Analysis of Morphological, Physiological, and Biochemical Traits of Salt Stress Tolerance in Asian Rice Cultivars at Seedling and Early Vegetative Stages
Abstract
1. Introduction
2. Results
2.1. Morphological Responses of Asian Rice Cultivars to Salt Stress at Seedling and Early Vegetative Stages
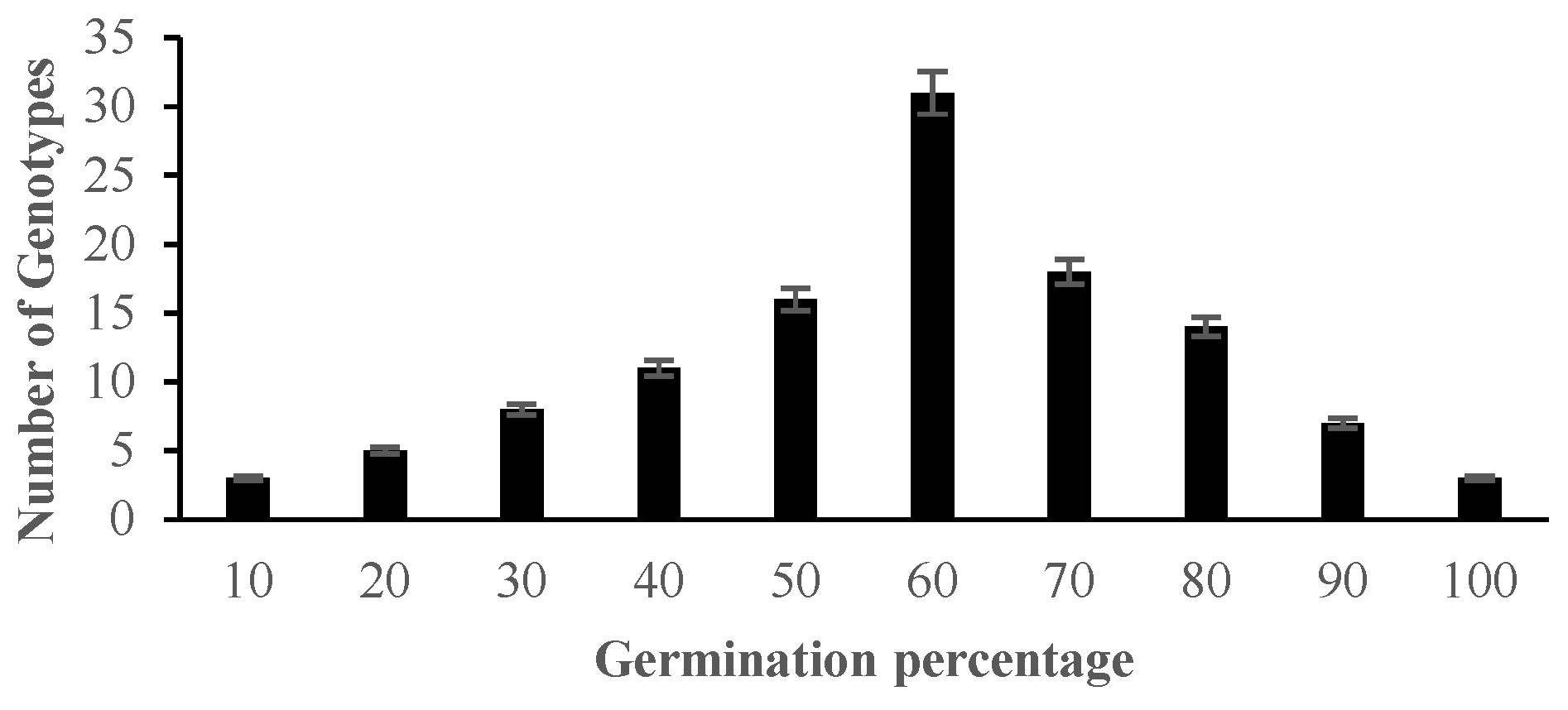
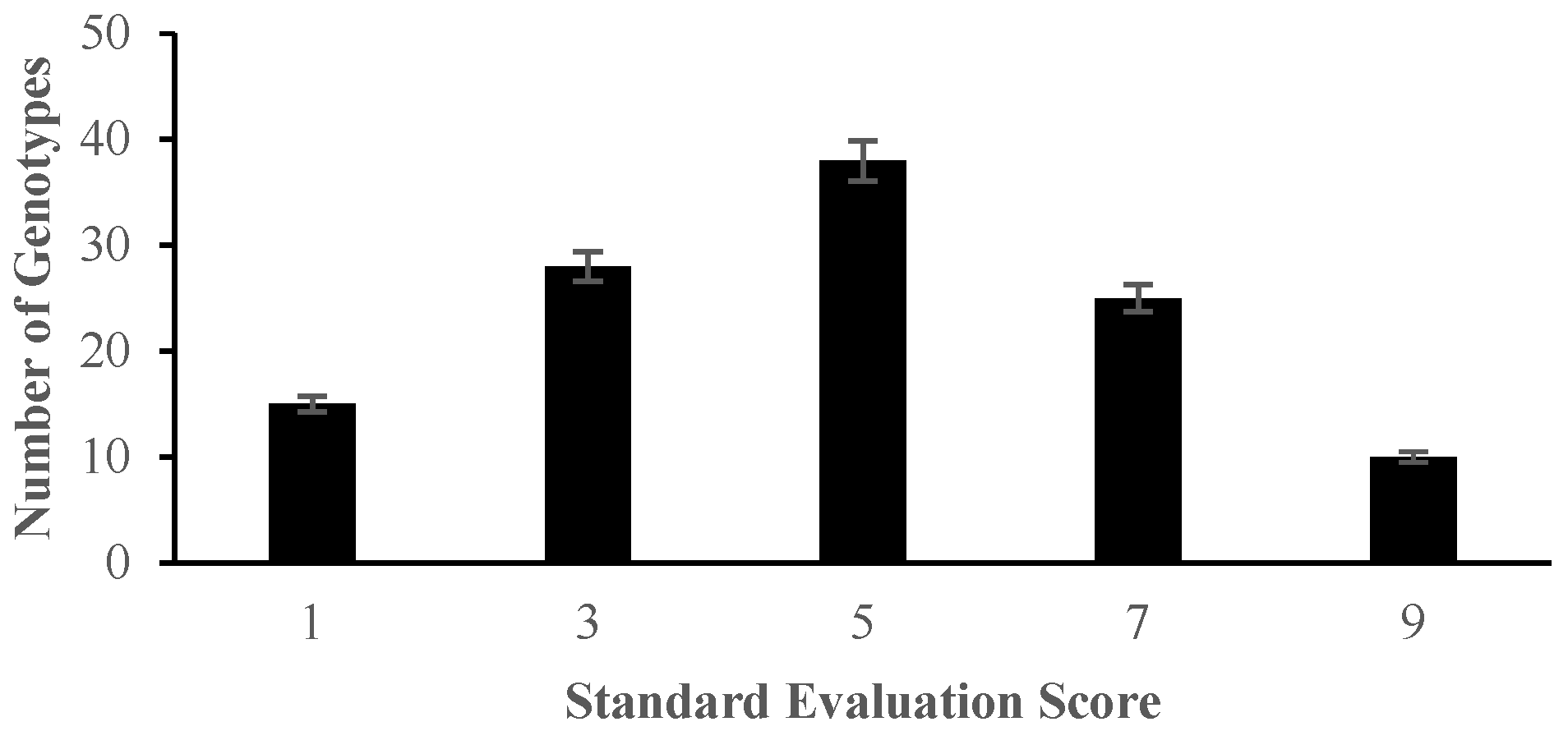
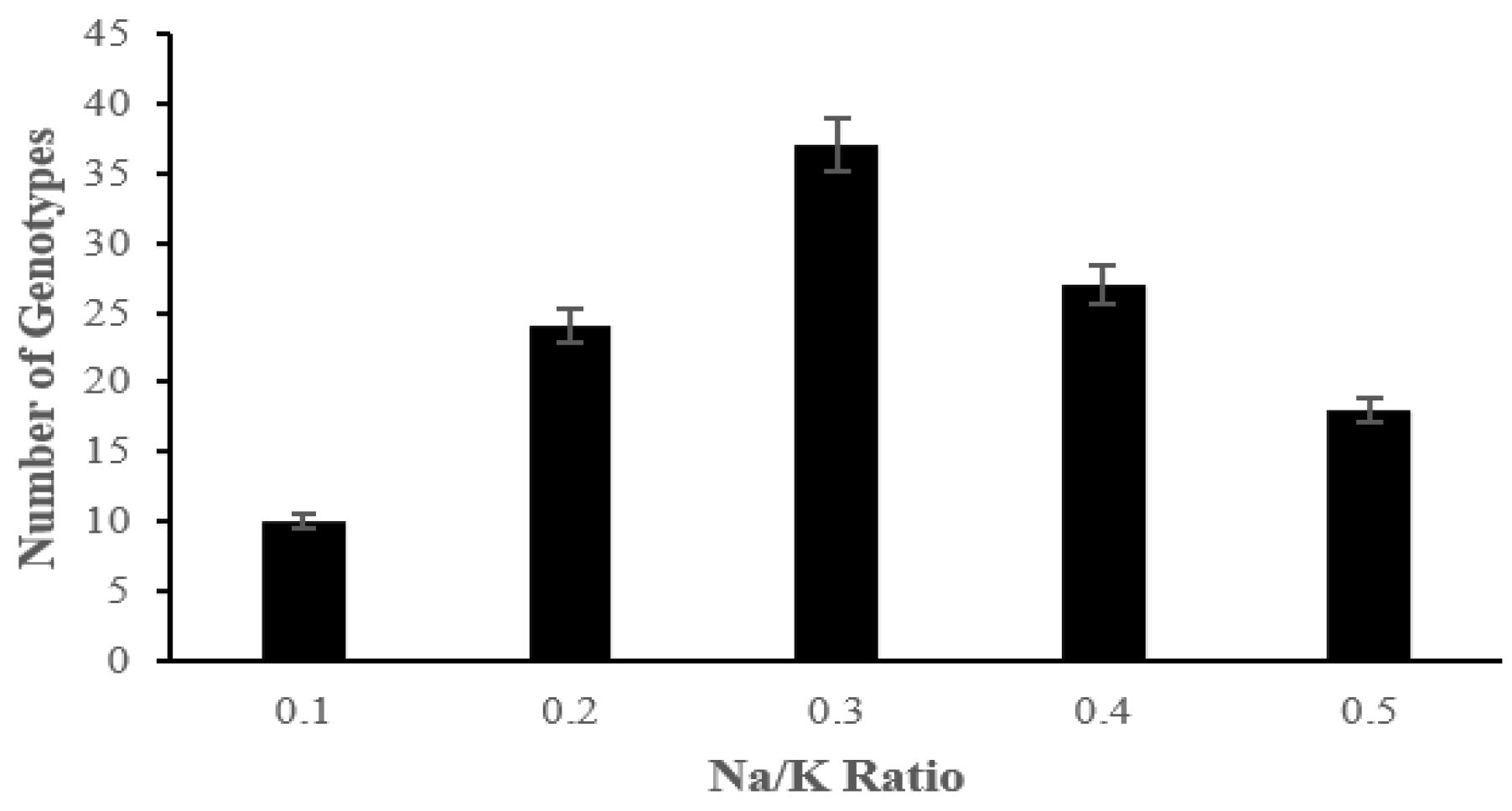
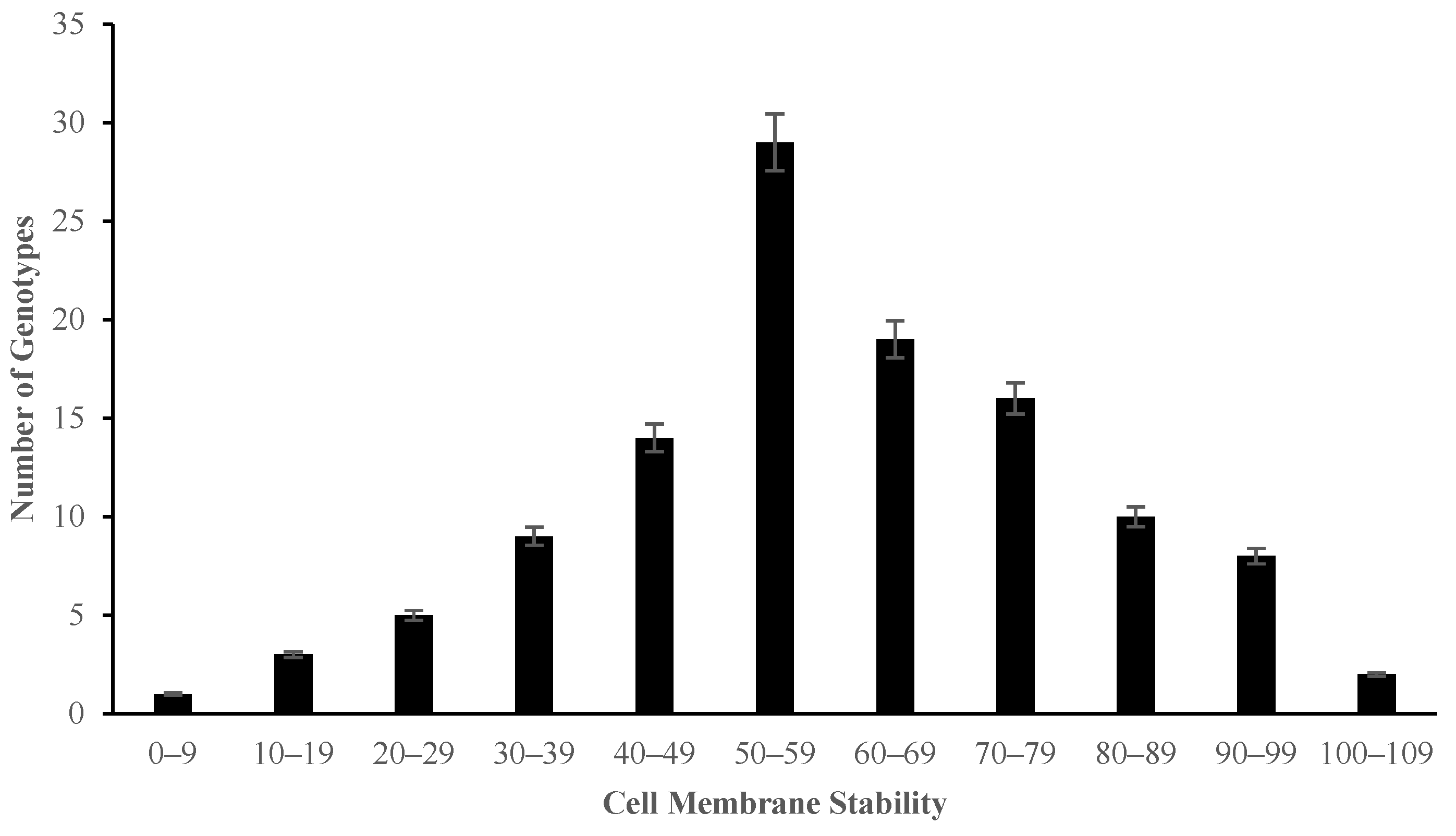
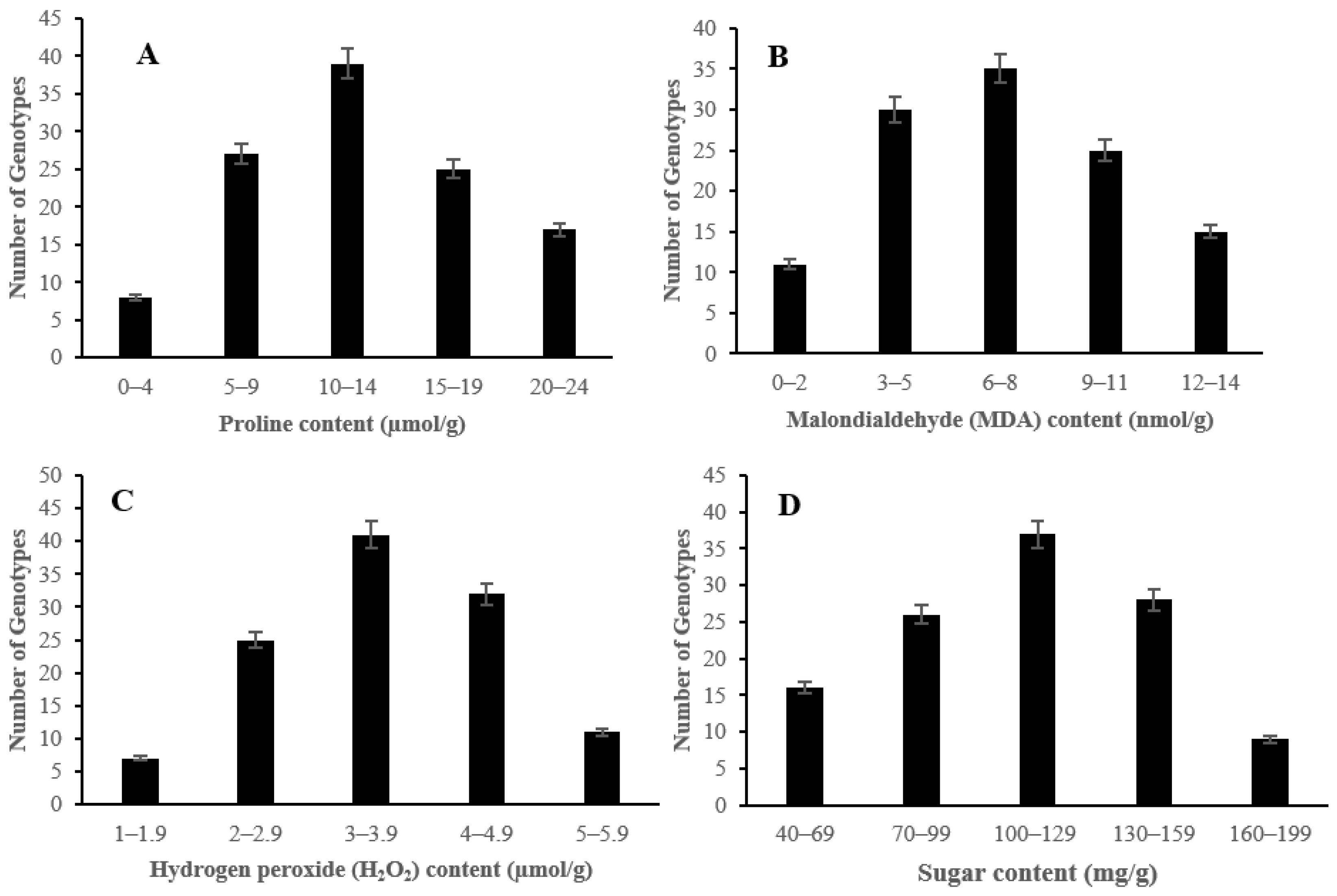
2.2. The Effect of Salinity Stress on Physiological and Biochemical Characteristics of Asian Rice Cultivars at Seedling and Early Vegetative Stages
2.3. Correlation among Morphological, Physiological, and Biochemical Traits under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Salinity Screening at Seedling and Early Vegetative Stage
