Role of Lignin in Wheat Plant for the Enhancement of Resistance against Lodging and Biotic and Abiotic Stresses
Abstract
1. Importance of Wheat Crop
2. Role of Lignin in Wheat Plant
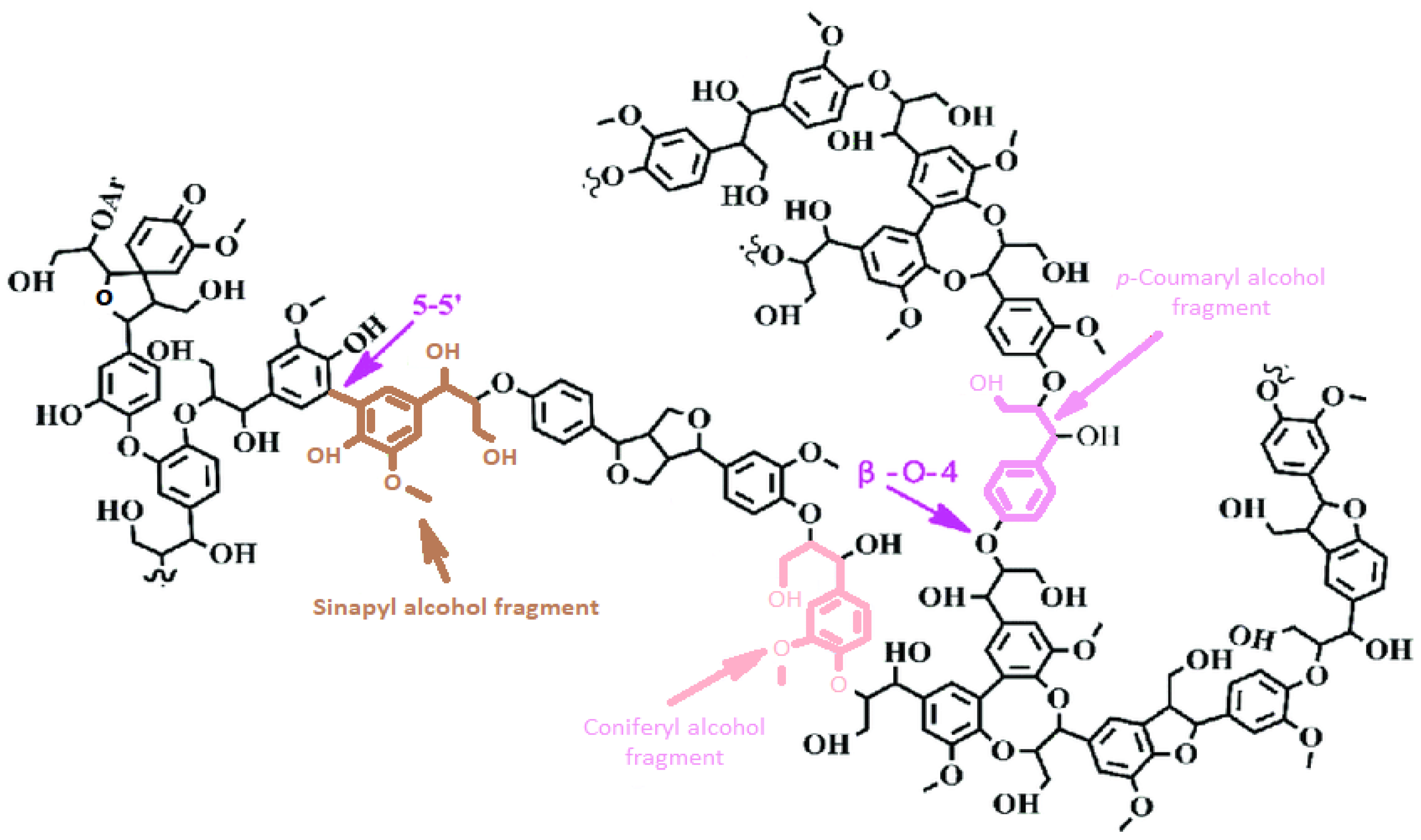
3. Structural and Chemical Composition of Lignin
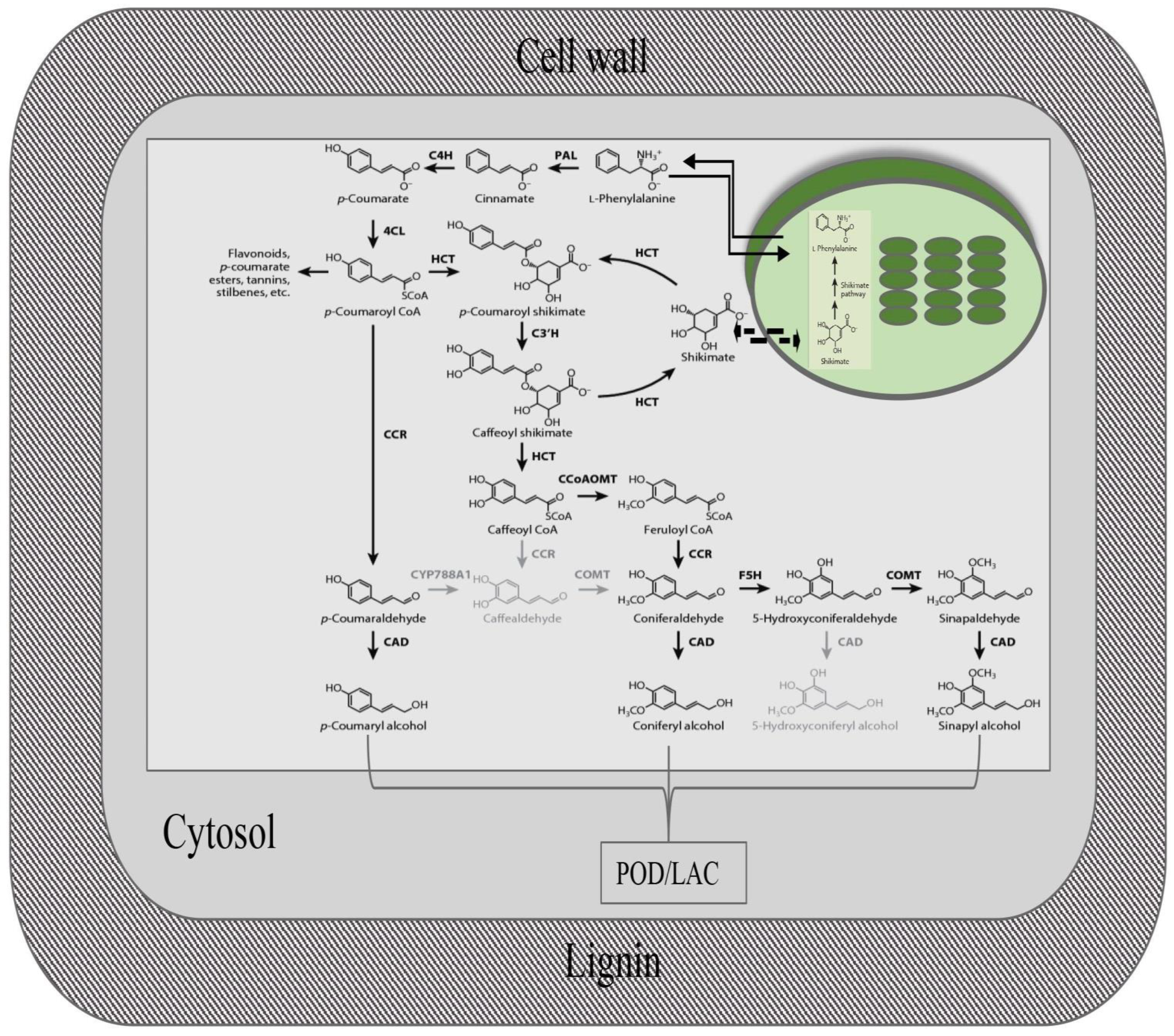
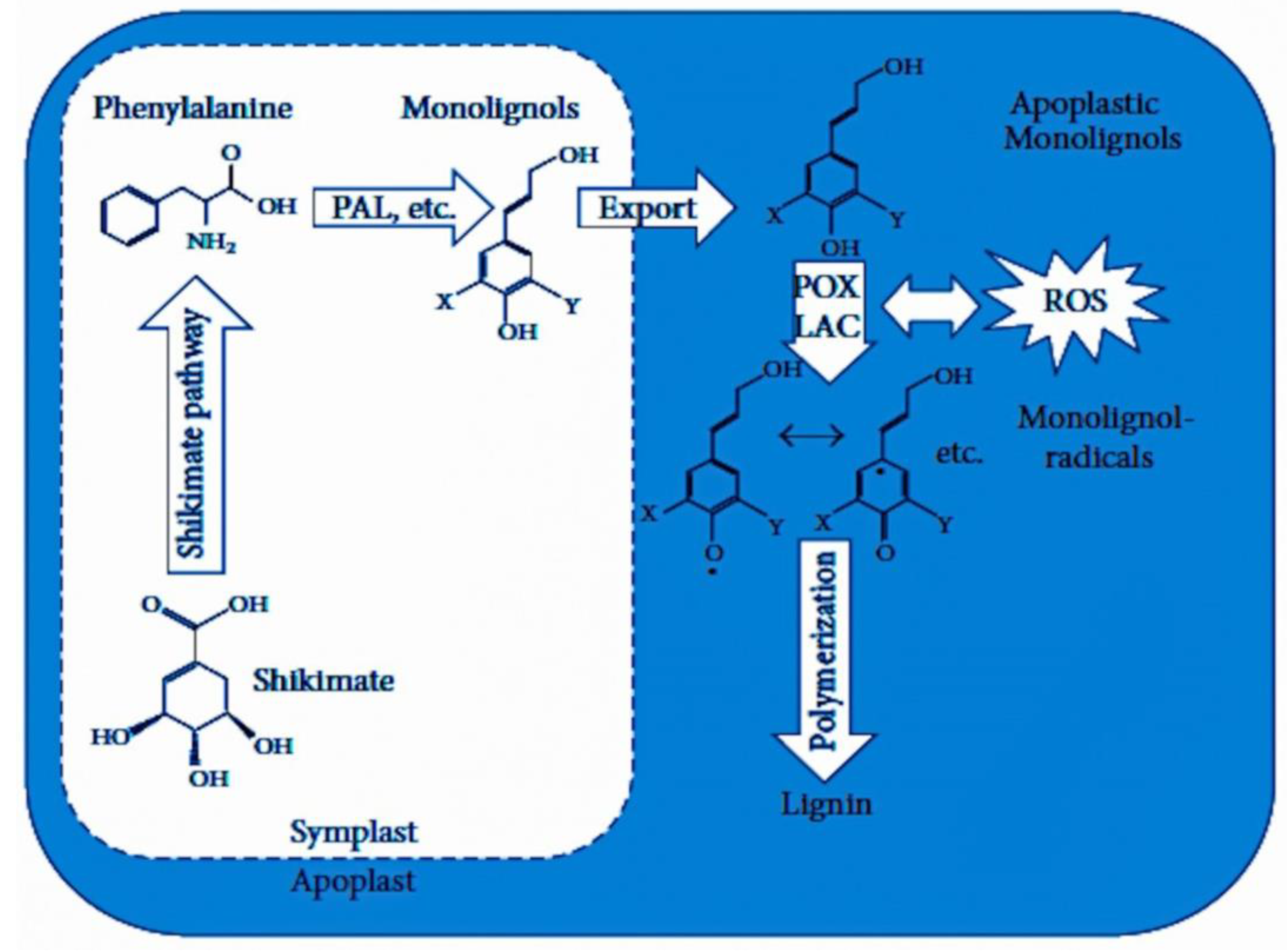
4. Lignin Biosynthesis (Monomers, Transport, and Polymerization)
5. Lignin Decomposition in the Soil Profile
6. Correlation between Lignin Biosynthesis and Plant Stress Response
6.1. Lignin Biosynthesis’s Role in Response to Biotic Stresses (Insect Pests and Diseases)
6.2. Lignin Biosynthesis’ Role in Response to Abiotic Stresses (Drought, Salinity, and Low and High Temperatures)
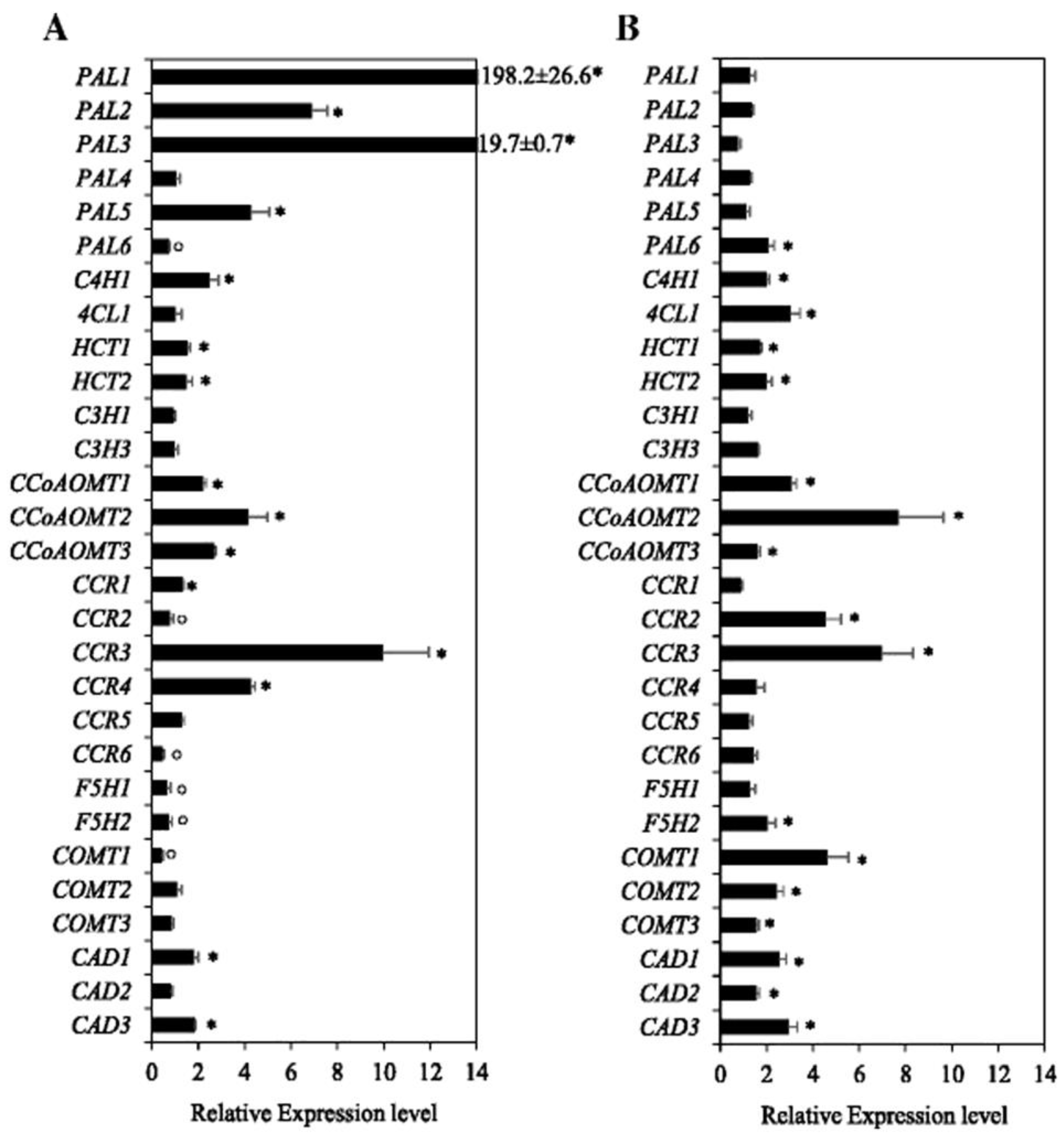
6.3. Gene Expression under Waterlogging, and Molecular Mechanisms for the Control of Lignin Biosynthesis
7. Plant Heavy Metal Stress Tolerance: The Role of Lignin
8. Role of Lignin against Lodging Resistance in Wheat
9. Interrelationship between Cellulose and Sterols Pathways
10. Modification of Lignin Synthesis using Genetic Engineering Technology
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Barros-Rios, J.; Malvar, R.A. Impact of Cell Wall Composition on Maize Resistance to Pests and Diseases. Int. J. Mol. Sci. 2013, 14, 6960–6980. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.C. BREAD WHEAT Improvement and Productiontle; FAO Plant Production and Protection Series; FAO: Rome, Italy, 2002; ISBN 92-5-104809-6. [Google Scholar]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Park, S.-C.; Kim, Y.-H.; Jeong, J.C.; Kim, C.Y.; Lee, H.-S.; Bang, J.-W.; Kwak, S.-S. Sweetpotato Late Embryogenesis Abundant 14 (IbLEA14) Gene Influences Lignification and Increases Osmotic-and Salt Stress-Tolerance of Transgenic Calli. Planta 2011, 233, 621–634. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Mulla, S.I.; Pant, D.; Sharma, T.; Kumar, A. Lignin as Potent Industrial Biopolymer: An Introduction. Lignin, Biosynthesis and Transformation for Industrial Applications; Springer Series on Polymer and Composite Materials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar]
- Bi, C.; Chen, F.; Jackson, L.; Gill, B.S.; Li, W. Expression of Lignin Biosynthetic Genes in Wheat during Development and upon Infection by Fungal Pathogens. Plant Mol. Biol. Rep. 2011, 29, 149–161. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P Stoichiometry in Soil: Is There a “Redfield Ratio” for the Microbial Biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of Their Candidacy for Alternate Renewable Fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Cheng, L.-M.; Liu, Z.-H. Rapid Effect of Cadmium on Lignin Biosynthesis in Soybean Roots. Plant Sci. 2007, 172, 632–639. [Google Scholar] [CrossRef]
- Moura-Sobczak, J.; Souza, U.; Mazzafera, P. Drought Stress and Changes in the Lignin Content and Composition in Eucalyptus. In Proceedings of the BMC Proceedings, Porto Seguro, Brazil, 26 June–2 July 2011; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, p. 1. [Google Scholar]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Ma, R. Erwinia Carotovora Ssp. Carotovora Infection Induced “Defense Lignin” Accumulation and Lignin Biosynthetic Gene Expression in Chinese Cabbage (Brassica Rapa L. Ssp. Pekinensis). J. Integr. Plant Biol. 2007, 49, 993–1002. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Zhang, C.; Li, S.; Huang, Z.; Ruan, W. Synergistic and Pretreatment Effect on Anaerobic Co-Digestion from Rice Straw and Municipal Sewage Sludge. BioResources 2014, 9, 5871–5882. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Lignin in Straw and Its Applications as an Adhesive. Int. J. Adhes. Adhes. 2014, 48, 92–101. [Google Scholar] [CrossRef]
