep. 2021, 11, 279–290. [Google Scholar]
- Evdokimova, G.A.; Mozgova, N.P.; Korneikova, M.V. The content and toxicity of heavy metals in soils affected by aerial emissions from the Pechenganikel plant. Eurasian Soil Sci. 2014, 47, 504–510. [Google Scholar]
- Nikonov, V.; Goryainova, V.; Lukina, N. Ni and Cu migration and accumulation in forest ecosystems on the Kola peninsula. Chemosphere 2001, 42, 93–100. [Google Scholar] [PubMed]
- Steinnes, E.; Lukina, N.; Nikonov, V.; Aamlid, D.; Royset, O. A gradient study of 34 elements in the vicinity of a copper-nickel smelter in the Kola peninsula. Environ. Monit. Assess. 2000, 60, 71–88. [Google Scholar]
- Makkonen, H.V.; Halkoaho, T.; Konnunaho, J.; Rasilainen, K.; Kontinen, A.; Eilu, P. Ni-(Cu-PGE) deposits in Finland—Geology and exploration potential. Ore Geol. Rev. 2017, 90, 667–696. [Google Scholar]
- Golubkina, N.; Sheshnitsan, S.; Koshevarov, A.; Pirogov, N.; Plotnikova, U.; Tallarita, A.V.; Murariu, O.C.; Merlino, L.; Caruso, G. Peculiarities of plant mineral composition in semi-desert conditions. Int. J. Plant Biol. 2024, 15, 1229–1249. [Google Scholar] [CrossRef]
- Falandysz, J. Selenium in edible mushrooms. J. Environ. Sci. Health C 2008, 26, 256–299. [Google Scholar]
- Leonhardt, T.; Borovička, J.; Sácký, J.; Šantrůček, J.; Kameník, J.; Kotrba, P. Zn overaccumulating Russula species clade together and use the same mechanism for the detoxification of excess Zn. Chemosphere 2019, 225, 618–626. [Google Scholar]
- Singh, P.; Singh, A.; D’Souza, L.M.; Roy, U.; Singh, S.M. Chemical constituents and antioxidant activity of the Arctic mushroom Pers. Polar Res. 2012, 31, 17329. [Google Scholar]
- Buruleanu, L.C.; Radulescu, C.; Georgescu, A.A.; Dulama, I.D.; Nicolescu, K.M.; Olteanu, R.L.; Stanescu, G. Chemometric assessment of the interactions between the metal contents, antioxidant activity, total phenolics, and flavonoids in mushrooms. Anal. Lett. 2019, 52, 1195–1214. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020. (In Russian) [Google Scholar] [CrossRef]
- Golubkina, N.; Skrypnik, L.; Logvinenko, L.; Zayachkovsky, V.; Smirnova, A.; Krivenkov, L.; Romanov, V.; Kharchenko, V.; Poluboyarinov, P.; Sekara, A.; et al. The ‘Edge Effect’ phenomenon in plants: Morphological, biochemical and mineral characteristics of border tissues. Diversity 2023, 15, 123. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Asgher, M.; Rehaman, A.; Islam, S.N.; Arshad, M.; Khan, N.A. Appraisal of functions and role of selenium in heavy metal stress adaptation in plants. Agriculture 2023, 13, 1083. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Naozuka, J.; Landero-Figuera, J.A. The protective role of selenium against uptake and accumulation of cadmium and lead in white oyster (Pleurotus ostreatus) and pink oyster (Pleurotus djamor) mushrooms. Food Addit. Contam. A 2022, 39, 508–524. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Naozuka, J.; Figueroa, J.A.L. Feasibility study for mercury remediation by selenium competition in Pleurotus mushrooms. J. Hazard. Mater. 2023, 451, 131098. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Mycoremediation potential of Pleurotus species for heavy metals: A review. Bioresour. Bioprocess. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Park, Y.-D.; Lee, J.-R.; Hahn, H.-S.; Lee, S.-J.; Bae, C.-D.; Yang, J.-M.; Kim, D.-E.; Hahn, M.-J. Inhibition kinetics of mushroom tyrosinase by copper-chelating ammonium tetrathiomolybdate. Biochim. Biophys. Acta (BBA) Gen. Subj. 2005, 1726, 115–120. [Google Scholar] [CrossRef]
- Hartikainen, S.; Lankinen, P.; Rajasärkkä, J.; Hilkka, K.; Virta, M.; Hatakka, A.; Kähkönen, M.A. Impact of copper and zinc on the growth of saprotrophic fungi and the production of extracellular enzymes. Boreal Environ. Res. 2012, 17, 210–218. [Google Scholar]
- Anyachor, C.P.; Dooka, D.B.; Orish, C.N.; Amadi, C.N.; Bocca, B.; Ruggieri, F.; Senofonte, M.; Frazzoli, C.; Orisakwe, O.E. Mechanistic considerations and biomarkers level in nickel-induced neurodegenerative diseases: An updated systematic review. IBRO Neurosci. Rep. 2022, 13, 136–146. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenium and selenium-antagonistic elements in nutritional cancer prevention. Crit. Rev. Biotechnol. 2009, 29, 10–17. [Google Scholar] [PubMed]
- Courtecuisse, R.; Duhem, B. (Eds.) Mushrooms and Toadstools of Britain and Europe; HarperCollins: London, UK, 1995. [Google Scholar]
- Gorlenko, M.V.; Bondartseva, M.A.; Garibova, L.V.; Sidorova, I.I.; Sizova, T.P. Mushrooms of USSR; Misl: Moscow, Russia, 1980. [Google Scholar]
- Kidin, V.V.; Derugin, I.P.; Kobzarenko, V.I. Workshop on Agrochemistry; Kolos: Moscow, Russia, 2008. [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America: Madison WI, USA, 1982; Chapter 2. [Google Scholar]
- Alonso, J.; Garcia, M.A.; Parez-Lopez, M.; Melgar, M.J. The concentrations and bioconcentration factors of copper and zinc in edible mushrooms. Arch. Environ. Contam. Toxicol. 2003, 44, 180–188. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).