Beyond the Classical Janzen–Connell Hypothesis: The Role of the Area Under the Parent Tree Crown of Manilkara zapota
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Sampling
4.3. Leaf Asymmetry
4.4. Insect Herbivory and Pathogen Damage
4.5. Data Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Price, P.W.; Bouton, C.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weis, A.E. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Ann. Rev. Ecol. Syst. 1980, 11, 41–65. Available online: https://www.jstor.org/stable/2096902 (accessed on 10 October 2024). [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In Dynamics of Populations; Den Boer, P.J., Grandwell, G.R., Eds.; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 2014; pp. 298–312. [Google Scholar]
- Weis, A.E.; Berenbaum, M.R. Herbivorous insects and green plants. In Plant Animal Interactions; Abrahamson, W.G., Ed.; McGraw Hill: New York, NY, USA, 1989; pp. 123–162. [Google Scholar]
- Huntly, N. Herbivores and the dynamics of communities and ecosystems. Ann. Rev. Ecol. Syst. 1991, 22, 477–503. [Google Scholar] [CrossRef]
- Dalling, J.W.; Hubbell, S.P. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. J. Ecol. 2002, 90, 557–568. [Google Scholar] [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Hanula, J.L.; Mayfield, A.E., III; Fraedrich, S.W.; Rabaglia, R.J. Biology and host associations of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae), exotic vector of laurel wilt killing redbay trees in the southeastern United States. J. Econ. Entomol. 2008, 101, 1276–1286. [Google Scholar] [CrossRef]
- Gong, B.; Zhang, G. Interactions between plants and herbivores: A review of plant defense. Acta Ecol. Sin. 2014, 34, 325–336. [Google Scholar] [CrossRef]
- Díaz, M.; Pulido, F.J.; Møller, A.P. Herbivore effects on developmental instability and fecundity of holm oaks. Oecologia 2004, 139, 224–234. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Novais-Pereira, G.C.; Gélvez-Zúñiga, I.; Fernandes, G.W.; Venâncio, H.; Santos, J.C.; Maldonado-López, Y. Effects of ferric soils on arthropod abundance and herbivory on Tibouchina heteromalla (Melastomataceae): Is fluctuating asymmetry a good indicator of environmental stress? Plant Ecol. 2018, 219, 69–78. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Do defoliating insects distinguish between symmetric and asymmetric leaves within a plant? Ecol. Entomol. 2018, 43, 656–664. [Google Scholar] [CrossRef]
- Carson, W.P.; Anderson, J.T.; Leigh, E.G.; Schnitzer, S.A. Challenges associated with testing and falsifying the Janzen-Connell hypothesis: A review and critique. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Wiley-Blackwell: West Sussex, UK, 2008; pp. 210–241. ISBN 978-1-4051-1897-2. [Google Scholar]
- Levi, T.; Barfield, M.; Barrantes, S.; Sullivan, C.; Holt, R.D.; Terborgh, J. Tropical forests can maintain hyperdiversity because of enemies. Proc. Natl. Acad. Sci. USA 2019, 116, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Clark, D.B. Spacing dynamics of a tropical rain forest tree: Evaluation of the Janzen-Connell model. Am. Nat. 1984, 124, 769–788. [Google Scholar] [CrossRef]
- Burkey, T.V. Tropical tree species diversity: A test of the Janzen-Connell model. Oecologia 1994, 97, 533–540. [Google Scholar] [CrossRef]
- Hammond, D.S.; Brown, V.K. Disturbance, phenology and life-history characteristics: Factors influencing distance/density-dependent attack on tropical seeds and seedlings. In Dynamics of Tropical Communities; Newberry, D.M., Prins, H.H.T., Brown, N., Eds.; Blackwell Science Ltd.: Oxford, UK, 1998; pp. 51–78. ISBN 0-632-04944-8. [Google Scholar]
- Romo, M.; Tuomisto, H.; Loiselle, B.A. On the density-dependence of seed predation in Dipteryx micrantha, a bat dispersed rainforest tree. Oecologia 2004, 140, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Packer, A.; Clay, K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 2000, 404, 278–281. [Google Scholar] [CrossRef]
- Lambers, J.H.R.; Clark, J.S.; Beckage, B. Density-dependent mortality and the latitudinal gradient in species diversity. Nature 2002, 417, 732–735. [Google Scholar] [CrossRef]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the Janzen–Connell hypothesis: A meta-analysis of experimental evidence for distance-and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef]