- Ralph, J.; Brunow, G.; Harris, P.J.; Dixon, R.A.; Schatz, P.F.; Boerjan, W. Lignification: Are Lignins Biosynthesized via Simple Combinatorial Chemistry or via Proteinaceous Control and Template Replication. Recent Adv. Polyphen. Res. 2008, 1, 36–66. [Google Scholar]
- Legay, S.; Lacombe, E.; Goicoechea, M.; Briere, C.; Séguin, A.; Mackay, J.; Grima-Pettenati, J. Molecular Characterization of EgMYB1, a Putative Transcriptional Repressor of the Lignin Biosynthetic Pathway. Plant Sci. 2007, 173, 542–549. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H. Lignins: Natural Polymers from Oxidative Coupling of 4-Hydroxyphenyl-Propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- van Parijs, F.R.D.; Morreel, K.; Ralph, J.; Boerjan, W.; Merks, R.M.H. Modeling Lignin Polymerization. I. Simulation Model of Dehydrogenation Polymers. Plant Physiol. 2010, 153, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Vane, C.H.; Abbott, G.D.; Head, I.M. The Effect of Fungal Decay (Agaricus Bisporus) on Wheat Straw Lignin Using Pyrolysis–GC–MS in the Presence of Tetramethylammonium Hydroxide (TMAH). J. Anal. Appl. Pyrolysis 2001, 60, 69–78. [Google Scholar] [CrossRef]
- Vane, C.H.; Drage, T.C.; Snape, C.E. Biodegradation of Oak (Quercus Alba) Wood during Growth of the Shiitake Mushroom (Lentinula Edodes): A Molecular Approach. J. Agric. Food Chem. 2003, 51, 947–956. [Google Scholar] [CrossRef]
- Tanaka, K.; Murata, K.; Yamazaki, M.; Onosato, K.; Miyao, A.; Hirochika, H. Three Distinct Rice Cellulose Synthase Catalytic Subunit Genes Required for Cellulose Synthesis in the Secondary Wall. Plant Physiol. 2003, 133, 73–83. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin Biosynthesis in Wheat (Triticum aestivum L.): Its Response to Waterlogging and Association with Hormonal Levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef]
- Dixon, R.A.; Barros, J. Lignin Biosynthesis: Old Roads Revisited and New Roads Explored. Open Biol. 2019, 9, 190215. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Storme, V.; Vanholme, B.; Sundin, L.; Christensen, J.H.; Goeminne, G.; Halpin, C.; Rohde, A.; Morreel, K.; Boerjan, W. A Systems Biology View of Responses to Lignin Biosynthesis Perturbations in Arabidopsis. Plant Cell 2012, 24, 3506–3529. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Deshmukh, P.S.; Srivastava, G.C. Physiology and Biochemistry of Waterlogging Tolerance in Plants. Biol. Plant. 2008, 52, 401. [Google Scholar] [CrossRef]
- Richards, E.H.; Norman, A.G. The Biological Decomposition of Plant Materials: Some Factors Determining the Quantity of Nitrogen Immobilised during Decomposition. Biochem. J. 1931, 25, 1769–1778. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen Deficiency and Root Metabolism: Injury and Acclimation under Hypoxia and Anoxia. Annu. Rev. Plant Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Van deáWouwer, D. A Click Chemistry Strategy for Visualization of Plant Cell Wall Lignification. Chem. Commun. 2014, 50, 12262–12265. [Google Scholar]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer Lignins: Harnessing the Plasticity of Lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Frei, M. Lignin: Characterization of a Multifaceted Crop Component. Sci. World J. 2013, 2013, 436517. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Berstis, L.; Elder, T.; Crowley, M.; Beckham, G.T. Radical Nature of C-Lignin. ACS Sustain. Chem. Eng. 2016, 4, 5327–5335. [Google Scholar] [CrossRef]