4.3. Measurement of Morphological, Physiological, and Biochemical Traits
4.3.1. Determination of Germination Percentage and Standard Evaluation Score for Salt Injury
4.3.2. Measurement of Root Length, Root Fresh Weight, Shoot Length, and Plant Biomass
4.3.3. Determination of Leaf Rolling
4.3.4. Analysis of Chlorophyll Content
4.3.5. Measurement of Na/K Ratio
4.3.6. Measurement of Cell Membrane Stability
4.3.7. Measurement of Proline Content
4.3.8. Measurement of Malondialdehyde (MDA) Content
4.3.9. Measurement of Hydrogen Peroxide (H2O2) Content
4.3.10. Measurement of Sugar Content
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopalan, C.; Rama Sastri, B.V.; Balasubramanian, S. Nutritive Value of Indian Foods. In National Institute of Nutrition (NIN); ICMR: New Delhi, India, 2007. [Google Scholar]
- Khush, G.S.; Virk, P.S. Rice Breeding: Achievements and Future Strategies. Crop Improv. 2000, 27, 115–144. [Google Scholar]
- GRiSP. Rice Almanac, 4th ed.; International Rice Research Institute: Los Banos, Philippines, 2013. [Google Scholar]
- ANU. Technological Transformation of Productivity, Profitability and Sustainability: Rice. In The First Ten K R Narayanan Orations: Essays by Eminent Persons on the Rapidly Transforming Indian Economy; Australia South Asia Research Centre the Australian National University: Canberra, Australia, 2006; Available online: http://epress.anu.edu.au/narayanan/mobile_devices/pr01.html (accessed on 11 February 2023).