- Hyatt, L.A.; Rosenberg, M.S.; Howard, T.G.; Bole, G.; Fang, W.; Anastasia, J.; Brown, K.; Grella, R.; Hinman, K.; Kurdziel, J.P.; et al. The distance dependence prediction of the Janzen-Connell hypothesis: A meta-analysis. Oikos 2003, 103, 590–602. [Google Scholar] [CrossRef]
- Song, X.; Lim, J.Y.; Yang, J.; Luskin, M.S. When do Janzen–Connell effects matter? A phylogenetic meta-analysis of conspecific negative distance and density dependence experiments. Ecol. Lett. 2021, 24, 608–620. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Similar responses of insect herbivores to leaf fluctuating asymmetry. Arthropod-Plant Interact. 2011, 5, 59–69. [Google Scholar] [CrossRef]
- Downey, H.; Lewis, O.T.; Bonsall, M.B.; Fernandez, D.C.; Gripenberg, S. Insect herbivory on seedlings of rainforest trees: Effects of density and distance of conspecific and heterospecific neighbors. Ecol. Evol. 2018, 8, 12702–12711. [Google Scholar] [CrossRef] [PubMed]
- Bayandala; Fukasawa, Y.; Seiwa, K. Roles of pathogens on replacement of tree seedlings in heterogeneous light environments in a temperate forest: A reciprocal seed sowing experiment. J. Ecol. 2016, 104, 765–772. [Google Scholar] [CrossRef]
- Calderón-Sanou, I.; Ríos, L.D.; Cascante-Marín, A.; Barrantes, G.; Fuchs, E.J. The effect of conspecific density, herbivory, and bamboo on seedling dynamics of a dominant oak in a Neotropical highland forest. Biotropica 2019, 51, 817–825. [Google Scholar] [CrossRef]
- Bayandala; Masaka, K.; Seiwa, K. Leaf diseases drive the Janzen–Connell mechanism regardless of light conditions: A 3-year field study. Oecologia 2017, 183, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Loayza, P.; Terborgh, J. Fates of seedling carpets in an Amazonian floodplain forest: Intra-cohort competition or attack by enemies? J. Ecol. 2011, 99, 1045–1054. [Google Scholar] [CrossRef]
- Malik, N.; Edwards, D.; Freckleton, R.P. Distance and density dependence in two native Bornean dipterocarp species. Ecol. Evol. 2023, 13, e10004. [Google Scholar] [CrossRef]
- Adler, P.B.; Smull, D.; Beard, K.H.; Choi, R.T.; Furniss, T.; Kulmatiski, A.; Meiners, J.M.; Tredennick, A.T.; Veblen, K.E. Competition and coexistence in plant communities: Intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018, 21, 1319–1329. [Google Scholar] [CrossRef]
- Pablo-Rodríguez, J.L.; Bravo-Monzón, Á.E.; Montiel-González, C.; Benítez-Malvido, J.; Álvarez-Betancourt, S.; Ramírez-Sánchez, O.; Oyama, K.; Arena-Ortiz, M.A.; Alvarez-Añorve, M.A.; Avila-Cabadilla, L.D. Linking Anthropogenic landscape perturbation to herbivory and pathogen leaf amage in tropical tree communities. Plants 2023, 12, 3839. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Ann. Rev. Ecol. Syst. 1986, 17, 391–421. Available online: https://www.jstor.org/stable/2097002 (accessed on 12 October 2024). [CrossRef]
- Turmukhametova, N.V. Evaluation of the state of the environment in Yoshkar-Ola using morphometric indicators of Betula pendula Roth. Biol. Bull. Russ. Acad. Sci. 2020, 47, 191–197. [Google Scholar] [CrossRef]
- Shadrina, E.; Soldatova, V.; Turmukhametova, N. Fluctuating asymmetry as a measure of stress in natural populations of woody plants: Influence of ecological and geographical factors on developmental stability. Symmetry 2023, 15, 700. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Vaca-Sánchez, M.S.; Canché-Delgado, A.; García-Jaín, S.E.; González-Rodríguez, A.; Cornelissen, T.; Cuevas-Reyes, P. Leaf herbivory and fluctuating asymmetry as indicators of mangrove stress. Wetl. Ecol. Manag. 2019, 27, 571–580. [Google Scholar] [CrossRef]
- Mattson, W.J.; Haack, R.A. The role of drought in outbreaks of plant-eating insects. BioScience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Perfect is best: Low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 2005, 142, 46–56. [Google Scholar] [CrossRef]
