Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior
Abstract
:1. Introduction
2. Results
2.1. Motility Characteristics
2.2. Oxidative Profile
2.3. Protein Expression
3. Discussion
4. Materials and Methods
4.1. Semen Collection and Cryopreservation
4.2. Sperm Motility
4.3. Oxidative Profile
4.4. Western Blot
- -
- Rabbit anti-BAX antibody (BCL2-Associated X Protein) N-Term, 1:1000 in 5% milk/TBS/0.1% Tween-20 (Antibodies Online; Dunwoody, GA, USA);
- -
- Rabbit anti-Bcl-2 antibody (B-Cell CLL/lymphoma 2) N-Term, 1:1000 in 5% milk/TBS/0.1% Tween-20 (Antibodies Online; Dunwoody, GA, USA);
- -
- Rabbit HSP70 Antibody, 1:1000 in 5% BSA/TBS/0.1% Tween-20 (Cell Signaling Technology; Danvers, MA, USA);
- -
- Rabbit HSP90α (D1A7) mAb, 1:1000 in 5% BSA/TBS/0.1% Tween-20 (Cell Signaling Technology; Danvers, MA, USA).
4.5. Statistics
- -
- Native control (CtrlN) was compared to the cryopreserved control (CtrlC);
- -
- Experimental groups were compared to both controls.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Ozimic, S.; Ban-Frangez, H.; Stimpfel, M. Sperm Cryopreservation Today: Approaches, Efficiency, and Pitfalls. Curr. Issues Mol. Biol. 2023, 45, 4716–4734. [Google Scholar] [CrossRef]
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: From past practical difficulties to present success. Reprod. Biomed. Online 2003, 6, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. Int. J. Mol. Sci. 2023, 24, 2510. [Google Scholar] [CrossRef]
- Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, A.; Governini, L.; et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants 2022, 11, 10. [Google Scholar] [CrossRef]
