Role of Hormones and the Potential Impact of Multiple Stresses on Infertility
Abstract
1. Introduction
2. Possible Mediators of Infertility
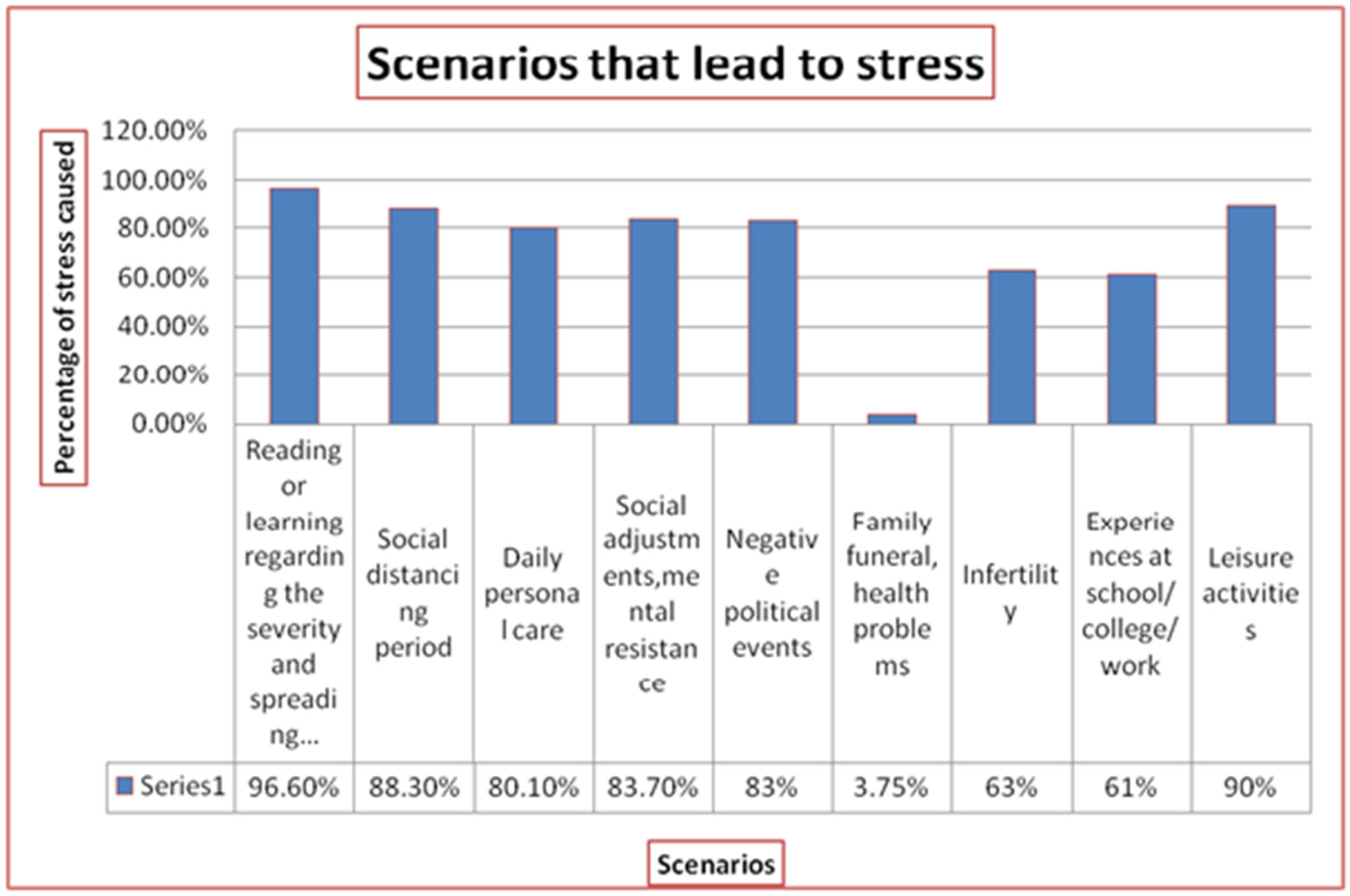
2.1. Psychological Stress
2.2. Emotional Stress
2.3. Metabolic Stress
2.4. Oxidative Stress
2.5. Preconception Stress
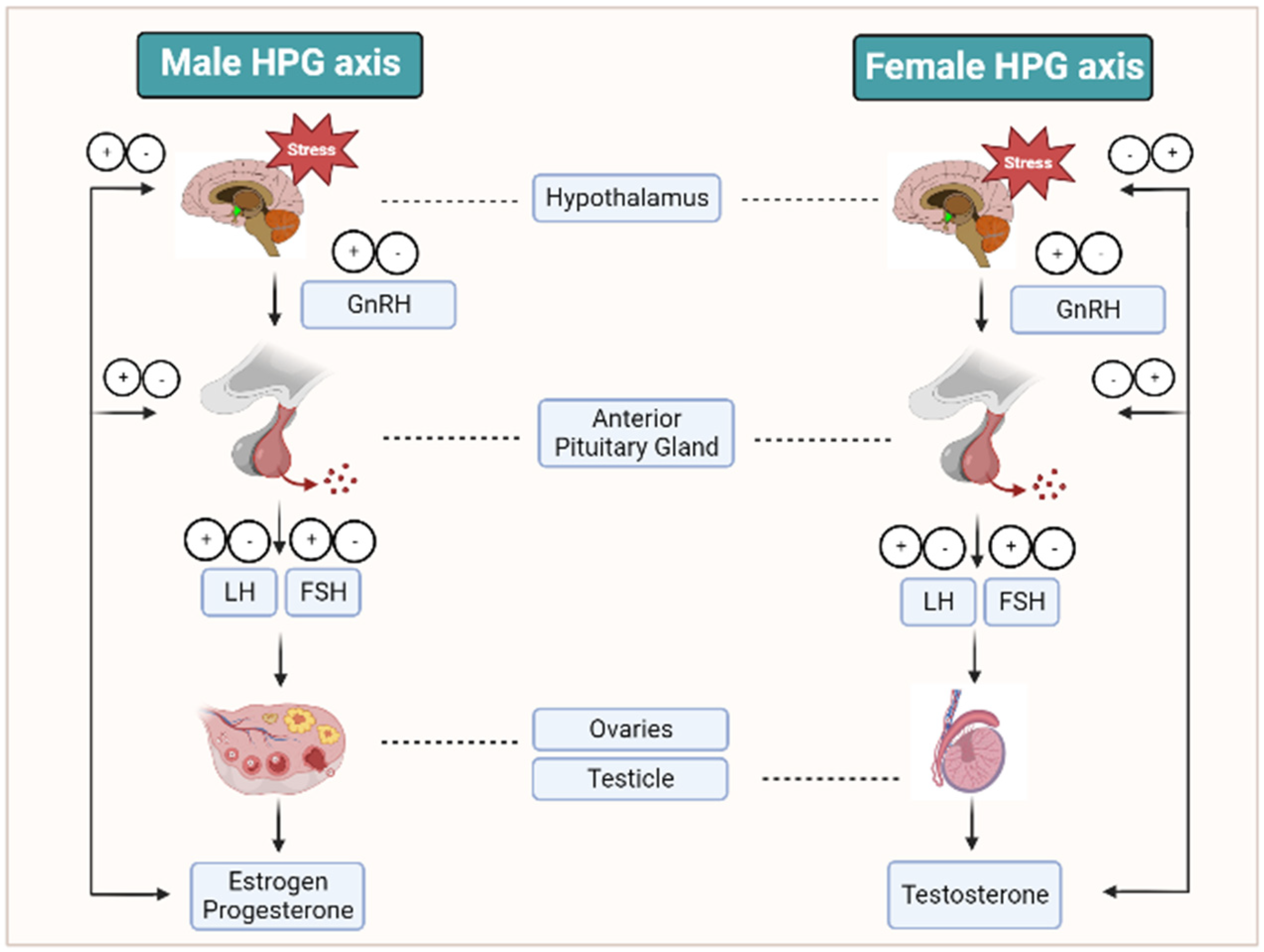
3. Reproductive Hormonal Changes during Stress and Infertility Conditions
3.1. GnRh
3.2. Inhibin
3.3. Testosterone
3.4. FSH
3.5. LH
3.6. Prolactin
3.7. Estrogen
3.8. Progesterone
3.9. Anti-Mullerian Hormone (AMH)
3.10. Prolactin
3.11. Thyroid Hormones
4. Various Biological Mechanisms Related to Stress That Leads to Infertility
5. Stress and Other Reproductive Ailments
6. Impact of Stress on Infertility Treatment
7. Factors That Cause Decreased Infertility Rate
7.1. Nutrition
7.2. Smoking
7.3. Caffeine Consumption
7.4. Gadgets
7.5. Age
7.6. Marijuana
7.7. Obesity
7.8. Stress
7.9. Anxiety
7.10. Depression
8. Infertility Management
8.1. Supplementations
8.2. Health Management
8.3. Therapeutics
8.4. Psychological Support
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aldhuwayhi, S.; Shaikh, S.A.; Mallineni, S.K.; Kumari, V.V.; Thakare, A.A.; Ahmed Khan, A.R.; Mustafa, M.Z.; Manva, M.Z. Occupational Stress and Stress Busters Used Among Saudi Dental Practitioners During the COVID-19 Pandemic Outbreak. Disaster Med. Public Health Prep. 2022, 16, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Singh, A.; Singh, A.K.; Sharma, V.; Kotwal, A. Coping Strategies and Perception toward Drugs, Electronic Gadgets, and Media in Relation to Stress: A Cross-sectional Study among Residents of a Suburban Area. Indian J. Community Med. 2021, 46, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol, K.; Tigges, J.; Freundl, G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005, 20, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef]
- Chu, K.Y.; Patel, P.; Ramasamy, R. Consideration of gender differences in infertility evaluation. Curr. Opin. Urol. 2019, 29, 267–271. [Google Scholar] [CrossRef]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Wu, J.X.; Lin, S.; Kong, S.B. Psychological Stress and Functional Endometrial Disorders: Update of Mechanism Insights. Front. Endocrinol. 2021, 3, 12–690255. [Google Scholar] [CrossRef]
- Lei, A.; You, H.; Luo, B.; Ren, J. The associations between infertility-related stress, family adaptability, and family cohesion in infertile couples. Sci. Rep. 2021, 11, 24220. [Google Scholar] [CrossRef]
- Dobson, H.; Smith, R.F. What is stress, and how does it affect reproduction? Anim. Reprod. Sci. 2000, 60–61, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Dragoş, D.; Tănăsescu, M.D. The effect of stress on the defense systems. J. Med. Life 2010, 3, 10–18. [Google Scholar]
- Mintziori, G.; Duntas, L.H.; Veneti, S.; Goulis, D.G. Metabolic, oxidative and psychological stress as mediators of the effect of COVID-19 on male infertility: A literature review. Int. J. Environ. Res. Public Health 2022, 19, 5277. [Google Scholar] [CrossRef]
- Brauner, E.V.; Nordkap, L.; Priskorn, L.; Hansen, A.M.; Bang, A.K.; Holmboe, S.A.; Schmidt, L.; Jensen, T.K.; Jorgensen, N. Psychological stress, stressful life events, male factor infertility, and testicular function: A cross-sectional study. Fertil. Steril. 2020, 113, 865–875. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.; Laganà, A.S. Psychology of infertility and assisted reproductive treatment: The Italian situation. J. Psychosom. Obstet. Gynaecol. 2017, 38, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Negris, O.; Lawson, A.; Brown, D.; Warren, C.; Galic, I.; Bozen, A.; Swanson, A.; Jain, T. Emotional stress and reproduction: What do fertility patients believe? J. Assist. Reprod. Genet. 2021, 38, 877–887. [Google Scholar] [CrossRef]