- De Souza Amorim, D.; Brown, B.V.; Boscolo, D.; Ale-Rocha, R.; Alvarez-Garcia, D.M.; Balbi, M.I.P.; Barbosa, A.D.M.; Soares, C.R.; Barros de Carvalho, C.J.; Souto, C.M.; et al. Vertical stratification of insect abundance and species richness in an Amazonian tropical forest. Sci. Rep. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Novotny, V.; Miller, S.E.; Hrcek, J.; Baje, L.; Basset, Y.; Lewis, O.T.; Stewart, A.J.A.; Weiblen, G.D. Insects on plants: Explaining the paradox of low diversity within specialist herbivore guilds. Am. Nat. 2012, 179, 351–362. [Google Scholar] [CrossRef][Green Version]
- Stiegel, S.; Entling, M.H.; Mantilla-Contreras, J. Reading the leaves’ palm: Leaf traits and herbivory along the microclimatic gradient of forest layers. PLoS ONE 2017, 12, e0169741. [Google Scholar] [CrossRef]
- Fokunang, C.N.; Akem, C.N.; Ikotun, T.; Dixon, A.G.O.; Tembe, E.A. Role of the insect vector, Pseudotheraptus devastans, in cassava anthracnose disease development. Eur. J. Plant Pathol. 2000, 106, 319–327. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology; Elsevier Academic Press: Burlington, MA, USA, 2005; ISBN 90-12-044565-4. [Google Scholar]
- Abdala-Roberts, L.; Berny-Mier y Terán, J.C.; Vázquez-González, C.; Cohuo, A.; León, J.; Valle, L.; Mooney, K.A.; Reyes-Novelo, E.; Moreira, X. Effects of seedling conspecific density and heterospecific frequency on insect herbivory in a tropical dry forest. Agric. For. Entomol. 2023, 25, 549–557. [Google Scholar] [CrossRef]
- Carabias-Lillo, J.; Provencio, E.; de la Maza Elvira, J.; Rodriguez de la Gala Mendez, J.B. Programa de Manejo Reserva de la Biosfera de Calakmul; Instituto Nacional de Ecología: Camp, Mexico, 2000. [Google Scholar]
- Martínez, E.; Souza, S.M.; Ramos-Álvarez, C.H. Listados Florísticos de México: Región de Calakmul, Campeche; Instituto de Biología, Universidad Nacional Autónoma de Mexico: México City, Mexico, 2001. [Google Scholar]
- Martínez, E.; Galindo-Leal, C. La vegetación de Calakmul, Campeche, México: Clasificación, descripción y distribución. Bol. Soc. Bot. México 2002, 71, 7–32. Available online: http://www.redalyc.org/articulo.oa?id=57707101 (accessed on 13 October 2024). [CrossRef]
- Vidal-Zepeda, R. Las Regiones Climáticas de México (1.2.2); Instituto de Geografía, Universidad Nacional Autónoma de Mexico: México City, Mexico, 2005; Volume 2. [Google Scholar]
- Mardero, S.; Nickl, E.; Schmook, B.; Schneider, L.; Rogan, J.; Christman, Z.; Lawrence, D. Sequías en el sur de la península de Yucatán: Análisis de la variabilidad anual y estacional de la precipitación. Investig. Geogr. 2012, 78, 19–33. [Google Scholar] [CrossRef]
- Vester, H.F.M.; Lawrence, D.; Eastman, J.R.; Turner, B.L., II; Calmé, S.; Dickson, R.; Pozo, C.; Sangermano, F. Land change in the Southern Yucatan and Calakmul Biosphere Reserve: Effects on habitat and biodiversity. Ecol. Appl. 2007, 17, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T. Composition, floristic affinities, and origins of the canopy tree flora of the Mexican Atlantic slope rain forest. In Biological Diversity of Mexico: Origins and Distribution; Ramamoorthy, T.P.R., Bye, R., Lot, A., Fa, J., Eds.; Oxford University: New York, NY, USA, 1993; pp. 595–680. [Google Scholar]
- Cruz-Rodríguez, J.A.; Lopez-Mata, L. Demography of the seedling bank of Manilkara zapota (L.) Royen, in a subtropical rain forest of Mexico. Plant Ecol. 2004, 172, 227–235. [Google Scholar] [CrossRef]
- Hunt, R. Basic Growth Analysis: Plant Growth Analysis for Beginners; Unwin Hyman Ltd.: London, UK, 1978; p. 112. ISBN 13:978-0-04-4453-73-4. [Google Scholar]
- Ruiz-Santiago, R.R.; Ballina-Gómez, H.S.; Ruiz-Sánchez, E. Características morfológicas foliares y su relación con la defoliación en tres especies de plantas forrajeras. Acta Biol. Colomb. 2023, 28, 12–22. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig, Digitize Landmarks and Outlines, Version 2.05, Windows; Stony Brook, Department of Ecology and Evolution, State University of New York: New York, NY, USA, 2006. [Google Scholar]
- Lamari, L. Assess: Image Analysis Software for Plant Disease Quantification, version 2.0, Windows; The American Phytopathological Society: Saint Paul, MN, USA, 2008. [Google Scholar]
- Hull, R. Plant Virology; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123848710. [Google Scholar]
- The Jamovi Project. Jamovi, Version 2.6, iOS. 2024. Available online: https://www.jamovi.org. (accessed on 9 October 2024).
