The Preventive and Curative Potential of Morinda citrifolia Essential Oil for Controlling Anthracnose in Cassava Plants: Fungitoxicity, Phytotoxicity and Target Site
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Morinda citrifolia Essential Oil
2.2. In Vitro Sensitivity of Colletotrichum spp. to Morinda citrifolia Essential Oil
2.3. Phytotoxity of Morinda citrifolia Essential Oil in Cassava Plants
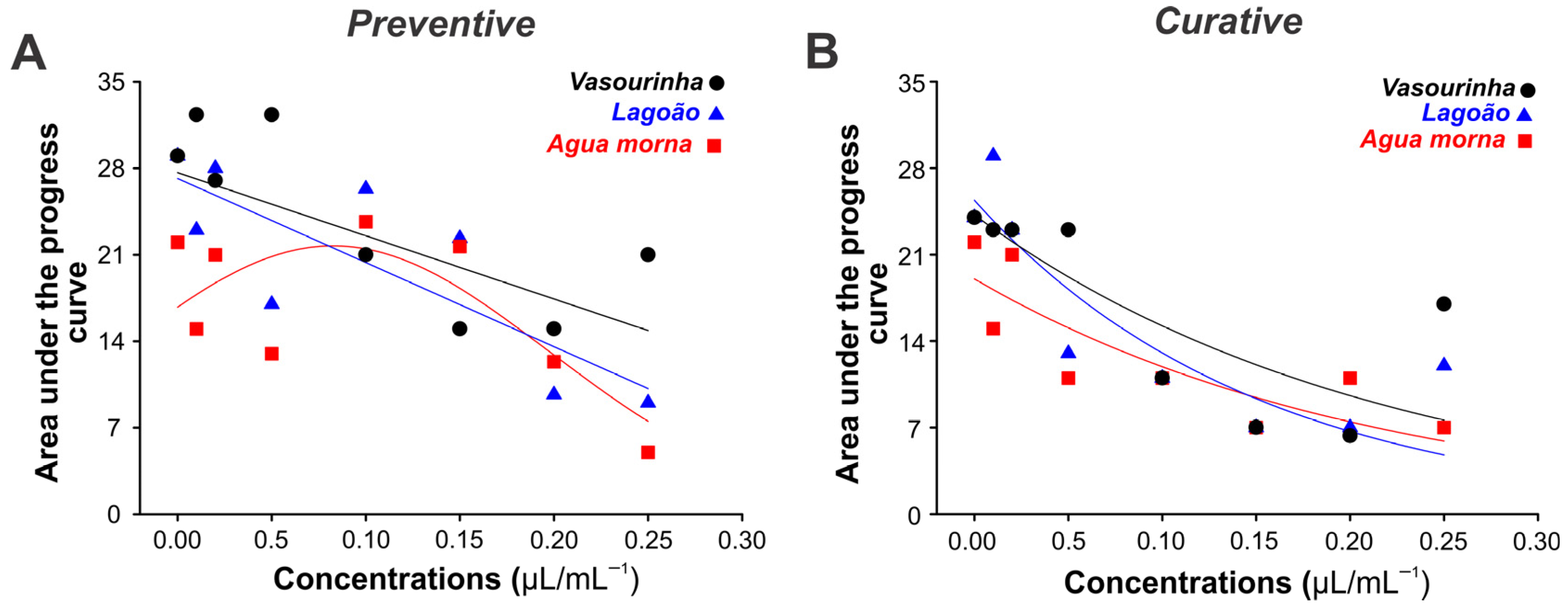
2.4. In Vivo Control of Cassava Anthracnose
2.5. Identification of the Interaction Between the Target Receptor and the Morinda citrifolia Essential Oil
3. Discussion
4. Materials and Methods
4.1. Sampling, Isolation, Species Identification, and Pathogenicity
4.2. Noni Essential Oil Extraction
4.3. Gas Chromatography (GC) Analysis
4.4. In Vitro Fungitoxicity of Morinda citrifolia Essential Oil in Colletotrichum spp.
4.5. Phytotoxity of Morinda citrifolia Essential oil in Cassava Plants
4.6. Preventive and Curative Disease Control of Noni Essential Oil
4.7. In Silico Analysis
4.7.1. Ligand Modeling
4.7.2. Preparing the Molecular Target
4.7.3. Molecular Docking Calculations
4.8. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Cassava Statistics Division. 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 20 June 2022).
