Abscisic Acid: Metabolism, Signaling, and Crosstalk with Other Phytohormones under Heavy Metal Stress
Abstract
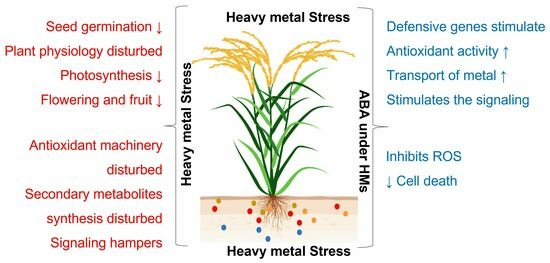
:1. Introduction
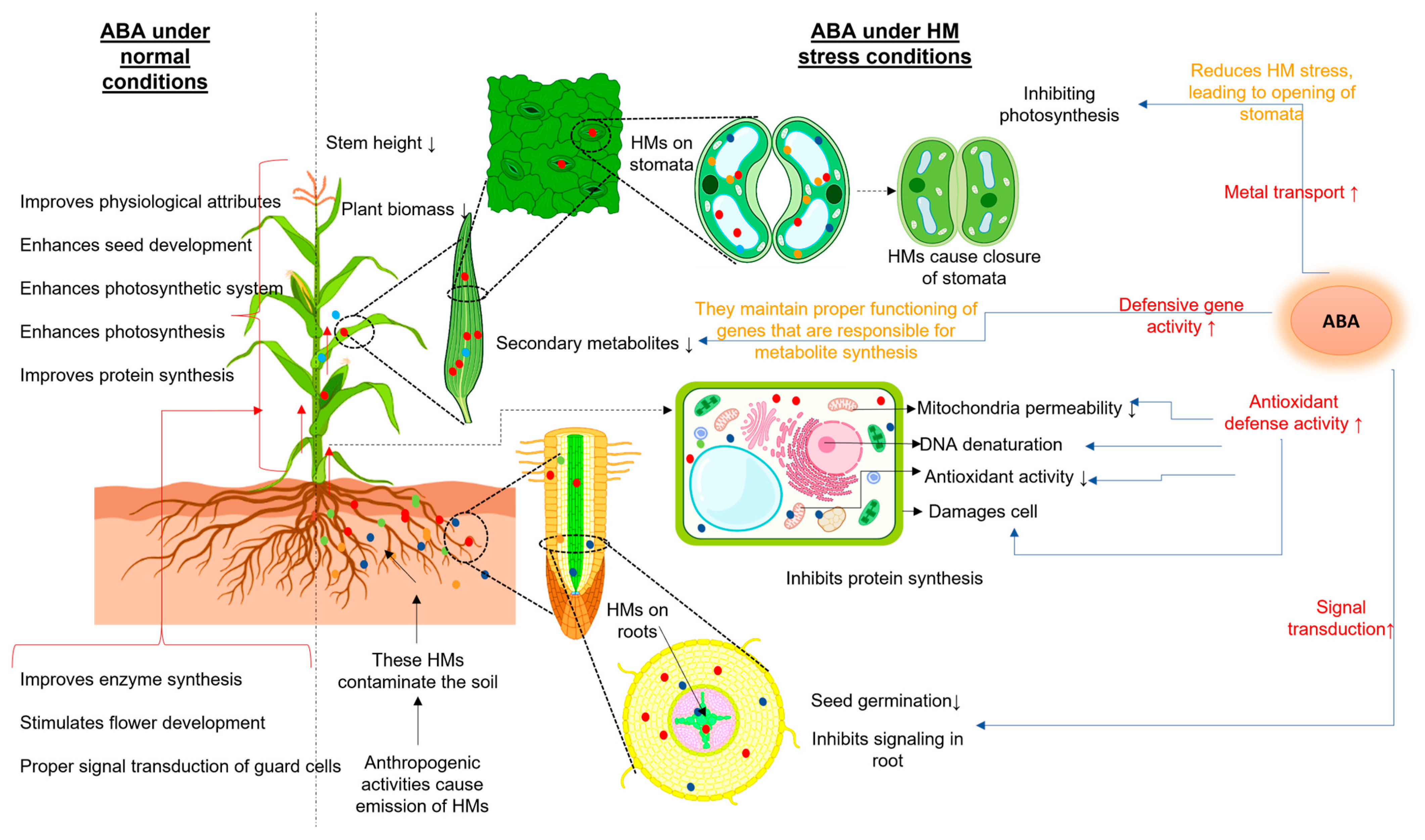
2. Abscisic Acid
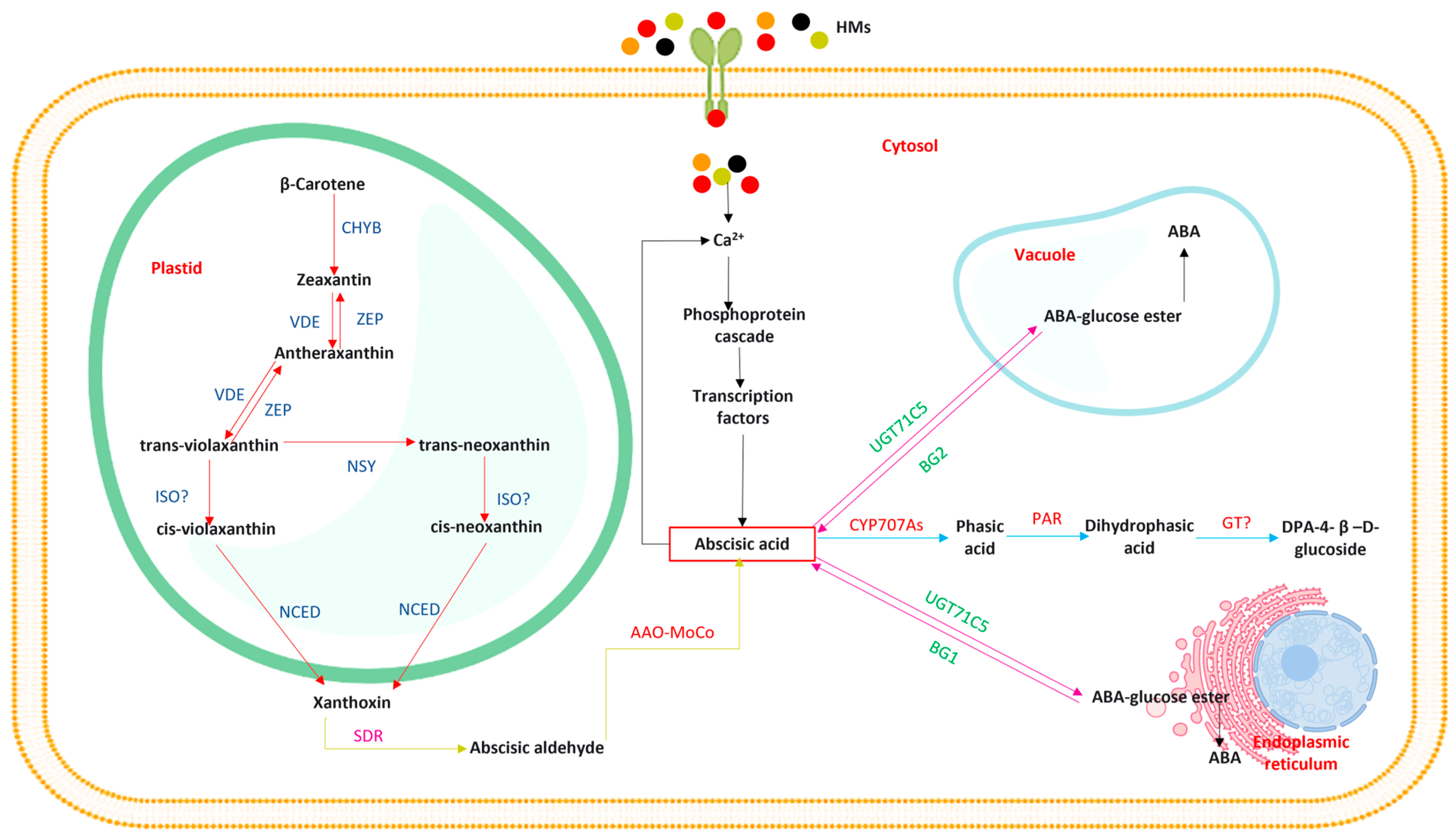
3. Abscisic Acid Biosynthesis
4. Transport of Abscisic Acid
5. Signaling of Abscisic Acid
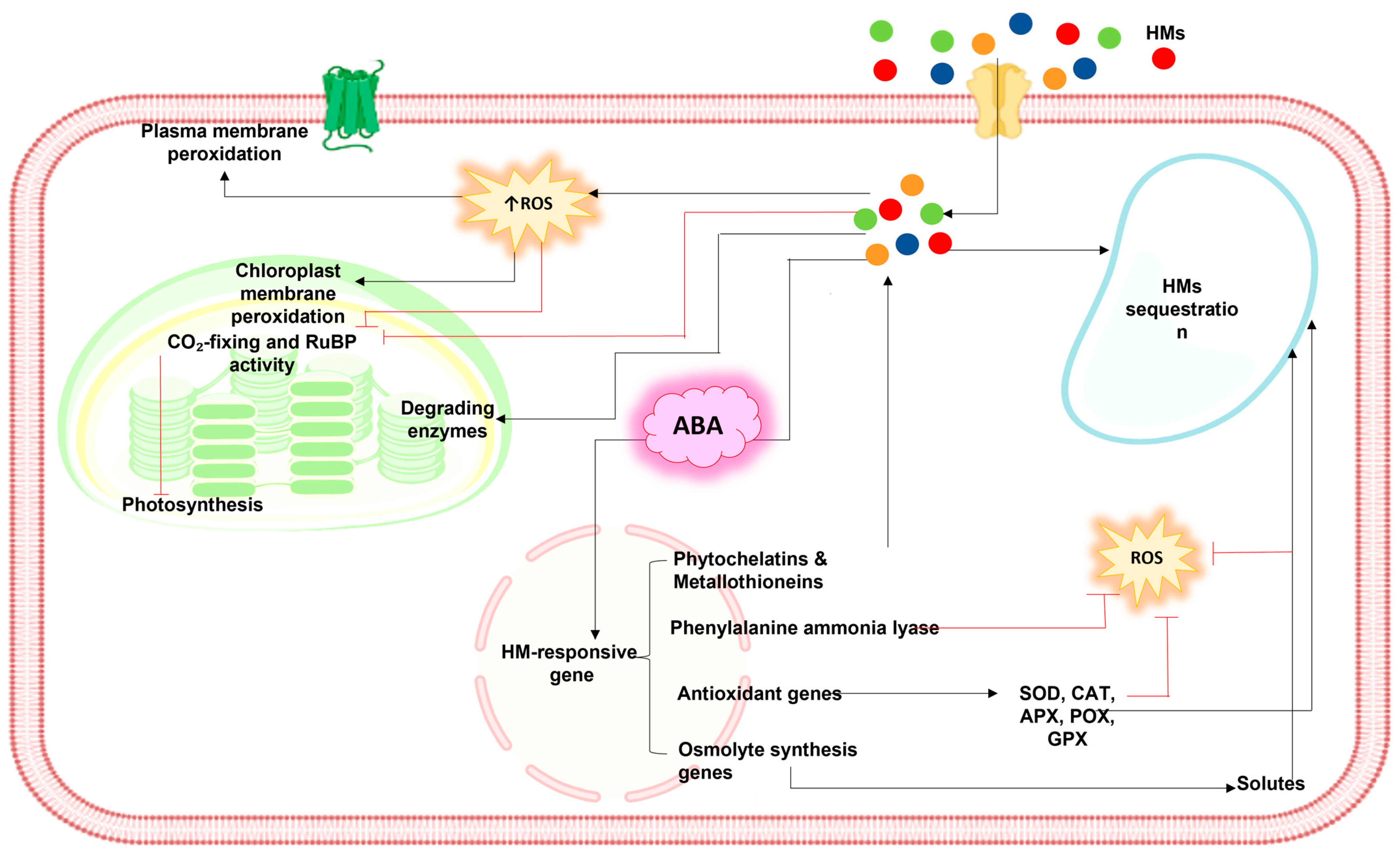
6. Regulation of ABA-Responsive Detoxification of Heavy Metals
| HM and Metalloid Type | Plant Species | Growth Environment | Dose of ABA | Concentration of HMs | Response | Reference |
|---|---|---|---|---|---|---|
| As | Oryza sativa L. | Culture medium | 10 µM | 50 µM | Improved glutathione content, non-protein thiol, osmolyte concentrations, as well as phytochelatins reductase activity | [117] |
| Cd | Brassica campestris L. | Hydroponic | 5 µM | 100 µM L−1 | Cd presence in plant roots leads to increased soluble protein and chlorophyll content, as well as enhanced antioxidant defense systems. | [86] |
| Cd | Lactuca sativa L. | Soil | 5 µM | 10 µM L−1 | The plant exhibited enhancements in biomass, proline content, stomatal conductance, antioxidant enzyme activity, internal CO2 concentration, and soluble protein content. | [123] |
| Cd, Cr, Ni, Hg, Se, Pb, Sn | Fragaria × ananassa | Soil | 40 μmol L−1 | Cr (8.53), Cd (5.16), Se (6.06), Hg (4.55), Ni (2.18), Sn (1.23) Pb (32.16), in mg kg −1 | The addition of exogenous ABA led to a reduction in the translocation of Cr, Hg, Cd, and Sn into Fragaria× ananassa leaves. Moreover, the concurrent increase in antioxidant capacity significantly mitigated the harmful impact of HM stress on the chlorophyll concentration in the leaves of strawberries. | [124] |
| Cd | Brassica napus L. | Hydroponic | 10 µM | 100 µM | Decreased malondialdehyde and accumulation of Cd content and increased plant biomass | [105] |
| Cd | Populus euphratica L. | Culture medium | 5 µM | 100 µM | Antioxidant activities and cell proliferation showed improvement. | [108] |
| Cd | Vigna radiata L. | Seed tray | 10 µM | 100 µM | By inhibiting lipid peroxidation and stimulating antioxidant enzyme activity, the plant’s tolerance was affected | [125] |
| Cd | Perilla frutescens L. | Soil | 5 µM | 10 mg kg−1 | Increased plant antioxidant activities, photosynthetic pigments, and biomass | [126] |
| Co | Solanum lycopersicum L. | Hydroponic | 10 µM | 400 µM L−1 | Decreased translocation of Co from roots to shoots and improved antioxidant enzyme activities and proline content | [82] |
| Ni | Trigonella foenum-graecum L. | Soil | 40 µM | 80 mg kg−1 | Improved secondary metabolites | [127] |
| Pb | Populus alba L. | Soil | 10 µM | 3 mM | Enhanced root biomass, glutathione, ascorbate content, and photosynthetic rate | [112] |
| Zn | Arabidopsis thaliana L. | Culture medium | 15 µM | 200 mg L−1 | Increased activities of antioxidant enzyme, proline accumulation, and ABA endogenous level | [115] |
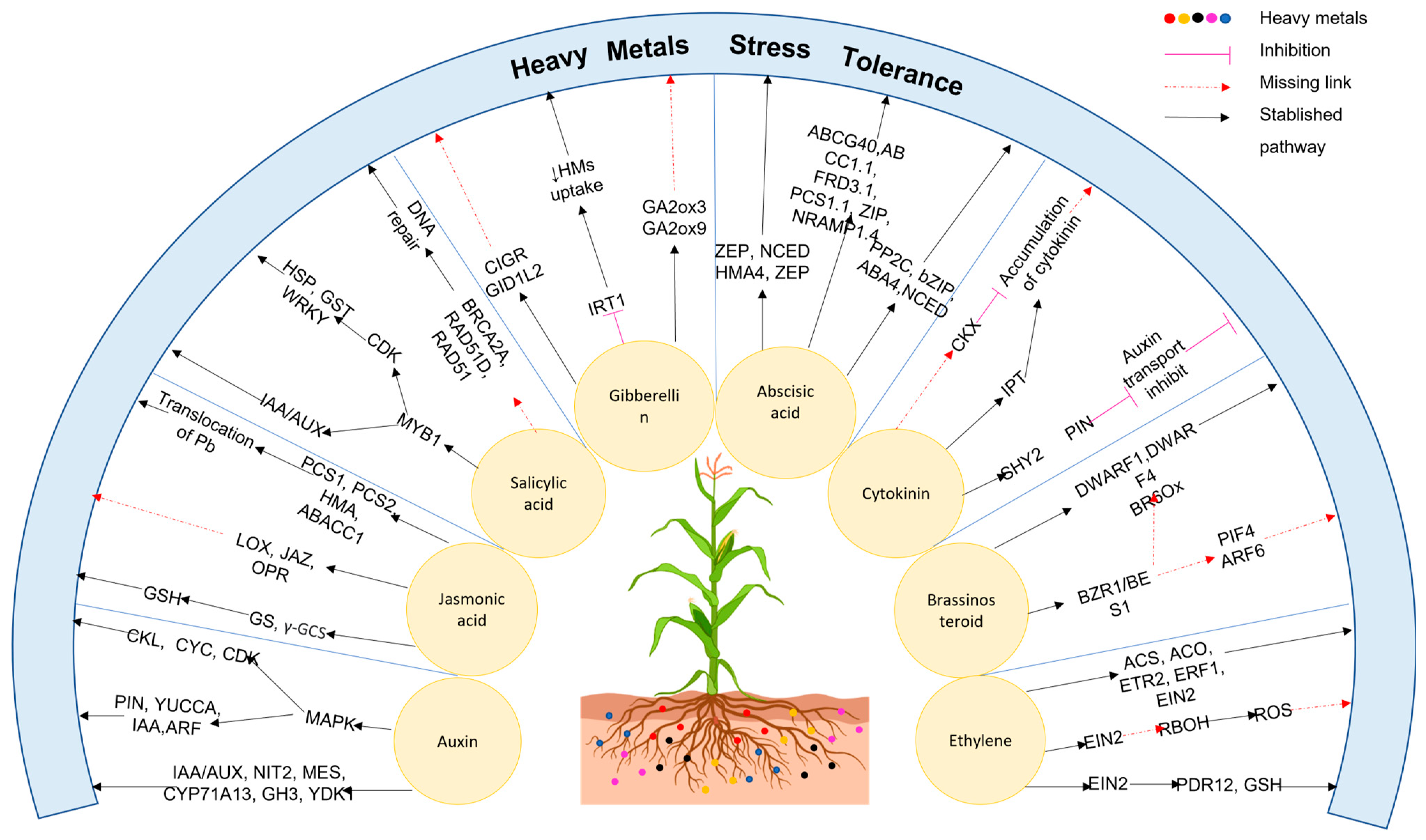
7. Abscisic Acid Crosstalk with Phytohormones
| Heavy Metal and Metalloid | Model Plant | Concentration of HMs | Gene Alteration | Plant Response | Reference |
|---|---|---|---|---|---|
| As | Oryza sativa L. | 50 and 25 mmol L− 1 | Proline and antioxidant biosynthesis gene, alkaline phosphatase, phosphatase, H+/ATPase, and ROS | Antioxidative biosynthesis genes upregulated and downregulated ROS biosynthesis genes and regulated H+/ATPase phosphatase | [117] |
| As | Oryza sativa L. | ABA4, PP2C4, PP2C5NCED2, NCED3, bZIP10, 12 | Increased biosynthesis and signaling of ABA in response to As exposure resulted in decreased root growth | [154] | |
| Cd | Sedum alfredii | NCED, ZEP, AAO | Increased endogenous ABA levels, alleviated Cd toxicity | [114] | |
| Cd | Malus hupehensis | 100 μmol L−1 CdSO4 | Biosynthesis genes of ROS | Downregulated MDA and H2O2 expression to decrease the Cd2+ influx rate | [43] |
| Cd | Pisum sativum | 50 µM CdCl2 | Proline biosynthesis gene, PsPDH1, PsP5CS2, PsProT1 and 2 | In the leaves of pea plants, ABA played a regulatory role in Cd toxicity by downregulating the expression of genes while upregulating proline biosynthesis genes | [155] |
| Cd | Vigna radiate | CdCl2 100 μmol L−1 and 50 μmol L−1 | Stress, ABA-responsive genes, proline, and antioxidant biosynthesis gene | Enzymatic antioxidant genes to regulate Cd toxicity | [125] |
| Cd | Sedum alfredii | Cd(NO3)2: 5 and 25 mmol L−1 | Aquaporin genes | In roots, to regulate Cd toxicity, ABA upregulated the SaPIP genes | [119] |
| Cd | Brassica campestris L. | 100 μmol L−1 | ROS-mediated biosynthesis genes for antioxidative biosynthesis, and proline gene | ABA upregulates antioxidative biosynthesis and proline gene expression to regulate the toxicity of Cd | [86] |
| Cd | Oryza sativa L. | NCED4 | Enhanced HM alleviation and ABA biosynthesis | [156] | |
| Co | Solanum lycopersicum L. | 400 μmol L−1 | CAT, APX, POD, SOD | ROS detoxification increased | [82] |
| Cu | Artemisia annua | 40 and 20 mg kg−1 | Antioxidant biosynthesis genes | Upregulated antioxidative biosynthesis gene expression, promoting the homeostasis of ROS mediated by Cu. | [157] |
| Pb | Populus x canescens | - | PCS1.1, NRAMP1.4, FRD3.1, ABCG40, ABCC1.1 | Enhanced antioxidant enzyme activity and improved uptake, transport, and detoxification of Pb | [112] |
