Canine and Human Red Blood Cells: Biochemical Mechanisms for the Control of Heat Dissipation
Abstract
1. Introduction
2. Results
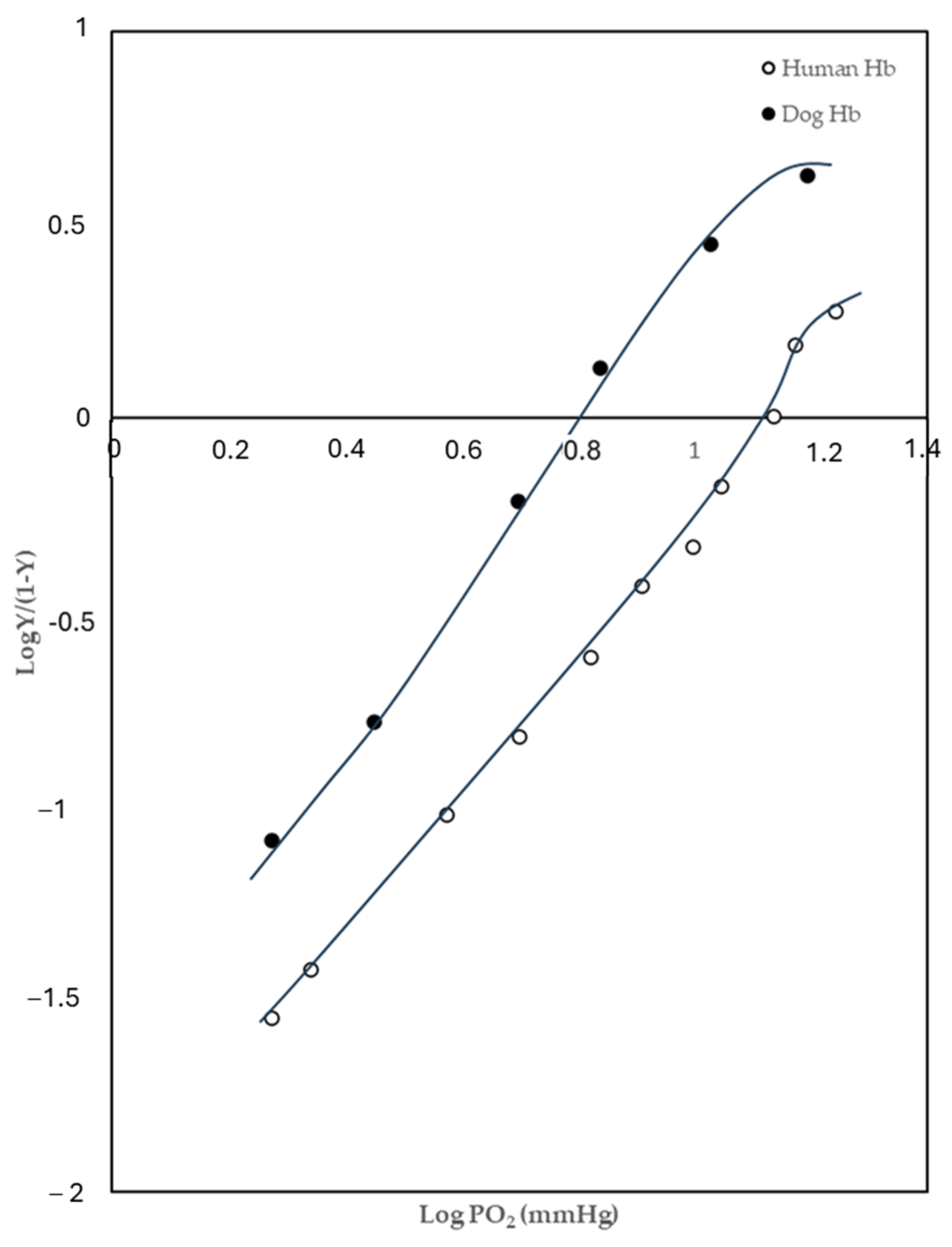
2.1. Oxygen-Binding Behaviours
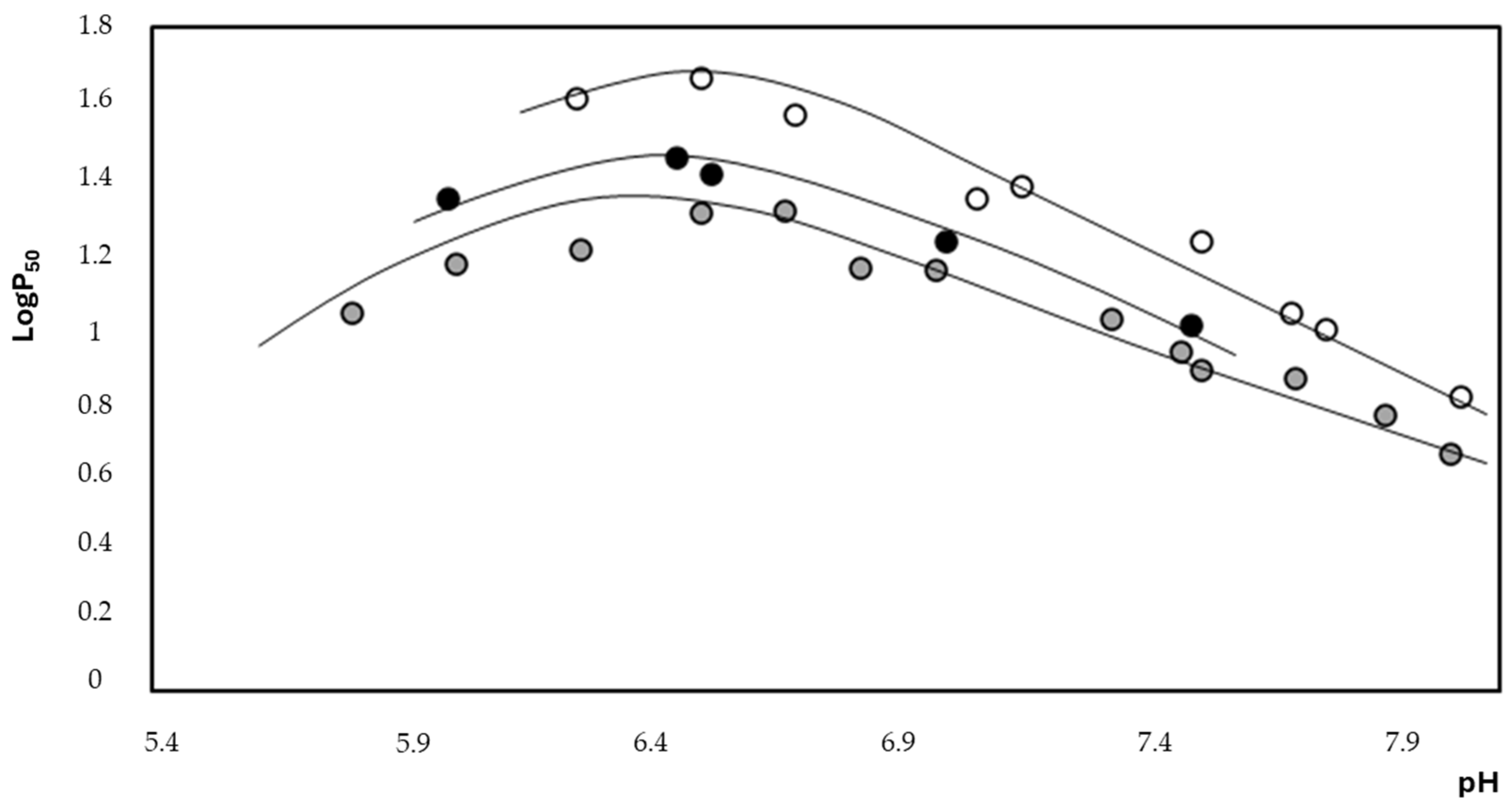