- Orek, C. A review of management of major arthropod pests affecting cassava production in Sub-Saharan Africa. Crop Prot. 2024, 175, 106465. [Google Scholar] [CrossRef]
- Shan, Z.; Luo, X.; Wu, M.; Wei, L.; Fan, Z.; Zhu, Y. Genome-wide identification and expression of GRAS gene family members in Cassava. BMC Plant Biol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Ono, L.T.; Silva, J.J.; Doná, S.; Martins, L.M.; Iamanaka, B.T.; Fungaro, M.H.P.; Pitt, J.I.; Taniwaki, M.H. Aspergillus section flavi and aflatoxins in Brazilian cassava (Manihot esculenta Crantz) and products. Mycotoxin Res. 2021, 37, 221–228. [Google Scholar] [CrossRef]
- Ekeleme, F.; Dixon, A.; Atser, G.; Hauser, S.; Chikoye, D.; Korie, S.; Olojede, A.; Agada, M.; Olorunmaiye, P.M. Increasing cassava root yield on farmers’ fields in Nigeria through appropriate weed management. Crop Prot. 2021, 150, 105810. [Google Scholar] [CrossRef]
- Jolayemi, O.L.; Opabode, J.T. Responses of cassava (Manihot esculenta Crantz) varieties to in vitro mannitol-induced drought stress. J. Crop Improv. 2018, 32, 566–578. [Google Scholar] [CrossRef]
- More, S.J.; Bardhan, K.; Ravi, V.; Pasala, R.; Chaturvedi, A.K.; Lal, M.K.; Siddique, K.H.M. Morphophysiological responses and tolerance mechanisms in cassava (Manihot esculenta Crantz) under drought stress. J. Soil Sci. Plant Nutr. 2023, 23, 71–91. [Google Scholar] [CrossRef]
- Changtor, P.; Jaroenpol, W.; Buddhachat, K.; Wattanachaiyingcharoen, W.; Yimtragool, N. Rapid detection of Sclerotium rolfsii causing dry stem and root rot disease in cassava by recombinase polymerase amplification technique (RPA) combined with CRISPR/Cas12a. Crop Prot. 2023, 172, 106340. [Google Scholar] [CrossRef]
- Venturini, M.T.; Araújo, T.d.S.; Abreu, E.F.M.; Andrade, E.C.d.; Santos, V.d.S.; Silva, M.R.d.; Oliveira, E.J.d. Crop losses in Brazilian cassava varieties induced by the Cassava common mosaic virus. Sci. Agric. 2016, 73, 520–524. [Google Scholar] [CrossRef]
- William, M.N.M.; Mbega, E.R.; Mabagala, R.B. An Outbreak of Anthracnose Caused by Colletotrichum gloesporioides f.sp. manihotis in Cassava in North Western Tanzania. Am. J. Plant Sci. 2012, 3, 4. [Google Scholar] [CrossRef]
- Martins, S.d.S.; Souza, G.J.T.d.; Netto, M.d.S.B.; Vieira, W.A.d.S.; Assunção, I.P.; Neto, F.d.A.; Filho, F.d.A.C.R.; de Melo, M.P. Colletotrichum truncatum causing anthracnose of Catharanhthus roseus in Brazil. Crop Prot. 2024, 175, 106449. [Google Scholar] [CrossRef]
- Machado, S.d.C.S.; Veloso, J.S.; Câmara, M.P.S.; Vieira, W.A.S.; Jumbo, L.O.V.; Aguiar, R.W.S.; Cangussu, A.S.R.; Giongo, M.V.; Moraes, C.B.; Campos, F.S.; et al. Diversity, prevalence and virulence of Colletotrichum species causing anthracnose on cassava leaves in the northern region of Brazil. J. Fungi 2024, 10, 367. [Google Scholar] [CrossRef]
- Wokocha, R.; Nneke, N.; Umechuruba, C. Screening Colletotrichum gloeosporioides f. sp Manihotis isolates for virulence on cassava in Akwa Ibom state of Nigeria. Agric. Sci 2010, 9. [Google Scholar] [CrossRef]
- Kunkeaw, S.; Worapong, J.; Smith, D.R.; Triwitayakorn, K. An in vitro detached leaf assay for pre-screening resistance to anthracnose disease in cassava (Manihot esculenta Crantz). Australas. Plant Pathol. 2010, 39, 547–550. [Google Scholar] [CrossRef]