| Pb, Cd, and Zn | Hylotelephium spectabile, Sedum alfredii | - | Antioxidative enzymatic genes, stress-responsive genes, proline, and antioxidant biosynthesis genes | antioxidative biosynthesis genes in both plants under HM toxicities and ABA upregulated antioxidative enzymatic genes | [116] |
| Zn | Vitis vinifera | - | ZIP | Regulated the uptake and accumulation of Zn | [111] |
| Zn | Vitis vinifera | - | NRAMP3, YSL, PCR2 | The co-application of Zn and ABA led to increases in the multiple detoxification-related gene expression | [111] |
8. Conclusions and Future Scope
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azameti, M.K.; Imoro, A.M. Nanotechnology: A Promising Field in Enhancing Abiotic Stress Tolerance in Plants. Crop Des. 2023, 100037, in press. [Google Scholar] [CrossRef]
- Raj, V.; Chauhan, M.S.; Pal, S.L. Potential of Sugarcane Bagasse in Remediation of Heavy Metals: A Review. Chemosphere 2022, 307, 135825. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y.; Wang, X.; Zhou, Y.; Shi, B.; Kwon, B.O.; Choi, K.; et al. Accumulation and Ecological Risk of Heavy Metals in Soils Along the Coastal Areas of the Bohai Sea and the Yellow Sea: A Comparative Study of China and South Korea. Environ. Int. 2020, 137, 105519. [Google Scholar] [CrossRef]
- Rahman, S.U.; Nawaz, M.F.; Gul, S.; Yasin, G.; Hussain, B.; Li, Y.; Cheng, H. State-of-the-Art OMICS Strategies against Toxic Effects of Heavy Metals in Plants: A Review. Ecotoxicol. Environ. Saf. 2022, 242, 113952. [Google Scholar] [CrossRef]
- Ahmad, W.; Najeeb, U.; Zia, M.H. Soil Contamination with Metals: Sources, Types and Implications. In Soil Remediation and Plants. Prospects and Challenges; Hakeem, K.R., Sabir, M., Öztürk, M., Mermut, A.R., Eds.; Academic Press: London, UK, 2015; pp. 37–56. [Google Scholar]
- Sharma, P.; Bano, A.; Singh, S.P.; Sharma, S.; Xia, C.; Nadda, A.K.; Lam, S.S.; Tong, Y.W. Engineered Microbes as Effective Tools for the Remediation of Polyaromatic Aromatic Hydrocarbons and Heavy Metals. Chemosphere 2022, 306, 135538. [Google Scholar] [CrossRef]
- Asgari Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Yadav, S.; Singh, S.P. Biocatalytic Degradation of Emerging Micropollutants. Top. Catal. 2023, 66, 676–690. [Google Scholar] [CrossRef]
- Raklami, A.; Meddich, A.; Oufdou, K.; Baslam, M. Plants—Microorganisms-Based Bioremediation for Heavy Metal Cleanup: Recent Developments, Phytoremediation Techniques, Regulation Mechanisms, and Molecular Responses. Int. J. Mol. Sci. 2022, 23, 5031. [Google Scholar] [CrossRef]
- Sikdar, A.; Jeyasundar, P.G.S.A.; Debnath, B.; Hossain, M.S.; Islam, M.A.; Ahammed, G.J. Cadmium Contamination in the Soil Environment: Impact on Plant Growth and Human Health. In Agrochemicals in Soil and Environment: Impacts and Remediation; Springer Nature: Singapore, 2022; pp. 367–408. [Google Scholar]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions Between Plant Hormones and Heavy Metals Responses. Genet. Mol. Biol. 2017, 40 (Suppl. S1), 373–386. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium Stress in Rice: Toxic Effects, Tolerance Mechanisms, and Management: A Critical Review. Environ. Sci. Pollut. Res. Int. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links Between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M. Adsorptive Removal of Noxious Cadmium Ions from Aqueous Medium Using Activated Carbon/Zirconium Oxide Composite: Isotherm and Kinetic Modelling. J. Mol. Liq. 2020, 310, 113025. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Zia Ur Rehman, M.Z.; Zubair, M.; Riaz, M.; Qayyum, M.F.; Alharby, H.F.; Bamagoos, A.A.; Ali, S. Application of co-Composted Farm Manure and Biochar Increased the Wheat Growth and Decreased Cadmium Accumulation in Plants under Different Water Regimes. Chemosphere 2020, 246, 125809. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Qayyum, M.F.; Nawaz, R.; Ahmad, A.; Asrar, M.; Ahmad, S.R.; Alsahli, A.A.; et al. Combined Use of Different Nanoparticles Effectively Decreased Cadmium (Cd) Concentration in Grains of Wheat Grown in a Field Contaminated with Cd. Ecotoxicol. Environ. Saf. 2021, 215, 112139. [Google Scholar] [CrossRef]
- Khan, A.R.; Wakeel, A.; Muhammad, N.; Liu, B.; Wu, M.; Liu, Y.; Ali, I.; Zaidi, S.H.R.; Azhar, W.; Song, G.; et al. Involvement of Ethylene Signaling in Zinc Oxide Nanoparticle-Mediated Biochemical Changes in Arabidopsis thaliana Leaves. Environ. Sci. Nano 2019, 6, 341–355. [Google Scholar] [CrossRef]
- Ur Rehman, M.Z.; Waqar, M.; Bashir, S.; Rizwan, M.; Ali, S.; El Baroudy, A.A.E.F.; Khalid, H.; Ayub, M.A.; Usman, M.; Jahan, S. Effect of Biochar and Compost on Cadmium Bioavailability and Its Uptake by Wheat–Rice Cropping System Irrigated with Untreated Sewage Water: A Field Study. Arab. J. Geosci. 2021, 14, 135. [Google Scholar] [CrossRef]
- Thudi, M.; Palakurthi, R.; Schnable, J.C.; Chitikineni, A.; Dreisigacker, S.; Mace, E.; Srivastava, R.K.; Satyavathi, C.T.; Odeny, D.; Tiwari, V.K.; et al. Genomic Resources in Plant Breeding for Sustainable Agriculture. J. Plant Physiol. 2021, 257, 153351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Asami, T. Chemical Regulators of Plant Hormones and Their Applications in Basic Research and Agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 1265–1300. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef]