- Teklemicheal, A.G.; Kassa, E.M.; Weldetensaye, E.K. Prevalence and correlates of infertility related psychological stress in women with infertility: A cross-sectional hospital-based survey. BMC Psychol. 2022, 10, 91. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R. The international glossary on Infertility and fertility care. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Westerman, R.; Kuhnt, A.K. Metabolic risk factors and fertility disorders: A narrative review of the female perspective. Reprod. Biomed. Online 2022, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, H.; Pan, P.; Yang, D.; Zhang, Q. Impact of central obesity on women with polycystic ovary syndrome undergoing in vitro fertilization. Biores. Open Access 2018, 7, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Rajkhowa, M. Effect of obesity on assisted reproductive treatment outcomes and its management: A literature review. Obstet. Gynecol. 2017, 19, 47–54. [Google Scholar] [CrossRef]
- Boutari, C.; Pappas, P.D.; Mintziori, G.; Nigdelis, M.P.; Athanasiadis, L.; Goulis, D.G.; Mantzoros, C.S. The effect of underweight on female and male reproduction. Metabolism 2020, 107, 154229. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Kshetrimayum, C. Environmental & occupational exposure & female reproductive dysfunction. Indian J. Med. Res. 2019, 150, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Bruno, C.; Vergani, E.; d’Abate, C.; Giacchi, E.; Silvestrini, A. Oxidative Stress and Low-Grade Inflammation in Polycystic Ovary Syndrome: Controversies and New Insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Liu, H.; Bai, H.; Hu, K.; Zhang, R.; Liu, Q.; Fan, P. Oxidative stress promotes hyperandrogenism by reducing sex hormone-binding globulin in polycystic ovary syndrome. Fertil. Steril. 2021, 116, 1641–1650. [Google Scholar] [CrossRef]
- Wunder, D.; Kretschmer, R.; Bersinger, N.A. Concentrations of leptin and C-reactive protein in serum and follicular fluid during assisted reproductive cycles. Hum. Reprod. 2005, 20, 1266–1271. [Google Scholar] [CrossRef]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef]
- Din, H.N.; Strong, D.; Singh-Carlson, S.; Corliss, H.L.; Hartman, S.J.; Madanat, H.; Su, H.I. Association between pregnancy intention and preconception health behaviors. Cancer 2022, 128, 615–623. [Google Scholar] [CrossRef]
- Bhat, A.; Byatt, N. Infertility and Perinatal Loss: When the Bough Breaks. Curr. Psychiatry Rep. 2016, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Frey, K.A.; Navarro, S.M.; Kotelchuck, M.; Lu, M.C. The clinical content of preconception care: Preconception care for men. Am. J. Obstet. Gynecol. 2008, 199, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lynch, C.D.; Kim, S.; Sundaram, R.; Sapra, K.J.; Buck Louis, G.M. Preconception stress and the secondary sex ratio in a population-based preconception cohort. Fertil. Steril. 2017, 107, 714–722. [Google Scholar] [CrossRef]
- Jungwirth, A.; Diemer, T.; Kopa, Z.; Krausz, C.; Minhas, S.; Tournaye, H. EAU guidelines on male infertility. Eur. Urol. 2018, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Daolio, J.; Romeo, S.; Battaglia, F.A.; Marci, R.; La Sala, G.B. Lifestyle and fertility: The influence of stress and quality of life on female fertility. Reprod. Biol. Endocrinol. 2018, 16, 113. [Google Scholar] [CrossRef]
- Rehman, R.; Amjad, S.; Tariq, H.; Zahid, N.; Akhter, M.; Ashraf, M. Oxidative stress and male infertility: A cross-sectional study. J. Pak. Med. Assoc. 2020, 70, 461–466. [Google Scholar] [CrossRef]