- Sangha, A.K.; Davison, B.H.; Standaert, R.F.; Davis, M.F.; Smith, J.C.; Parks, J.M. Chemical Factors That Control Lignin Polymerization. J. Phys. Chem. B 2014, 118, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Jensen, L.S.; Nielsen, N.E.; Magid, J. Turnover of Carbon and Nitrogen in a Sandy Loam Soil Following Incorporation of Chopped Maize Plants, Barley Straw and Blue Grass in the Field. Soil Biol. Biochem. 1998, 30, 561–571. [Google Scholar] [CrossRef]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant Cell Wall Lignification and Monolignol Metabolism. Front. Plant Sci. 2013, 4, 220. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Miao, Y.-C.; Zhang, K.-W. Sequestration and Transport of Lignin Monomeric Precursors. Molecules 2011, 16, 710–727. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Lewis, N.; Davin, L.; Sarkanen, S. The Nature and Function of Lignins. In Comprehensive Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 1999; Volume 3, pp. 617–745. [Google Scholar]
- Sieling, K.; Kage, H. N Balance as an Indicator of N Leaching in an Oilseed Rape–Winter Wheat–Winter Barley Rotation. Agric. Ecosyst. Environ. 2006, 115, 261–269. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Shi, Y.; Li, Y.; Yin, Y.; Yang, D.; Luo, Y.; Pang, D.; Xu, X.; Li, W. Manipulation of Lignin Metabolism by Plant Densities and Its Relationship with Lodging Resistance in Wheat. Sci. Rep. 2017, 7, 41805. [Google Scholar] [CrossRef]
- Demirbas, A. Adsorption of Lead and Cadmium Ions in Aqueous Solutions onto Modified Lignin from Alkali Glycerol Delignication. J. Hazard. Mater. 2004, 109, 221–226. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are Microorganisms More Effective than Plants at Competing for Nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Cheshire, M.V.; Bedrock, C.N.; Williams, B.L.; Chapman, S.J.; Solntseva, I.; Thomsen, I. The Immobilization of Nitrogen by Straw Decomposing in Soil. Eur. J. Soil Sci. 1999, 50, 329–341. [Google Scholar] [CrossRef]
- Henke, J.; Böttcher, U.; Neukam, D.; Sieling, K.; Kage, H. Evaluation of Different Agronomic Strategies to Reduce Nitrate Leaching after Winter Oilseed Rape (Brassica napus L.) Using a Simulation Model. Nutr. Cycl. Agroecosystems 2008, 82, 299–314. [Google Scholar] [CrossRef]
- Turlapati, P.V.; Kim, K.-W.; Davin, L.B.; Lewis, N.G. The Laccase Multigene Family in Arabidopsis Thaliana: Towards Addressing the Mystery of Their Gene Function (S). Planta 2011, 233, 439–470. [Google Scholar] [CrossRef]
- Mielenz, J.R. Ethanol Production from Biomass: Technology and Commercialization Status. Curr. Opin. Microbiol. 2001, 4, 324–329. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, L.; Zhang, H.; Du, X.; An, C.; Xia, X.; Chen, F.; Jiang, J.; Chen, S. CmMYB19 Over-Expression Improves Aphid Tolerance in Chrysanthemum by Promoting Lignin Synthesis. Int. J. Mol. Sci. 2017, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, L.; He, F.; Zhao, F.-J.; Shen, Z.; Zheng, L. Transcriptional and Physiological Analyses Identify a Regulatory Role for Hydrogen Peroxide in the Lignin Biosynthesis of Copper-Stressed Rice Roots. Plant Soil 2015, 387, 323–336. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and Biotic Stresses and Changes in the Lignin Content and Composition in Plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Schutzendubel, A.; Polle, A. Plant Responses to Abiotic Stresses: Heavy Metal-induced Oxidative Stress and Protection by Mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Morel, O.; Spriet, C.; Lion, C.; Baldacci-Cresp, F.; Pontier, G.; Baucher, M.; Biot, C.; Hawkins, S.; Neutelings, G. Chapter 21 Ratiometric Fluorescent Safranin-O Staining Allows the Quantification of Lignin Contents In Muro. In Histochemistry of Single Molecules. Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Hu, D.; Liu, X.B.; She, H.Z.; Gao, Z.; Ruan, R.W.; Wu, D.Q.; Yi, Z.L. The Lignin Synthesis Related Genes and Lodging Resistance of Fagopyrum Esculentum. Biol. Plant. 2017, 61, 138–146. [Google Scholar] [CrossRef]
