Mycorrhizal Symbiosis and Water Deficit: Morphophysiological and Gene Expression Responses in Caatinga Passion Fruit
Abstract
1. Introduction
2. Results
2.1. Morpho-Agronomic and Physiological Descriptors of P. cincinnata with Different Inoculation Treatments Under Water Stress
2.1.1. Morpho-Agronomic Descriptors
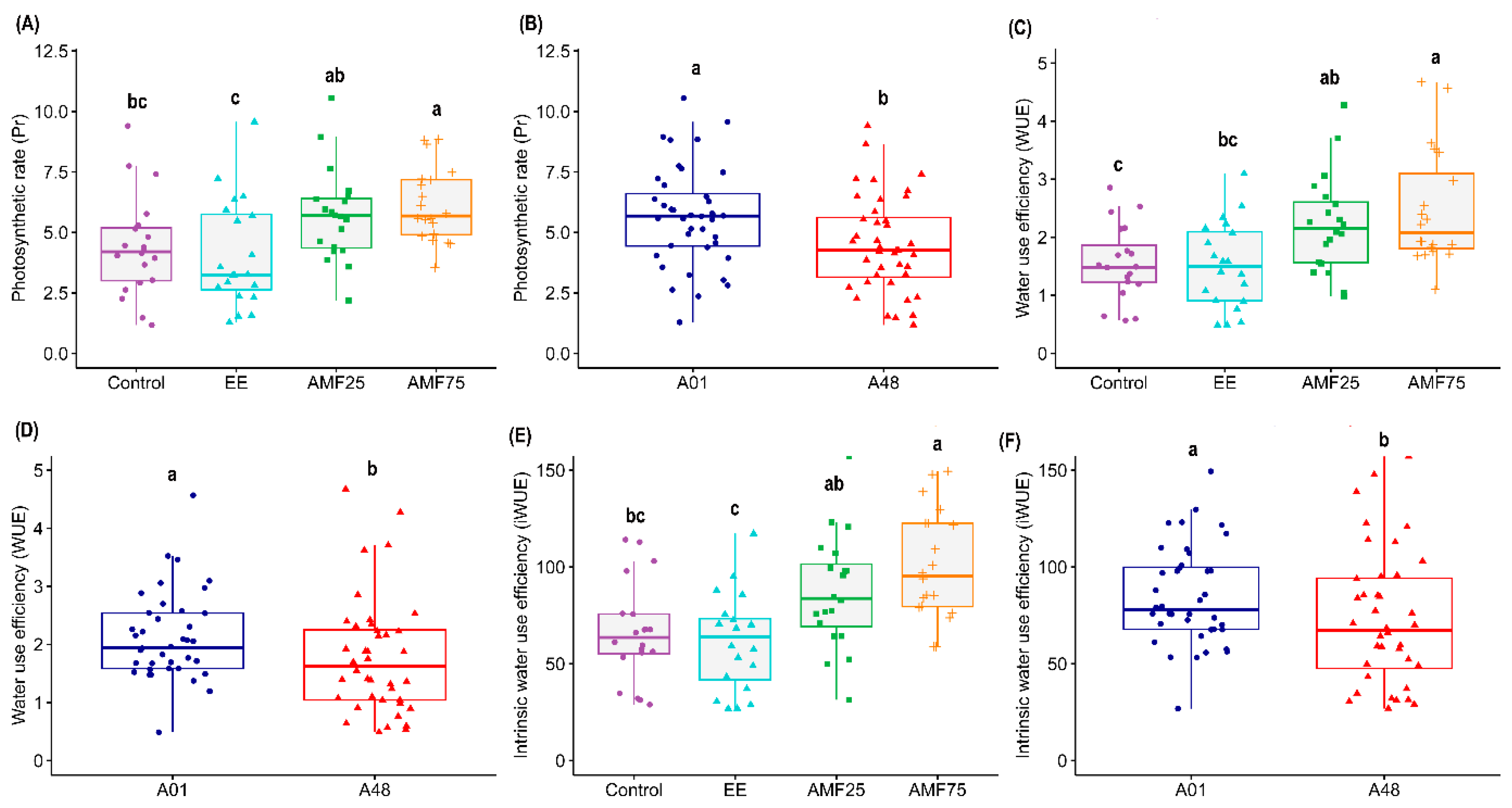
2.1.2. Physiological Descriptors
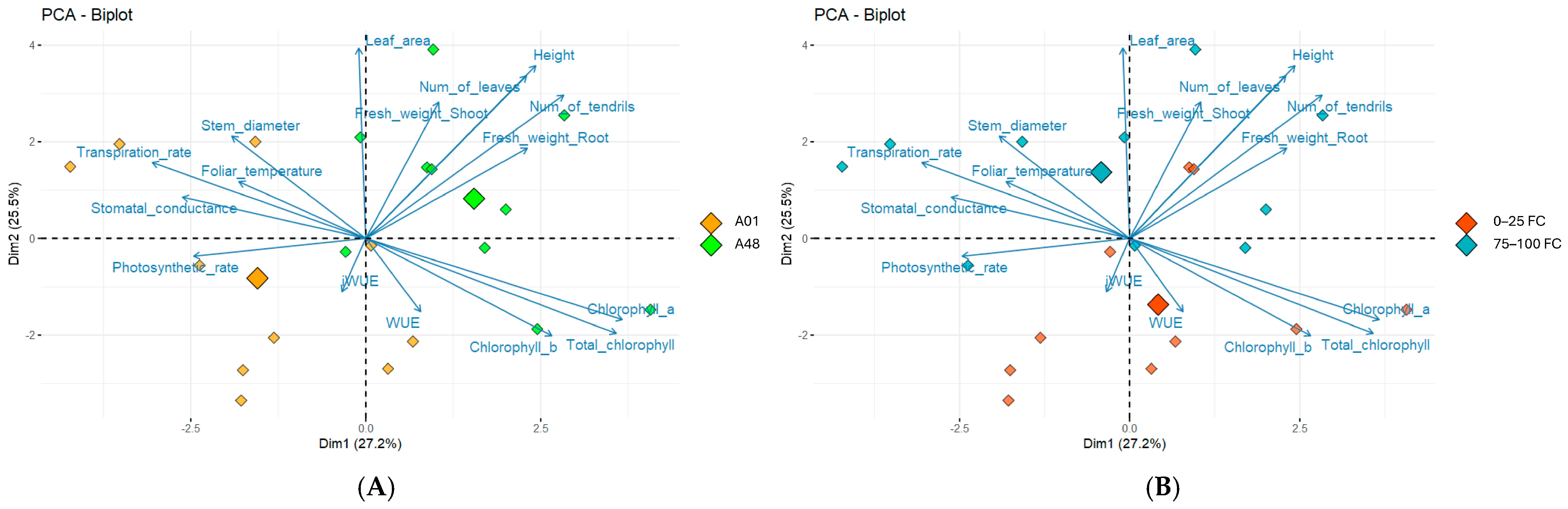
2.1.3. PCA Analysis of Morpho-Agronomic and Physiological Descriptors
2.1.4. Relative Mycorrhizal Responsiveness
2.2. Gene Expression, Colonization, and Mycorrhizal Abundance of P. cincinnata Inoculated with AMF Under Water Deficit Condition
2.2.1. Differential Gene Expression in P. cincinnata Accessions Inoculated with Native AMF Communities from Water Deficit Conditions (AMF25)
2.2.2. Mycorrhizal Colonization in P. cincinnata Accessions Inoculated with AMF Communities from Water Deficit Conditions (AMF25)
2.2.3. PCA in P. cincinnata Accessions Inoculated with AMF Communities from Water Deficit Conditions (AMF25)
2.2.4. Glomerospore Abundance and Native AMF Communities in P. cincinnata Accessions Inoculated with AMF Communities from Water Deficit Conditions (AMF25)
3. Discussion
3.1. Morpho-Agronomic and Physiological Descriptors of P. cincinnata Inoculated with AMF or Not, Under Contrasting Water Availability Conditions
3.2. Differential Gene Expression in P. cincinnata Inoculated with AMF Communities Originated from Water Deficit Conditions
4. Materials and Methods
4.1. Accessions of P. cincinnata
4.2. Inoculation with AMF
4.3. Microbial Filtrate
4.4. Microcosm Experiment Imposing Water Deficit on P. cincinnata Accessions
4.5. Morpho-Agronomic Descriptors
4.6. Physiological Descriptors
4.7. Mycorrhizal Responsiveness
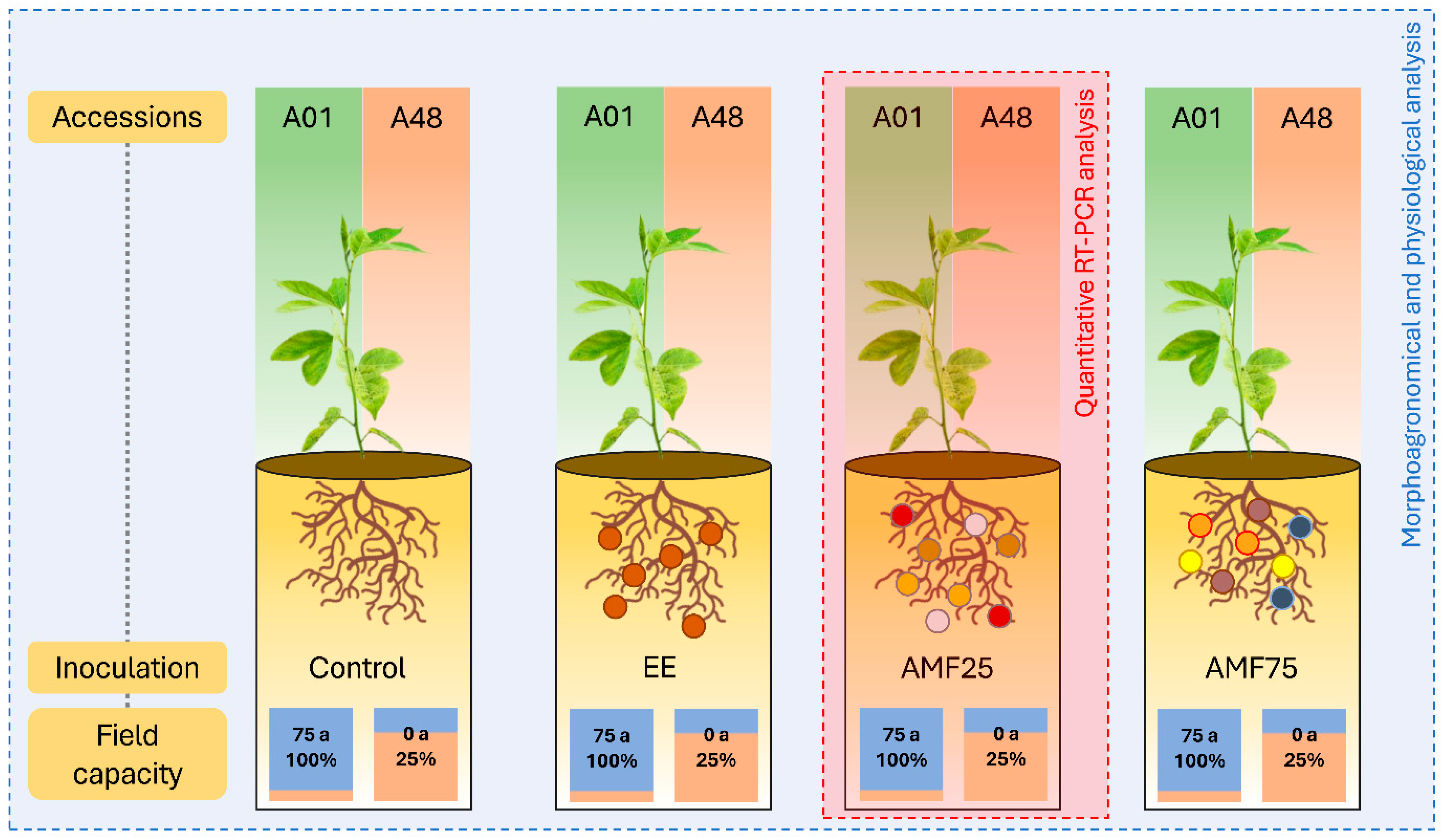
4.8. Experimental Design
4.8.1. Morpho-Agronomic and Physiological Characteristics and Mycorrhizal Responsiveness of P. cincinnata
4.8.2. Analysis of Differential Gene Expression in P. cincinnata
4.9. Differential Gene Expression Analysis and Its Relationship with Mycorrhizal Colonization and AMF Community
4.10. Molecular Analyses and Validation by Quantitative Real-Time PCR (qPCR)
4.11. Communities of Arbuscular Mycorrhizal Fungi and Mycorrhizal Colonization
4.12. Statistical Analyses
| Gene (Access Number) | Description | Function | Primers | Amplicon (bp) | Reference |
|---|---|---|---|---|---|
| RG—PcNDID (AB304270.1) | NADP-Dependent Isocitrate Dehydrogenase (IDH) | Responses to abiotic stress and associated with drought tolerance 1 | F: GTCGTCACTCTCTCTTTACG R: TCATTTCATCACCGTCCATC | 155 | [90] |
| RG—PcEF1α1 (DQ447160.1) | Translation Elongation Factor 1α-1 | Exhibits stable expression in drought and oxidative stress experiments 2 | F: GTTAAGGATTTGAAGCGTGG R: ATGTGTGATGTGTGGCAGT | 172 | [90] |
| PcSIP (JAEPBF010000225.1) | Small and basic intrinsic protein | Mobilizes water and responds to drought stress 3 | F: CGTGTCTCTCTTGTCGATGG R: TCACTTGCAGAATTGCCTTG | 83 | This study |
| PcLEA (JAEPBF010000087.1) | Late Embryogenesis Abundant | Involved in signaling pathways for abiotic stress responses 4 | F: GCAACAGGAGGGTCAAAATC R: ACCGTTGTCTTTGTGTCGTG | 118 | This study |
| PcbZIP (JAEPBF010000054.1) | Basic leucine zipper | Enhances expression of genes related to abiotic stress tolerance 5 | F: CAAAACGTGTGAGGAGGATG R: CAGATGGGCTTGCTTTCTTC | 74 | This study |
| PcCAT (JAEPBF010000191.1) | Catalase | Induced by ABA and linked to drought stress tolerance 6 | F: GAACAACACGCTCAGGGATG R: GCCCTATTCTGCTCGAGGAC | 81 | This study |
| PcSOD (JAEPBF010000343.1) | Superoxide dismutases | Responses to drought stress 7 | F: CAAAACCCATGGTGCTCCTG R: GCAGTGCCATCATCACCAAC | 81 | This study |
| PcSTK (JAEPBF010000187.1) | Serine/threonine protein kinase | Regulates drought and osmotic-stress tolerance 8 | F: AGTCGGCTCTATTGGCCTTC R: ACCGGGAAGGCTACAACAAG | 90 | This study |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.B.; Kim, S.H.; Bae, D.H. The impacts of global warming on arid climate and drought features. Theor. Appl. Climatol. 2023, 152, 693–708. [Google Scholar] [CrossRef]
- Nguvava, M.; Abiodun, B.J. Potential impacts of 1.5 °C and 2 °C global warming levels on drought modes over Eastern Africa. Clim. Change 2023, 176, 163. [Google Scholar] [CrossRef]