| Environmental Variable | Under Crown | Free Crown | BMTest | ||
|---|---|---|---|---|---|
| (mean ± se) | (min–max) | (mean ± se) | (min–max) | ||
| PPFD (μmol m2 s−1) | 12.6 ± 0.47 | 4.46–37.1 | 11.9 ± 0.38 | 4.22–40.4 | −1.58 ns |
| Temperature (°C) | 26.5 ± 0.03 | 25–27.6 | 26.6 ± 0.06 | 25.1–39.1 | −0.02 ns |
| Relative humidity (%) | 85.2 ± 0.28 a | 64.4–90.6 b | 84.8 ± 0.22 a | 64.9–89.8 b | −2.01 |
| Model Effects | Parameter Estimates | ||||||
|---|---|---|---|---|---|---|---|
| Wald χ2 | df | p | β | SE | Wald χ2 | p | |
| Mortality | |||||||
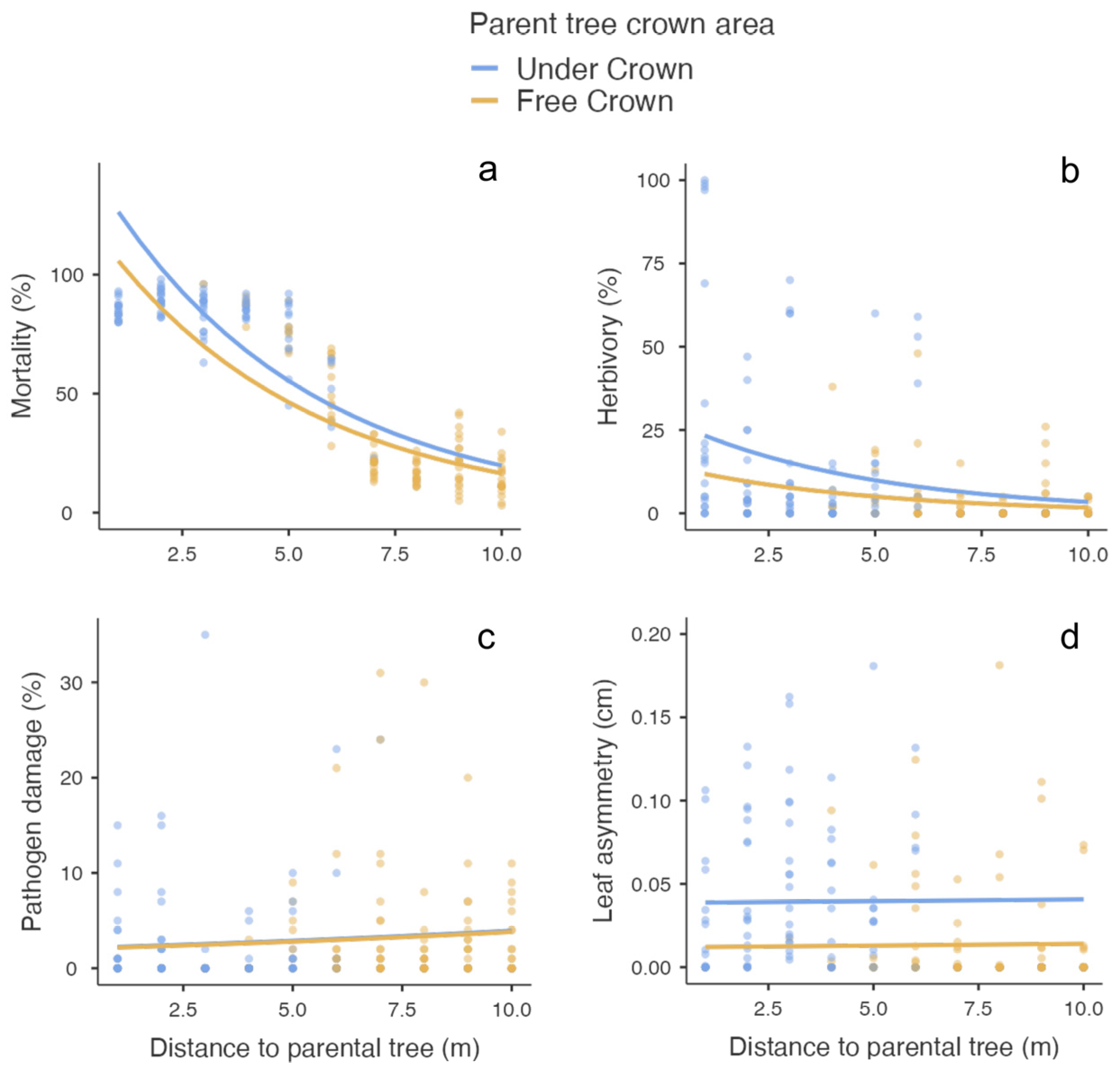
| Distance to parent tree | 190.2 | 1 | <0.001 | −0.206 | 0.015 | −13.1 | <0.001 |
| Crown area | 4.96 | 1 | 0.02 | −0.178 | 0.088 | −2.01 | 0.04 |