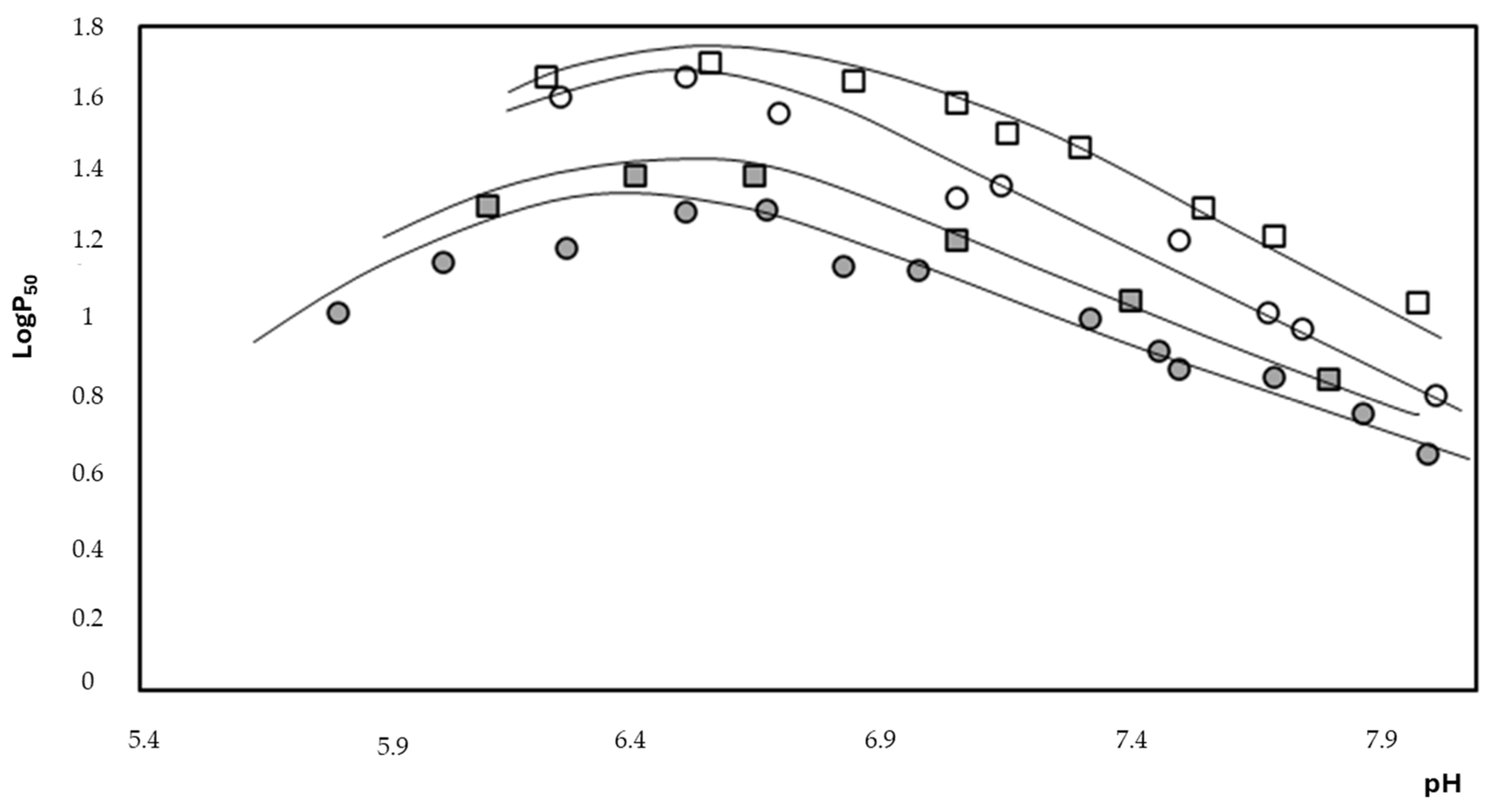
2.2. Thermodynamic Evaluation
2.3. Anion Exchange Evaluation
2.4. Intra- and Extracellular ATP Measurements
3. Discussion
4. Materials and Methods
4.1. Reagents and Compounds
4.2. Preparation of Erythrocytes
4.3. Haemolysis Percentage and Methaemoglobin Calculation
4.4. Kinetic Measurements
- -
- c(t) is the concentration of sulfate at time t;
- -
- c∞ is the concentration of intracellular sulfate at equilibrium;
- -
- k is the rate constant of the sulfate inflow.