- Hadebe, S.T.; Modi, A.T.; Mabhaudhi, T. Drought tolerance and water use of cereal crops: A focus on sorghum as a food security crop in sub-Saharan Africa. J. Agron. Crop Sci. 2017, 203, 177–191. [Google Scholar] [CrossRef]
- Rosero, A.; Granda, L.; Berdugo-Cely, J.A.; Šamajová, O.; Šamaj, J.; Cerkal, R. A dual strategy of breeding for drought tolerance and introducing drought-tolerant, underutilized crops into production systems to enhance their resilience to water deficiency. Plants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Tsiupka, V.; Tsiupka, S.; Plugatar, Y.; Bulavin, I.; Komar-Tyomnaya, L. Assessment of the drought-tolerance criteria for screening peach cultivars. Horticulturae 2023, 9, 1045. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Ahmed, J.U.; Hasan, M.; Mohi-Ud-Din, M. Assessment of genetic variation among wheat genotypes for drought tolerance utilizing microsatellite markers and morpho-physiological characteristics. Heliyon 2023, 9, e21629. [Google Scholar] [CrossRef]
- Mkhabela, S.S.; Shimelis, H.; Gerrano, A.S.; Mashilo, J. Drought tolerance assessment of okra (Abelmoschus esculentus [L.] Moench) accessions based on leaf gas exchange and chlorophyll fluorescence. Life 2023, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Arslan, M.; Golukcu, M.; Bera, S.K.; Uzun, B.; Yol, E. Assessment of drought tolerance of sesame germplasm with agronomic and quality traits. Crop Sci. 2023, 63, 2763–2777. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Cao, P.; Zeng, X.; Xu, B.; Luo, F.; Yang, X.; Wang, X.; Wang, X.; Xiao, X.; et al. Morphological structure and physiological and biochemical responses to drought stress of Iris japonica. Plants 2023, 12, 3729. [Google Scholar] [CrossRef]
- Zagoub, K.; Krichen, K.; Chaieb, M.; Mnif, L.F. Morphological and physiological responses to drought stress of carob trees in Mediterranean ecosystems. J. Arid. Land 2023, 15, 562–577. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Olvera-Carrillo, Y.; Campos, F.; Reyes, J.L.; Garciarrubio, A.; Covarrubias, A.A. Functional analysis of the group 4 late embryogenesis abundant proteins reveals their relevance in the adaptive response during water deficit in Arabidopsis. Plant Physiol. 2010, 154, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Liu, F.; Sun, L.; Hao, F. Versatile roles of aquaporins in plant growth and development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; El-Kereamy, A.; Kim, S.H.; Nambara, E.; Rothstein, S.J. ANAC032 positively regulates age-dependent and stress-induced senescence in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 2029–2046. [Google Scholar] [CrossRef]
- Zong, W.; Tang, N.; Yang, J.; Peng, L.; Ma, S.; Xu, Y.; Li, G.; Xiong, L. Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes. Plant Physiol. 2016, 171, 2810–2825. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Ruggiero, B.; Koiwa, H.; Manabe, Y.; Quist, T.M.; Inan, G.; Saccardo, F.; Joly, R.J.; Hasegawa, P.M.; Bressan, R.A.; Maggio, A. Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol. 2004, 136, 3134–3147. [Google Scholar] [CrossRef]
- Zupin, M.; Sedlar, A.; Kidrič, M.; Meglič, V. Drought-induced expression of aquaporin genes in leaves of two common bean cultivars differing in tolerance to drought stress. J. Plant Res. 2017, 130, 735–745. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Feng, H.; Yang, J.; Li, C. Characterization of the gene expression profile response to drought stress in Populus ussuriensis using PacBio SMRT and Illumina sequencing. Int. J. Mol. Sci. 2022, 23, 3840. [Google Scholar] [CrossRef]
- Xu, W.; Wuyun, T.; Chen, J.; Yu, S.; Zhang, X.; Zhang, L. Responses of Trollius chinensis to drought stress and rehydration: From photosynthetic physiology to gene expression. Plant Physiol. Biochem. 2023, 201, 107841. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Meng, P.P.; Dou, Q.; Zhang, S.X.; Wang, H.H.; Wang, C.Y. Advances in mechanisms of nutrient exchange between mycorrhizal fungi and host plants. J. Appl. Ecol. 2019, 30, 3596–3604. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of Vitis vinifera L. cv. Ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Souza, P.U.; Lima, L.K.S.; Soares, T.L.; de Jesus, O.N.; Coelho Filho, M.A.; Girardi, E.A. Biometric, physiological and anatomical responses of Passiflora. spp. to controlled water deficit. Sci. Hortic. 2018, 229, 77–90. [Google Scholar] [CrossRef]