- Pinto-Pinho, P.; Arantes-Rodrigues, R.; Gaivão, I.; Peixoto, F.; Gomes, Z.; Brito, M.; Moutinho, O.; Colaço, B.; Pinto-Leite, R. Mitochondrial Effects, DNA Damage, and Antioxidant Enzyme Activity in Cryopreserved Human Sperm Samples: A Pilot Study. Physiologia 2022, 2, 80–93. [Google Scholar] [CrossRef]
- Baňas, Š.; Ďuračka, M.; Benko, F.; Žiarovská, J.; Lukáč, N.; Tvrdá, E. Epicatechin improves frozen sperm vitality by its antioxidant and cryoprotective actions. J. Microbiol. Biotech. Food Sci. 2022, 12, e9490. [Google Scholar]
- Cabrita, E.; Ma, S.; Diogo, P.; Martínez-Páramo, S.; Sarasquete, C.; Dinis, M.T. The influence of certain aminoacids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim. Reprod. Sci. 2011, 125, 189–195. [Google Scholar] [CrossRef]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Sariozkan, S.; Tuncer, P.B.; Sakin, F.; Atessahin, A.; Kulaksiz, R.; Cevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rum. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Tvrdá, E.; Debacker, M.; Ďuračka, M.; Kováč, J.; Bučko, O. Quercetin and Naringenin Provide Functional and Antioxidant Protection to Stored Boar Semen. Animals 2020, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Hassan, M.A.E.; Mohammed, A.K.; Alhimaidi, A.R.; Al-Gabri, N.; Al-Khaldi, K.O.; Swelum, A.A. The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm. Animals 2020, 10, 1508. [Google Scholar] [CrossRef]
- Tvrda, E.; Straka, P.; Galbavy, D.; Ivanic, P. Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules 2019, 24, 3226. [Google Scholar] [CrossRef]