- Nandeesha, C.V.; Akbari, L.F.; Jaiswal, A.; Harsha, B.R.; Patil, B.; Bhaliya, C.M.; Kumar, N.; Singh, T.; Jain, S. Control efficacy and yield response of different fungicides evaluated against anthracnose of green gram. Crop Prot. 2023, 174, 106432. [Google Scholar] [CrossRef]
- Morais, M.d.S.; Nascimento, L.C.d.; Moreira, K.A.; Cavalcanti, M.d.S.; Oliveira, N.T.d. Levantamento e avaliação da incidência das doenças da mandioca no estado da Paraíba. Summa Phytopathol. 2013, 39, 204–206. [Google Scholar] [CrossRef]
- Pelaez, V.M.; da Silva, L.R.; Guimarães, T.A.; Dal Ri, F.; Teodorovicz, T. A (des) coordenação de políticas para a indústria de agrotóxicos no Brasil. Rev. Bras. Inovação 2015, 14, 153–178. [Google Scholar] [CrossRef]
- Souza, A.E.; Araújo, E.; Nascimento, L.C. Atividade antifúngica de extratos de alho e capim-santo sobre o desenvolvimento de Fusarium proliferatum isolado de grãos de milho. Fitopatol. Bras. 2007, 32, 465–471. [Google Scholar] [CrossRef]
- Santos, G.d.; Monteiro, M. Sistema orgânico de produção de alimentos. Alim. Nutr. Araraquara 2008, 15, 73–86. [Google Scholar]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The use of essential oils from thyme, sage and peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef]
- Weisany, W.; Samadi, S.; Amini, J.; Hossaini, S.; Yousefi, S.; Maggi, F. Enhancement of the antifungal activity of thyme and dill essential oils against Colletotrichum nymphaeae by nano-encapsulation with copper NPs. Ind. Crops Prod. 2019, 132, 213–225. [Google Scholar] [CrossRef]
- Bazie, S.; Ayalew, A.; Woldetsadik, K. Integrated management of postharvest banana anthracnose (Colletotrichum musae) through plant extracts and hot water treatment. Crop Prot. 2014, 66, 14–18. [Google Scholar] [CrossRef]
- Barros, A.M.; de Souza Ferreira, T.P.; Mourão, D.d.S.C.; de Souza Ferreira, T.P.; Lima, C.S.L.; dos Santos Barbosa, R.; Battistelli, L.C.; dos Santos, G.R. Utilização da farinha e óleo de noni no controle alternativo de Rhizoctonia solani em soja. Braz. J. Dev. 2021, 7, 36069–36079. [Google Scholar] [CrossRef]
- Silva, J.C.E.; Mourão, D.D.S.C.; Lima, F.S.d.O.; Sarmento, R.D.A.; Dalcin, M.S.; Aguiar, R.W.d.S.; Santos, G.R.d. The efficiency of noni (Morinda citrifolia L.) essential oil on the control of leaf spot caused by Exserohilum turcicum in maize culture. Medicines 2017, 4, 60. [Google Scholar] [CrossRef]
- Veloso, R.A.; de Souza Ferreira, T.P.; Dias, B.L.; MourÃ, D.d.S.C.; de Araújo Filho, R.N.; Glória, R.S.L.; Barros, A.M.; de Souza Ferreira, T.P.; Chapla, V.M.; Cangussu, A.S.R. Chemical composition and bioactivity of essential oil from Morinda citrifolia L. fruit. J. Med. Plant Res. 2020, 14, 208–214. [Google Scholar] [CrossRef]
- Dalcin, M.S.; CafÃ, A.C.a.; de Almeida Sarmento, R.; do Nascimento, I.R.; de Souza Ferreira, T.P.; de Sousa Aguiar, R.W.; dos Santos, G.R. Evaluation of essential oils for preventive or curative management of melon gummy stem blight and plant toxicity. J. Med. Plant Res. 2017, 11, 426–432. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential oils as green pesticides: Potential and constraints. Biopestic. Int 2008, 4, 63–84. [Google Scholar]