- Lozano-Montaña, P.A.; Sarmiento, F.; Mejía-Sequera, L.M.; Álvarez-Flórez, F.; Melgarejo, L.M. Physiological, biochemical and transcriptional responses of Passiflora edulis Sims f. edulis under progressive drought stress. Sci. Hortic. 2021, 275, 109655. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, L.; Ghani, M.I.; Peng, Q.; Fan, R.; Hu, X.; Chen, X. Effects of drought stress induced by hypertonic polyethylene glycol (PEG-6000) on Passiflora edulis Sims physiological properties. Plants 2023, 12, 2296. [Google Scholar] [CrossRef]
- Song, S.; Zhang, D.; Ma, F.; Xing, W.; Huang, D.; Wu, B.; Chen, J.; Chen, D.; Xu, B.; Xu, Y. Genome-wide identification and expression analyses of the aquaporin gene family in passion fruit (Passiflora edulis), revealing PeTIP3-2 to be involved in drought stress. Int. J. Mol. Sci. 2022, 23, 5720. [Google Scholar] [CrossRef]
- Sazegari, S.; Niazi, A.; Ahmadi, F.S. A study on the regulatory network with promoter analysis for Arabidopsis DREB-genes. Bioinformation 2015, 11, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Downie, B.; Gurusinghe, S.; Dahal, P.; Thacker, R.R.; Snyder, J.C.; Nonogaki, H.; Yim, K.; Fukanaga, K.; Alvarado, V.; Bradford, K.J. Expression of a GALACTINOL SYNTHASE gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented. Plant Physiol. 2003, 131, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Chekol, H.; Warkineh, B.; Shimber, T.; Mierek-Adamska, A.; Dąbrowska, G.B.; Degu, A. Drought Stress Responses in Arabica Coffee Genotypes: Physiological and Metabolic Insights. Plants 2024, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Resmi, L.; Nair, A.S. Evaluation of drought stress responses in two different banana genotypes from India. S. Afr. J. Bot. 2024, 165, 282–289. [Google Scholar] [CrossRef]
- de Araújo, F.P.; da Silva, N.; de Queiroz, M.A. Divergência genética entre acessos de Passiflora cincinnata Mast. com base em descritores morfoagronômicos. Rev. Bras. Frutic. 2008, 30, 723–730. [Google Scholar] [CrossRef]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; dos Santos, O.J.A.P.; Moreira, R.S.; Moreira, A.F.P.; Gonçalves, L.S.A. Physiological, biochemical and morphoagronomic characterization of drought-tolerant and drought-sensitive bean genotypes under water stress. Physiol. Mol. Biol. Plants 2018, 24, 1059–1067. [Google Scholar] [CrossRef]
- Luo, Z.; Brock, J.; Dyer, J.M.; Kutchan, T.; Schachtman, D.; Augustin, M.; Ge, Y.; Fahlgren, N.; Abdel-Haleem, H. Genetic diversity and population structure of a Camelina sativa spring panel. Front. Plant Sci. 2019, 10, 184. [Google Scholar] [CrossRef]
- Mengistu, G.; Shimelis, H.; Laing, M.; Lule, D.; Assefa, E.; Mathew, I. Genetic diversity assessment of sorghum (Sorghum bicolor (L.) Moench) landraces using SNP markers. S. Afr. J. Plant Soil 2020, 37, 220–226. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Al-Atar, A.A.; Abdel-Salam, E.M.; El-Sheikh, M.A.; Gaafar, A.Z.; Faisal, M. Genetic Diversity and Structure Analysis of a Worldwide Collection of Faba bean (Vicia. faba) Genotypes using ISSR Markers. Int. J. Agric. Biol. 2021, 25, 683–691. [Google Scholar] [CrossRef]
- Dantas, L.V.A.; Yano-Melo, A.M.; de Melo, N.F. Water availability and accessions of Passiflora cincinnata Mast. can shape the communities of arbuscular mycorrhizal fungi. Rhizosphere 2024, 31, 100945. [Google Scholar] [CrossRef]
- Welles, S.R.; Funk, J.L. Patterns of intraspecific trait variation along an aridity gradient suggest both drought escape and drought tolerance strategies in an invasive herb. Ann. Bot. 2021, 127, 461–471. [Google Scholar] [CrossRef]
- Valladares, F.; Vilagrosa, A.; Peñuelas, J.; Ogaya, R.; Camarero, J.J.; Corcuera, L.; Sisó, S.; Gil-Pelegrín, E. Estrés hídrico: Ecofisiología y escalas de la sequía. In Ecología del Bosque Mediterráneo en un Mundo Cambiante; Valladares, F., Ed.; Asociación Española de Ecología Terrestre: Madrid, Spain, 2004; pp. 165–192. [Google Scholar]
- Pappula-Reddy, S.P.; Pang, J.; Chellapilla, B.; Kumar, S.; Dissanayake, B.M.; Pal, M.; Millar, A.H.; Siddique, K.H.M. Insights into chickpea (Cicer arietinum L.) genotype adaptations to terminal drought stress: Evaluating water-use patterns, root growth, and stress-responsive proteins. Environ. Exp. Bot. 2024, 218, 105579. [Google Scholar] [CrossRef]