- Hossain, A.; Pamanick, B.; Venugopalan, V.K.; Ibrahimova, U.; Rahman, M.A.; Siyal, A.L.; Maitra, S.; Chatterjee, S.; Aftab, T. Emerging Roles of Plant Growth Regulators for Plants Adaptation to Abiotic Stress–Induced Oxidative Stress. In Emerging Plant Growth Regulators in Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 1–72. [Google Scholar]
- Sonkar, S.; Sharma, L.; Singh, R.K.; Pandey, B.; Rathore, S.S.; Singh, A.K.; Porwal, P.; Singh, S.P.; Singh, S.P. Plant Stress Hormones Nanobiotechnology. In Nanobiotechnology: Mitigation of Abiotic Stress in Plants; Springer: Cham, Switzerland, 2021; pp. 349–373. [Google Scholar]
- Addicott, F.T.; Lyon, J.L. Physiology of Abscisic Acid and Related Substances. Annu. Rev. Plant Physiol. 1969, 20, 139–164. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A. Seed Dormancy: The Complex Process Regulated by Abscisic Acid, Gibberellins, and Other Phytohormones That Makes Seed Germination Work. In Phytohormones-Signaling Mechanisms and CrossTalk in Plant Development and Stress Responses; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arabidopsis Book. Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA Transport and Transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Addicott, F.T. Plant Hormones in the Control of Abscission. Biol. Rev. 1970, 45, 485–524. [Google Scholar] [CrossRef]
- Cracker, L.E.; Abeles, F.B. Abscission: Role of Abscisic Acid. Plant Physiol. 1969, 44, 1144–1149. [Google Scholar] [CrossRef]
- Ogawa, M.; Kay, P.; Wilson, S.; Swain, S.M. Arabidopsis Dehiscence Zone Polygalacturonase1 (ADPG1), ADPG2, and QUARTET2 Are Polygalacturonases Required for Cell Separation during Reproductive Development in Arabidopsis. Plant Cell 2009, 21, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.J.; Morgan, P.W. Abscission. Crit. Rev. Plant Sci. 1989, 8, 103–129. [Google Scholar] [CrossRef]
- Djilianov, D.L.; Dobrev, P.I.; Moyankova, D.P.; Vankova, R.; Georgieva, D.T.; Gajdošová, S.; Motyka, V. Dynamics of Endogenous Phytohormones During Desiccation and Recovery of the Resurrection Plant Species Haberlea Rhodopensis. J. Plant Growth Regul. 2013, 32, 564–574. [Google Scholar] [CrossRef]
- Hu, B.; Deng, F.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, J.; Chen, Z.H. Evolution of Abscisic Acid Signaling for Stress Responses to Toxic Metals and Metalloids. Front. Plant Sci. 2020, 11, 909. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.H.; Vimala, Y.; Jatav, H.S.; Ahmad, P.; Chen, Y.; Siddique, K.H.M. Abscisic Acid: Metabolism, Transport, Crosstalk with Other Plant Growth Regulators, and Its Role in Heavy Metal Stress Mitigation. Front. Plant Sci. 2022, 13, 972856. [Google Scholar] [CrossRef]
- Gonzalez-Villagra, J.; Figueroa, C.; Luengo-Escobar, A.; Morales, M.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Abscisic Acid and Plant Response under Adverse Environmental Conditions. In Plant Performance under Environmental Stress; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 17–47. [Google Scholar]
- Deng, B.; Zhang, W.; Yang, H. Abscisic Acid Decreases Cell Death in Malus hupehensis Rehd. Under Cd Stress by Reducing Root Cd2+ Influx and Leaf Transpiration. J. Plant Growth Regul. 2022, 41, 639–646. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Song, J.; Yue, S. CD2+ Uptake Inhibited by MhNCED3 from Malus hupehensis Alleviates Cd-Induced Cell Death. Environ. Exp. Bot. 2019, 166, 103802. [Google Scholar] [CrossRef]
- Humplík, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To Stimulate or Inhibit? That Is the Question for the Function of Abscisic Acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Asgher, M.; Rehaman, A.; Islam, S.N.U.; Arshad, M.; Khan, N.A. Appraisal of Functions and Role of Selenium in Heavy Metal Stress Adaptation in Plants. Agriculture 2023, 13, 1083. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.; Luo, J.; Li, J.; Kováč, J.; Li, B.; Li, Q.; Wu, K.; Liang, Y.; et al. Abscisic Acid-Mediated Modifications of Radial Apoplastic Transport Pathway Play a Key Role in Cadmium Uptake in Hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Lei, X.J.; Wang, Y.Y.; Liu, B.C.; Wang, D.N.; Liu, Z.Y.; Gao, C.Q. Transcriptomic Analysis of Cadmium Stressed Tamarix hispida Revealed Novel Transcripts and the Importance of Abscisic Acid Network. Front. Plant Sci. 2022, 13, 843725. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid Metabolism and Regulation in Horticultural Crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Dong, X.; Li, Q.; Chen, Z.; Liu, L. An Update on the Function and Regulation of Methylerythritol Phosphate and Mevalonate Pathways and Their Evolutionary Dynamics. J. Integr. Plant Biol. 2021, 63, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, W.C.; Xiao, Y.; Liu, H.W.; Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Frank, A.; Groll, M. The Methylerythritol Phosphate Pathway to Isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid Biosynthesis in Flowering Plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Elucidation of the Indirect Pathway of Abscisic Acid Biosynthesis by Mutants, Genes, and Enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.P.; Mi, J.; Ali, S.; Ohyanagi, H.; Moreno, J.C.; Ablazov, A.; Balakrishna, A.; Berqdar, L.; Fiore, A.; Diretto, G.; et al. An Alternative, Zeaxanthin Epoxidase-Independent Abscisic Acid Biosynthetic Pathway in Plants. Mol. Plant 2022, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Park, Y.; Hwang, I. Abscisic Acid: Biosynthesis, Inactivation, Homoeostasis and Signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Todoroki, Y. ABA 8′-Hydroxylase and Its Chemical Inhibitors. Phytochem. Rev. 2006, 5, 385–404. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Kang, J. ABA Transport Assay in Plant Single-Cell System. In Abscisic Acid: Methods and Protocols; Humana: New York, NY, USA, 2022; pp. 71–84. [Google Scholar] [CrossRef]
- Kanno, Y.; Hanada, A.; Chiba, Y.; Ichikawa, T.; Nakazawa, M.; Matsui, M.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of an Abscisic Acid Transporter by Functional Screening Using the Receptor Complex as a Sensor. Proc. Natl. Acad. Sci. USA 2012, 109, 9653–9658. [Google Scholar] [CrossRef]
- Merilo, E.; Jalakas, P.; Laanemets, K.; Mohammadi, O.; Hõrak, H.; Kollist, H.; Brosché, M. Abscisic Acid Transport and Homeostasis in the Context of Stomatal Regulation. Mol. Plant. 2015, 8, 1321–1333. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A DTX/MATEtype Transporter Facilitates Abscisic Acid Efflux and Modulates ABA Sensitivity and Drought Tolerance in Arabidopsis. Mol. Plant. 2014, 7, 1522–1532. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-Type ABC Transporter Mediates Cellular Uptake of the Phytohormone Abscisic Acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC Transporter AtABCG25 Is Involved in Abscisic Acid Transport and Responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef]
- Andolfo, G.; Ruocco, M.; Di Donato, A.; Frusciante, L.; Lorito, M.; Scala, F.; Ercolano, M.R. Genetic Variability and Evolutionary Diversification of Membrane ABC Transporters in Plants. BMC Plant Biol. 2015, 15, 51. [Google Scholar] [CrossRef]
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Arabidopsis Mutants of AtABCG22, an ABC Transporter Gene, Increase Water Transpiration and Drought Susceptibility. Plant J. 2011, 67, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Dahuja, A.; Kumar, R.R.; Sakhare, A.; Watts, A.; Singh, B.; Goswami, S.; Sachdev, A.; Praveen, S. Role of ATP-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses. Physiol. Plantarum. 2021, 171, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fang, C.; Li, Z.; Wang, Y.; Pan, S.; Wu, Y.; An, X.; Long, Y.; Wan, X. ATP-Binding Cassette G Transporters and Their Multiple Roles Especially for Male Fertility in Arabidopsis, Rice and Maize. Int. J. Mol. Sci 2022, 23, 9304. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kanno, Y.; Suzuki, H.; Watanabe, S.; Seo, M. Arabidopsis NPF4. 6 and NPF5. 1 Control Leaf Stomatal Aperture by Regulating Abscisic Acid Transport. Genes 2021, 12, 885. [Google Scholar] [CrossRef]
- Ali, A.; Pardo, J.M.; Yun, D.J. Desensitization of ABA-Signaling: The Swing from Activation to Degradation. Front. Plant Sci. 2020, 11, 379. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, L.; Liu, X.; Liu, H.; Li, D.; Cao, M.; Chen, H.; Xu, L.; Zhu, J.K.; Zhao, Y. A Novel Chemical Inhibitor of ABA Signaling Targets All ABA Receptors. Plant Physiol. 2017, 173, 2356–2369. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Tian, J.; Xiang, X.; Zheng, H.; Liu, H.; Fang, Z.; Tian, Z.; Liu, L.; Zhu, Y.; et al. Synergistic Interplay between ABA-Generating Bacteria and Biochar in the Reduction of Heavy Metal Accumulation in Radish, Pakchoi, and Tomato. Environ. Pollut. 2023, 333, 122084. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Lozano-Juste, J.; Albert, A. PYR/PYL/RCAR ABA Receptors. In Advances in Botanical Research; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 92, pp. 51–82. [Google Scholar]
- Yang, Q.; Liu, K.; Niu, X.; Wang, Q.; Wan, Y.; Yang, F.; Li, G.; Wang, Y.; Wang, R. Genome-Wide Identification of PP2C Genes and Their Expression Profiling in Response to Drought and Cold Stresses in Medicago truncatula. Sci. Rep. 2018, 8, 12841. [Google Scholar] [CrossRef]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic Acid Perception and Signaling: Structural Mechanisms and Applications. Acta Pharmacol. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of Abscisic Acid and 6-Benzylaminopurine Modulated Morpho-physiological and Antioxidative Defense Responses of Tomato (Solanum lycopersicum L.) by Minimizing Cobalt Uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.J.; Kim, M.H.; Kwak, J.M. MAPK Cascades in Guard Cell Signal Transduction. Front. Plant Sci. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Alemán, F.; et al. Calcium Specificity Signaling Mechanisms in Abscisic Acid Signal Transduction in Arabidopsis Guard Cells. eLife 2015, 4, e03599. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the Plasma Membrane H+-ATPase AHA2 by BAK1 Is Required for ABA-Induced Stomatal Closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef]