- Chan-Blanco, Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.-M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Holanda, L.; Bezerra, G.B.; Ramos, C.S. Potent antifungal activity of essential oil from Morinda citrifolia fruits rich in short-chain fatty acids. Int. J. Fruit Sci. 2020, 20, S448–S454. [Google Scholar] [CrossRef]
- Dalcin, M.S.; Dias, B.L.; Viteri Jumbo, L.O.; Oliveira, A.C.S.S.; Araújo, S.H.C.; Moura, W.S.; Mourão, D.S.C.; Ferreira, T.P.S.; Campos, F.S.; Cangussu, A.S.R.; et al. Potential action mechanism and inhibition efficacy of Morinda citrifolia essential oil and octanoic acid against Stagonosporopsis cucurbitacearum infestations. Molecules 2022, 27, 5173. [Google Scholar] [CrossRef]
- Dias, B.L.; Sarmento, R.A.; Venzon, M.; Jumbo, L.O.V.; dos Santos, L.S.S.; de Souza Moura, W.; Mourão, D.d.S.C.; Fernandes, P.R.d.S.; Neitzke, T.R.; Oliveira, J.V.d.A.; et al. Morinda citrifolia Essential Oil: A plant resistance biostimulant and a sustainable alternative for controlling phytopathogens and insect pests. Biology 2024, 13, 479. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Ismail, S.; Man, C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012, 92, 593–597. [Google Scholar] [CrossRef]
- Brophy, J.; Devi, R.; Ali, S.; Rao, D.; Sotheeswaran, S. Chemistry and antimicrobial activity of the essential oils from ripe and unripe fruits of the Fijian Morinda citrifolia (noni/kura) Rubiaceae. J. Essent. Oil-Bear. Plants 2008, 11, 598–602. [Google Scholar] [CrossRef]
- Natheer, S.E.; Sekar, C.; Amutharaj, P.; Rahman, M.S.A.; Khan, K.F. Evaluation of antibacterial activity of Morinda citrifolia, Vitex trifolia and Chromolaena odorata. Afr. J. Pharm 2012, 6, 783–788. [Google Scholar] [CrossRef]
- Passos Braga, S.; Lundgren, G.A.; Macedo, S.A.; Tavares, J.F.; dos Santos Vieira, W.A.; Câmara, M.P.S.; de Souza, E.L. Application of coatings formed by chitosan and Mentha essential oils to control anthracnose caused by Colletotrichum gloesporioides and C. brevisporum in papaya (Carica papaya L.) fruit. Int. J. Biol. Macromol. 2019, 139, 631–639. [Google Scholar] [CrossRef]
- Lima Oliveira, P.D.; de Oliveira, K.Á.R.; Vieira, W.A.d.S.; Câmara, M.P.S.; de Souza, E.L. Control of anthracnose caused by Colletotrichum species in guava, mango and papaya using synergistic combinations of chitosan and Cymbopogon citratus (D.C. ex Nees) Stapf. essential oil. Int. J. Food Microbiol. 2018, 266, 87–94. [Google Scholar] [CrossRef]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal activity of three essential oils against Colletotrichum acutatum, the causal agent of strawberry anthracnose. J. Essent. Oil-Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Pedrotti, C.; Marcon, Â.R.; Delamare, A.P.L.; Echeverrigaray, S.; da Silva Ribeiro, R.T.; Schwambach, J. Alternative control of grape rots by essential oils of two Eucalyptus species. J. Sci. Food Agric. 2019, 99, 6552–6561. [Google Scholar] [CrossRef]