- Javed, M.; Tunio, M.T.; Abdul Rauf, H.; Bhutta, M.F.; Naz, S.; Iqbal, S. Addition of pomegranate juice (Punica granatum) in tris-based extender improves post-thaw quality, motion dynamics and in vivo fertility of Nili Ravi buffalo (Bubalus bubalis) bull spermatozoa. Andrologia 2019, 51, e13322. [Google Scholar] [CrossRef]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics Versus Natural Biomolecules: The Case of In Vitro Induced Bacteriospermia by Enterococcus faecalis in Rabbit Semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.F.; Gao, N.; Zhao, J.; Xu, J. Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharmacother. 2018, 108, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018, 13, 1827–1832. [Google Scholar] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Gouveia, B.B.; Macedo, T.J.S.; Santos, J.M.S.; Barberino, R.S.; Menezes, V.G.; Müller, M.C.; Almeida, J.R.G.S.; Figueiredo, J.R.; Matos, M.H.T. Supplemented base medium containing Amburana cearensis associated with FSH improves in vitro development of isolated goat preantral follicles. Theriogenology 2016, 86, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Keyhanian, S.; Stahl-Biskup, E. Phenolic constituents in dried flowers of aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. 2007, 73, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Terreaux, C.; Wang, Q.; Ioset, J.R.; Ndjoko, K.; Grimminger, W.; Hostettmann, K. Complete LC/MS analysis of a Tinnevelli senna pod extract and subsequent isolation and identification of two new benzophenone glucosides. Planta Med. 2002, 68, 349–354. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Leung, H.W.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol. 2007, 45, 2005–2013. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Parmar, K.H.; Singh, V.; Savsani, H.H.; Kavani, F.S.; Rajoriya, J.S.; Lone, S.A. Seasonal variation in heat shock proteins (hsp70 and hsp90) and their association with frozen semen quality and fertility in buffaloes. Cryoletters 2021, 42, 261–266. [Google Scholar] [PubMed]