- Doddaraju, P.; Dharmappa, P.M.; Thiagarayaselvam, A.; Vijayaraghavareddy, P.; Bheemanahalli, R.; Basavaraddi, P.A.; Malagondanahalli, M.K.V.; Kambalimath, S.; Thulasiram, H.V.; Sreeman, S.M. Comprehensive analysis of physiological and metabolomic responses to drought reveals specific modulation of acquired tolerance mechanisms in rice. Physiol. Plant. 2023, 175, 13917. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Fang, X.; Ren, Y.; You, Z.; Xia, J.; Hakeem, A.; Yang, Y.; Wang, L.; Fang, J.; et al. The physiology of drought stress in two grapevine cultivars: Photosynthesis, antioxidant system, and osmotic regulation responses. Physiol. Plant 2023, 175, 14005. [Google Scholar] [CrossRef]
- Conti, V.; Mareri, L.; Faleri, C.; Nepi, M.; Romi, M.; Cai, G.; Cantini, C. Drought stress affects the response of Italian local tomato (Solanum lycopersicum L.) varieties in a genotype-dependent manner. Plants 2019, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, J.L.; Aguayo, P.; Conejera, D.; Rubilar, R.; Balocchi, C.; Valenzuela, S. Transcriptomic response in foliar and root tissues of a drought-tolerant Eucalyptus globulus genotype under drought stress. Trees 2022, 36, 697–709. [Google Scholar] [CrossRef]
- Abdel-Salam, E.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018, 25, 1772–1780. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Ait-Rahou, Y.; Meddich, A. Assemblage of indigenous arbuscular mycorrhizal fungi and green waste compost enhance drought stress tolerance in carob (Ceratonia siliqua. L.) trees. Sci. Rep. 2021, 11, 22835. [Google Scholar] [CrossRef]
- Pedone-Bonfim, M.V.L.; da Silva, D.K.A.; Maia, L.C.; Yano-Melo, A.M. Mycorrhizal benefits on native plants of the Caatinga, a Brazilian dry tropical forest. Symbiosis 2018, 74, 79–88. [Google Scholar] [CrossRef]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Lounès-Hadj Sahraoui, A.; Ben Jeddi, F. Contribution of native and exotic arbuscular mycorrhizal fungi in improving the physiological and biochemical response of hulless barley (Hordeum vulgare ssp. nudum L.) to drought. J. Soil Sci. Plant Nutr. 2022, 22, 2187–2204. [Google Scholar] [CrossRef]
- Soares, A.C.F.; Martins, M.A. Influência de fungos micorrízicos arbusculares, associada à adição de compostos fenólicos, no crescimento de mudas de maracujazeiro amarelo (Passiflora edulis f. flavicarpus). Rev. Bras. Cienc. Solo 2000, 24, 731–740. [Google Scholar] [CrossRef]
- Cavalcante, U.M.T.; Maia, L.C.; Costa, C.M.C.; Santos, V.F. Mycorrhizal dependency of passion fruit (Passiflora edulis f. flavicarpa). Fruits 2001, 56, 317–324. [Google Scholar] [CrossRef]
- Gil, J.G.R.; Agudelo, M.M.; Bedoya, L.O.; Osorio, N.W.; Osorio, J.G.M. Germination and growth of purple passion fruit seedlings under pre-germination treatments and mycorrhizal inoculation. Pesqui. Agropec. Trop. 2015, 45, 257–265. [Google Scholar] [CrossRef][Green Version]
- Anjos, E.C.T. Mycorrhizal Dependency of Sweet Passion Fruit (Passiflora alata) and Behavior of Mycorrhizal Seedlings to Parasitism by The Root-Knot Nematode Meloidogyne Incognita Race 1. Master’s Dissertation, Federal University of Pernambuco, Pernambuco, Brazil, 2004. UFPE Campus Repository. Available online: https://repositorio.ufpe.br/bitstream/123456789/691/1/arquivo4507_1.pdf (accessed on 4 January 2025).
- Silva, E.M.; de Melo, N.F.; Mendes, A.M.S.; de Araújo, F.P.; Maia, L.C.; Yano-Melo, A.M. Response of Passiflora setacea to mycorrhization and phosphate fertilization in a semiarid region of Brazil. J. Plant Nutr. 2015, 38, 431–442. [Google Scholar] [CrossRef]
- Cavagnaro, T.R. Soil moisture legacy effects: Impacts on soil nutrients, plants and mycorrhizal responsiveness. Soil Biol. Biochem. 2016, 95, 173–179. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Parke, J.L.; Mueller, S.M.; Senior, L.; Stuber, C.; Tracy, W.F. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 2000, 40, 358–364. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, X.; Yang, Y.; Chen, F.; Zhang, F.; Feng, G. Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza 2013, 23, 497–505. [Google Scholar] [CrossRef]
- Erdinc, C.; Durak, E.D.; Ekincialp, A.; Sensoy, S.; Demir, S. Variations in response of determinate common bean (Phaseolus vulgaris L.) genotypes to arbuscular mycorrhizal fungi (AMF) inoculation. Turk. J. Agric. For. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Sensoy, S.; Demir, S.; Turkmen, O.; Erdinc, C.; Savur, O.B. Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci. Hortic. 2007, 113, 92–95. [Google Scholar] [CrossRef]