- Jannoey, P.; Pongprasert, W.; Lumyong, S.; Roytrakul, S.; Nomura, M. Comparative Proteomic Analysis of Two Rice Cultivars (‘Oryza sativa’ L.) Contrasting in Brown Planthopper (BPH) Stress Resistance. Plant Omics 2015, 8, 96–105. [Google Scholar]
- Kärkönen, A.; Koutaniemi, S. Lignin Biosynthesis Studies in Plant Tissue Cultures. J. Integr. Plant Biol. 2010, 52, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The Role of the Secondary Cell Wall in Plant Resistance to Pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef]
- Mandal, S.; Kar, I.; Mukherjee, A.K.; Acharya, P. Elicitor-Induced Defense Responses in Solanum Lycopersicum against Ralstonia Solanacearum. Sci. World J. 2013, 2013, 561056. [Google Scholar] [CrossRef]
- Ma, Q.-H.; Zhu, H.-H.; Han, J.-Q. Wheat ROP Proteins Modulate Defense Response through Lignin Metabolism. Plant Sci. 2017, 262, 32–38. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Shukla, P.S.; Gupta, K.; Jha, B. Bioengineering for Salinity Tolerance in Plants: State of the Art. Mol. Biotechnol. 2013, 54, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Mafa, M.S.; Rufetu, E.; Alexander, O.; Kemp, G.; Mohase, L. Cell-Wall Structural Carbohydrates Reinforcements Are Part of the Defence Mechanisms of Wheat against Russian Wheat Aphid (Diuraphis Noxia) Infestation. Plant Physiol. Biochem. 2022, 179, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging Resistance of Winter Wheat (Triticum Aestivum L.): Lignin Accumulation and Its Related Enzymes Activities Due to the Application of Paclobutrazol or Gibberellin Acid. Field Crop. Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Sharma, N.K.; Gupta, S.K.; Irulappan, V.; Yadav, S. MicroRNA397 Regulates Tolerance to Drought and Fungal Infection by Regulating Lignin Deposition in Chickpea Root. bioRxiv 2022. [Google Scholar]
- Bonello, P.; Blodgett, J.T. Pinus Nigra–Sphaeropsis Sapinea as a Model Pathosystem to Investigate Local and Systemic Effects of Fungal Infection of Pines. Physiol. Mol. Plant Pathol. 2003, 63, 249–261. [Google Scholar] [CrossRef]
- Moerschbacher, B.M.; Noll, U.; Gorrichon, L.; Reisener, H.-J. Specific Inhibition of Lignification Breaks Hypersensitive Resistance of Wheat to Stem Rust. Plant Physiol. 1990, 93, 465–470. [Google Scholar] [CrossRef]
- Menden, B.; Kohlhoff, M.; Moerschbacher, B.M. Wheat Cells Accumulate a Syringyl-Rich Lignin during the Hypersensitive Resistance Response. Phytochemistry 2007, 68, 513–520. [Google Scholar] [CrossRef]
- Kofalvi, S.A.; Nassuth, A. Influence of Wheat Streak Mosaic Virus Infection on Phenylpropanoid Metabolism and the Accumulation of Phenolics and Lignin in Wheat. Physiol. Mol. Plant Pathol. 1995, 47, 365–377. [Google Scholar] [CrossRef]
- Parrott, D.L.; Anderson, A.J.; Carman, J.G. Agrobacterium Induces Plant Cell Death in Wheat (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2002, 60, 59–69. [Google Scholar] [CrossRef]
- Smith, A.H.; Gill, W.M.; Pinkard, E.A.; Mohammed, C.L. Anatomical and Histochemical Defence Responses Induced in Juvenile Leaves of Eucalyptus Globulus and Eucalyptus Nitens by Mycosphaerella Infection. For. Pathol. 2007, 37, 361–373. [Google Scholar] [CrossRef]