- Gregorio, G.B. Tagging Salinity Tolerant Genes in Rice Using Amplified Fragment Length Polymorphism (AFLP). Ph.D. Thesis, University of the Philippines Los Baños College, Los Baños, Philippines, 1997. [Google Scholar]
- Abdullah, Z.; Khan, M.A.; Flowers, T.J. Causes of Sterility in Seed Set of Rice under Salinity Stress. J. Agron. Crop Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Dwiningsih, Y. Molecular Genetic Analysis of Drought Resistance and Productivity Traits of Rice Genotypes; University of Arkansas: Fayetteville, NC, USA, 2020. [Google Scholar]
- Aref, F.; Rad, H.E. Physiological Characterization of Rice under Salinity Stress during Vegetative and Reproductive Stages. Indian J. Sci. Technol. 2012, 5, 2578–2586. [Google Scholar] [CrossRef]
- Bhowmik, S.K.; Titov, S.; Islam, M.M.; Siddika, A.; Sultana, S.; Haque, M. Phenotypic and genotypic screening of rice genotypes at seedling stage for salt tolerance. Afr. J. Biotechnol. 2009, 8, 6490–6494. [Google Scholar]
- Bado, S.; Forster, B.P.; Ghanim, A.; Jankowicz-Cieslak, J.; Berthold, G.; Luxiang, L. Protocols for Pre-Field Screening of Mutants for Salt Tolerance in Rice, Wheat and Barley; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Ueda, A.; Yahagi, H.; Fujikawa, Y.; Nagaoka, T.; Esaka, M.; Calcaño, M.; González, M.; Martich, J.; Saneoka, H. Comparative physiological analysis of salinity tolerance in rice. Soil Sci. Plant Nutr. 2013, 59, 896–903. [Google Scholar] [CrossRef]
- Ge, X.; Khan, Z.I.; Chen, F.; Akhtar, M.; Ahmad, K.; Ejaz, A.; Ashraf, M.A.; Nadeem, M.; Akhtar, S.; Alkahtani, J.; et al. A study on the contamination assessment, health risk and mobility of two heavy metals in the soil-plants-ruminants system of a typical agricultural region in the semi-arid environment. Environ. Sci. Pollut. Res. 2022, 29, 14584–14594. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Choi, W.Y.; Ko, J.C.; Kim, T.S.; Gregorio, G.B. Salinity tolerance of japonica and indica rice (Oryza sativa L.) at the seedling stage. Planta 2003, 216, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Kordrostami, M.; Rabiei, B.; Kumleh, H. Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol. Mol. Biol. Plants 2017, 23, 529–544. [Google Scholar] [CrossRef]
- Maqsood, A.; Khan, Z.I.; Ahmad, K.; Akhtar, S.; Ashfaq, A.; Malik, I.S.; Sultana, R.; Nadeem, M.; Alkahtani, J.; Dwiningsih, Y.; et al. Quantitative evaluation of zinc metal in meadows and ruminants for health assessment: Implications for humans. Environ. Sci. Pollut. Res. 2022, 29, 21634–21641. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.M.L.; Williams, B.; Khanna, H.; Dale, J.; Mundree, S.G. Physiological basis of salt stress tolerance in rice expressing the antiapoptotic gene SfIAP. Funct. Plant Biol. 2014, 41, 1168–1177. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Sahi, C.; Singh, A.; Kumar, K. Salt stress response in rice: Genetics, molecular biology and comparative genomics. Funct. Integr. Genom. 2006, 6, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, P.G.; Miller, A.J.; Mathew, M.K.; Maathuis, F.J.M. Rice cultivars with differing salt tolerance contain similar cation channels in their root cells. J. Exp. Bot. 2012, 63, 3289–3296. [Google Scholar] [CrossRef]
- Hoang, T.; Tran, T.; Nguyen, T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Sitrarasi, R.; Nallal, U.M.; Razia, M.; Chung, W.J.; Shim, J.; Chandrasekaran, M.; Dwiningsih, Y.; Rasheed, R.A.; Alkahtani, J.; Elshikh, M.S.; et al. Inhibition of multi-drug resistant microbial pathogens using an ecofriendly root extract of Furcraea foetida silver nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 101794. [Google Scholar] [CrossRef]
- De Leon, T.B.; Linscombe, S.; Gregorio, G.; Subudhi, P.K. Genetic variation in Southern USA rice genotypes for seedling salinity tolerance. Front. Plant Sci. 2015, 6, 374. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Nejad, G.; Singh, R.K.; Arzanic, A.; Rezaiec, A.M.; Sabourid, H.; Gregorio, G.B. Evaluation of Salinity Tolerance in Rice Genotypes. Int. J. Plant Prod. 2010, 4, 199–207. [Google Scholar]
- Morales, S.G.; Tellez, L.I.T.; Merino, F.C.G.; Caldana, C.; Victoria, D.E.; Cabrera, B.E.H. Growth, Photosynthetic Activity, and Potassium and Sodium Concentration in Rice Plants under Salt Stress. Acta Sci. Agron. 2012, 34, 317–324. [Google Scholar] [CrossRef]
- Asch, F.; Dingkuhn, M.; Dorffling, K.; Miezan, K. Leaf K/Na ratio predicts salinity induced yield loss in irrigated rice. Euphytica 2000, 113, 109–118. [Google Scholar] [CrossRef]
- Yeo, A.R.; Flowers, T.J. Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. Aust. J. Plant Physiol. 1986, 13, 161–173. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Lauchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Dwiningsih, Y.; Alkahtani, J. Genetics, Biochemistry and Biophysical Analysis of Anthocyanin in Rice (Oryza sativa L.). Adv. Sustain. Sci. Eng. Technol. 2022, 4, 1. [Google Scholar] [CrossRef]