- Shen, G.; Niu, J.; Deng, Z. Abscisic Acid Treatment Alleviates Cadmium Toxicity in Purple Flowering Stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) Seedlings. Plant Physiol. Biochem. 2017, 118, 471–478. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Role of Abscisic Acid in Cadmium Tolerance of Rice (Oryza sativa L.) Seedlings. Plant Cell Environ. 2003, 26, 867–874. [Google Scholar] [CrossRef]
- Stroinski, A.; Giżewska, K.; Zielezińska, M. Abscisic acid is required in Transduction of Cadmium Signal to Potato Roots. Biol. Plant. 2013, 57, 121–127. [Google Scholar] [CrossRef]
- Lu, Q.; Weng, Y.; You, Y.; Xu, Q.; Li, H.; Li, Y.; Liu, H.; Du, S. Inoculation with Abscisic Acid (ABA)-Catabolizing Bacteria Can Improve Phytoextraction of Heavy Metal in Contaminated Soil. Environ. Pollut. 2020, 257, 113497. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Ju, Q.; Li, W.; Tran, L.-S.P.; Xu, J. The R2R3- MYB Transcription Factor MYB49 Regulates Cadmium Accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.E.; Eide, D.J.; Guerinot, M.L. Altered Selectivity in an Arabidopsis Metal Transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 12356–12360. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Tsednee, M.; Lo, Y.C.; Yang, S.C.; Hu, J.M.; Ishizaki, K.; Kohchi, T.; Lee, D.C.; Yeh, K.C. Evolutionary Analysis of Iron (Fe) Acquisition System in Marchantia polymorpha. New Phytol. 2016, 211, 569–583. [Google Scholar] [CrossRef]
- Wang, F.-Z.; Chen, M.-X.; Yu, L.-J.; Xie, L.-J.; Yuan, L.-B.; Qi, H.; Xiao, M.; Guo, W.; Chen, Z.; Yi, K.; et al. OsARM1, an R2R3 MYB transcription factor, is involved in regulation of the response to arsenic stress in rice. Front. Plant Sci. 2017, 8, 1868. [Google Scholar] [CrossRef]
- Lindsay, E.R.; Maathuis, F.J.M. New Molecular Mechanisms to Reduce Arsenic in Crops. Trends Plant Sci. 2017, 22, 1016–1026. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Diehn, T.A.; Bernhardt, N.; Bienert, M.D.; Mitani-Ueno, N.; Fuge, J.; Bieber, A.; Spitzer, C.; Bräutigam, A.; Ma, J.F.; et al. Functional Evolution of Nodulin 26-Like Intrinsic Proteins: From Bacterial Arsenic Detoxification to Plant Nutrient Transport. New Phytol. 2020, 225, 1383–1396. [Google Scholar] [CrossRef]
- Léran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A Unified Nomenclature of nitrate transporter 1/peptide transporter Family Members in Plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, J.; Jing, H.; Wu, Q.; Shen, R.; Zhu, X. Exogenous abscisic acid alleviates Cd toxicity in Arabidopsis thaliana by inhibiting Cd uptake, translocation and accumulation, and promoting Cd chelation and efflux. Plant Sci. 2022, 325, 111464. [Google Scholar] [CrossRef]
- Clemens, S. Evolution and Function of Phytochelatin Synthases. J. Plant Physiol. 2006, 163, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. A Family of Phytochelatin Synthase Genes from Plant, Fungal and Animal Species. Trends Plant Sci. 1999, 4, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-Responsive Phytochelatin Synthase Is Constitutively Present in the Ancient Liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, A.; De Benedictis, M.; Degola, F.; Pastore, G.; Calcagno, M.; Ruotolo, R.; Mengoni, A.; Sanità di Toppi, L. The Capability to Synthesize Phytochelatins and the Presence of Constitutive and Functional Phytochelatin Synthases Are Ancestral (Plesiomorphic) Characters for Basal Land Plants. J. Exp. Bot. 2014, 65, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fan, T.; Zhu, X.; Wu, X.; Ouyang, J.; Jiang, L.; Cao, S. WRKY12 Represses GSH1 Expression to Negatively Regulate Cadmium Tolerance in Arabidopsis. Plant Mol. Biol. 2019, 99, 149–159. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-Finger Transcription Factor ZAT6 Positively Regulates Cadmium Tolerance through the Glutathione-Dependent Pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef]
- Meng, H.; Hua, S.; Shamsi, I.H.; Jilani, G.; Li, Y.; Jiang, L. Cadmiuminduced Stress on the Seed Germination and Seedling Growth of Brassica napus L., and Its Alleviation through Exogenous Plant Growth Regulators. Plant Growth Regul. 2009, 58, 47–59. [Google Scholar] [CrossRef]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous Abscisic Acid Application Decreases Cadmium Accumulation in Arabidopsis Plants, Which Is Associated with the Inhibition of IRT1-Mediated Cadmium Uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef]
- Pan, W.; You, Y.; Shentu, J.L.; Weng, Y.N.; Wang, S.T.; Xu, Q.R.; Liu, H.J.; Du, S.T. Abscisic Acid (ABA)-Importing Transporter 1 (AIT1) Contributes to the Inhibition of Cd Accumulation via Exogenous ABA Application in Arabidopsis. J. Hazard. Mater. 2020, 391, 122189. [Google Scholar] [CrossRef]