- Clarke, I.J.; Arbabi, L. New concepts of the central control of reproduction, integrating the influence of stress, metabolic state, and season. Domest. Anim. Endocrinol. 2016, 56, 165–179. [Google Scholar] [CrossRef]
- Ohlander, S.J.; Lindgren, M.C.; Lipshultz, L.I. Testosterone and Male Infertility. Urol. Clin. N. Am. 2016, 43, 195–202. [Google Scholar] [CrossRef]
- Semercioz, A.; Baltaci, A.K.; Mogulkoc, R.; Avunduk, M.C. Effect of Zinc and Melatonin on Oxidative Stress and Serum Inhibin-B Levels in a Rat Testicular Torsion-Detorsion Model. Biochem. Genet. 2017, 55, 395–409. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar] [CrossRef]
- Bhongade, M.B.; Prasad, S.; Jiloha, R.C.; Ray, P.C.; Mohapatra, S.; Koner, B.C. Effect of psychological stress on fertility hormones and seminal quality in male partners of infertile couples. Andrologia 2015, 47, 336–342. [Google Scholar] [CrossRef]
- Samplaski, M.K.; Loai, Y.; Wong, K.; Lo, K.C.; Grober, E.D.; Jarvi, K.A. Testosterone use in the male infertility population: Prescribing patterns and effects on semen and hormonal parameters. Fertil. Steril. 2014, 101, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Panchendrabose, K.; Mann, U.; Patel, P. An update on male infertility and intratesticular testosterone-insight into novel serum biomarkers. Int. J. Impot. Res. 2022, 34, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Fábregues, F.; Peñarrubia, J.; Creus, M.; Manau, D.; Casals, G.; Carmona, F.; Balasch, J. Transdermal testosterone may improve ovarian response to gonadotrophins in low-responder IVF patients: A randomized, clinical trial. Hum. Reprod. 2009, 24, 349–359. [Google Scholar] [CrossRef]
- Bakun, O.V.; Yurkiv, O.I.; Slobodian, K.V.; Kolesnik, O.V.; Maruschak, A.V. The level of some hormones in the blood women with endometriosis which associated with infertility. Wiadomości Lek. 2019, 72, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Retana-Márquez, S.; Juárez-Rojas, L.; Ávila-Quintero, A.; Rojas-Maya, S.; Perera, G.; Casillas, F.; Betancourt, M.; Gómez-Quiroz, L. Neuroendocrine disruption is associated to infertility in chronically stressed female rats. Reprod. Biol 2020, 20, 474–483. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002, 82, 825–874. [Google Scholar] [CrossRef]
- Goto, T.; Hirabayashi, M.; Watanabe, Y.; Sanbo, M.; Tomita, K.; Inoue, N.; Tsukamura, H.; Uenoyama, Y. Testosterone Supplementation Rescues Spermatogenesis and In Vitro Fertilizing Ability of Sperm in Kiss1 Knockout Mice. Endocrinology 2020, 161, bqaa092. [Google Scholar] [CrossRef]
- Allan, C.M.; Garcia, A.; Spaliviero, J. Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: Evidence from genetic models of isolated FSH action. Endocrinology 2004, 145, 1587–1593. [Google Scholar] [CrossRef]
- Conneely, O.M.; Mulac-Jericevic, B.; DeMayo, F.; Lydon, J.P.; O’Malley, B.W. Reproductive functions of progesterone receptors. Recent Prog. Horm. Res. 2002, 57, 339–355. [Google Scholar] [CrossRef]
- Bolyakov, A.; Paduch, D.A. Prolactin in men’s health and disease. Curr. Opin. Urol. 2011, 21, 527–534. [Google Scholar] [CrossRef]
- McIntyre, C.; Li, X.F.; de Burgh, R.; Ivanova, D.; Lass, G.; O’Byrne, K.T. GABA Signaling in the Posterodorsal Medial Amygdala Mediates Stress-induced Suppression of LH Pulsatility in Female Mice. Endocrinology 2022, 164, 197. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Peng, G.; Liao, M.; Zhang, L.; Hu, H.; Long, L.; Tang, X.; Qu, H.; Shao, J.; et al. Pituitary P62 deficiency leads to female infertility by impairing luteinizing hormone production. Exp. Mol. Med. 2021, 53, 1238–1249. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Lennartsson, A.K.; Billig, H.; Jonsdottir, I.H. Burnout is associated with elevated prolactin levels in men but not in women. J. Psychosom. Res. 2014, 76, 380–383. [Google Scholar] [CrossRef]