- Lauvergeat, V.; Lacomme, C.; Lacombe, E.; Lasserre, E.; Roby, D.; Grima-Pettenati, J. Two Cinnamoyl-CoA Reductase (CCR) Genes from Arabidopsis Thaliana Are Differentially Expressed during Development and in Response to Infection with Pathogenic Bacteria. Phytochemistry 2001, 57, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Solla, A.; Woodward, S.; Gil, L. Detection of Differential Changes in Lignin Composition of Elm Xylem Tissues Inoculated with Ophiostoma Novo-ulmi Using Fourier Transform-infrared Spectroscopy. For. Pathol. 2007, 37, 187–191. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Bensaddek, L.; Crônier, D.; Baltora-Rosset, S.; Doussot, J.; Maury, S.; Mesnard, F.; Chabbert, B.; Hawkins, S. Differential Accumulation of Monolignol-Derived Compounds in Elicited Flax (Linum Usitatissimum) Cell Suspension Cultures. Planta 2006, 223, 975–989. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Liu, W.; Liu, G.; Selvaraj, G.; Wei, Y.; King, J. Transcriptional Regulation of Genes Involved in the Pathways of Biosynthesis and Supply of Methyl Units in Response to Powdery Mildew Attack and Abiotic Stresses in Wheat. Plant Mol. Biol. 2007, 64, 305–318. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Cathala, B.; Monties, B. Influence of Pectins on the Solubility and the Molar Mass Distribution of Dehydrogenative Polymers (DHPs, Lignin Model Compounds). Int. J. Biol. Macromol. 2001, 29, 45–51. [Google Scholar] [CrossRef]
- Fan, L.; Linker, R.; Gepstein, S.; Tanimoto, E.; Yamamoto, R.; Neumann, P.M. Progressive Inhibition by Water Deficit of Cell Wall Extensibility and Growth along the Elongation Zone of Maize Roots Is Related to Increased Lignin Metabolism and Progressive Stelar Accumulation of Wall Phenolics. Plant Physiol. 2006, 140, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Vishwakarma, R.K.; Arafat, Y.A.; Gupta, S.K.; Khan, B.M. Abiotic Stress Induces Change in Cinnamoyl CoA Reductase (CCR) Protein Abundance and Lignin Deposition in Developing Seedlings of Leucaena Leucocephala. Physiol. Mol. Biol. Plants 2015, 21, 197–205. [Google Scholar] [CrossRef]
- Kováčik, J.; Grúz, J.; Klejdus, B.; Štork, F.; Marchiosi, R.; Ferrarese-Filho, O. Lignification and Related Parameters in Copper-Exposed Matricaria Chamomilla Roots: Role of H2O2 and NO in This Process. Plant Sci. 2010, 179, 383–389. [Google Scholar] [CrossRef]
- Jain, R.; Singh, A.; Singh, P.; Solomon, S.; Tripathi, P.; Chandra, A.; Solomon, S. Study on physio-biochemical attributes and metallothionein geneexpression affected by chromium (VI) in sugarcane (Saccharum spp. hybrid). J. Environ. Biol. 2016, 37, 375. [Google Scholar]
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX Genes Positively Regulates Secondary Cell Wall Biosynthesis and Promotes Plant Growth and Yield in Arabidopsis under Salt Stress. Plant Mol. Biol. 2015, 87, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Wang, L.; Hu, P.; Wang, Y.; Jia, Y.; Zhang, C.; Zhang, Y.; Zhang, Y.; Wang, C. Expression of the MYB Transcription Factor Gene Bpl MYB 46 Affects Abiotic Stress Tolerance and Secondary Cell Wall Deposition in Betula Platyphylla. Plant Biotechnol. J. 2017, 15, 107–121. [Google Scholar] [CrossRef]
- Christensen, J.H.; Christensen, O.B. A Summary of the PRUDENCE Model Projections of Changes in European Climate by the End of This Century. Clim. Chang. 2007, 81, 7–30. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of Temperature Stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Zhang, X.; Gou, M.; Liu, C.-J. Arabidopsis Kelch Repeat F-Box Proteins Regulate Phenylpropanoid Biosynthesis via Controlling the Turnover of Phenylalanine Ammonia-Lyase. Plant Cell 2013, 25, 4994–5010. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.; Zhang, J.; Ge, H.; Yin, X.; Chen, K. Regulation of Loquat Fruit Low Temperature Response and Lignification Involves Interaction of Heat Shock Factors and Genes Associated with Lignin Biosynthesis. Plant. Cell Environ. 