- Martin, G.; Cagnon, N.; Sabido, O.; Sion, B.; Grizard, G.; Durand, P.; Levy, R. Kinetics of occurrence of some features of apoptosis during the cryopreservation process of bovine spermatozoa. Hum. Reprod. 2007, 22, 380–388. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Azab, R.E. The Effect of Kaempferol on Buffalo Semen Freezability and Redox State. Benha Vet. Med. J. 2022, 42, 164–169. [Google Scholar]
- Ďuračka, M.; Debacker, M.; Bučko, O.; Lukáč, N.; Tvrdá, E. The effect of kaempferol and naringenin may improve the in vitro quality of stored boar semen. J. Cent. Eur. Agric. 2019, 20, 1069–1075. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215–223. [Google Scholar]
- Montero, M.; Lobatón, C.D.; Hernández-Sanmiguel, E.; Santodomingo, J.; Vay, L.; Moreno, A.; Alvarez, J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004, 384, 19–24. [Google Scholar] [CrossRef]
- Seddiki, Y.; da Silva, H.M.; da Silva, F. Antioxidant Properties of Polyphenols and Their Potential Use in Improvement of Male Fertility: A Review. Biomed. J. Sci. Technol. Res. 2017, 1, 612–616. [Google Scholar]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation on the Antioxidant and Pro-Oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Wysokiński, D.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol derivatives isolated from Lens culinaris Medik. reduce DNA damage induced by etoposide in peripheral blood mononuclear cells. Toxicol. Res. 2019, 8, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Ma, F.; Wei, J.; Li, H.; Ma, H.; Sun, P. Physiological