- Hong, J.K.; Yang, H.J.; Jung, H.; Yoon, D.J.; Sang, M.K.; Jeun, Y.C. Application of volatile antifungal plant essential oils for controlling pepper fruit anthracnose by Colletotrichum gloeosporioides. Plant Pathol. J. 2015, 31, 269–277. [Google Scholar] [CrossRef]
- Albarracín, L.T.; Delgado, W.; Cuca, L.E.; Ávila, M.C. New butyrolactone and other metabolites from the bark of Endlicheria arenosa against of the phytopathogen Colletotrichum tamarilloi. Nat. Prod. Res. 2019, 33, 687–694. [Google Scholar] [CrossRef]
- Robles-Yerena, L.; Rodríguez-Mendoza, J.; Santoyo, G.; Ochoa-Alvarado, X.I.; Medina-Estrada, R.I.; Jiménez-Mejía, R.; Loeza-Lara, P.D. Phylogenetic identification of fungi isolated from strawberry and papaya fruits and their susceptibility to fatty acids. Can. J. Plant Pathol. 2022, 44, 828–835. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, L.-T.; Yuan, E.-L.; Ding, H.-X.; Ye, H.-C.; Zhang, Z.-K.; Yan, C.; Liu, Y.-Q.; Feng, G. Antifungal cctivity of compounds extracted from Cortex Pseudolaricis against Colletotrichum gloeosporioides. J. Agric. Food Chem. 2014, 62, 4905–4910. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Leyva, M.O.; Vicedo, B.; Finiti, I.; Flors, V.; Del Amo, G.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Preventive and post-infection control of Botrytis cinerea in tomato plants by hexanoic acid. Plant Pathol. 2008, 57, 1038–1046. [Google Scholar] [CrossRef]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.; Pereira, E.J.; Aguiar, R.W.; Oliveira, E.E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the Twenty-First century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A review. Sci. Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 3, 61–71. [Google Scholar]
- Matejić, J.S.; Stojanović-Radić, Z.Z.; Ristić, M.S.; Veselinović, J.B.; Zlatković, B.K.; Marin, P.D.; Džamić, A.M. Chemical characterization, in vitro biological activity of essential oils and extracts of three Eryngium L. species and molecular docking of selected major compounds. J. Food Sci. Technol. 2018, 55, 2910–2925. [Google Scholar] [CrossRef]
- Francklyn, C.; Perona, J.J.; Puetz, J.; Hou, Y.-M. Aminoacyl-tRNA synthetases: Versatile players in the changing theater of translation. RNA 2002, 8, 1363–1372. [Google Scholar] [CrossRef]
- Sun, J.; Lv, P.-C.; Zhu, H.-L. Tyrosyl-tRNA synthetase inhibitors: A patent review. Expert Opin. Ther. Pat. 2017, 27, 557–564. [Google Scholar] [CrossRef]
- Pang, L.; Weeks, S.D.; Van Aerschot, A. Aminoacyl-tRNA synthetases as valuable targets for antimicrobial drug discovery. Int. J. Mol. Sci. 2021, 22, 1750. [Google Scholar] [CrossRef]
- Elkolli, M.; Elkolli, H.; Alam, M.; Benguerba, Y. In silico study of antibacterial tyrosyl-tRNA synthetase and toxicity of main phytoconstituents from three active essential oils. J. Biomol. Struct. Dyn. 2023, 42, 1404–1416. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef]
- Grover, A. Plant Chitinases: Genetic diversity and physiological roles. Crit. Rev. Plant Sci. 2012, 31, 57–73. [Google Scholar] [CrossRef]