- Arowojolu, A.O.; Akinloye, O.; Shittu, O.B. Serum and seminal plasma prolactin levels in male attenders of an infertility clinic in Ibadan. J. Obstet. Gynaecol. 2004, 24, 306–309. [Google Scholar] [CrossRef]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Carreau, S.; Hess, R.A. Oestrogens and spermatogenesis. Philos. Trans. R Soc. Lond. B Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef]
- Lambard, S.; Carreau, S. Aromatase and oestrogens in human male germ cells. Int. J. Androl. 2005, 28, 254–259. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid. Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef]
- Bai, J.; Qi, Q.R.; Li, Y. Estrogen Receptors and Estrogen-Induced Uterine Vasodilation in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone receptor signaling mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Petousis, S.; Prapas, Y.; Margioula-Siarkou, C. Unexplained infertility patients present the mostly impaired levels of progesterone receptors: Prospective observational study. Am. J. Reprod. Immunol. 2018, 79, 12828. [Google Scholar] [CrossRef]
- Poorasamy, J.; Sengupta, J.; Patil, A.; Ghosh, D. Progesterone resistance in endometriosis. Eur. Med. J. 2022, 8, 51–63. [Google Scholar] [CrossRef]
- Kim, H.H.; Schlegel, P.N. Endocrine manipulation in male infertility. Urol. Clin. N. Am. 2008, 35, 303–318. [Google Scholar] [CrossRef]
- Jeppesen, J.V.; Anderson, R.A.; Kelsey, T.W.; Christiansen, S.L.; Kristensen, S.G.; Jayaprakasan, K.; Raine-Fenning, N.; Campbell, B.K.; Yding Andersen, C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol. Hum. Reprod. 2013, 19, 519–527. [Google Scholar] [CrossRef]
- Silva, M.S.B.; Giacobini, P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell. Mol. Life Sci. 2021, 78, 1–16. [Google Scholar] [CrossRef]
- Luo, W.; Mao, P.; Zhang, L.; Chen, X.; Yang, Z. Assessment of ovarian reserve by serum antiMullerian hormone in patients with systemic lupus erythematosus: A meta-analysis. Ann. Palliat. Med. 2020, 9, 207–215. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Defining ovarian reserve to better understand ovarian aging. Reprod. Biol. Endocrinol. 2011, 9, 23. [Google Scholar] [CrossRef]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment ofovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Binart, N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef]
- Studerus, E.; Ittig, S.; Beck, K.; Del Cacho, N.; Vila-Badia, R.; Butjosa, A.; Usall, J.; Riecher-Rössler, A. Relation between self-perceived stress, psychopathological symptoms and the stress hormone prolactin in emerging psychosis. J. Psychiatr. Res. 2021, 136, 428–434. [Google Scholar] [CrossRef]
- Sobrinho, L.G. Prolactin, psychological stress and environment in humans: Adaptation and maladaptation. Pituitary 2003, 6, 35–39. [Google Scholar] [CrossRef]
- Chu, Y.L.; Xu, Y.R.; Yang, W.X.; Sun, Y. The role of FSH and TGF-β superfamily in follicle atresia. Aging 2018, 10, 305–321. [Google Scholar] [CrossRef]
- Torner, L.; Neumann, I.D. The brain prolactin system: Involvement in stress response adaptations in lactation. Stress 2002, 5, 249–257. [Google Scholar] [CrossRef]
- Lennartsson, A.K.; Jonsdottir, I.H. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology 2011, 36, 1530–1539. [Google Scholar] [CrossRef]
- Quintino-Moro, A.; Zantut-Wittmann, D.E.; Tambascia, M.; Machado, H.C.; Fernandes, A. High Prevalence of Infertility among Women with Graves’ Disease and Hashimoto’s Thyroiditis. Int. J. Endocrinol. 2014, 2014, 982705. [Google Scholar] [CrossRef]