2016, 39, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tobimatsu, Y.; Zhou, R.; Pattathil, S.; Gallego-Giraldo, L.; Fu, C.; Jackson, L.A.; Hahn, M.G.; Kim, H.; Chen, F. Loss of Function of Cinnamyl Alcohol Dehydrogenase 1 Leads to Unconventional Lignin and a Temperature-Sensitive Growth Defect in Medicago Truncatula. Proc. Natl. Acad. Sci. USA 2013, 110, 13660–13665. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, Y.; Wang, R.; Zhang, J.; Owens, G. Cadmium Adsorption by Willow Root: The Role of Cell Walls and Their Subfractions. Environ. Sci. Pollut. Res. 2013, 20, 5665–5672. [Google Scholar] [CrossRef]
- Whetten, R.; Sederoff, R. Lignin Biosynthesis. Plant Cell 1995, 7, 1001. [Google Scholar] [CrossRef] [PubMed]
- Raes, J.; Rohde, A.; Christensen, J.H.; Van de Peer, Y.; Boerjan, W. Genome-Wide Characterization of the Lignification Toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Riabova, A.; Skrypnik, L.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Assessment of the Role of Pal in Lignin Accumulation in Wheat (Tríticum aestívum L.) at the Early Stage of Ontogenesis. Int. J. Mol. Sci. 2021, 22, 9848. [Google Scholar] [CrossRef] [PubMed]
- Gindl, W.; Grabner, M.; Wimmer, R. The Influence of Temperature on Latewood Lignin Content in Treeline Norway Spruce Compared with Maximum Density and Ring Width. Trees 2000, 14, 409–414. [Google Scholar] [CrossRef]
- Akgül, M.; Çöpür, Y.; Temiz, S. A Comparison of Kraft and Kraft-Sodium Borohydrate Brutia Pine Pulps. Build. Environ. 2007, 42, 2586–2590. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Ghanati, F.; Morita, A.; Yokota, H. Deposition of Suberin in Roots of Soybean Induced by Excess Boron. Plant Sci. 2005, 168, 397–405. [Google Scholar] [CrossRef]
- Constabel, C.P.; Yoshida, K.; Walker, V. Diverse Ecological Roles of Plant Tannins: Plant Defense and Beyond. Recent Adv. Polyphen. Res. 2014, 4, 115–142. [Google Scholar]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. In The Arabidopsis Book/American Society Plant Biologist; National Library of Medicine: Bethesda, MD, USA, 2011; Volume 9. [Google Scholar]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Eloy, N.B.; Voorend, W.; Lan, W.; de Lyra Soriano Saleme, M.; Cesarino, I.; Vanholme, R.; Smith, R.A.; Goeminne, G.; Pallidis, A.; Morreel, K.; et al. Silencing CHALCONE SYNTHASE in Maize Impedes the Incorporation of Tricin into Lignin and Increases Lignin Content. Plant Physiol. 2017, 173, 998–1016. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory Networks of Cadmium Stress in Plants. Plant Signal. Behav. 2010, 5, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Bačkor, M. Phenylalanine Ammonia-Lyase and Phenolic Compounds in Chamomile Tolerance to Cadmium and Copper Excess. Water. Air Soil Pollut. 2007, 185, 185–193. [Google Scholar] [CrossRef]
- Tahara, K.; Norisada, M.; Hogetsu, T.; Kojima, K. Aluminum Tolerance and Aluminum-Induced Deposition of Callose and Lignin in the Root Tips of Melaleuca and Eucalyptus Species. J. For. Res. 2005, 10, 325–333. [Google Scholar] [CrossRef]
- Lima, J.D.; Mazzafera, P.; da Silva Moraes, W.; da Silva, R.B. Aspectos Relacionados à Qualidade e Perspectivas. Ciência Rural 2009, 39, 1258–1266. [Google Scholar] [CrossRef]
- Mao, C.; Yi, K.; Yang, L.; Zheng, B.; Wu, Y.; Liu, F.; Wu, P. Identification of Aluminium-regulated Genes by CDNA-AFLP in Rice (Oryza sativa L.): Aluminium-regulated Genes for the Metabolism of Cell Wall Components. J. Exp. Bot. 2004, 55, 137–143. [Google Scholar] [CrossRef]