- Han, Y.; Wang, S.; Zhao, N.; Deng, S.; Zhao, C.; Li, N.; Sun, J.; Zhao, R.; Yi, H.; Shen, X.; et al. Exogenous Abscisic Acid Alleviates Cadmium Toxicity by Restricting Cd2+ Influx in Populus euphratica Cells. J. Plant Growth Regul. 2016, 35, 827–837. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Luo, L.; Liao, M.A.; Lv, X.; Wang, Z.; Liang, D.; Xia, H.; Wang, X.; Lai, Y.; et al. The Effects of Abscisic Acid (ABA) Addition on Cadmium Accumulation of Two Ecotypes of Solanum photeinocarpum. Environ. Monit. Assess. 2016, 188, 182. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ge, Y.; Zhang, W.; Zhao, Y.; Yang, G. The Walnut JrVHAG1 Gene Is Involved in Cadmium Stress Response through ABA-Signal Pathway and MYB Transcription Regulation. BMC Plant Biol. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yan, Y.; Rosado, A.; Zhang, Z.; Castellarin, S.D. ABA Alleviates Uptake and Accumulation of Zinc in Grapevine (Vitis vinifera L.) by Inducing Expression of ZIP and Detoxification-Related Genes. Front. Plant Sci. 2019, 10, 872. [Google Scholar] [CrossRef]
- Shi, W.G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.B. Abscisic Acid Enhances Lead Translocation from the Roots to the Leaves and Alleviates Its Toxicity in Populus × canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderón-Urrea, A.; Wu, Y.; Tang, Z. Foliar Application of Abscisic Acid Mitigates Cadmium Stress and Increases Food Safety of Cadmium-Sensitive Lettuce (Lactuca sativa L.) Genotype. PeerJ 2020, 8, e9270. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, S.; Li, Y.; Zheng, F.; He, B.; Gu, M. Exogenous Abscisic Acid (ABA) Promotes Cadmium (Cd) Accumulation in Sedum alfredii Hance by Regulating the Expression of Cd Stress Response Genes. Environ. Sci. Pollut. Res. Int. 2020, 27, 8719–8731. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Song, L.L. The Role of ABA in the Responses of Wildtype and Abscisic Acid Mutants of Arabidopsis thaliana to Excess Zinc. Acta Physiol. Plant. 2020, 42, 74. [Google Scholar] [CrossRef]
- Cheng, L.; Pu, L.; Li, A.; Zhu, X.; Zhao, P.; Xu, X.; Lei, N.; Chen, J. Implication of Exogenous Abscisic Acid (ABA) Application on Phytoremediation: Plants Grown in Co-contaminated Soil. Environ. Sci. Pollut. Res. 2022, 29, 8684–8693. [Google Scholar] [CrossRef]
- Saha, I.; Hasanuzzaman, M.; Adak, M.K. Abscisic Acid Priming Regulates Arsenite Toxicity in Two Contrasting Rice (Oryza sativa L.) Genotypes through Differential Functioning of sub1A Quantitative Trait Loci. Environ. Pollut. 2021, 287, 117586. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, X.; Zhang, Y.; Tang, M. The Synergy of Arbuscular Mycorrhizal Fungi and Exogenous Abscisic Acid Benefits Robinia pseudoacacia L. Growth through Altering the Distribution of Zn and Endogenous Abscisic Acid. J. Fungi 2021, 7, 671. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Dong, Q.; Yang, X.; Liu, Y.; Li, B.; Yuan, S.; Yin, J.; Xu, Q.; Li, T.; et al. Abscisic Acidmediated Modifications in Water Transport Continuum Are Involved in Cadmium Hyperaccumulation in Sedum alfredii. Chemosphere 2021, 268, 129339. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Q.; Chen, S.; Zhang, S.; Wang, M.; Mujtaba Munir, M.A.M.; Feng, Y.; He, Z.; Yang, X. Roles of Exogenous Plant Growth Regulators on Phytoextraction of Cd/Pb/Zn by Sedum alfredii Hance in Contaminated Soils. Environ. Pollut. 2022, 293, 118510. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tang, Y.; Wang, S.; Su, H.; Chen, J.; Zhang, D.; Wu, J.; Zhou, D.; Yan, M.; Liu, L. Abscisic acid modulates differential physiological and biochemical responses to cadmium stress in Brassica napus. Environ. Pollut. Bioavailab. 2023, 35, 2168216. [Google Scholar] [CrossRef]
- Yu, X.; Yang, L.; Fan, C.; Hu, J.; Zheng, Y.; Wang, Z.; Liu, Y.; Xiao, X.; Yang, L.; Lei, T.; et al. Abscisic acid (ABA) alleviates cadmium toxicity by enhancing the adsorption of cadmium to root cell walls and inducing antioxidant defense system of Cosmos bipinnatus. Ecotoxicol. Environ. Saf. 2023, 261, 115101. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, L.; Xie, Y.; Yu, X.; Li, H.; Lin, L.; Liao, M.; Wang, Z.; Sun, G.; Wang, X.; et al. Effects of Exogenous Abscisic Acid on the Growth and Cadmium Accumulation of Lettuce under Cadmium-Stress Conditions. Int. J. Environ. Anal. Chem. 2020, 100, 720–731. [Google Scholar] [CrossRef]
- Kocaman, A. Effects of Foliar Application of Abscisic Acid on Antioxidant Content, Phytohormones in Strawberry Shoots, and Translocation of Various Heavy Metals. Sci. Hortic. 2023, 314, 111943. [Google Scholar] [CrossRef]
- Leng, Y.; Li, Y.; Ma, Y.H.; He, L.F.; Li, S.W. Abscisic Acid Modulates Differential Physiological and Biochemical Responses of Roots, Stems, and Leaves in Mung Bean Seedlings to Cadmium Stress. Environ. Sci. Pollut. Res. Int. 2021, 28, 6030–6043. [Google Scholar] [CrossRef]
- Shu, J.; He, Q.; Zheng, H.; Liao, M.A.; Kuang, N.; Huang, Z. Abscisic Acid Improves the Ability of Accumulator Plant Perilla frutescens to Perform Phytoremediation in Cadmium-Contaminated Soils. Int. J. Environ. Anal. Chem. 2021, 18, 2182–2191. [Google Scholar] [CrossRef]
- Parwez, R.; Aftab, T.; Khan, M.M.A.; Naeem, M. Exogenous Abscisic Acid Fine-Tunes Heavy Metal Accumulation and Plant’s Antioxidant Defence Mechanism to Optimize Crop Performance and Secondary Metabolite Production in Trigonella foenum-Graecum L. Under Nickel Stress. Plant Sci. 2023, 332, 111703. [Google Scholar] [CrossRef]