- Maraka, S.; Ospina, N.M.; O’Keeffe, D.T.; Espinosa De Ycaza, A.E.; Gionfriddo, M.R.; Erwin, P.J.; Coddington, C.C.; Stan, M.N.; Murad, M.H.; Montori, V.M. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 580–590. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Prummel, M.F.; Wiersinga, W.M. Thyroid autoimmunity and miscarriage. Eur. J. Endocrinol. 2004, 150, 751–755. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Poppe, K.; Glinoer, D.; Tournaye, H.; Devroey, P.; Velkeniers, B. Impact of the ovarian hyperstimulation syndrome on thyroid function. Thyroid 2008, 18, 801–802. [Google Scholar] [CrossRef]
- Wagner, M.S.; Wajner, S.M.; Maia, A.L. The role of thyroid hormone in testicular development and function. J. Endocrinol. 2008, 199, 351–365. [Google Scholar] [CrossRef]
- Capel, B. The battle of the sexes. Mech. Dev. 2000, 92, 89–103. [Google Scholar] [CrossRef]
- La Vignera, S.; Vita, R.; Condorelli, R.A. Impact of thyroid disease on testicular function. Endocrine 2017, 58, 397–407. [Google Scholar] [CrossRef]
- Rijntjes, E.; Gomes, M.L.M.; Zupanic, N. Transient hypothyroidism: Dual effect on adult-type Leydig cell and Sertoli cell development. Front. Physiol. 2017, 8, 323. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, 22823. [Google Scholar] [CrossRef]
- Alahmar, A.; Dutta, S.; Sengupta, P. Thyroid hormones in male reproduction and infertility. Asian Pac. J. Reprod. 2019, 8, 203. [Google Scholar] [CrossRef]
- Romano, R.M.; Gomes, S.N.; Cardoso, N.C.S.; Schiessl, L.; Romano, M.A.; Oliveira, C.A. New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine 2017, 55, 607–617. [Google Scholar] [CrossRef]
- Louis, G.M.; Lum, K.J.; Sundaram, R.; Chen, Z.; Kim, S.; Lynch, C.D.; Schisterman, E.F.; Pyper, C. Stress reduces conception probabilities across the fertile window: Evidence in support of relaxation. Fertil. Steril. 2011, 95, 2184–2189. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia 2021, 53, 13595. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. SARS-CoV-2 and Male Infertility: Possible Multifaceted Pathology. Reprod. Sci. 2021, 28, 23–26. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gupta, A.; Tiwari, M.; Prasad, S.; Pandey, A.N.; Yadav, P.K.; Sharma, A.; Sahu, K.; Asrafuzzaman, S.; Vengayil, D.T.; et al. Impact of stress on female reproductive health disorders: Possible beneficial effects of shatavari (Asparagus racemosus). Biomed. Pharmacother. 2018, 103, 46–49. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Mayila, Y.; Irahara, M. The roles of kisspeptin and gonadotropin inhibitory hormone in stress-induced reproductive disorders. Endocr. J. 2018, 65, 133–140. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Wojsiat, J.; Korczyński, J.; Borowiecka, M.; Żbikowska, H.M. The role of oxidative stress in female infertility and in vitro fertilization. Postep. Hig. Med. Dosw. 2017, 71, 359–366. [Google Scholar] [CrossRef]
- Alkadi, H. A review on free radicals and antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Boers, S.A.; Jansen, R.; Hays, J.P. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1059–1070. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sangwan, N.; Parekh, N.V. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur. Urol. 2021, 79, 826–836. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, 13126. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef]
- Ma, F.; Feng, Y.; Zhang, Y.; Wang, R.H.; Su, D. The Roles of Stress-Induced Immune Response in Female Reproduction. Adv. Exp. Med. Biol. 2021, 1300, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Kalantaridou, S.N.; Zoumakis, E.; Makrigiannakis, A.; Lavasidis, L.G.; Vrekoussis, T.; Chrousos, G.P. Corticotropin-releasing hormone, stress and human reproduction: An update. J. Reprod. Immunol. 2010, 85, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Vrekoussis, T.; Zoumakis, E.; Navrozoglou, I.; Kalantaridou, S.N. CRH Receptors in Human Reproduction. Curr. Mol. Pharmacol. 2018, 11, 81–87. [Google Scholar] [CrossRef]