- Ferreira, L.J.; Azevedo, V.; Maroco, J.; Oliveira, M.M.; Santos, A.P. Salt Tolerant and Sensitive Rice Varieties Display Differential Methylome Flexibility under Salt Stress. PLoS ONE 2015, 10, e0124060. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.L.; Levesley, A.; Koebner, R.; Flowers, T.J.; Yeo, A.R. Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol. 2001, 125, 406–422. [Google Scholar] [CrossRef]
- Gregorio, G.B.; Senadhira, D. Genetic analysis of salinity tolerance in rice (Oryza sativa L.). Theor. Appl. Genet. 1993, 86, 333–338. [Google Scholar] [CrossRef]
- Bashir, S.; Gulshan, A.B.; Iqbal, J.; Husain, A.; Alwahibi, M.S.; Alkahtani, J.; Dwiningsih, Y.; Bakhsh, A.; Ahmed, N.; Khan, M.J.; et al. Comparative role of animal manure and vegetable waste induced compost for polluted soil restoration and maize growth. Saudi J. Biol. Sci. 2021, 28, 2534–2539. [Google Scholar] [CrossRef]
- Ali, M.; Yeasmin, L.; Gantait, S.; Goswami, R.; Chakraborty, S. Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol. Mol. Biol. Plants 2014, 20, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Yeo, A.R. Breeding for salinity resistance in crop plants: Where next? Aust. J. Plant Physiol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Ali, M.H.; Khan, M.I.; Bashir, S.; Azam, M.; Naveed, M.; Qadri, R.; Bashir, S.; Mehmood, F.; Shoukat, M.A.; Li, Y.; et al. Biochar and Bacillus sp. MN54 Assisted Phytoremediation of Diesel and Plant Growth Promotion of Maize in Hydrocarbons Contaminated Soil. Agronomy 2021, 11, 1795. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and Challenges in the Breeding of Salt-Tolerant Rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Dwiningsih, Y.; Rahmaningsih, M.; Alkahtani, J. Development of single nucleotide polymorphism (SNP) markers in tropical crops. Adv. Sustain. Sci. Eng. Technol. 2020, 2, 2. [Google Scholar] [CrossRef]
- Thomson, M.J.; de Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.L.; Tumimbang-Raiz, E.; Blumwald, E.; Seraj, Z.I.; Singh, R.K.; et al. Characterizing the saltol quantitative trait locus for salinity tolerance in Rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Zhao, Y.; Khan, N.U.; Zhao, Z.; Zhang, Y.; Wen, X.; Tang, F.; Wang, F.; Li, Z. Genetic basis and identification of candidate genes for salt tolerance in rice by GWAS. Sci. Rep. 2020, 10, 9958. [Google Scholar] [CrossRef] [PubMed]
- Lekklar, C.; Pongpanich, M.; Suriya-arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Liu, C.; Chen, K.; Zhao, X.Q.; Wang, X.Q.; Shen, C.C.; Zhu, Y.J.; Dai, M.L.; Qiu, X.J.; Yang, R.W.; Xing, D.Y.; et al. Identification of genes for salt tolerance and yield-related traits in rice plants grown hydroponically and under saline field conditions by genome-wide association study. Rice 2019, 12, 88. [Google Scholar] [CrossRef]
- Mansuri, R.M.; Shobbar, Z.S.; Jelodar, N.B.; Ghaffari, M.R.; Nematzadeh, G.A.; Asari, S. Dissecting molecular mechanisms underlying salt tolerance in rice: A comparative transcriptional profiling of the contrasting genotypes. Rice 2019, 12, 13. [Google Scholar] [CrossRef]
- Alkahtani, J.; Elshikh, M.S.; Dwiningsih, Y.; Rathi, M.A.; Sathya, R.; Vijayaraghavan, P. In-vitro antidepressant property of methanol extract of Bacopa monnieri. J. King Saud Univ.-Sci. 2022, 34, 102299. [Google Scholar] [CrossRef]
- Yildirim, B.; Yaser, F.; Ozpay, T.; Ozpay, D.; Turkozu, D.; Terziodlu, O.; Tamkoc, A. Variations in response to salt stress among field pea genotypes (Pisum sativum sp. arvense L.). J. Anim. Vet. Adv. 2008, 7, 907–910. [Google Scholar]
- Raza, S.H.; Athar, H.U.R.; Ashraf, M. Influence of exogenously applied glycinebetaine on the photosynthetic capacity of two differently adapted wheat cultivars under salt stress. Pak. J. Bot. 2006, 38, 341–351. [Google Scholar]
- Mukhtar, E.; Siddiqi, K.; Bhatti, K.H.; Nawaz, K.; Hussain, K. Gas exchange attributes can be valuable selection criteria for salinity tolerance in canola cultivars (Brassica napus L.). Pak. J. Bot. 2013, 45, 35–40. [Google Scholar]
- Siddiqi, E.H.; Ashraf, M.; Hussain, M.; Jamil, A. Assessment of intercultivar variation for salt tolerance in safflower (Carthamus tinctorius L.) using gas exchange characteristics as selection criteria. Pak. J. Bot. 2009, 41, 2251–2259. [Google Scholar]
- Mano, Y.; Takeda, K. Diallel analysis of salt tolerance at germination and the seedling stage in barley (Hordeum vulgare L.). Breed. Sci. 1997, 47, 203–209. [Google Scholar] [CrossRef]
- Jamil, M.; Lee, K.B.; Jung, K.Y.; Lee, D.B.; Han, M.S.; Rha, E.S. Salt stress inhibits germination and early seedling growth in cabbage (Brassica oleracea capitata L.). Pak. J. Biol. Sci. 2007, 10, 910–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guan, B.; Yu, J.; Lu, Z.; Japhet, W.; Chen, X.; Xie, W. Salt tolerance in two Suaeda species: Seed germination and physiological response. Asian J. Plant Sci. 2010, 9, 194–199. [Google Scholar] [CrossRef][Green Version]