- Taylor, A.; Pereira, N.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Bending, G.D. Growth and nutritional responses to arbuscular mycorrhizal fungi are dependent on onion genotype and fungal species. Biol. Fert. Soils 2015, 51, 801–813. [Google Scholar] [CrossRef]
- Abdelhalim, T.; Jannoura, R.; Joergensen, R.G. Arbuscular mycorrhizal dependency and phosphorus responsiveness of released, landrace and wild Sudanese sorghum genotypes. Arch. Agron. Soil Sci. 2020, 66, 706–716. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Ahmadi, J.; Golkari, S.; Sadeghzadeh, B. Expression profiling of PAP3, BZIP, and P5CS genes in soybean underdrought stress conditions. Turk. J. Bot. 2015, 39, 952–961. [Google Scholar] [CrossRef]
- Tu, M.; Wang, X.; Feng, T.; Sun, X.; Wang, Y.; Huang, L.; Gao, M.; Wang, Y.; Wang, X. Expression of a grape (Vitis vinifera) bZIP transcription factor, VlbZIP36, in Arabidopsis thaliana confers tolerance of drought stress during seed germination and seedling establishment. Plant Sci. 2016, 252, 311–323. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Xu, J.; Duan, S.; Wang, Q.; Li, G.; Jin, L. Transcriptome profiling reveals effects of drought stress on gene expression in diploid potato genotype P3-198. Int. J. Mol. Sci. 2019, 20, 852. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Zhao, Q.; Han, Y.; Li, L.; Sun, C.; Zhang, M. Basic leucine zipper (bZIP) transcription factor genes and their responses to drought stress in ginseng, Panax ginseng C.A. Meyer. BMC Genom. 2021, 22, 316. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, C.; Yin, H.; Liu, H.; Luo, H.; He, M.; Zhou, Y. CgbZIP1: A bZIP transcription factor from Chrysanthemum grandiflora confers plant tolerance to salinity and drought stress. Agronomy 2022, 12, 556. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, T.; Kang, Y.; Li, W.; Wang, J.; Yu, W.; Zhou, Y. Identification of the bZIP gene family and investigation of their response to drought stress in Dendrobium catenatum. Agronomy 2023, 13, 236. [Google Scholar] [CrossRef]
- Liu, J.; Qin, G.; Liu, C.; Liu, X.; Zhou, J.; Li, J.; Lu, B.; Zhao, J. Genome-wide identification of candidate aquaporins involved in water accumulation of pomegranate outer seed coat. PeerJ 2021, 9, e11810. [Google Scholar] [CrossRef]
- He, H.; Wang, Q.; Wang, L.; Yang, K.; Yang, R.; You, C.; Ke, J.; Wu, L. Photosynthetic physiological response of water-saving and drought-resistant rice to severe drought under wetting-drying alternation irrigation. Physiol. Plant 2021, 173, 2191–2206. [Google Scholar] [CrossRef] [PubMed]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, Z.S.; Yusop, M.R.; Ismail, M.R.; Mohamed, M.T.M.; Harun, A.R.; Yusuff, O.; Magaji, U.; Fatai, A. LEA gene expression assessment in advanced mutant rice genotypes under drought stress. Int. J. Genom. 2019, 2019, 8406036. [Google Scholar] [CrossRef]
- Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Wang, J.; Yu, Q. The LEA gene family in tomato and its wild relatives: Genome-wide identification, structural characterization, expression profiling, and role of SlLEA6 in drought stress. BMC Plant Biol. 2022, 22, 596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lei, X.; Wang, Y.; Di, P.; Meng, X.; Peng, W.; Rong, J.; Wang, Y. Genome-wide identification of the LEA gene family in Panax ginseng: Evidence for the role of PgLEA2-50 in plant abiotic stress response. Plant Physiol. Biochem. 2024, 212, 108742. [Google Scholar] [CrossRef]
- Sanchita, R.S.; Mishra, A.; Dhawan, S.S.; Shirke, P.A.; Gupta, M.M.; Sharma, A. Physiological performance, secondary metabolite and expression profiling of genes associated with drought tolerance in Withania somnifera. Protoplasma 2015, 252, 1439–1450. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhang, J.; Ma, Y.; Li, Y.; Zhang, F.; Zhang, Y.; Liang, Y. Overexpression of a Mitogen-Activated Protein Kinase SlMAPK3 Positively Regulates Tomato Tolerance to Cadmium and Drought Stress. Molecules 2019, 24, 556. [Google Scholar] [CrossRef]
- Liu, X.; Cui, Y.; Wu, Z.; Zhao, Y.; Hu, X.; Bi, Q.; Yang, S.; Wang, L. Transcriptome and Co-Expression Network Analyses Identify the Molecular Signatures Underlying Drought Resistance in Yellowhorn. Forests 2020, 11, 840. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhao, X.; Liu, L.; Tang, Q.; Fu, J.; Tang, X.; Yang, R.; Lin, J.; Liu, X.; et al. Receptor-like cytoplasmic kinase STK confers salt tolerance in rice. Rice 2023, 16, 21. [Google Scholar] [CrossRef]