Functions of Heat Shock Proteins. Curr. Protein Pept. Sci. 2020, 21, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.L.; Wang, Y.X.; Xiang, Z.Q.; Li, Z. Cryopreservation-induced decrease in heat-shock protein 90 in human spermatozoa and its mechanism. Asian J. Androl. 2003, 5, 43–46. [Google Scholar] [PubMed]
- Wang, P.; Shu, Z.; He, L.; Cui, X.; Wang, Y.; Gao, D. The pertinence of expression of heat shock proteins (HSPs) to the efficacy of cryopreservation in HELAs. Cryoletters 2005, 26, 7–16. [Google Scholar]
- Zhang, X.G.; Hu, S.; Han, C.; Zhu, Q.C.; Yan, G.J.; Hu, J.H. Association of heat shock protein 90 with motility of post-thawed sperm in bulls. Cryobiology 2015, 70, 164–169. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Hong, J.-Y.; Yan, G.-J.; Wang, Y.-F.; Li, Q.-W.; Hu, J.-H. Association of heat shock protein 70 with motility of frozen-thawed sperm in bulls. Czech J. Anim. Sci. 2015, 60, 256–262. [Google Scholar] [CrossRef]
- Tvrda, E.; Mackovich, A.; Greifova, H.; Hashim, F.; Lukac, N. Antioxidant effects of lycopene on bovine sperm survival and oxidative profile following cryopreservation. Vet. Med. 2018, 62, 429–436. [Google Scholar] [CrossRef]
- Arispe, N.; Doh, M.; De Maio, A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones 2002, 7, 330–338. [Google Scholar] [CrossRef]
- Aboagla, E.M.; Terada, T. Trehalose-enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol. Reprod. 2003, 69, 1245–1250. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Liu, S.; Yang, J.; Chen, C.; Sun, Z. Grape seed extract supplementation attenuates the heat stress-induced responses of jejunum epithelial cells in Simmental × Qinchuan steers. Br. J. Nutr. 2014, 112, 347–357. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hirayoshi, K.; Kudo, H.; Takechi, H.; Aoike, A.; Kawai, K.; Nagata, K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol. Cell. Biol. 1992, 12, 3490–3498. [Google Scholar] [PubMed]
- Burkitt, M.J.; Duncan, J. Effects of trans-Resveratrol on Copper-Dependent Hydroxyl-Radical Formation and DNA Damage: Evidence for Hydroxyl-Radical Scavenging and a Novel, Glutathione-Sparing Mechanism of Action. Arch. Biochem. Biophys. 2000, 381, 253–263. [Google Scholar] [CrossRef]
- Putics, A.; Végh, E.M.; Csermely, P.; Soti, C. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antioxid. Redox Signal. 2008, 10, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Anzar, M.; He, L.; Buhr, M.M.; Kroetsch, T.G.; Pauls, K.P. Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol. Reprod. 2002, 66, 354–360. [Google Scholar] [CrossRef]
- Karabulut, S.; Demiroğlu-Zergeroğlu, A.; Yılmaz, E.; Kutlu, P.; Keskin, İ. Effects of human sperm cryopreservation on apoptotic markers in normozoospermic and non-normozoospermic patients. Zygote 2018, 26, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Atreja, S.K. Early detection of Buffalo sperm apoptosis at various stages of cryopreservation. Int. Adv. Res. J. Sci. Eng. Technol. 2018, 5, 50–56. [Google Scholar]
- Llambi, F.; Moldoveanu, T.; Tait, S.W.; Bouchier-Hayes, L.; Temirov, J.; McCormick, L.L.; Dillon, C.P.; Green, D.R. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol. Cell 2011, 44, 517–531. [Google Scholar] [CrossRef]
- Dalal, J.; Kumar, A.; Honparkhe, M.; Deka, D.; Singh, N. Minimization of apoptosis-like changes in cryopreserved buffalo bull sperm by supplementing extender with Bcl-2 protein. Vet. World 2016, 9, 432–436. [Google Scholar] [CrossRef]