- Dwiningsih, Y.; Alkahtani, J. Phenotypic Variations, Environmental Effects and Genetic Basis Analysis of Grain Elemental Concentrations in Rice (Oryza sativa L.) for Improving Human Nutrition. Preprints 2022, 2022090263. [Google Scholar] [CrossRef]
- Zhang, R.; Hussain, S.; Wang, Y.; Liu, Y.; Li, Q.; Chen, Y.; Wei, H.; Gao, P.; Dai, Q. Comprehensive Evaluation of Salt Tolerance in Rice (Oryza sativa L.) Germplasm at the Germination Stage. Agronomy 2021, 11, 1569. [Google Scholar] [CrossRef]
- Dwiningsih, Y.; Alkahtani, J. Rojolele: A Premium Aromatic Rice Variety in Indonesia. Preprints 2022, 2022100373. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. Role of cultivar tolerance in increasing rice production in saline lands. In Salinity Tolerance in Plants. Strategies for Crop Improvement; Staples, R.C., Toenniessen, G.H., Eds.; Wiley-Interscience: New York, NY, USA, 1984; pp. 255–271. [Google Scholar]
- Dwiningsih, Y.; Al-Kahtani, J. Genome-Wide Association Study of Complex Traits in Maize Detects Genomic Regions and Genes for Increasing Grain Yield and Grain Quality. Adv. Sustain. Sci. Eng. Technol. 2022, 4, 0220209. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Khafagy, M.A.; Arafa, A.A.; El-Banna, M.F. Glycinebetaine and ascorbic acid can alleviate the harmful effects of NaCl salinity in sweet pepper. Aust. J. Crop Sci. 2009, 3, 257–267. [Google Scholar]
- Santos, V.C. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 130, 93–99. [Google Scholar] [CrossRef]
- Lone, J.; Shikari, A.; Sofi, N.; Ganie, S.; Sharma, M.; Sharma, M.; Kumar, M.; Saleem, M.H.; Almaary, K.S.; Elshikh, M.S.; et al. Screening technique based on seed and early seedling parameters for cold tolerance of selected F2-derived F3 rice genotypes under controlled conditions. Sustainability 2022, 14, 8447. [Google Scholar] [CrossRef]
- Kishor, P.B.; Sangam, S.; Amrutha, R.N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Ghosh, N.; Adak, M.K.; Ghosh, P.D.; Gupta, S.; Sen Gupta, D.N.; Mandal, C. Differential responses of two rice varieties to salt stress. Plant Biotechnol. Rep. 2011, 5, 89–103. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khalil, R.R.A.E.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef]
- Saha, P.; Chatterjee, P.; Biswas, A.K. NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian J. Exp. Biol. 2010, 48, 593–600. [Google Scholar]
- Abu-Muriefah, S.S. Effect of sitosterol on growth, metabolism and protein pattern of pepper (Capsicum Annuum L.) plants grown under salt stress conditions. Int. J. Agric. Crop Sci. 2015, 8, 94–106. [Google Scholar]
- Azuma, R.; Ito, N.; Nakayama, N.; Suwa, R.; Nguyen, N.T.; Larrinaga Mayoral, J.A.; Esaka, M.; Fujiyama, H.; Saneoka, H. Fruits are more sensitive to salinity than leaves and stems in pepper plants (Capsicum annuum L.). Sci. Hortic. 2010, 125, 171–178. [Google Scholar] [CrossRef]
- Stepien, P.; Klobus, G. Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol. Plantarum. 2005, 125, 31–40. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Chang, J.; Cheong, B.; Natera, S.; Roessner, U. Morphological and metabolic responses to salt stress of rice (Oryza sativa L.) cultivars which differ in salinity tolerance. Plant Physiol. Biochem. 2019, 144, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yang, Z.Z.; Li, F.; Yan, C.R.; Zhong, X.L.; Liu, Q.; Xia, X.; Li, H.R.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Ismanto, A.; Hadibarata, T.; Widada, S.; Indrayanti, E.; Ismunarti, D.; Safinatunnajah, N.; Kusumastuti, W.; Dwiningsih, Y.; Alkahtani, J. Groundwater contamination status in Malaysia: Level of heavy metal, source, health impact, and remediation technologies. Bioprocess Biosyst. Eng. 2022, 46, 467–482. [Google Scholar] [CrossRef]
- Peng, S.; Cassman, K.G.; Virmani, S.S.; Sheehy, J.; Khush, G.S. Yield potential trends of tropical rice since the release of IR8 and the challenge of increasing rice yield potential. Crop Sci. 1999, 39, 1552–1559. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, P.; Wang, L.X.; Hu, Z.L.; Zhu, L.H.; Zhu, Y.G. Genetic dissection of the relationships of biomass production and partitioning with yield and yield related traits in rice. Plant Sci. 2004, 167, 1–8. [Google Scholar] [CrossRef]
- Dwiningsih, Y.; Alkahtani, J. Agronomics, Genomics, Breeding and Intensive Cultivation of Ciherang Rice Variety. Preprints 2022, 2022110489. [Google Scholar] [CrossRef]
- Dwiningsih, Y.; Alkahtani, J. Potential of Pigmented Rice Variety Cempo Ireng in Rice Breeding Program for Improving Food Sustainability. Preprints 2023, 2023020039. [Google Scholar] [CrossRef]
- IRRI [International Rice Research Institute]. Annual Report for 1967; International Rice Research Institute: Manila, Philippines, 1967; Volume 308. [Google Scholar]