- Parwez, R.; Aftab, T.; Gill, S.S.; Naeem, M. Abscisic Acid Signaling and Crosstalk with Phytohormones in Regulation of Environmental Stress Responses. Environ. Exp. Bot. 2022, 199, 104885. [Google Scholar] [CrossRef]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin Response Factor2 (ARF2) and Its Regulated Homeodomain Gene HB33 Mediate Abscisic Acid Response in Arabidopsis. PLoS Genet. 2011, 7, e1002172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Ye, T.; Lu, Y.; Chen, X.; Wu, Y. The Inhibitory Effect of ABA on Floral Transition Is Mediated by ABI5 in Arabidopsis. J. Exp. Bot. 2013, 64, 675–684. [Google Scholar] [CrossRef]
- Rock, C.D.; Sun, X. Crosstalk Between ABA and Auxin Signaling Pathways in Roots of Arabidopsis thaliana (L.) Heynh. Planta 2005, 222, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin Controls Seed Dormancy Through Stimulation of Abscisic Acid Signaling by Inducing ARF-Mediated ABI3 Activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J. Auxin: Hormonal Signal Required for Seed Development and Dormancy. Plants 2020, 9, 705. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic Acid Accumulation Modulates Auxin Transport in the Root Tip to Enhance Proton Secretion for Maintaining Root Growth under Moderate Water Stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Zhao, X.; Xu, S.; Xia, Y.; Sun, X. Nitric Oxide Acts Downstream of Abscisic Acid in Molybdenum-Induced Oxidative Tolerance in Wheat. Plant Cell Rep. 2018, 37, 599–610. [Google Scholar] [CrossRef]
- Yuan, K.; Rashotte, A.M.; Wysocka-Diller, J.W. ABA and GA Signaling Pathways Interact and Regulate Seed Germination and Seedling Development under Salt Stress. Acta Physiol. Plant. 2011, 33, 261–271. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Tuan, P.A.; Ayele, B.T. Jasmonate Regulates Seed Dormancy in Wheat via Modulating the Balance between Gibberellin and Abscisic Acid. J. Exp. Bot. 2022, 73, 2434–2453. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Pati, P.K. Versatile Roles of Brassinosteroid in Plants in the Context of Its Homeostasis, Signaling and Crosstalks. Front. Plant Sci. 2015, 6, 950. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Y.; Shang, J.; Xin, R.; Tang, W. The Antagonistic Regulation of Abscisic Acid-Inhibited Root Growth by Brassinosteroids Is Partially Mediated via Direct Suppression of Abscisic Acid Insensitive 5 Expression by Brassinazole Resistant 1. Plant Cell Environ. 2016, 39, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hadi, F.; Ali, N. Effective Phytoextraction of Cadmium (Cd) with Increasing Concentration of Total Phenolics and Free Proline in Cannabis sativa (L.) Plant Under Various Treatments of Fertilizers, Plant Growth Regulators and Sodium Salt. Int. J. Phytoremediat. 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Z.; Wang, X. The Primary Signaling Outputs of Brassinosteroids Are Regulated by Abscisic Acid Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef]
- Avalbaev, A.M.; Somov, K.A.; Yuldashev, R.A.; Shakirova, F.M. Cytokinin Oxidase Is Key Enzyme of Cytokinin Degradation. Biochemistry 2012, 77, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Sirko, A.; Wawrzyńska, A.; Brzywczy, J.; Sieńko, M. Control of ABA Signaling and Crosstalk with Other Hormones by the Selective Degradation of Pathway Components. Int. J. Mol. Sci. 2021, 22, 4638. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Köllmer, I.; Bartrina, I.; Holst, K.; Schmülling, T. New Insights into the Biology of Cytokinin Degradation. Plant Biol. 2006, 8, 371–381. [Google Scholar] [CrossRef]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of AHK1/ATHK1 and Cytokinin Receptor Histidine Kinases in Response to Abscisic Acid, Drought, and Salt Stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef]
- Chen, L.I.N.; Dodd, I.C.; Davies, W.J.; Wilkinson, S. Ethylene Limits Abscisic Acid-Or Soil Drying-Induced Stomatal Closure in Aged Wheat Leaves. Plant Cell Environ. 2013, 36, 1850–1859. [Google Scholar] [CrossRef]
- Harrison, M.A. Cross-Talk between Phytohormone Signaling Pathways under Both Optimal and Stressful Environmental Conditions. In Phytohormones and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 49–76. [Google Scholar]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA Crosstalk with Ethylene and Nitric Oxide in Seed Dormancy and Germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Yu, Y.; Quan, R.; Zhang, Z.; Zhang, H.; Huang, R. The Ethylene Response Factor AtERF11 That Is Transcriptionally Modulated by the bZIP Transcription Factor HY5 Is a Crucial Repressor for Ethylene Biosynthesis in Arabidopsis. Plant J. 2011, 68, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a Key Factor in the Regulation of Seed Dormancy. Front. Plant Sci. 2014, 5, 539. [Google Scholar] [CrossRef] [PubMed]
- Kovács, V.; Gondor, O.K.; Szalai, G.; Darkó, E.; Majláth, I.; Janda, T.; Pál, M. Synthesis and Role of Salicylic Acid in Wheat Varieties with Different Levels of Cadmium Tolerance. J. Hazard. Mater. 2014, 280, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic Acid-Induced Protection against Cadmium Toxicity in Wheat Plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Huang, T.L.; Nguyen, Q.T.T.; Fu, S.F.; Lin, C.Y.; Chen, Y.C.; Huang, H.J. Transcriptomic Changes and Signalling Pathways Induced by Arsenic Stress in Rice Roots. Plant Mol. Biol. 2012, 80, 587–608. [Google Scholar] [CrossRef]