| Insect herbivory | |||||||
| Distance to parent tree | 7.66 | 1 | 0.006 | −0.214 | 0.087 | −2.46 | 0.01 |
| Crown area | 2.44 | 1 | 0.11 | −0.679 | 0.498 | 0.173 | 0.17 |
| Pathogen damage | |||||||
| Distance to parent tree | 0.57 | 1 | 0.44 | 0.062 | 0.086 | 0.722 | 0.47 |
| Crown area | 0.004 | 1 | 0.94 | −0.032 | 0.496 | −0.656 | 0.94 |
| Leaf asymmetry | |||||||
| Distance to parent tree | 0.017 | 1 | 0.89 | 2.1−4 | 0.001 | 0.132 | 0.89 |
| Crown area | 8.015 | 1 | 0.005 | −0.026 | 0.009 | −2.831 | 0.005 |
| Model Effects | Parameter Estimates | ||||||
|---|---|---|---|---|---|---|---|
| Wald χ2 | df | p | β | SE | Wald χ2 | p | |
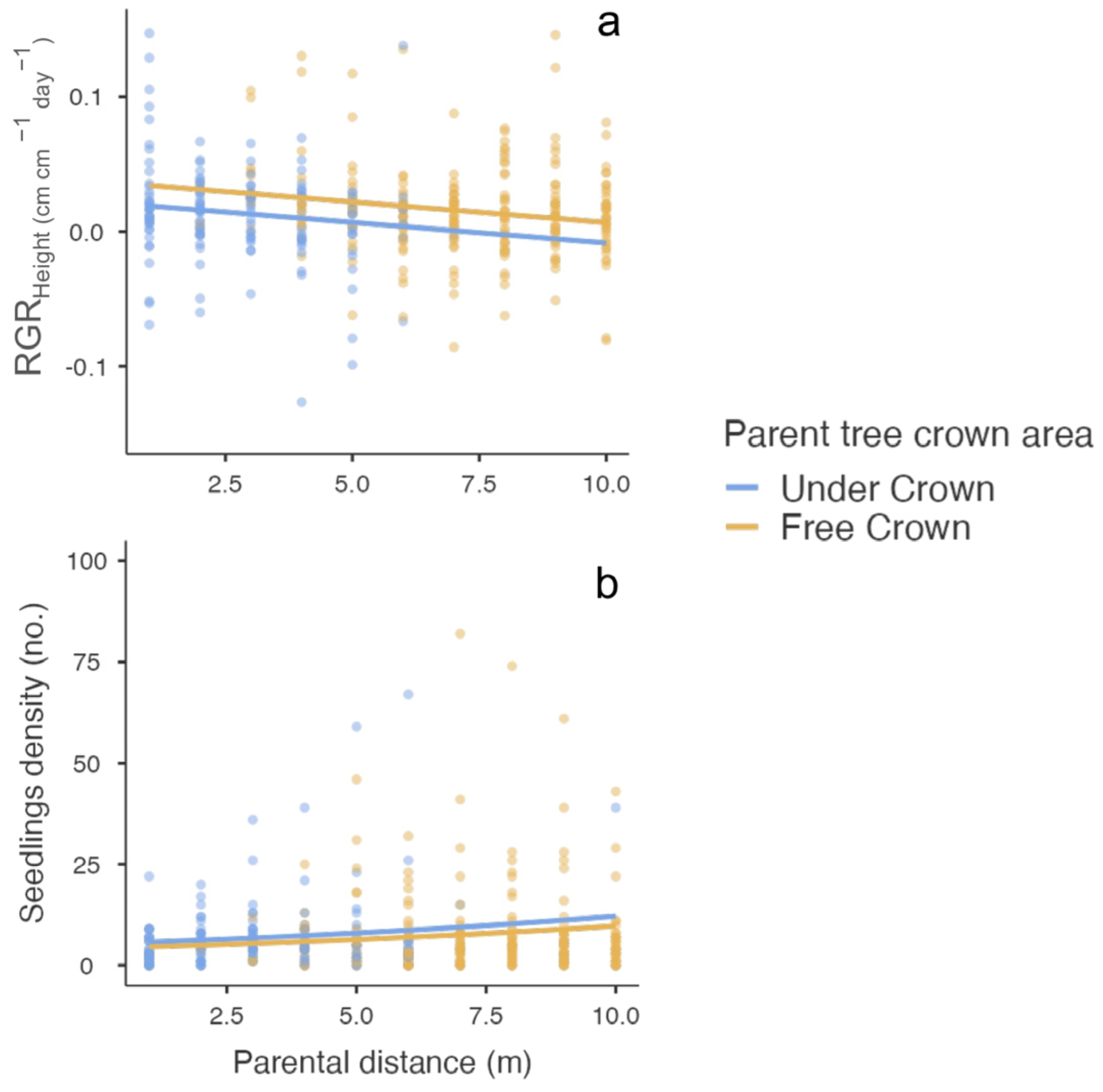
| RGRHeight | |||||||