- Gryndler, M.; Šmilauer, P.; Püschel, D.; Bukovská, P.; Hršelová, H.; Hujslová, M.; Gryndlerová, H.; Beskid, O.; Konvalinková, T.; Jansa, J. Appropriate nonmycorrhizal controls in arbuscular mycorrhiza research: A microbiome perspective. Mycorrhiza 2018, 28, 435–450. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants Without Soils; California Agricultural Experimental Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Jarstfer, A.G.; Sylvia, D.M. Inoculum production and inoculation strategies for vesicular-arbuscular mycorrhizal fungi. In Soil Microbial Ecology: Applications in Agricultural and Environmental Management; Metting, F.B., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1992; pp. 349–377. [Google Scholar]
- Mujawamariya, M.; Manishimwe, A.; Ntirugulirwa, B.; Zibera, E.; Ganszky, D.; Bahati, E.N.; Nyirambangutse, B.; Nsabimana, D.; Wallin, G.; Uddling, J. Climate sensitivity of tropical trees along an elevation gradient in Rwanda. Forests 2018, 9, 647. [Google Scholar] [CrossRef]
- Tavares, C.J.; Junior, W.Q.R.; Ramos, M.L.G.; Pereira, L.F.; Casari, R.A.d.C.N.; Pereira, A.F.; de Sousa, C.A.F.; da Silva, A.R.; Neto, S.P.d.S.; Mertz-Henning, L.M. Water stress alters morphophysiological, grain quality and vegetation indices of soybean cultivars. Plants 2022, 11, 559. [Google Scholar] [CrossRef]
- Baon, J.B.; Smith, S.A.; Alston, A.M. Mycorrhizal responses of barley cultivars differing in P efficiency. Plant Soil 1993, 157, 97–105. [Google Scholar] [CrossRef]
- Kokkoris, V.; Hamel, C.; Hart, M.M. Mycorrhizal response in crop versus wild plants. PLoS ONE 2019, 14, e0221037. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, C.F. Identification of Differentially Expressed Genes During the Yellow Passion Fruit-Xanthomonas axonopodis Interaction. Ph.D. Dissertation, University of São Paulo, São Paulo, Brazil, 2013. Available online: https://www.teses.usp.br/teses/disponiveis/11/11137/tde-30102013-135202/publico/Carla_de_Freitas_Munhoz.pdf (accessed on 25 February 2025).
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone. species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Jenkins, W.R. A Rapid Centrifugal-Flotation Technique for Separating Nematodes from Soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Silva, G.A.; Maia, L.C.; Sturmer, S.L. A dichotomous key to Scutellospora. species (Gigasporaceae, Glomeromycota) using morphological characters. Mycotaxon 2005, 94, 293–302. [Google Scholar]
- Blaszkowski, J.; Glomeromycota, W. Szafer Institute of Botany; Polish Academy of Sciences: Warsaw, Poland, 2012. [Google Scholar]
- Lin, T.C.; da Silva, G.A.; Oehl, F. Acaulospor. tsugae a new species in the Glomeromycetes from Taiwan, and a key to species in Acaulosporaceae. Nova Hedwig. 2019, 108, 475–488. [Google Scholar] [CrossRef]
- Oehl, F.; Sánchez-Castro, I.; da Silva, D.K.A.; Santos, V.M.; Palenzuela, J.; da Silva, G.A. Septoglomus nigrum, a new arbuscular mycorrhizal fungus from France, Germany and Switzerland. Nova Hedwig. 2019, 109, 121–134. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Vallejos-Torres, G.; Vallejos-Tapullima, A.; Tenorio-Cercado, M.Á.; Caballero, W.M.; Marín, C.; Santos, V.M.; da Silva, A.; Oehl, F. Rhizoglomus cacao, a new species of the Glomeraceae from the rhizosphere of Theobroma cacao in Peru, with an updated identification key for all species attributed to Rhizoglomus. Nova Hedwig. 2022, 115, 99–115. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Palenzuela, J.; Ineichen, K.; Alves da Silva, G. Advances in Glomeromycota taxonomy and classification. IMA Fungus 2011, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Blaszkowski, J.; Sánchez-García, M.; Niezgoda, P.; Zubek, S.; Fernández, F.; Vila, A.; Al-Yahya’ei, M.N.; Symanczik, S.; Milczarski, P.; Malinowski, R.; et al. A new order, Entrophosporales, and three new Entrophospora. species in Glomeromycota. Front. Microbiol. 2022, 13, 962856. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.A. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Conway, M. gsheet: Download Google Sheets Using Just the URL, R Package Version 0.4.5; R Foundation for Statistic Computing: Vienna, Austria, 2020. Available online: https://CRAN.R-project.org/package=gsheet (accessed on 20 June 2024).