- Geraghty, A.C.; Kaufer, D. Glucocorticoid Regulation of Reproduction. Adv. Exp. Med. Biol. 2015, 872, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.L.; Ubuka, T.; Tsutsui, K. Regulation of stress response on the hypothalamic-pituitary-gonadal axis via gonadotropin-inhibitory hormone. Front. Neuroendocrinol. 2022, 64, 100953. [Google Scholar] [CrossRef]
- El Hayek, S.; Bitar, L.; Hamdar, L.H.; Mirza, F.G.; Daoud, G. Poly Cystic Ovarian Syndrome: An Updated Overview. Front. Physiol. 2016, 7, 124. [Google Scholar] [CrossRef]
- Collée, J.; Mawet, M.; Tebache, L.; Nisolle, M.; Brichant, G. Polycystic ovarian syndrome and infertility: Overview and insights of the putative treatments. Gynecol. Endocrinol. 2021, 37, 869–874. [Google Scholar] [CrossRef]
- Bachelot, A. Polycystic ovarian syndrome: Clinical and biological diagnosis. Ann. Biol. Clin. 2016, 74, 661–667. [Google Scholar] [CrossRef]
- Anagnostis, P.; Tarlatzis, B.C.; Kauffman, R.P. Polycystic ovarian syndrome (PCOS): Long-term metabolic consequences. Metabolism 2018, 86, 33–43. [Google Scholar] [CrossRef]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Kuczmierczyk, A.R.; Hardiman, P.J. Anxiety and depression in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2011, 26, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Z.; Zhao, S.; Cheng, L.; Man, Y.; Gao, X.; Zhao, H. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J. Assist. Reprod. Genet. 2021, 38, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Peker, N.; Turan, G.; Ege, S.; Bademkıran, M.H.; Karaçor, T.; Erel, Ö. The effect of clomiphene citrate on oxidative stress parameters in polycystic ovarian syndrome. J. Obstet. Gynaecol. 2021, 41, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Sun, W.; Wang, C. Improvement of anti-Müllerian hormone and oxidative stress through regular exercise in Chinese women with polycystic ovary syndrome. Hormones 2021, 20, 339–345. [Google Scholar] [CrossRef]
- Simic, D.; Nikolic Turnic, T.; Dimitrijevic, A.; Zivadinovic, A.; Milosevic-Stevanovic, J.; Djuric, D.; Zivkovic, V.; Jakovljevic, V. Potential role of d-chiro-inositol in reducing oxidative stress in the blood of nonobese women with polycystic ovary syndrome. Can. J. Physiol. Pharmacol. 2022, 100, 629–636. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Jamilian, M.; Bahmani, F.; Asemi, Z. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 5. [Google Scholar] [CrossRef]
- Xing, L.; Xu, J.; Wei, Y.; Chen, Y.; Zhuang, H.; Tang, W.; Yu, S.; Zhang, J.; Yin, G.; Wang, R.; et al. Depression in polycystic ovary syndrome: Focusing on pathogenesis and treatment. Front. Psychiatry 2022, 13, 1001484. [Google Scholar] [CrossRef]
- Zehravi, M.; Maqbool, M.; Ara, I. Depression and anxiety in women with polycystic ovarian syndrome: A literature survey. Int. J. Adolesc. Med. Health 2021, 33, 367–373. [Google Scholar] [CrossRef]
- Damone, A.L.; Joham, A.E.; Loxton, D.; Earnest, A.; Teede, H.J.; Moran, L.J. Depression, anxiety and perceived stress in women with and without PCOS: A community-based study. Psychol. Med. 2019, 49, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.A.; Pasch, L.A.; Cedars, M.I.; Legro, R.S.; Eisenberg, E.; Huddleston, H.G. Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil. Steril. 2018, 110, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dai, Y.; Tong, X.; Xu, W.; Huang, Q.; Jin, X.; Li, C.; Zhou, F.; Zhou, H.; Lin, X.; et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox. Biol. 2020, 30, 101431. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.M. Drugs in the treatment of female infertility. Recent advances. Drugs 1986, 32, 169–177. [Google Scholar] [CrossRef]