- Osorio, P.R.A.; Dias, F.R.; Mourão, D.S.C.; Araujo, S.H.C.; Toledo, P.F.S.; Silva, A.C.F.; Viera, W.A.S.; Câmara, M.P.S.; Moura, W.S.; Aguiar, R.W.A.; et al. Essential oil of Noni, Morinda citrifolia L., fruits controls the rice stem-rot disease without detrimentally affect beneficial fungi and ladybeetles. Ind. Crops Prod. 2021, 170, 113728. [Google Scholar] [CrossRef]
- Castellani, A. Maintenance and cultivation of common pathogenic fungi of man in sterile distilled water. Further researches. J. Trop. Med. Hyg. 1967, 70, 181–184. [Google Scholar]
- Sutton, B. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980; p. 696. [Google Scholar]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Machado, S.d.C.S.; Veloso, J.S.; Câmara, M.P.S.; Campos, F.; Sarmento, R.d.A.; Giongo, M.V.; dos Santos, G.R. First report of Colletotrichum chrysophillum causing cassava anthracnose in Brazil. Plant Dis. 2021, 105, 1196. [Google Scholar] [CrossRef]
- Soliman, M.H.; El-Mohamedy, R.S.R. Induction of defense-related physiological and antioxidant enzyme response against powdery mildew disease in Okra (Abelmoschus esculentus L.) plant by using chitosan and potassium salts. Mycobiology 2017, 45, 409–420. [Google Scholar] [CrossRef]
- González, A.M.; Yuste-Lisbona, F.J.; Rodiño, A.P.; De Ron, A.M.; Capel, C.; García-Alcázar, M.; Lozano, R.; Santalla, M. Uncovering the genetic architecture of Colletotrichum lindemuthianum resistance through QTL mapping and epistatic interaction analysis in common bean. Front. Plant Sci. 2015, 6, 141. [Google Scholar] [CrossRef]
- Guimarães, L.G.d.L.; Cardoso, M.d.G.; Zacaroni, L.M.; Lima, R.K.d.; Pimentel, F.A.; Morais, A.R.d. Influência da luz e da temperatura sobre a oxidação do óleo essencial de capim-limão (Cymbopogon citratus (DC) Stapf). Química Nova 2008, 31, 1476–1480. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th online ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Freitas, S.; Moreira, J.; Freitas, I.; Freitas Júnior, S.; Amaral Júnior, A.d.; Silva, V. Fitotoxicidade de herbicidas a diferentes cultivares de milho-pipoca. Planta Daninha 2009, 27, 1095–1103. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons.: New York, NY, USA, 1990; p. 532. [Google Scholar]
- Alencar, M.S.R.; Solino, A.J.d.S.; Oliveira, J.S.B.; Pascholati, S.F.; Schwan-Estrada, K.R.F. Induction of defense mechanisms in tomato plants by saprobic fungi filtrates against early blight disease. Rev. Catinga 2020, 33, 671–678. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Sasisekharan, V. Conformation of Polypeptides and Proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Anson, M.L., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 23, pp. 283–437. [Google Scholar]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous Automated Model Evaluation (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2010, 27, 343–350. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. 1999, 17, 57–61. [Google Scholar]
- Souza Moura, W.; Souza, S.R.; Campos, F.S.; Sander Rodrigues Cangussu, A.; Macedo Sobrinho Santos, E.; Silva Andrade, B.; Borges Gomes, C.H.; Fernandes Viana, K.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crops Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrodinger, Inc. The PyMOL Molecular Graphics System, ver. 2.0.1; Schrodinger: New York, NY, USA, 2018.