4.5. Measurement of ATP
4.6. Purification of Haemoglobin
4.7. Measurement of Oxygen Dissociation Curves (ODC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yeo, J.H.; Lam, Y.W.; Fraser, S.T. Cellular dynamics of mammalian red blood cell production in the erythroblastic island niche. Biophys. Rev. 2019, 11, 873–894. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kelm, M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, M.; Messana, I.; Sanna, M.T.; Giardina, B. Oxygen-linked modulation of erythrocyte metabolism: State of the art. Blood Transfus. 2010, 8, s53–s58. [Google Scholar] [PubMed]
- Messana, I.; Misiti, F.; el-Sherbini, S.; Giardina, B.; Castagnola, M. Quantitative determination of the main glucose metabolic f luxes in human erythrocytes by 13C- and 1H-MR spectroscopy. J. Biochem. Biophys. Methods 1999, 39, 63–84. [Google Scholar] [CrossRef]
- Campanella, M.E.; Chu, H.; Low, P.S. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. USA 2005, 102, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.C.; Carelli Alinovi, C.; Galtieri, A.; Russo, A.; Giardina, B. Allosteric properties of hemoglobin and the plasma membrane of the erythrocyte: New insights in gas transport and metabolic modulation. IUBMB Life 2008, 60, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Galtieri, A.; Tellone, E.; Romano, L.; Misiti, F.; Bellocco, E.; Ficarra, S.; Russo, A.; Di Rosa, D.; Castagnola, M.; Giardina, B.; et al. Band-3 protein function in human erythrocytes: Effect of oxygenation-deoxygenation. Biochim. Biophys. Acta 2002, 1564, 214–218. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Y. The effect of red blood cells on blood heat transfer. Int. J. Heat. Mass. Transf. 2017, 113, 840–849. [Google Scholar] [CrossRef]
- Farsaci, F.; Tellone, E.; Galtieri, A.; Ficarra, S. A new model for thermodynamic characterization of hemoglobin. Fluids 2019, 4, 135. [Google Scholar] [CrossRef]
- Tellone, E.; Clementi, M.E.; Russo, A.M.; Ficarra, S.; Lania, A.; Lupi, A.; Giardina, B.; Galtieri, A. Oxygen Transport and Diving Behaviour: The Haemoglobin from Dolphin Tursiops truncatus. In Hemoglobin Function in Vertebrates; Springer: Milan, Italy, 2000; pp. 77–82. [Google Scholar]
- Giardina, B.; Scatena, R.; Clementi, M.E.; Cerroni, L.; Nuutinen, M.; Brix, O.; Sletten, S.N.; Castagnola, M.; Condò, S.G. Physiological relevance of the overall delta H of oxygen binding to fetal human hemoglobin. J. Mol. Biol. 1993, 229, 512–516. [Google Scholar] [CrossRef]
- Giardina, B.; Condò, S.G.; Petruzzelli, R.; Bardgard, A.; Brix, O. Thermodynamics of oxygen binding to arctic hemoglobins. The case of reindeer. Biophys. Chem. 1990, 37, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Giardina, B.; Corda, M.; Pellegrini, M.G.; Sanna, M.T.; Brix, O.; Clementi, M.E.; Condo, S.G. Flight and heat dissipation in birds. A possible molecular mechanism. FEBS Lett. 1990, 270, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Cataldi, E.; Capo, C.; Petruzzelli, R.; Tellone, E.; Giardina, B. Purification and characterization of the hemoglobin components of Adriatic sturgeon (Acipenser naccarii) blood. J. Appl. Ichthyol. 1999, 15, 78–80. [Google Scholar] [CrossRef]