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes.pt: Pacote Experimental Designs (Portuguese), R Package Version 1.2.2; R Foundation for Statistic Computing: Vienna, Austria, 2021. Available online: https://CRAN.R-project.org/package=ExpDes.pt (accessed on 20 June 2024).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, Package Version 2.6-4; R Foundation for Statistic Computing: Vienna, Austria, 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 June 2024).
- Simpson, G. Permute: Functions for Generating Restricted Permutations of Data, R Package Version 0.9-7; R Foundation for Statistic Computing: Vienna, Austria, 2022. Available online: https://CRAN.R-project.org/package=permute (accessed on 20 June 2024).
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. Available online: http://lmdvr.r-forge.r-project.org (accessed on 20 June 2024).
- Chiapello, M.; Das, D.; Gutjahr, C. Ramf: An Open-Source R Package for Statistical Analysis and Display of Quantitative Root Colonization by Arbuscular Mycorrhiza Fungi. Front. Plant Sci. 2019, 10, 1184. [Google Scholar] [CrossRef]
- Morgan, M.; Ramos, M. BiocManager: Access the Bioconductor Project Package Repository, R Package Version 1.30.22; R Foundation for Statistic Computing: Vienna, Austria, 2023. Available online: https://CRAN.R-project.org/package=BiocManager (accessed on 20 June 2024).
- Wickham, H.; Hester, J.; Chang, W.; Bryan, J. Devtools: Tools to Make Developing R Packages Easier, R Package Version 2.4.5; R Foundation for Statistic Computing: Vienna, Austria, 2022. Available online: https://CRAN.R-project.org/package=devtools (accessed on 20 June 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Wei, N.; Zhang, Z.; Yang, H.; Hu, D.; Wu, Y.; Xue, J.; Guo, D.; Xu, S. Characterization of the isocitrate dehydrogenase gene family and their response to drought stress in maize. Plants 2023, 12, 3466. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R.; Pradhan, S.K.; Shivaraj, S.M.; Deshmukh, R.; Sreevathsa, R.; Singh, N.K. Drought and oxidative stress in flax (Linum usitatissimum L.) entails harnessing non-canonical reference gene for precise quantification of qRT-PCR gene expression. Antioxidants 2023, 12, 950. [Google Scholar] [CrossRef]
- Salvatierra, A.; Mateluna, P.; Toro, G.; Solís, S.; Pimentel, P. Genome-wide identification and gene expression analysis of sweet cherry aquaporins (Prunus avium L.) under abiotic stresses. Genes 2023, 14, 940. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Yoshida, R.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 17306–17311. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, L.; Shang, J.; Hu, X.; Yu, H.; Wu, H.; Lv, W.; Zhao, Y. Genome-wide analysis of the maize superoxide dismutase (SOD) gene family reveals important roles in drought and salt responses. Genet. Mol. Biol. 2021, 44, 35. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, N.; Dong, T.; Zhang, H.; Xue, T.; Zhao, F.; Zhao, F.; Duan, Y.; Xue, J. A novel Pinellia ternata catalase gene PtCAT2 regulates drought tolerance in Arabidopsis by modulating ROS balance. Front. Plant Sci. 2023, 14, 1206798. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, T.-F.; Ma, J.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Wei, W.-L.; Xu, Z.-S. The soybean bZIP transcription factor gene GmbZIP2 confers drought and salt resistances in transgenic plants. Int. J. Mol. Sci. 2020, 21, 670. [Google Scholar] [CrossRef]

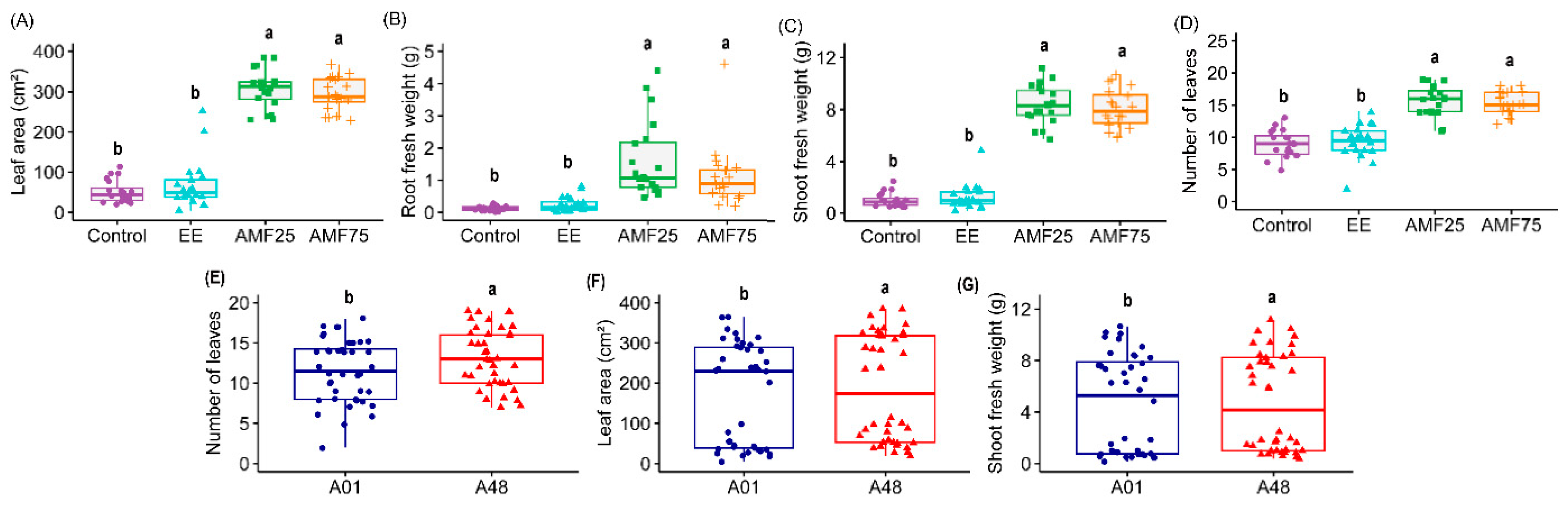



| Morphoagronomic | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| A | I | FC | A × I | A × FC | I × FC | A × I × FC | |
| Height (cm) | 5.671 * | 67.57 *** | 17.10 *** | 5.05 ** | 1.58 ns | 1.10 ns | 0.89 ns |
| Number of leaves | 13.91 *** | 77.15 *** | 14.81 *** | 2.67 ns | 0.15 ns | 1.67 ns | 0.70 ns |