- Al-Inany, H.; Johnson, N. Drugs for anovulatory infertility in polycystic ovary syndrome. BMJ 2006, 332, 1461–1462. [Google Scholar] [CrossRef]
- Elizur, S.E.; Tulandi, T. Drugs in infertility and fetal safety. Fertil. Steril. 2008, 89, 1595–1602. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Augoulea, A.; Mastorakos, G.; Lambrinoudaki, I.; Christodoulakos, G.; Creatsas, G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol. Endocrinol. 2009, 25, 75–81. [Google Scholar] [CrossRef]
- Harley, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Waqar, A.; Jain, A.; Joseph, C.; Srivastava, K.; Ochuba, O.; Alkayyali, T.; Ruo, S.W.; Poudel, S. Oxidative Stress in Polycystic Ovarian Syndrome and the Effect of Antioxidant N-Acetylcysteine on Ovulation and Pregnancy Rate. Cureus 2021, 13, 17887. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; de Rooij, S.R. Long-term effects of prenatal stress and glucocorticoid exposure. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Mate, A.; Reyes-Goya, C.; Santana-Garrido, Á.; Vázquez, C.M. Lifestyle, Maternal Nutrition and Healthy Pregnancy. Curr. Vasc. Pharmacol. 2021, 19, 132–140. [Google Scholar] [CrossRef]
- Bisconti, M.; Simon, J.F.; Grassi, S.; Leroy, B.; Martinet, B.; Arcolia, V.; Isachenko, V.; Hennebert, E. Influence of Risk Factors for Male Infertility on Sperm Protein Composition. Int. J. Mol. Sci. 2021, 22, 13164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Osadchuk, L.V.; Osadchuk, A.V. Individual Lifestyle, and Male Fertility. Hum Physiol. 2023, 49, 196–207. [Google Scholar] [CrossRef]
- Kumar, M.; Zilate, S.; Gupta, C. Effect of Stress and Caffeine on Male Infertility. Cureus 2022, 14, 28487. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Barazani, Y.; Katz, B.F.; Nagler, H.M.; Stember, D.S. Lifestyle, environment, and male reproductive health. Urol. Clin. N. Am. 2014, 41, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Brown, S.I. Effects of lifestyle factors on fertility: Practical recommendations for modification. Reprod. Fertil. 2021, 2, R13–R26. [Google Scholar] [CrossRef]
- Hammoud, A.O.; Meikle, A.W.; Reis, L.O.; Gibson, M.; Peterson, C.M.; Carrell, D.T. Obesity and male infertility: A practical approach. Semin. Reprod. Med. 2012, 30, 486–495. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluationof the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Klemetti, R.; Raitanen, J.; Sihvo, S.; Saarni, S.; Koponen, P. Infertility, mental disorders and well-being—A nationwide survey. Acta Obstet. Gynecol. Scand. 2010, 89, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Maroufizadeh, S.; Karimi, E.; Vesali, S.; Samani, R.O. Anxiety and depression after failure of assisted reproductive treatment among patients experiencing infertility. Int. J. Gynecol. Obstet. 2015, 130, 253–256. [Google Scholar] [CrossRef]
- Donarelli, Z.; Lo Coco, G.; Gullo, S.; Marino, A.; Volpes, A.; Allegra, A. Are attachment dimensions associated with infertility-related stress in couples undergoing their first IVF treatment? A study on the individual and cross-partner effect. Hum. Reprod. 2012, 27, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, A.; Basirat, Z.; Nasiri-Amiri, F. Sexual dysfunction in women undergoing fertility treatment in Iran: Prevalence and associated risk factors. J. Reprod. Infertil. 2016, 17, 26–33. [Google Scholar]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, 13162. [Google Scholar] [CrossRef]