- Gao, L.; Peng, K.; Chen, Y.; Wang, G.; Shen, Z. Roles of Apoplastic Peroxidases, Laccases, and Lignification in the Manganese Tolerance of Hyperaccumulator Phytolacca Americana. Acta Physiol. Plant. 2012, 34, 151–159. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chen, L.-M.; Liu, Z.-H. Rapid Effect of Copper on Lignin Biosynthesis in Soybean Roots. Plant Sci. 2005, 168, 855–861. [Google Scholar] [CrossRef]
- van de Mortel, J.E.; Schat, H.; Moerland, P.D.; Van Themaat, E.V.L.; Van Der Ent, S.; Blankestijn, H.; Ghandilyan, A.; Tsiatsiani, S.; Aarts, M.G.M. Expression Differences for Genes Involved in Lignin, Glutathione and Sulphate Metabolism in Response to Cadmium in Arabidopsis Thaliana and the Related Zn/Cd-hyperaccumulator Thlaspi Caerulescens. Plant Cell Environ. 2008, 31, 301–324. [Google Scholar] [CrossRef]
- Ederli, L.; Reale, L.; Ferranti, F.; Pasqualini, S. Responses Induced by High Concentration of Cadmium in Phragmites Australis Roots. Physiol. Plant. 2004, 121, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, J.; Li, D.; Zhu, C. Comparative Transcriptome Combined with Morpho-physiological Analyses Revealed Key Factors for Differential Cadmium Accumulation in Two Contrasting Sweet Sorghum Genotypes. Plant Biotechnol. J. 2018, 16, 558–571. [Google Scholar] [CrossRef]
- Legay, S.; Sivadon, P.; Blervacq, A.; Pavy, N.; Baghdady, A.; Tremblay, L.; Levasseur, C.; Ladouce, N.; Lapierre, C.; Séguin, A. EgMYB1, an R2R3 MYB Transcription Factor from Eucalyptus Negatively Regulates Secondary Cell Wall Formation in Arabidopsis and Poplar. New Phytol. 2010, 188, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, N.; Nakamura, T.; Komatsu, S. Differential Responses of Microsomal Proteins and Metabolites in Two Contrasting Cadmium (Cd)-Accumulating Soybean Cultivars under Cd Stress. Amino Acids 2012, 42, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.M.; Sterling, M.; Spink, J.H.; Baker, C.J.; Sylvester-Bradley, R.; Mooney, S.J.; Tams, A.R.; Ennos, A.R. Understanding and Reducing Lodging in Cereals. Adv. Agron. 2004, 84, 215–269. [Google Scholar]
- Islam, M.S.; Peng, S.; Visperas, R.M.; Ereful, N.; Bhuiya, M.S.U.; Julfiquar, A.W. Lodging-Related Morphological Traits of Hybrid Rice in a Tropical Irrigated Ecosystem. Field Crop. Res. 2007, 101, 240–248. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, Y. Rice Brittleness Mutants: A Way to Open the ‘Black Box’of Monocot Cell Wall Biosynthesis Free Access. J. Integr. Plant Biol. 2011, 53, 136–142. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Sayre, K.D.; Kaul, J.N.; Narang, R.S. Growth and Morphology of Spring Wheat (Triticum aestivum L.) Culms and Their Association with Lodging: Effects of Genotypes, N Levels and Ethephon. Field Crop. Res. 2003, 84, 271–290. [Google Scholar] [CrossRef]
- Kuai, J.; Sun, Y.; Zhou, M.; Zhang, P.; Zuo, Q.; Wu, J.; Zhou, G. The Effect of Nitrogen Application and Planting Density on the Radiation Use Efficiency and the Stem Lignin Metabolism in Rapeseed (Brassica napus L.). Field Crop. Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Ding, Y.; Yao, X.; Wu, X.; Weng, F.; Li, G.; Liu, Z.; Tang, S.; Ding, C. Nitrogen Fertilizer Application Affects Lodging Resistance by Altering Secondary Cell Wall Synthesis in Japonica Rice (Oryza sativa). J. Plant Res. 2017, 130, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bouchabke-Coussa, O.; Lebris, P.; Antelme, S.; Soulhat, C.; Gineau, E.; Dalmais, M.; Bendahmane, A.; Morin, H.; Mouille, G. LACCASE5 Is Required for Lignification of the Brachypodium Distachyon Culm. Plant Physiol. 2015, 168, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Sun, M.; Wang, F.; Liu, J.; Feng, B.; Si, J.; Zhang, B.; Li, S.; Li, H. Effects of High NH4+ on K+ Uptake, Culm Mechanical Strength and Grain Filling in Wheat. Front. Plant Sci. 2014, 5, 703. [Google Scholar] [CrossRef]