- Pearson, G.A.; Ayers, S.D.; Eberhard, D.L. Relative salt tolerance of rice during germination and early seedling development. Soil Sci. 1966, 102, 151–156. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last Generation Genome–Environment Associations Reveal the Genetic Basis of Heat Tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.J.; Monserrate, F.A.; Ramı’rez-Villegas, J.; Madrinan, S.; Blair, M.W. Drought Tolerance in Wild Plant Populations: The Case of Common Beans (Phaseolus vulgaris L.). PLoS ONE 2013, 8, e62898. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.J.; Chavaro, M.; Madrinan, S.; This, D.; Blair, M. Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet. 2012, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Cortés, A.J.; This, D. Identification of an ERECTA gene and its drought adaptation associations with wild and cultivated common bean. Plant Sci. 2016, 242, 250–259. [Google Scholar] [CrossRef]
- Cortes, A.J.; This, D.; Chavarro, C.; Madrinan; Blair, M. Nucleotide diversity patterns at the drought-related DREB2 encoding genes in wild and cultivated common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2012, 125, 1069–1085. [Google Scholar] [CrossRef]
- Buitrago-Bitar, M.A.; Cortés, A.J.; López-Hernández, F.; Londoño-Caicedo, J.M.; Muñoz-Florez, J.E.; Muñoz, L.C.; Blair, M.W. Allelic Diversity at Abiotic Stress Responsive Genes in Relationship to Ecological Drought Indices for Cultivated Tepary Bean, Phaseolus acutifolius A. Gray, and Its Wild Relatives. Genes 2021, 12, 556. [Google Scholar] [CrossRef]
- Cortés, A.J.; Blair, M.W. Genotyping by Sequencing and Genome–Environment Associations in Wild Common Bean Predict Widespread Divergent Adaptation to Drought. Front. Plant Sci. 2018, 9, 128. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F. Harnessing Crop Wild Diversity for Climate Change Adaptation. Genes 2021, 12, 783. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F.; Osorio-Rodriguez, D. Predicting Thermal Adaptation by Looking into Populations’ Genomic Past. Front. Genet. 2020, 11, 564515. [Google Scholar] [CrossRef] [PubMed]
- Burbano-Erazo, E.; León-Pacheco, R.I.; Cordero-Cordero, C.C.; López-Hernández, F.; Cortés, A.J.; Tofiño-Rivera, A.P. Multi-Environment Yield Components in Advanced Common Bean (Phaseolus vulgaris L.) × Tepary Bean (P. acutifolius A. Gray) Interspecific Lines for Heat and Drought Tolerance. Agronomy 2021, 11, 1978. [Google Scholar] [CrossRef]
- Pathan, M.S.; Nguyen, H.T.; Subudhi, P.K.; Courtois, B. Molecular Dissection of Abiotic Stress Tolerance in Sorghum and Rice. In Physiology and Biotechnology Integration for Plant Breeding; Nguyen, H.T., Blum, A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 525–570. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; IRRI: Las Banos, Philippines, 1976; Volume 83. [Google Scholar]


| SS | PG | RL | RFW | SL | PB | PH | LR | CC | CMS | Na/K | Proline | MDA | H2O2 | Sugar | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | 1 | ||||||||||||||
| PG | −0.87 * | 1 | |||||||||||||
| RL | −0.91 * | 0.61 | 1 | ||||||||||||
| RFW | −0.93 * | 0.57 | 0.89 * | 1 | |||||||||||
| SL | −0.92 * | 0.68 | 0.83 | 0.75 | 1 | ||||||||||
| PB | −0.85 * | 0.73 | 0.87 * | 0.91 * | 0.92 * | 1 | |||||||||
| PH | −0.89 * | 0.67 | 0.88 * | 0.68 | 0.79 | 0.92 * | 1 | ||||||||
| LR | 0.95 * | 0.53 | 0.51 | 0.42 | 0.39 | 0.41 | 0.37 | 1 | |||||||
| CC | −0.87 * | 0.56 | −0.73 | 0.83 | 0.74 | 0.86 | 0.79 | −0.59 | 1 | ||||||
| CMS | −0.14 | 0.51 | 0.81 | 0.69 | 0.51 | 0.62 | 0.84 | 0.68 | 0.36 | 1 | |||||
| Na/K | 0.92 * | −0.47 | −0.61 | −0.47 | −0.68 | −0.57 | −0.62 | 0.55 | −0.27 | −0.35 | 1 | ||||
| Proline | −0.90 * | −0.67 | −0.58 | −0.53 | −0.53 | −0.41 | −0.68 | 0.61 | −0.22 | −0.29 | −0.47 | 1 | |||
| MDA | 0.57 | −0.61 | −0.51 | −0.42 | −0.41 | −0.47 | −0.79 | 0.69 | −0.22 | −0.41 | 0.97 * | −0.39 | 1 | ||
| H2O2 | 0.91 * | −0.73 | −0.62 | −0.39 | −0.36 | −0.58 | −0.61 | 0.54 | −0.27 | −0.32 | 0.96 * | 0.42 | 0.85 | 1 | |
| Sugar | −0.91 * | −0.64 | −0.64 | −0.54 | −0.31 | −0.64 | −0.57 | 0.62 | −3.48 | −0.37 | −0.51 | 0.38 | 0.41 | 0.25 | 1 |
| Rice Variety | Country | Sub-Population * | |
|---|---|---|---|
| 1 | Karang Serang | Indonesia | TRJ |
| 2 | Rojolele | Indonesia | TRJ |
| 3 | Cempo Ireng | Indonesia | TRJ |
| 4 | Ciherang | Indonesia | IND |
| 5 | Mayang Khang | Indonesia | IND |
| 6 | Sipirasikkam | Indonesia | TRJ |
| 7 | Mitak | Indonesia | TRJ |
| 8 | Dara | Indonesia | AUS |
| 9 | B805D-Mr-16-8-3 | Indonesia | IND |
| 10 | Tia Bura | Indonesia | TRJ |
| 11 | C 5560 | Thailand | TEJ/TRJ |
| 12 | Nam Dawk Mai | Thailand | IND |
| 13 | Bkn 6987-68-14 | Thailand | IND |
| 14 | Td 70 | Thailand | IND |
| 15 | Cntlr80076-44-1-1-1 | Thailand | IND |
| 16 | Nahng Sawn | Thailand | IND |
| 17 | Quinimpol | Philippines | TRJ |
| 18 | TCCP 266 | Philippines | IND |
| 19 | IR 4482-5-3-9-5 | Philippines | IND |
| 20 | IR 45427 | Philippines | IND |
| 21 | IR 9660-48-1-1-2 | Philippines | IND |
| 22 | IR 238 | Philippines | IND |
| 23 | IR 2061-214-2-3 | Philippines | IND |