- Bai, C.F.; Sun, J.W.; Li, J.; Jing, W.H.; Zhang, X.K.; Zhang, X.; Ma, L.L.; Yue, R.; Cao, F.L. Gender differences in factors associated with depression in infertility patients. J. Adv. Nurs. 2019, 75, 3515–3524. [Google Scholar] [CrossRef]
- Gdańska, P.; Drozdowicz-Jastrzębska, E.; Grzechocińska, B.; Radziwon-Zaleska, M.; Węgrzyn, P.; Wielgoś, M. Anxiety and depression in women undergoing infertility treatment. Ginekol. Pol. 2017, 88, 109–112. [Google Scholar] [CrossRef]
- Gil-Villa, A.M.; Cardona-Maya, W.; Agarwal, A.; Sharma, R.; Cadavid, A. Role of male factor in early recurrent embryo loss: Do antioxidants have any effect? Fertil. Steril. 2009, 92, 565–571. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Metals, I.; Skinner, M.K. Reference module in biomedical sciences: Encyclopedia. Asian Pac. J. Reprod. 2018, 99, 579–587. [Google Scholar] [CrossRef]
- Oron, G.; Allnutt, E.; Lackman, T.; Sokal-Arnon, T.; Holzer, H.; Takefman, J. A prospective study using Hatha Yoga for stress reduction among women waiting for IVF treatment. Reprod. Biomed. Online 2015, 30, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef] [PubMed]
- Lolak, S.; Rashid, N.; Wise, T.N. Interface of Women’s Mental and Reproductive Health. Curr. Psychiatry Rep. 2005, 7, 220–227. [Google Scholar] [CrossRef]
- Schmidt, L. Infertility and assisted reproduction in Denmark. Epidemiology and psychosocial consequences. Dan Med. Bull 2006, 53, 390–417. [Google Scholar]
- Elkina, Y.L.; Atroshchenko, M.M.; Bragina, E.E.; Muronetz, V.I.; Schmalhausen, E.V. Oxidation of glyceraldehyde-3-phosphate dehydrogenase decreases sperm motility. Biochemistry 2011, 76, 268–272. [Google Scholar] [CrossRef]
- Alkatout, I.; Mettler, L.; Beteta, C.; Hedderich, J.; Jonat, W.; Schollmeyer, T.; Salmassi, A. Combined surgical and hormone therapy for endometriosis is the most effective treatment: Prospective, randomized, controlled trial. J. Minim. Invasive Gynecol. 2013, 20, 473–481. [Google Scholar] [CrossRef]
- Calogero, A.E.; Condorelli, R.A.; Russo, G.I.; La Vignera, S. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. Biomed. Res. Int. 2017, 2017, 4650182. [Google Scholar] [CrossRef]
| Reproductive Hormones | Functions |
|---|---|
| Gonadotropin-Releasing Hormone |
|
| Follicle-stimulating Hormone |
|
| Luteinizing Hormone |
|
| Prolactin |
|
| Inhibin |
|
| Testosterone |
|
| Estrogen |
|
| Progesterone |
|
| Thyroid Hormone |
|
| Anti Mullerian Hormone |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramya, S.; Poornima, P.; Jananisri, A.; Geofferina, I.P.; Bavyataa, V.; Divya, M.; Priyanga, P.; Vadivukarasi, J.; Sujitha, S.; Elamathi, S.; et al. Role of Hormones and the Potential Impact of Multiple Stresses on Infertility. Stresses 2023, 3, 454-474. https://doi.org/10.3390/stresses3020033
Ramya S, Poornima P, Jananisri A, Geofferina IP, Bavyataa V, Divya M, Priyanga P, Vadivukarasi J, Sujitha S, Elamathi S, et al. Role of Hormones and the Potential Impact of Multiple Stresses on Infertility. Stresses. 2023; 3(2):454-474. https://doi.org/10.3390/stresses3020033
Chicago/Turabian StyleRamya, Shanmugam, Prasad Poornima, Arumugam Jananisri, Irudhayaraj Peatrise Geofferina, Venkataramanaravi Bavyataa, Murugan Divya, Palanisamy Priyanga, Jeganathan Vadivukarasi, Senthil Sujitha, Selvarasu Elamathi, and et al. 2023. "Role of Hormones and the Potential Impact of Multiple Stresses on Infertility" Stresses 3, no. 2: 454-474. https://doi.org/10.3390/stresses3020033
APA StyleRamya, S., Poornima, P., Jananisri, A., Geofferina, I. P., Bavyataa, V., Divya, M., Priyanga, P., Vadivukarasi, J., Sujitha, S., Elamathi, S., Anand, A. V., & Balamuralikrishnan, B. (2023). Role of Hormones and the Potential Impact of Multiple Stresses on Infertility. Stresses, 3(2), 454-474. https://doi.org/10.3390/stresses3020033