- Simmons, B.A.; Loqué, D.; Ralph, J. Advances in Modifying Lignin for Enhanced Biofuel Production. Curr. Opin. Plant Biol. 2010, 13, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Hao, H.; Xue, Y.; Alam, A.; Bai, S.; Hu, W.; Sajid, M.; Hu, Z.; Samad, R.A.; Li, Z. Survey of Wheat Straw Stem Characteristics for Enhanced Resistance to Lodging. Cellulose 2020, 27, 2469–2484. [Google Scholar] [CrossRef]
- Madina, B.R.; Sharma, L.K.; Chaturvedi, P.; Sangwan, R.S.; Tuli, R. Purification and Physico-Kinetic Characterization of 3β-Hydroxy Specific Sterol Glucosyltransferase from Withania somnifera (L) and Its Stress Response. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2007, 1774, 392–402. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Mishra, M.; Akhtar, N.; Gupta, P.; Mishra, P.; Tuli, R. Sterol Glycosyltransferases-Identification of Members of Gene Family and Their Role in Stress in Withania Somnifera. Mol. Biol. Rep. 2012, 39, 9755–9764. [Google Scholar] [CrossRef]
- Madina, B.R.; Sharma, L.K.; Chaturvedi, P.; Sangwan, R.S.; Tuli, R. Purification and Characterization of a Novel Glucosyltransferase Specific to 27β-Hydroxy Steroidal Lactones from Withania Somnifera and Its Role in Stress Responses. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 2007, 1774, 1199–1207. [Google Scholar] [CrossRef]
- Sharma, L.K.; Madina, B.R.; Chaturvedi, P.; Sangwan, R.S.; Tuli, R. Molecular Cloning and Characterization of One Member of 3β-Hydroxy Sterol Glucosyltransferase Gene Family in Withania Somnifera. Arch. Biochem. Biophys. 2007, 460, 48–55. [Google Scholar] [CrossRef]
- He, J.-X.; Fujioka, S.; Li, T.-C.; Kang, S.G.; Seto, H.; Takatsuto, S.; Yoshida, S.; Jang, J.-C. Sterols Regulate Development and Gene Expression in Arabidopsis. Plant Physiol. 2003, 131, 1258–1269. [Google Scholar] [CrossRef]
- Peng, L.; Kawagoe, Y.; Hogan, P.; Delmer, D. Sitosterol-β-Glucoside as Primer for Cellulose Synthesis in Plants. Science 2002, 295, 147–150. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Wagner, A.; Donaldson, L.; Kim, H.; Phillips, L.; Flint, H.; Steward, D.; Torr, K.; Koch, G.; Schmitt, U.; Ralph, J. Suppression of 4-Coumarate-CoA Ligase in the Coniferous Gymnosperm Pinus Radiata. Plant Physiol. 2009, 149, 370–383. [Google Scholar] [CrossRef]
- Rana, R.; Nanda, S.; Meda, V.; Dalai, A.K.; Kozinski, J.A. A Review of Lignin Chemistry and Its Biorefining Conversion Technologies. J. Biochem. Eng. Bioprocess. Technol. 2018, 1, 2. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, M.W.; Yousaf, M.I.; Hussain, Q.; Yasir, M.; Sajjad, M.; Shah, L. Role of Lignin in Wheat Plant for the Enhancement of Resistance against Lodging and Biotic and Abiotic Stresses. Stresses 2023, 3, 434-453. https://doi.org/10.3390/stresses3020032
Riaz MW, Yousaf MI, Hussain Q, Yasir M, Sajjad M, Shah L. Role of Lignin in Wheat Plant for the Enhancement of Resistance against Lodging and Biotic and Abiotic Stresses. Stresses. 2023; 3(2):434-453. https://doi.org/10.3390/stresses3020032
Chicago/Turabian StyleRiaz, Muhammad Waheed, Muhammad Irfan Yousaf, Quaid Hussain, Muhammad Yasir, Muhammad Sajjad, and Liaqat Shah. 2023. "Role of Lignin in Wheat Plant for the Enhancement of Resistance against Lodging and Biotic and Abiotic Stresses" Stresses 3, no. 2: 434-453. https://doi.org/10.3390/stresses3020032
APA StyleRiaz, M. W., Yousaf, M. I., Hussain, Q., Yasir, M., Sajjad, M., & Shah, L. (2023). Role of Lignin in Wheat Plant for the Enhancement of Resistance against Lodging and Biotic and Abiotic Stresses. Stresses, 3(2), 434-453. https://doi.org/10.3390/stresses3020032