| 24 | IR 2462 | Philippines | IND |
| 25 | IR 58614-B-B-8-2 | Philippines | IND |
| 26 | Pakkali | Philippines | ARO |
| 27 | IR64 | Philippines | IND |
| 28 | IR58 | Philippines | IND |
| 29 | IR29 | Philippines | IND |
| 30 | Taichu Mochi 59 | Taiwan | TRJ |
| 31 | Ai Chueh Ta Pai Ku | Taiwan | IND |
| 32 | Ragasu | Taiwan | TEJ/TRJ |
| 33 | Tobura | Taiwan | TEJ/TRJ |
| 34 | Kao Chio Lin Chou | Taiwan | IND |
| 35 | Taino 38 | Taiwan | IND/AUS |
| 36 | Nanton No. 131 | Taiwan | TRJ/(admix) |
| 37 | Hsin Hsing Pai Ku | Taiwan | IND |
| 38 | Tainung 45 | Taiwan | IND |
| 39 | Ao Chiu 2 Hao | China | IND |
| 40 | Chun 118-33 | China | IND |
| 41 | Kin Shan Zim | China | IND |
| 42 | Pan Ju | China | IND |
| 43 | Kechengnuo No. 4 | China | IND |
| 44 | 4484 | China | IND |
| 45 | 4595 | China | IND |
| 46 | You-I B | China | IND |
| 47 | Chunjiangzao No. 1 | China | TEJ |
| 48 | Shimizu Mochi | Japan | TEJ |
| 49 | Norin 11 | Japan | TEJ |
| 50 | Tamanishiki | Japan | TEJ |
| 51 | Niwahutaw Mochi | Japan | TEJ |
| 52 | Somewake | Japan | TEJ |
| 53 | A 5 | Japan | TEJ |
| 54 | C.B. Ii | Japan | AUS |
| 55 | Fujisaka 5 | Japan | IND |
| 56 | Nipponbare | Japan | TEJ |
| 57 | Khao Phoi | Laos | TEJ/TRJ |
| 58 | Khao Luang | Laos | TRJ/(admix) |
| 59 | Padi Pohon Batu | Malaysia | TRJ |
| 60 | Acheh | Malaysia | IND |
| 61 | Mahsuri | Malaysia | IND |
| 62 | Padi Tarab Arab | Malaysia | TRJ |
| 63 | Nc 1/536 | Pakistan | AUS |
| 64 | Red | Pakistan | AUS/(Admix) |
| 65 | Santhi 990 | Pakistan | IND/AUS |
| 66 | Daudzai Field Mix | Pakistan | AUS |
| 67 | Jp 5 | Pakistan | IND/AUS |
| 68 | Won Son Zo No. 11 | Republic of Korea | IND |
| 69 | Daegudo | Republic of Korea | TEJ |
| 70 | Guweoldo | Republic of Korea | TEJ |
| 71 | Namyang 7 | Republic of Korea | TEJ |
| 72 | Yong Chal Byo | Republic of Korea | TEJ |
| 73 | Thang 10 | Vietnam | IND |
| 74 | Cm1_ Haipong | Vietnam | IND |
| 75 | Nang Bang Bentre | Vietnam | AUS |
| 76 | Lua Chua Chan | Vietnam | TRJ |
| 77 | Soc Nau | Vietnam | IND |
| 78 | Heo Trang | Vietnam | IND |
| 79 | Pd 46 | Sri Lanka | IND |
| 80 | Bakiella 1 | Sri Lanka | IND |
| 81 | Gallawa | Sri Lanka | AUS |
| 82 | Ittikulama | Sri Lanka | AUS |
| 83 | Karayal | Sri Lanka | AUS |
| 84 | Amane | Sri Lanka | IND |
| 85 | Thavalu | Sri Lanka | AUS |
| 86 | Patnai 6 | Myanmar | AUS |
| 87 | Buphopa | Myanmar | TEJ/TRJ |
| 88 | Kaukkyi Ani | Myanmar | TRJ |
| 89 | A100943-R | Myanmar | AUS |
| 90 | Nsgc 5953 | Myanmar | IND |
| 91 | A 36-3 | Myanmar | IND |
| 92 | Jumli Dhan | Nepal | TEJ/TRJ |
| 93 | N-2703 | Nepal | AUS |
| 94 | Bhim Dhan | Nepal | TEJ/TRJ |
| 95 | Juppa | Nepal | IND |
| 96 | Tauli | Nepal | AUS |
| 97 | Darmali | Nepal | TEJ/ARO |
| 98 | Dhan | Nepal | IND |
| 99 | Tchampa | Iran | AUS |
| 100 | Phudugey | Bhutan | AUS |
| 101 | Jyanak | Bhutan | TEJ/TRJ |
| 102 | Wir 3039 | Tajikistan | TEJ |
| 103 | Ak Tokhum | Azerbaijan | ARO |
| 104 | Gasym Hany | Azerbaijan | ARO |
| 105 | Celiaj | Azerbaijan | TEJ |
| 106 | Shimla Early | Iraq | IND/AUS |
| 107 | A 152 | Bangladesh | IND/TRJ |
| 108 | Dnj 179 | Bangladesh | AUS |
| 109 | Dj 24 | Bangladesh | AUS |
| 110 | Dj 102 | Bangladesh | AUS |
| 111 | Dnj 121 | Bangladesh | AUS |
| 112 | Aswina 330 | Bangladesh | AUS |
| 113 | Tranoeup Beykher | Cambodia | IND |
| 114 | Srav Prapay | Cambodia | IND |
| 115 | Simpor | Brunei | TRJ |
| 116 | Pokkali | India | IND |
| Score | Observation | Tolerance |
|---|---|---|
| 1 | Normal growth, no leaf symptoms | Highly tolerant |
| 3 | Nearly normal growth, but leaf tips or few leaves whitish and rolled | Tolerant |
| 5 | Growth severely retarded; most leaves rolled; only a few are elongating | Moderately tolerant |
| 7 | Complete cessation of growth; most leaves dry; some plants dying | Susceptible |
| 9 | Almost all plants dead or dying | Highly susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkahtani, J.; Dwiningsih, Y. Analysis of Morphological, Physiological, and Biochemical Traits of Salt Stress Tolerance in Asian Rice Cultivars at Seedling and Early Vegetative Stages. Stresses 2023, 3, 717-735. https://doi.org/10.3390/stresses3040049
Alkahtani J, Dwiningsih Y. Analysis of Morphological, Physiological, and Biochemical Traits of Salt Stress Tolerance in Asian Rice Cultivars at Seedling and Early Vegetative Stages. Stresses. 2023; 3(4):717-735. https://doi.org/10.3390/stresses3040049
Chicago/Turabian StyleAlkahtani, Jawaher, and Yheni Dwiningsih. 2023. "Analysis of Morphological, Physiological, and Biochemical Traits of Salt Stress Tolerance in Asian Rice Cultivars at Seedling and Early Vegetative Stages" Stresses 3, no. 4: 717-735. https://doi.org/10.3390/stresses3040049
APA StyleAlkahtani, J., & Dwiningsih, Y. (2023). Analysis of Morphological, Physiological, and Biochemical Traits of Salt Stress Tolerance in Asian Rice Cultivars at Seedling and Early Vegetative Stages. Stresses, 3(4), 717-735. https://doi.org/10.3390/stresses3040049