- De Rosa, M.C.; Sanna, M.T.; Messana, I.; Castagnola, M.; Galtieri, A.; Tellone, E.; Scatena, R.; Botta, B.; Botta, M.; Giardina, B. Glycated human hemoglobin (HbA(1c)): Functional characteristics and molecular modeling studies. Biophys. Chem. 1998, 72, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Russo, A.; Giardina, B.; Galtieri, A.; Ficarra, S. Metabolic effects of endogenous and exogenous heterotropic hemoglobin modulators on anion transport: The case of pig erythrocytes. OALIB 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Atyabi, N.; Rahbarghazi, R.; Araghi, A.; Neqouiejahromi, O.A. Haemoglobin typing and its variations in Iranian domestic dogs. Comp. Clin. Pathol. 2012, 21, 1515–1519. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K.; Bretz, W.L.; Taylor, C.R. Panting in dogs: Unidirectional air flow over evaporative surfaces. Science 1970, 169, 1102–1104. [Google Scholar] [CrossRef]
- Goldberg, M.B.; Langman, V.A.; Taylor, R.C. Panting in dogs: Paths of air flow in response to heat and exercise. Respir. Physiol. 1981, 43, 327–338. [Google Scholar] [CrossRef]
- Pleschka, K.; Krönert, H. Thermoregulatory adjustment of lingual blood flow in the conscious dog at high ambient temperature. Isr. J. Med. Sci. 1976, 12, 1077–1078. [Google Scholar]
- Hales, J.R. Effects of exposure to hot environments on the regional distribution of blood flow and on cardiorespiratory function in sheep. Pflug. Arch. 1973, 344, 133–148. [Google Scholar] [CrossRef]
- Potter, A.W.; Berglund, L.G.; O'Brien, C. A canine thermal model for simulating temperature responses of military working dogs. J Therm. Biol. 2020, 91, 102651. [Google Scholar] [CrossRef] [PubMed]
- Krönert, H.; Pleschka, K. Lingual blood flow and its hypothalamic control in the dog during panting. Pflug. Arch. 1976, 367, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, H.; Brett, I. Embryonic and fetal hemoglobin in animals. Ann. N. Y. Acad. Sci. 1974, 241, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Rodriguez-Franco, F.; Tesouro-Diez, M.A.; Rodriguez-Sanchez, M. Concentrations of 2,3-diphosphoglycerate (2,3-DPG) in canine blood (healthy dogs, dogs with cardiopulmonary insufficiency and dogs with renal insufficiency). Zentralbl Vet. B 1994, 41, 9–16. [Google Scholar] [CrossRef]
- Böning, D.; Schünemann, H.J.; Maassen, N.; Busse, M.W. Reduction of oxylabile CO2 in human blood by lactate. J. Appl. Physiol. 1993, 74, 710–714. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Weber, R.E. Antagonistic interaction between oxygenation-linked lactate and CO2 binding to human hemoglobin. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 429–434. [Google Scholar] [CrossRef]
- Lukin, J.A.; Ho, C. The structure–function relationship of hemoglobin in solution at atomic resolution. Chem. Rev. 2004, 104, 1219–1230. [Google Scholar] [CrossRef]
- Garner, M.H.; Bogardt, R.A.; Gurd, F.R.N. Determination of the pK values for the α-amino groups of human hemoglobin. J. Biol. Chem. 1975, 250, 4398–4404. [Google Scholar] [CrossRef]
- Okonjo, K.O. Bohr effect of hemoglobins: Accounting for differences in magnitude. J. Theor. Biol. 2015, 380, 436–443. [Google Scholar] [CrossRef]