| Distance to parent tree | 11.05 | 1 | <0.001 | −0.003 | 0.0009 | −3.32 | <0.001 |
| Crown area | 7.92 | 1 | 0.005 | 0.015 | 0.005 | 2.81 | 0.005 |
| Seedlings density | |||||||
| Distance to parent tree | 8.86 | 1 | 0.003 | 0.083 | 0.028 | 2.93 | 0.003 |
| Crown area | 2.06 | 1 | 0.151 | −0.220 | 0.167 | −1.32 | 0.188 |
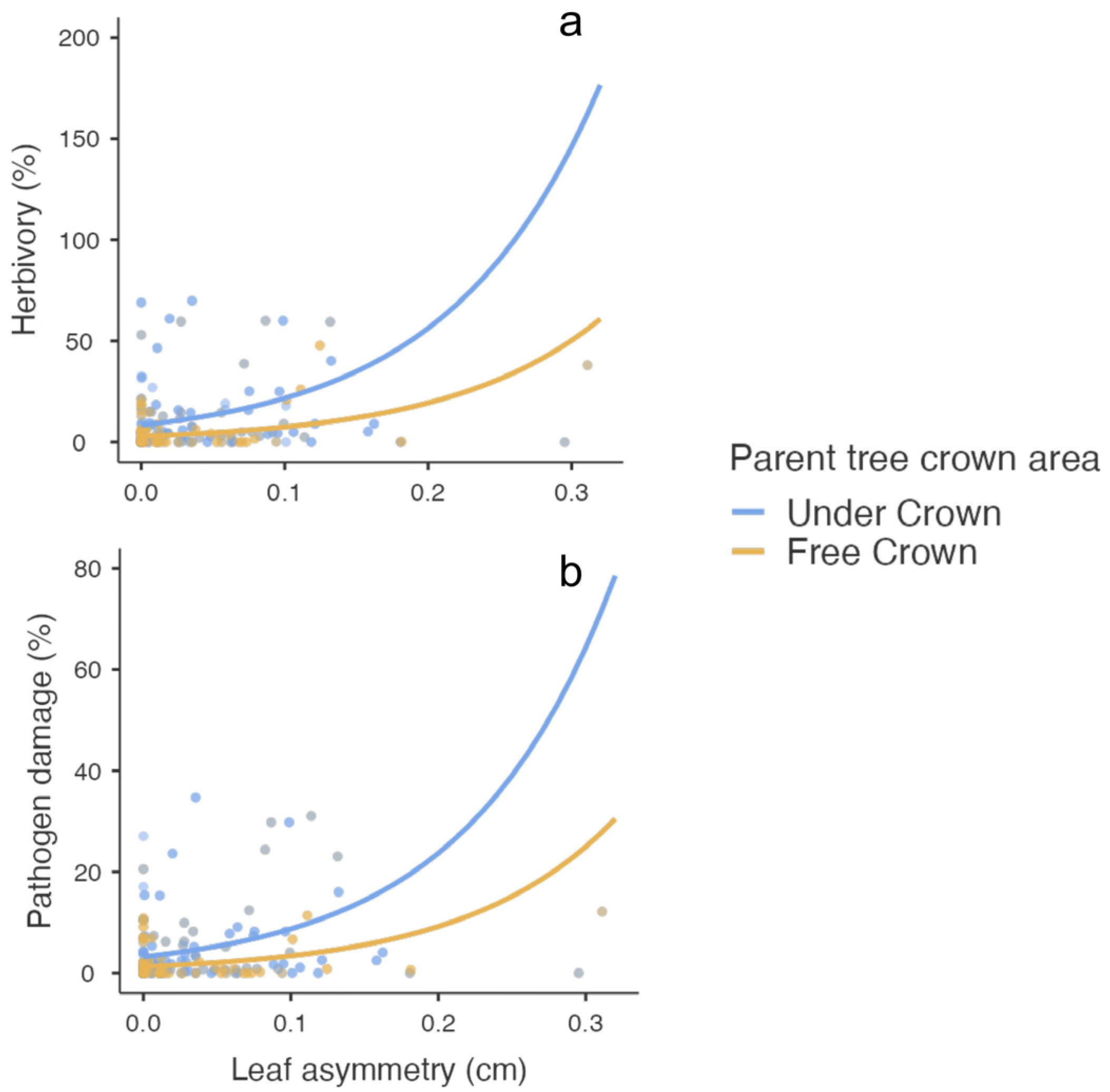
| Herbivory | |||||||
| Leaf asymmetry | 21.6 | 1 | <0.001 | 9.53 | 2.053 | 4.64 | <0.001 |
| Crown area | 28.8 | 1 | <0.001 | −1.07 | 0.205 | −5.19 | <0.001 |
| Pathogen damage | |||||||
| Leaf asymmetry | 24.5 | 1 | <0.001 | 9.996 | 1.923 | 5.20 | <0.001 |
| Crown area | 24.7 | 1 | <0.001 | −0.947 | 0.196 | −4.83 | <0.001 |
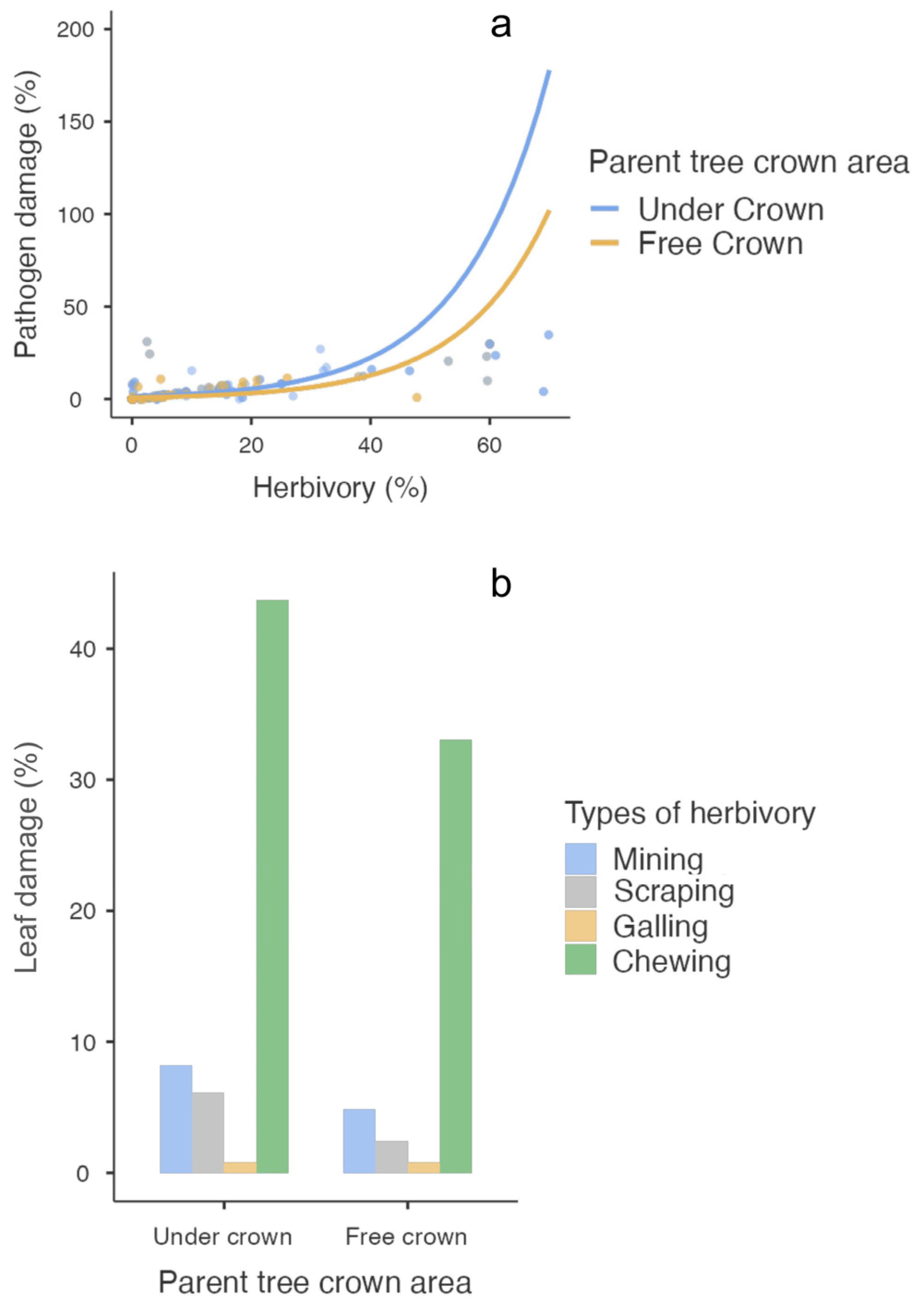
| Influence of herbivory on pathogen damage | |||||||