- Paasch, U.; Sharma, R.K.; Gupta, A.K.; Grunewald, S.; Mascha, E.J.; Thomas, A.J., Jr.; Glander, H.J.; Agarwal, A. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol. Reprod. 2004, 71, 1828–1837. [Google Scholar] [CrossRef]
- Dogan, S.; Mason, M.C.; Govindaraju, A.; Belser, L.; Kaya, A.; Stokes, J.; Rowe, D.; Memili, E. Interrelationships between apoptosis and fertility in bull sperm. J. Reprod. Dev. 2013, 59, 18–26. [Google Scholar] [CrossRef]
- Rezaei, N.; Mohammadi, M.; Mohammadi, H.; Khalatbari, A.; Zare, Z. Acrosome and chromatin integrity, oxidative stress, and expression of apoptosis-related genes in cryopreserved mouse epididymal spermatozoa treated with L-Carnitine. Cryobiology 2020, 95, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; de Gannes, M.K.; Luchetti, G.; Pilsner, J.R. Rapid method for the isolation of mammalian sperm DNA. BioTechniques 2015, 58, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Vorilhon, S.; Brugnon, F.; Kocer, A.; Dollet, S.; Bourgne, C.; Berger, M.; Janny, L.; Pereira, B.; Aitken, R.J.; Moazamian, A.; et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum. Reprod. 2018, 33, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Geschwandtnerová, A.; Žiarovská, J.; Lukáč, N.; Tvrdá, E. Expression of HSP90 Gene in the Cryopreserved Bovine Spermatozoa. Sci. Pap. Anim. Sci. Biotechnol. 2021, 54, 73–77. [Google Scholar]
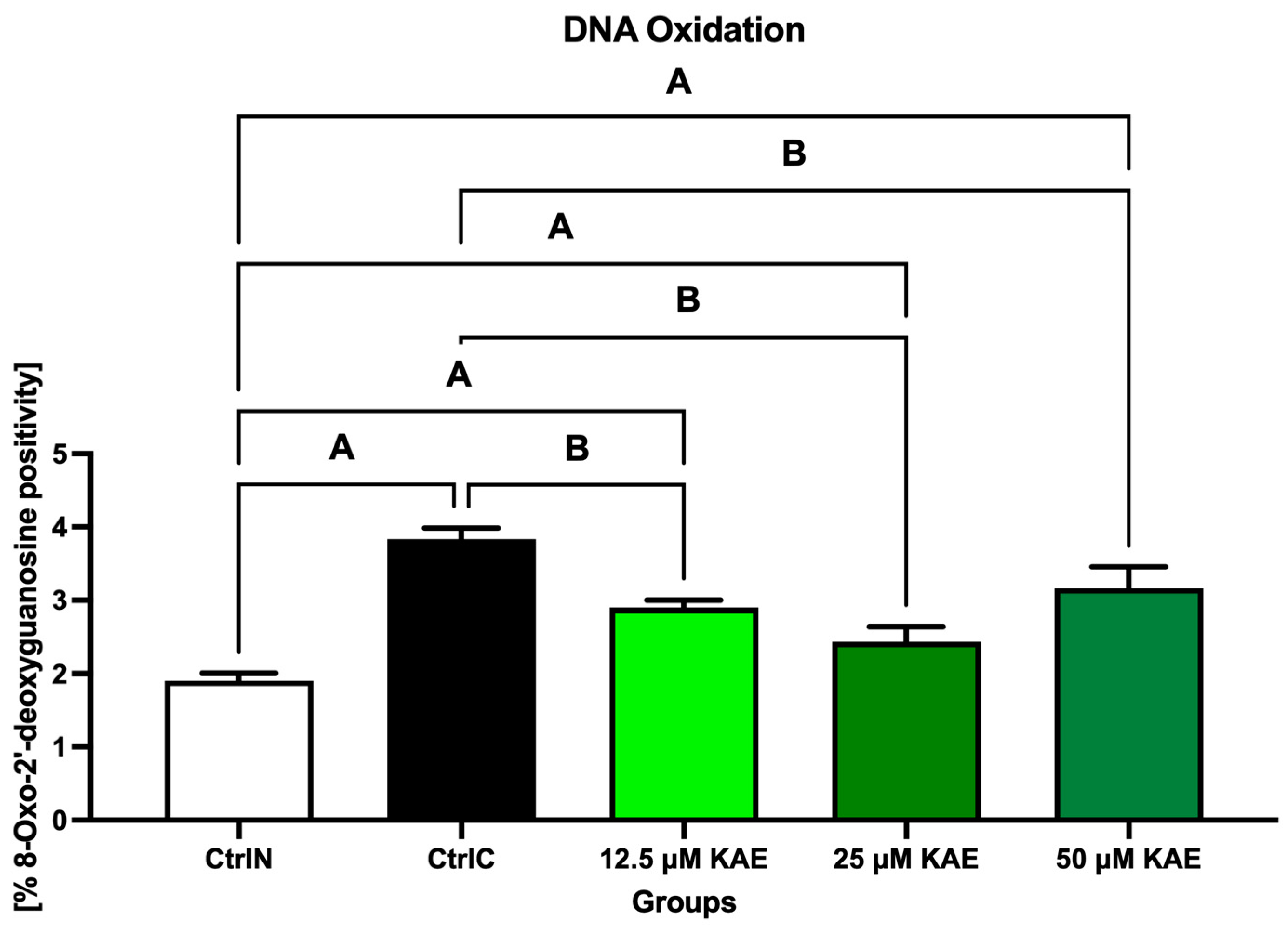
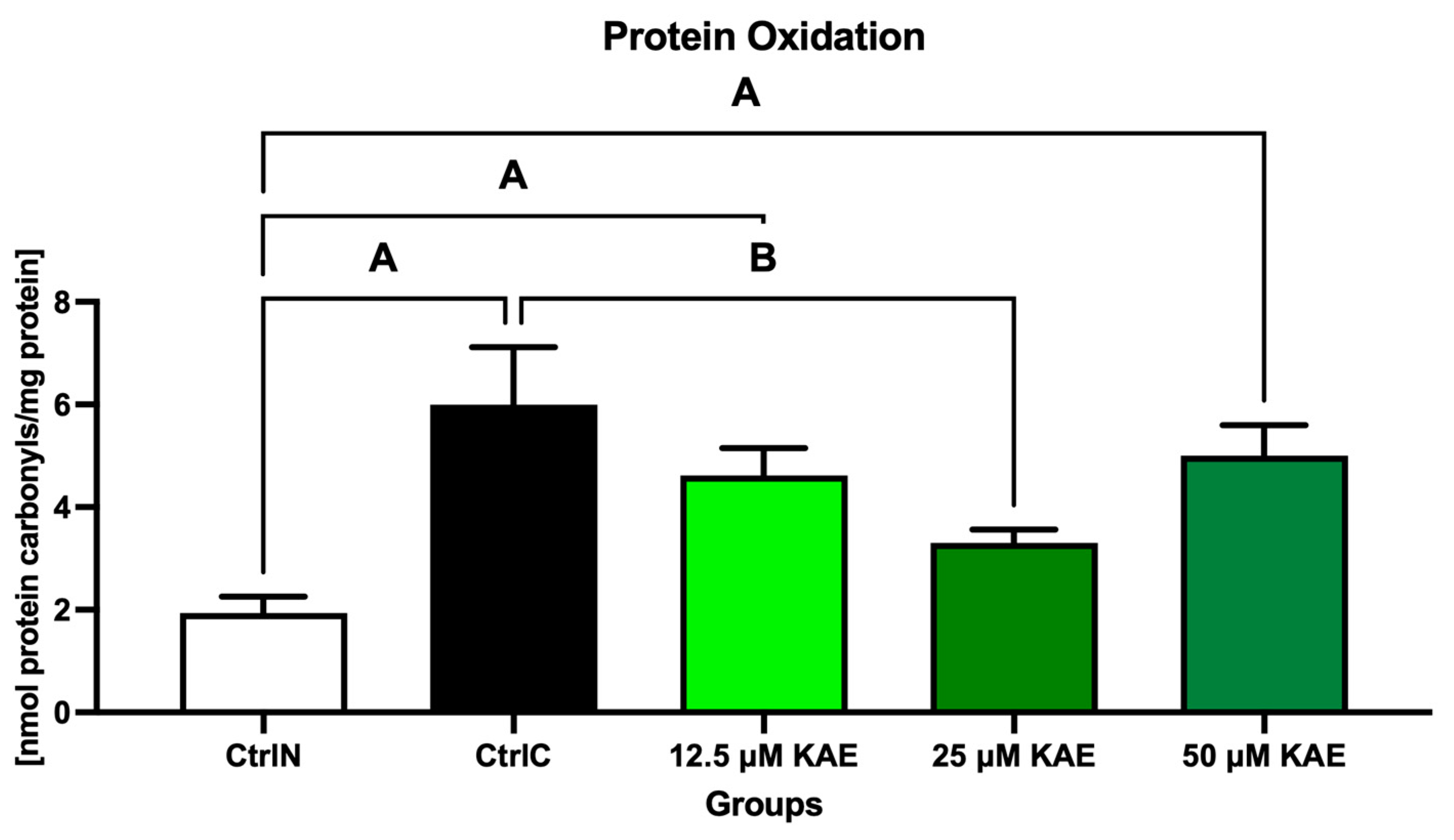
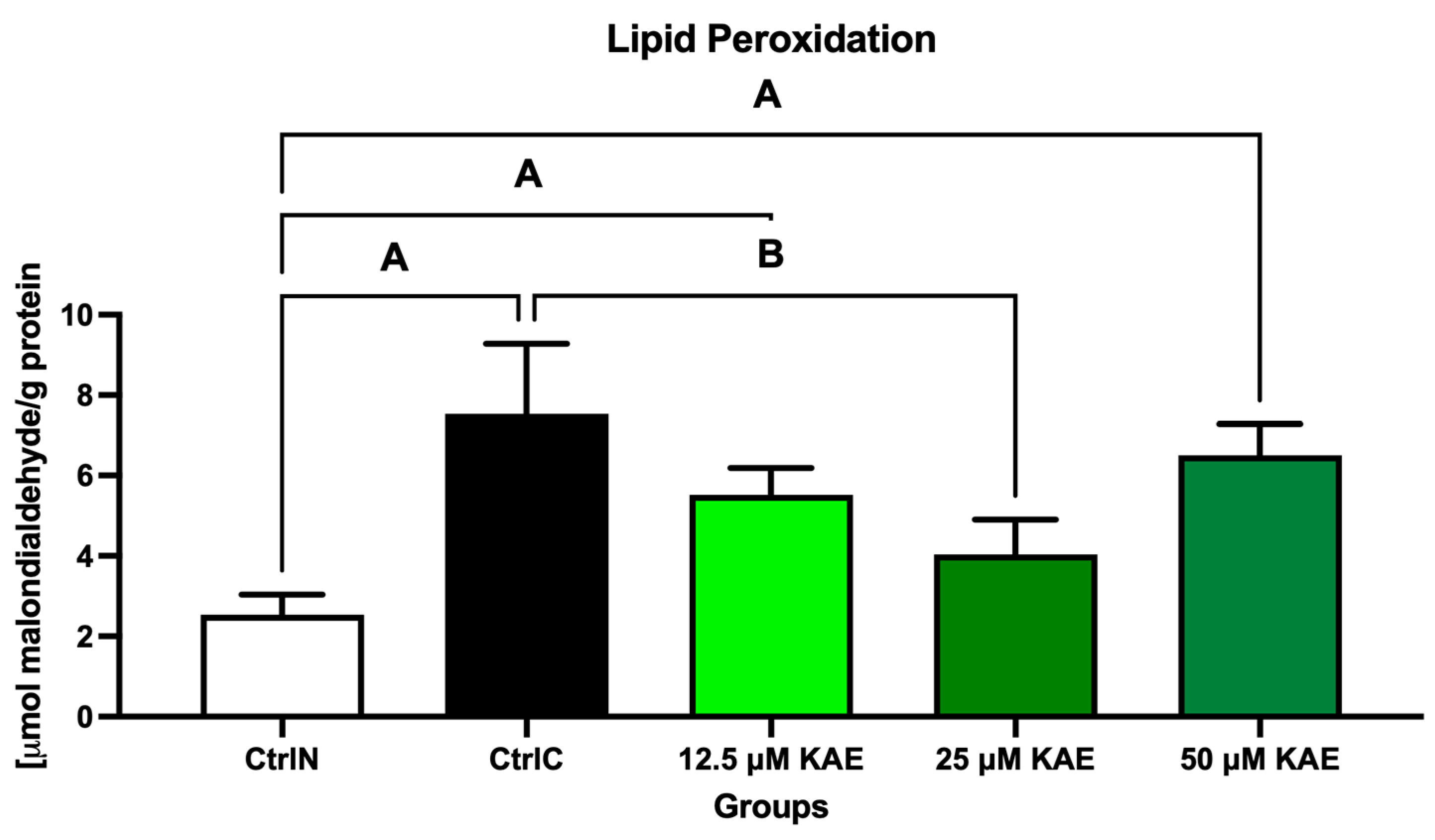


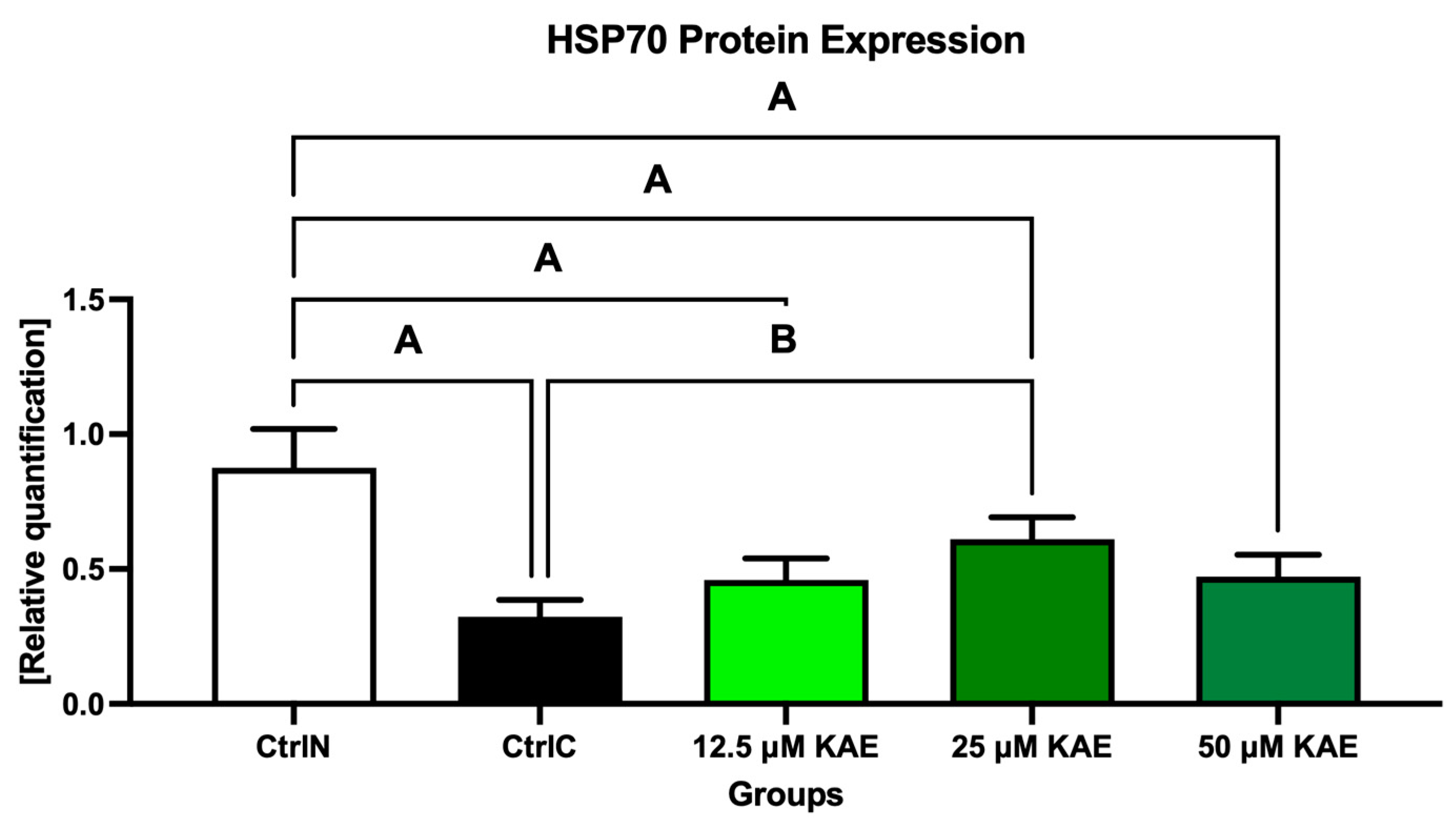
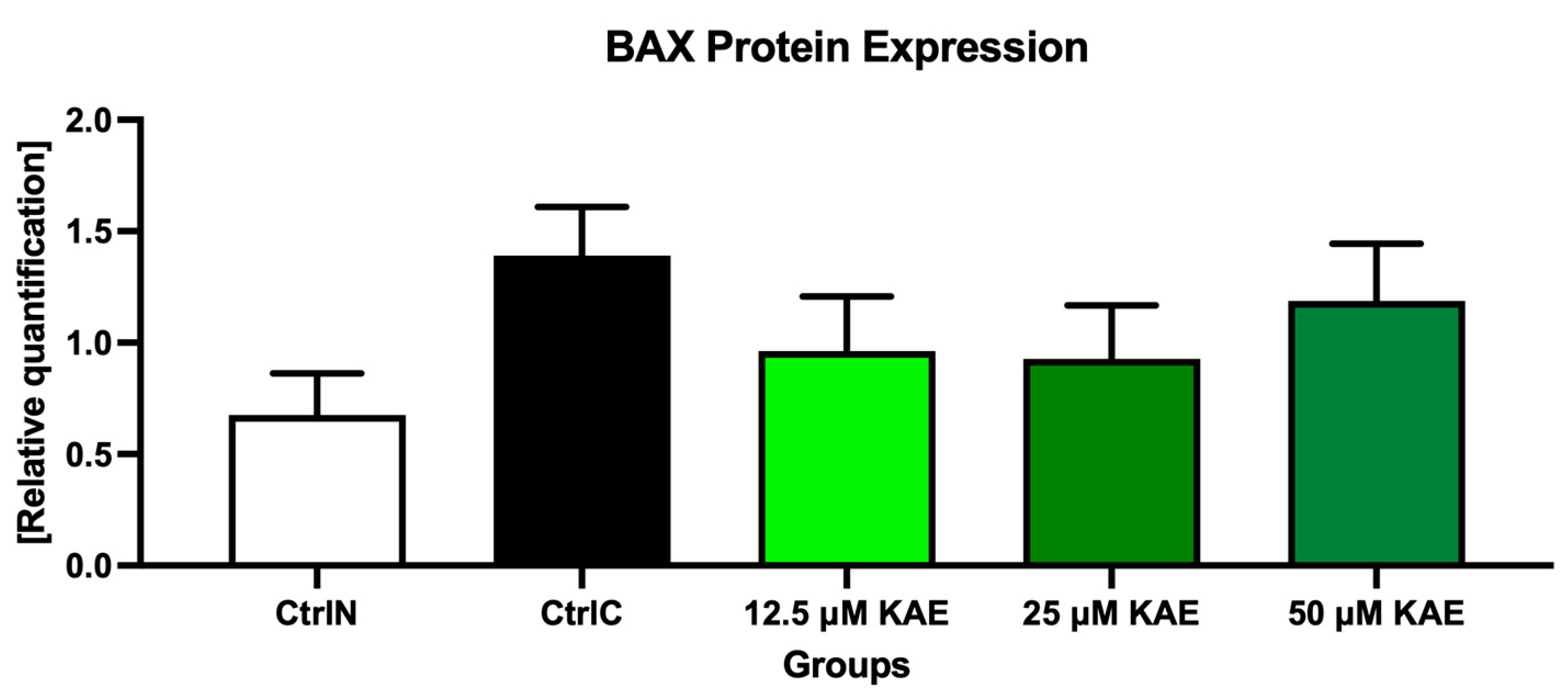
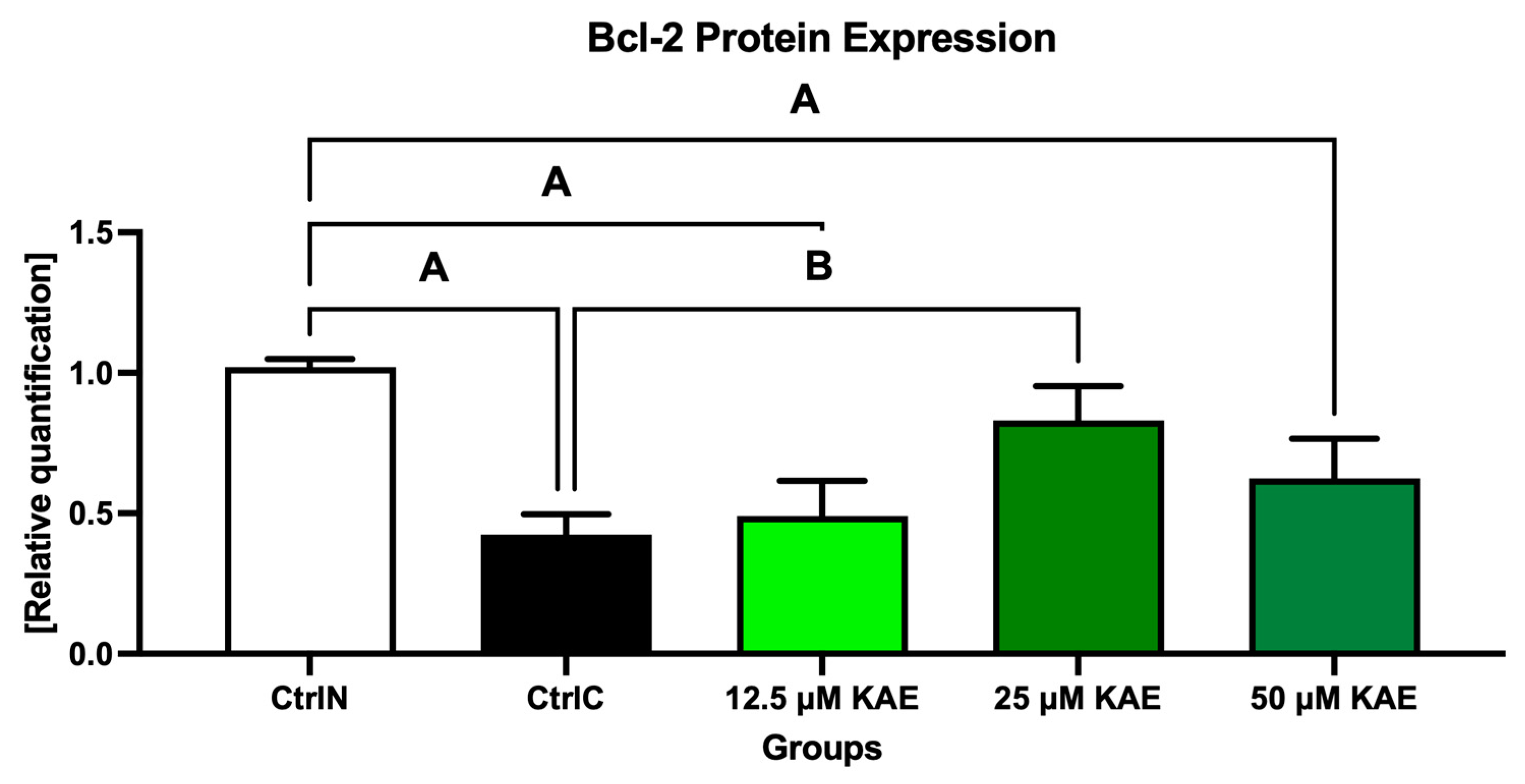