| Number of tendrils | 21.30 *** | 2.86 ns | 0.37 ns | 5.32 * | 2.36 ns | 0.59 ns | 0.59 ns |
| Stem diameter (mm) | 0.056 ns | 63.94 *** | 15.23 *** | 3.28 * | 0.03 ns | 0.56 ns | 2.57 ns |
| Total Chlorophyll (a + b) | 5.17 * | 11.68 *** | 6.03 * | 4.15 ** | 0.11 ns | 10.33 *** | 2.88 * |
| Fresh weight (aboveground part) (g) | 5.79 * | 90.43 *** | 63.30 ** | 1.65 ns | 0.07 ns | 1.35 ns | 1.60 ns |
| Fresh weight (root) (g) | 2.69 ns | 39.17 *** | 5.08 ns | 4.37 ns | 8.34 ns | 3.49 ns | 2.53 ns |
| Leaf area (cm2) | 14.43 *** | 101.07 *** | 83.35 *** | 1.14 ns | 0.51 ns | 0.36 ns | 1.12 ns |
| Physiological | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| A | I | FC | A × I | A × FC | I × FC | A × I × FC | |
| Photosynthetic rate | 8.29 ** | 5.95 ** | 5.87 * | 0.97 ns | 1.94 ns | 0.55 ns | 0.53 ns |
| Stomatal conductance | 0.69 ns | 0.46 ns | 5.73 * | 1.05 ns | 0.001 ns | 1.85 ns | 1.59 ns |
| Transpiration | 0.02 ns | 0.14 ns | 4.29 * | 1.02 ns | 0.43 ns | 0.16 ns | 1.04 ns |
| Leaf temperature | 0.45 ns | 0.50 ns | 0.001 ns | 0.23 ns | 0.49 ns | 0.93 ns | 1.29 ns |
| WUE | 4.57 * | 7.28 *** | 0.48 ns | 0.35 ns | 0.97 ns | 0.66 ns | 0.96 ns |
| iWUE | 4.05 *** | 9.15 *** | 9.37 ** | 0.33 ns | 1.60 ns | 0.45 ns | 0.42 ns |
| Accession | Inoculation | Height | Leaf Area | Shoot Fresh Biomass | Root Fresh Biomass |
|---|---|---|---|---|---|
| 01 | EE | 12.71 | 81.57 | 50.94 | 192.3 |
| C25 | 586.60 | 671.38 | 1102.76 | 2119.9 | |
| C75 | 790.46 | 680.8 | 1131.8 | 1990.9 | |
| 48 | EE | 10.09 | −13.38 | −12.35 | −38 |
| C25 | 987.54 | 479.57 | 769.92 | 1095.3 | |
| C75 | 719.5 | 429.07 | 679.6 | 352.36 |
| Genes | Accession | Relative Expression | Std. Error | 95% Confidence Interval | p | Result |
|---|---|---|---|---|---|---|
| PcbZIP | A01 | 2.433 | 1.613–3.598 | 1.261–5.521 | 0.001 *** | Up-regulated |
| PcCAT | A01 | 1.292 | 0.304–3.329 | 0.239–4.837 | 0.469 ns | Constitutive |
| PcLEA | A01 | 1.064 | 0.659–1.922 | 0.360–2.355 | 0.742 ns | Constitutive |
| PcSIP | A01 | 2.278 | 0.762–6.489 | 0.276–7.974 | 0.04 * | Up-regulated |
| PcSOD | A01 | 0.97 | 0.657–1.283 | 0.525–1.664 | 0.769 ns | Constitutive |
| PcSTK | A01 | 2.252 | 1.634–3.225 | 1.091–4.098 | 0.001 *** | Up-regulated |
| PcbZIP | A48 | 1.672 | 0.289–6.867 | 0.184–49.351 | 0.371 ns | Constitutive |
| PcCAT | A48 | 0.502 | 0.160–1.357 | 0.082–1.699 | 0.052 ns | Constitutive |
| PcLEA | A48 | 0.699 | 0.360–1.427 | 0.311–1.860 | 0.078 ns | Constitutive |
| PcSIP | A48 | 0.531 | 0.276–1.260 | 0.170–1.368 | 0.014 * | Down-regulated |
| PcSOD | A48 | 1.004 | 0.677–1.513 | 0.434–1.972 | 0.985 ns | Constitutive |
| PcSTK | A48 | 0.724 | 0.594–0.856 | 0.548–1.029 | 0.001 *** | Down-regulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dantas, L.V.d.A.; Silva, R.L.d.O.; Simões, W.L.; Yano-Melo, A.M.; Melo, N.F.d. Mycorrhizal Symbiosis and Water Deficit: Morphophysiological and Gene Expression Responses in Caatinga Passion Fruit. Stresses 2025, 5, 18. https://doi.org/10.3390/stresses5010018
Dantas LVdA, Silva RLdO, Simões WL, Yano-Melo AM, Melo NFd. Mycorrhizal Symbiosis and Water Deficit: Morphophysiological and Gene Expression Responses in Caatinga Passion Fruit. Stresses. 2025; 5(1):18. https://doi.org/10.3390/stresses5010018
Chicago/Turabian StyleDantas, Luiz Victor de Almeida, Roberta Lane de Oliveira Silva, Welson Lima Simões, Adriana Mayumi Yano-Melo, and Natoniel Franklin de Melo. 2025. "Mycorrhizal Symbiosis and Water Deficit: Morphophysiological and Gene Expression Responses in Caatinga Passion Fruit" Stresses 5, no. 1: 18. https://doi.org/10.3390/stresses5010018
APA StyleDantas, L. V. d. A., Silva, R. L. d. O., Simões, W. L., Yano-Melo, A. M., & Melo, N. F. d. (2025). Mycorrhizal Symbiosis and Water Deficit: Morphophysiological and Gene Expression Responses in Caatinga Passion Fruit. Stresses, 5(1), 18. https://doi.org/10.3390/stresses5010018