| Herbivory | 172.8 | 1 | <0.001 | 0.069 | 0.005 | 13.154 | <0.001 |
| Crown area | 11.8 | 1 | <0.001 | −0.554 | −0.168 | −3.298 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Euan-Quiñones, O.A.; Mena-Martín, H.; Herrera-Pérez, P.; Cetina-Pérez, R.A.; Bautista-Parra, S.G.; Ballina-Gomez, H.S. Beyond the Classical Janzen–Connell Hypothesis: The Role of the Area Under the Parent Tree Crown of Manilkara zapota. Stresses 2024, 4, 762-772. https://doi.org/10.3390/stresses4040050
Euan-Quiñones OA, Mena-Martín H, Herrera-Pérez P, Cetina-Pérez RA, Bautista-Parra SG, Ballina-Gomez HS. Beyond the Classical Janzen–Connell Hypothesis: The Role of the Area Under the Parent Tree Crown of Manilkara zapota. Stresses. 2024; 4(4):762-772. https://doi.org/10.3390/stresses4040050
Chicago/Turabian StyleEuan-Quiñones, Oscar Antonio, Helbert Mena-Martín, Patricia Herrera-Pérez, Ramiro Alexandro Cetina-Pérez, San German Bautista-Parra, and Horacio Salomon Ballina-Gomez. 2024. "Beyond the Classical Janzen–Connell Hypothesis: The Role of the Area Under the Parent Tree Crown of Manilkara zapota" Stresses 4, no. 4: 762-772. https://doi.org/10.3390/stresses4040050
APA StyleEuan-Quiñones, O. A., Mena-Martín, H., Herrera-Pérez, P., Cetina-Pérez, R. A., Bautista-Parra, S. G., & Ballina-Gomez, H. S. (2024). Beyond the Classical Janzen–Connell Hypothesis: The Role of the Area Under the Parent Tree Crown of Manilkara zapota. Stresses, 4(4), 762-772. https://doi.org/10.3390/stresses4040050