| Parameter | CtrlN | CtrlC | 12.5 µM KAE | 25 µM KAE | 50 µM KAE |
|---|---|---|---|---|---|
| MOT [%] | 96.17 ± 4.40 | 60.12 ± 5.83 A | 80.83 ± 4.16 A,B | 82.53 ± 3.14 A,B | 75.78 ± 4.65 A,B |
| PROG [%] | 76.09 ± 6.94 | 32.57 ± 5.35 A | 60.10 ± 5.98 A,B | 63.78 ± 7.02 B | 42.67 ± 5.76 A,B |
| VAP [μm/s] | 107.50 ± 8.44 | 70.43 ± 7.59 A | 86.97 ± 8.80 A | 92.17 ± 14.61 B | 74.93 ± 8.46 A |
| VSL [μm/s] | 91.23 ± 6.70 | 51.20 ± 6.62 A | 64.60 ± 8.33 A | 72.43 ± 9.77 A,B | 59.93 ± 6.49 A |
| VCL [μm/s] | 184.30 ± 16.85 | 153.90 ± 11.22 A | 169.10 ± 7.19 | 178.60 ± 21.61 B | 166.10 ± 8.86 |
| ALH [μm] | 7.86 ± 0.82 | 6.60 ± 0.36 A | 6.73 ± 0.53 A | 7.68 ± 0.54 B | 6.64 ± 0.69 A |
| BCF [Hz] | 40.80 ± 3.65 | 29.57 ± 2.32 A | 31.50 ± 2.71 A | 35.03 ± 3.53 A,B | 30.40 ± 3.06 A |
| STR [%] | 84.67 ± 7.70 | 72.44 ± 6.62 A | 80.69 ± 7.09 | 79.00 ± 6.16 A | 76.20 ± 7.10 A |
| LIN [%] | 51.00 ± 3.59 | 33.00 ± 2.94 A | 45.33 ± 3.70 B | 46.00 ± 5.65 B | 43.00 ± 3.82 A,B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior. Stresses 2023, 3, 687-700. https://doi.org/10.3390/stresses3040047
Baňas Š, Benko F, Ďuračka M, Lukáč N, Tvrdá E. Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior. Stresses. 2023; 3(4):687-700. https://doi.org/10.3390/stresses3040047
Chicago/Turabian StyleBaňas, Štefan, Filip Benko, Michal Ďuračka, Norbert Lukáč, and Eva Tvrdá. 2023. "Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior" Stresses 3, no. 4: 687-700. https://doi.org/10.3390/stresses3040047
APA StyleBaňas, Š., Benko, F., Ďuračka, M., Lukáč, N., & Tvrdá, E. (2023). Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior. Stresses, 3(4), 687-700. https://doi.org/10.3390/stresses3040047