- Dassault Systèmes. BIOVIA Discovery Studio; Dassault Systèmes: Waltham, MA, USA, 2017. [Google Scholar]


| Compounds | Retention Time (min) | RI (Calculated Retention Index) | % |
|---|---|---|---|
| 2-Heptanone | 5.01 | 927 | 0.14 |
| Methyl hexanoate | 5.78 | 944 | 1.08 |
| Ethyl-hexanoate | 7.99 | 991 | 0.50 |
| Hexanoic acid | 8.68 | 1006 | 10.16 |
| Butanoic, 4-pentenyl ester | 10.39 | 1043 | 0.19 |
| Methyl octanoate | 12.75 | 1094 | 5.35 |
| Octanoate acetate | 15.84 | 1163 | 3.58 |
| Octanoic acid | 17.41 | 1198 | 64.03 |
| 4-pentyl hexanoate | 18.60 | 1225 | 4.30 |
| Decanoic acid, methyl ester | 21.39 | 1288 | 0.19 |
| Butanoic acid | 26.99 | 1420 | 8.64 |
| Hexyl caprylate | 31.93 | 1540 | 0.37 |
| 1-Octanoyl-1H-imidazole | 28.30 | 1451 | 0.90 |
| Others | 0.57 | ||
| Total | 100 |
| Treatment (µL/mL) | Scale | Observed in Cassava Plants |
|---|---|---|
| Control (sterilized H2O + Tween 80) | 0 | No phytotoxicity |
| Control (sterilized H2O) | 0 | No phytotoxicity |
| 0.5 | 0 | No phytotoxicity |
| 1.0 | 0 | No phytotoxicity |
| 1.5 | 0 | No phytotoxicity |
| 2.0 | 0 | No phytotoxicity |
| 2.5 | 0 | No phytotoxicity |
| 5.0 | 75 | High chlorosis and necrosis |
| Organism | Target (Unipot Database) | Identity | Model Identity (%) | Ramachandran Favored (%) | Q Medium |
|---|---|---|---|---|---|
| Glomerella cingulata | Tyrosine-tRNA ligase (T0L8P8) | 4OJM | 61.62 | 97.46 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damascena, J.F.; Viteri, L.O.; Souza, M.H.P.; Aguiar, R.W.; Camara, M.P.; Moura, W.S.; Oliveira, E.E.; Santos, G.R. The Preventive and Curative Potential of Morinda citrifolia Essential Oil for Controlling Anthracnose in Cassava Plants: Fungitoxicity, Phytotoxicity and Target Site. Stresses 2024, 4, 663-675. https://doi.org/10.3390/stresses4040042
Damascena JF, Viteri LO, Souza MHP, Aguiar RW, Camara MP, Moura WS, Oliveira EE, Santos GR. The Preventive and Curative Potential of Morinda citrifolia Essential Oil for Controlling Anthracnose in Cassava Plants: Fungitoxicity, Phytotoxicity and Target Site. Stresses. 2024; 4(4):663-675. https://doi.org/10.3390/stresses4040042
Chicago/Turabian StyleDamascena, Jossimara F., Luis O. Viteri, Matheus H. P. Souza, Raimundo W. Aguiar, Marcos P. Camara, Wellington S. Moura, Eugênio E. Oliveira, and Gil R. Santos. 2024. "The Preventive and Curative Potential of Morinda citrifolia Essential Oil for Controlling Anthracnose in Cassava Plants: Fungitoxicity, Phytotoxicity and Target Site" Stresses 4, no. 4: 663-675. https://doi.org/10.3390/stresses4040042
APA StyleDamascena, J. F., Viteri, L. O., Souza, M. H. P., Aguiar, R. W., Camara, M. P., Moura, W. S., Oliveira, E. E., & Santos, G. R. (2024). The Preventive and Curative Potential of Morinda citrifolia Essential Oil for Controlling Anthracnose in Cassava Plants: Fungitoxicity, Phytotoxicity and Target Site. Stresses, 4(4), 663-675. https://doi.org/10.3390/stresses4040042