- Jagger, J.E.; Bateman, R.M.; Ellsworth, M.L.; Ellis, C.G. Role of erythrocyte in the regulation of local O2 release mediated by hemoglobin oxygenation. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.J.; Darrow, C.C.; Hoehn, B.A.; Zhu, H. Generation and export of red blood cell ATP in health and disease. Anterior Physiol. 2021, 12, 754638. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.J. The relationship between pH and aerobic glycolysis in human and canine erythrocytes. Comp. Biochem. Physiol. Part B Comp. Biochem. 1972, 41, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Miseta, A.; Bogner, P.; Berényi, E.; Kellermayer, M.; Galambos, C.; Wheatley, D.N.; Cameron, I.L. Relationship between cellular ATP, potassium, sodium and magnesium concentrations in mammalian and avian erythrocytes. Biochim. Biophys. Acta 1993, 1175, 133–139. [Google Scholar] [CrossRef]
- Nemeth, N.; Baskurt, O.K.; Meiselman, H.J.; Kiss, F.; Uyuklu, M.; Hever, T.; Sajtos, E.; Kenyeres, P.; Toth, K.; Furka, I.; et al. Storage of laboratory animal blood samples causes hemorheological alterations: Inter-species differences andthe effects of duration and temperature. Korea-Aust. Rheol. J. 2009, 21, 127–133. [Google Scholar]
- Matrai, A.A.; Varga, G.; Tanczos, B.; Barath, B.; Varga, A.; Horvath, L.; Bereczky, Z.; Deak, A.; Nemeth, N. In vitro effects of temperature on red blood cell deformability and membrane stability in human and various vertebrate species. Clin. Hemorheol. Microcirc. 2021, 78, 291–300. [Google Scholar] [CrossRef]
- Passow, H. Molecular aspects of band 3 protein-mediated anion transport across the red blood cell membrane. Rev. Physiol. Biochem. Pharmacol. 1986, 103, 61–203. [Google Scholar]
- Jennings, M.L. Proton fluxes associated with erythrocyte membrane anion exchange. J. Membr. Biol. 1976, 28, 187–205. [Google Scholar] [CrossRef]
- Russo, A.; Patanè, G.T.; Putaggio, S.; Lombardo, G.E.; Ficarra, S.; Barreca, D.; Giunta, E.; Tellone, E.; Laganà, G. Mechanisms Underlying the Effects of Chloroquine on Red Blood Cells Metabolism. Int. J. Mol. Sci. 2024, 25, 6424. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; North-Holland Pub. Co.: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Giardina, B.; Amiconi, G. Measurement of binding of gaseous and nongaseous ligands to hemoglobins by conventional spectrophotometric procedures. Methods Enzymol. 1981, 76, 417–427. [Google Scholar]





| Sample | pH | ΔH (Kcal/mol) |
|---|---|---|
| Hb | 6.5 | −5.55 |
| 7.5 | −15.31 | |
| Hb + 2,3-BPG (3 mM) | 6.5 | −2.49 |
| 7.5 | −5.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Patanè, G.T.; Putaggio, S.; Tellone, E.; Ficarra, S.; Barreca, D.; Laganà, G. Canine and Human Red Blood Cells: Biochemical Mechanisms for the Control of Heat Dissipation. Stresses 2024, 4, 787-799. https://doi.org/10.3390/stresses4040052
Russo A, Patanè GT, Putaggio S, Tellone E, Ficarra S, Barreca D, Laganà G. Canine and Human Red Blood Cells: Biochemical Mechanisms for the Control of Heat Dissipation. Stresses. 2024; 4(4):787-799. https://doi.org/10.3390/stresses4040052
Chicago/Turabian StyleRusso, Annamaria, Giuseppe Tancredi Patanè, Stefano Putaggio, Ester Tellone, Silvana Ficarra, Davide Barreca, and Giuseppina Laganà. 2024. "Canine and Human Red Blood Cells: Biochemical Mechanisms for the Control of Heat Dissipation" Stresses 4, no. 4: 787-799. https://doi.org/10.3390/stresses4040052
APA StyleRusso, A., Patanè, G. T., Putaggio, S., Tellone, E., Ficarra, S., Barreca, D., & Laganà, G. (2024). Canine and Human Red Blood Cells: Biochemical Mechanisms for the Control of Heat Dissipation. Stresses, 4(4), 787-799. https://doi.org/10.3390/stresses4040052