- Zdunek-Zastocka, E.; Grabowska, A.; Michniewska, B.; Orzechowski, S. Proline Concentration and Its Metabolism Are Regulated in a Leaf Age Dependent Manner but Not by Abscisic Acid in Pea Plants Exposed to Cadmium Stress. Cells 2021, 10, 946. [Google Scholar] [CrossRef]
- Tan, M.; Cheng, D.; Yang, Y.; Zhang, G.; Qin, M.; Chen, J.; Chen, Y.; Jiang, M. Co-expression Network Analysis of the Transcriptomes of Rice Roots Exposed to Various Cadmium Stresses Reveals Universal Cadmium-Responsive Genes. BMC Plant Biol. 2017, 17, 194. [Google Scholar] [CrossRef]
- Zehra, A.; Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Exogenous Abscisic Acid Mediates ROS Homeostasis and Maintains Glandular Trichome to Enhance Artemisinin Biosynthesis in Artemisia annua under Copper Toxicity. Plant Physiol. Biochem. 2020, 156, 125–134. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic Interaction Between Abscisic Acid and Jasmonate-Ethylene Signaling Pathways Modulates Defense Gene Expression and Disease Resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Proietti, S.; Caarls, L.; Coolen, S.; Van Pelt, J.A.; Van Wees, S.C.M.; Pieterse, C.M.J. Genome-Wide Association Study Reveals Novel Players in Defense Hormone Crosstalk in Arabidopsis. Plant Cell Environ. 2018, 41, 2342–2356. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The Basic Helixloop-Helix Transcription Factor MYC2 Directly Represses PLETHORA Expression During Jasmonate-Mediated Modulation of the Root Stem Cell Niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, C. Cross Talk of Signaling Pathways Between ABA and Other Phytohormones. In Abscisic Acid: Metabolism, Transport and Signaling; Springer: Dordrecht, The Netherlands, 2014; pp. 243–253. [Google Scholar]
- Hancock, J.T.; Neill, S.J.; Wilson, I.D. Nitric Oxide and ABA in the Control of Plant Function. Plant Sci. 2011, 181, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Crosstalk Between Nitric Oxide (NO) and Abscisic Acid (ABA) Signalling Molecules in Higher Plants. Environ. Exp. Bot. 2019, 161, 41–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Guo, Z.; Lu, S.; He, S.; Shu, W.; Zhou, B. Increased Abscisic Acid Levels in Transgenic Tobacco Over-Expressing 9-cis-epoxycarotenoid Dioxygenase Influence H2O2 and NO Production and Antioxidant Defenses. Plant Cell Environ. 2009, 32, 509–519. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Ye, N.; Liu, R.; Jia, W.; Zhang, J. Nitric Oxideinduced Rapid Decrease of Abscisic Acid Concentration Is Required in Breaking Seed Dormancy in Arabidopsis. New Phytol. 2009, 183, 1030–1042. [Google Scholar] [CrossRef]
- Signorelli, S.; Considine, M.J. Nitric Oxide Enables Germination by a Four-Pronged Attack on ABA-Induced Seed Dormancy. Front. Plant Sci. 2018, 9, 296. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-Induced NO Generation and Stomatal Closure in Arabidopsis Are Dependent on H2O2 Synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Saradadevi, R.; Bramley, H.; Siddique, K.H.M.; Edwards, E.; Palta, J.A. Reprint of ‘Contrasting Stomatal Regulation and Leaf ABA Concentrations in Wheat Genotypes When Split Root Systems Were Exposed to Terminal Drought’. Field Crops Res. 2014, 165, 5–14. [Google Scholar] [CrossRef]
- Hung, K.T.; Kao, C.H. Nitric Oxide Counteracts the Senescence of Rice Leaves Induced by Abscisic Acid. J. Plant Physiol. 2003, 160, 871–879. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Crosstalk Between Abscisic Acid and Nitric Oxide under Heat Stress: Exploring New Vantage Points. Plant Cell Rep. 2021, 40, 1429–1450. [Google Scholar] [CrossRef]
- Iqbal, N.; Sehar, Z.; Fatma, M.; Umar, S.; Sofo, A.; Khan, N.A. Nitric Oxide and Abscisic Acid Mediate Heat Stress Tolerance through Regulation of Osmolytes and Antioxidants to Protect Photosynthesis and Growth in Wheat Plants. Antioxidants 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Latif, H.H.; Hanafy, R.S. Influence of Nitric Oxide Application on Some Biochemical Aspects, Endogenous Hormones, Minerals and Phenolic Compounds of Vicia faba Plant Grown Under Arsenic Stress. Gesunde Pflanz. 2016, 68, 99–107. [Google Scholar] [CrossRef]
- Sadeghipour, O. Nitric Oxide Increases Pb Tolerance by Lowering Pb Uptake and Translocation as well as Phytohormonal Changes in Cowpea (Vigna unguiculata (L.) Walp.). Sains Malays. 2017, 46, 189–195. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bano, A.; Singh, K.; Singh, S.P.; Sharma, P. Abscisic Acid: Metabolism, Signaling, and Crosstalk with Other Phytohormones under Heavy Metal Stress. Stresses 2023, 3, 665-686. https://doi.org/10.3390/stresses3040046
Bano A, Singh K, Singh SP, Sharma P. Abscisic Acid: Metabolism, Signaling, and Crosstalk with Other Phytohormones under Heavy Metal Stress. Stresses. 2023; 3(4):665-686. https://doi.org/10.3390/stresses3040046
Chicago/Turabian StyleBano, Ambreen, Kratika Singh, Surendra Pratap Singh, and Pooja Sharma. 2023. "Abscisic Acid: Metabolism, Signaling, and Crosstalk with Other Phytohormones under Heavy Metal Stress" Stresses 3, no. 4: 665-686. https://doi.org/10.3390/stresses3040046
APA StyleBano, A., Singh, K., Singh, S. P., & Sharma, P. (2023). Abscisic Acid: Metabolism, Signaling, and Crosstalk with Other Phytohormones under Heavy Metal Stress. Stresses, 3(4), 665-686. https://doi.org/10.3390/stresses3040046