Lohmann Brown Rooster Semen: Intrinsic Bacteria and Their Impact on Sperm Progressive Motility and Seminal Biochemical Parameters—A Preliminary Study
Abstract
1. Introduction
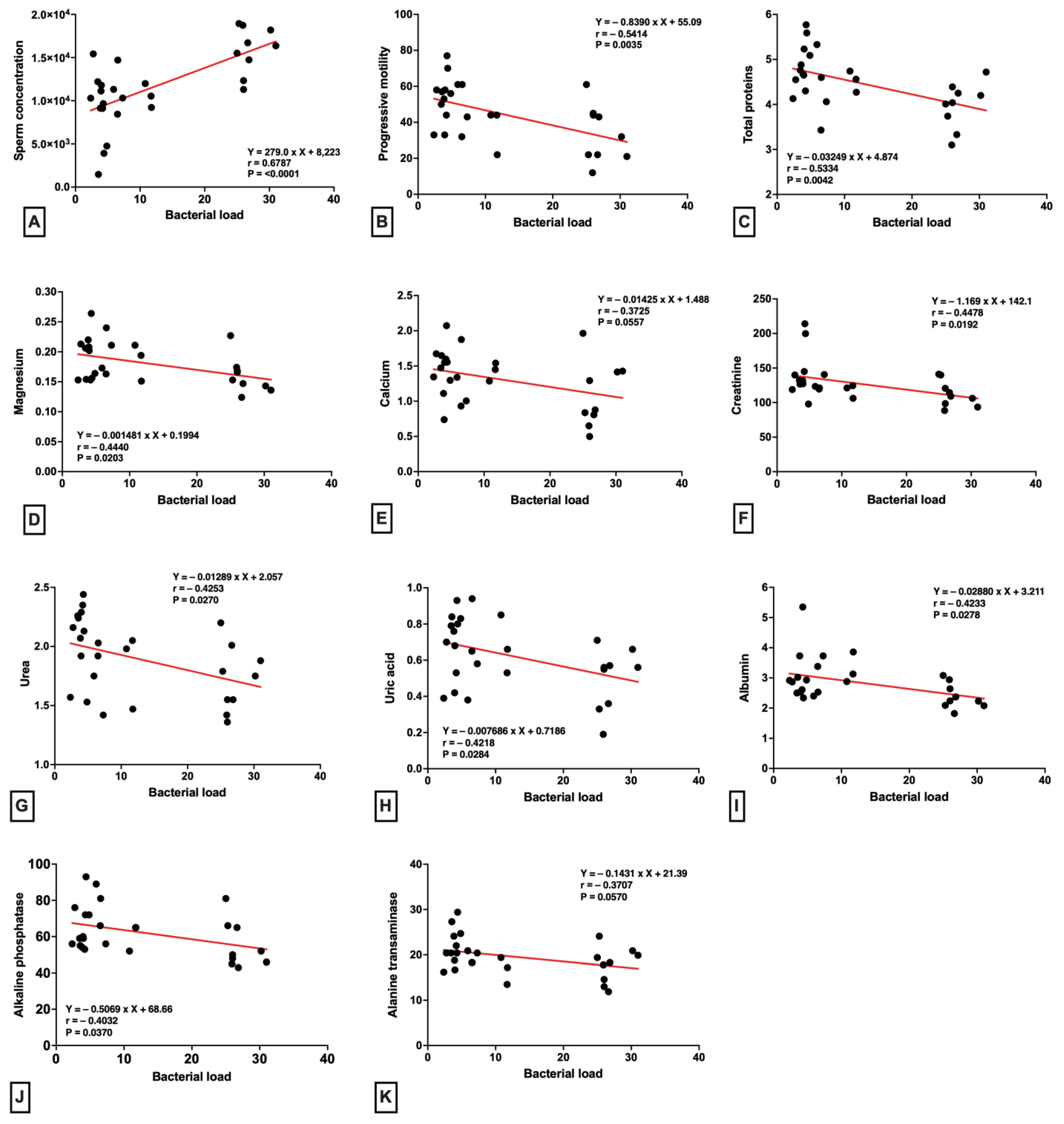
2. Results
3. Discussion
4. Materials and Methods
4.1. Collection of Biological Material
4.2. Bacteriological Cultures and Identification
4.3. Sperm Concentration and Progressive Motility Assessment
4.4. Biochemical Analysis of Seminal Plasma
4.5. Statistical Evaluations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Alkan, S.; Baran, A.; Özdaş, Ö.B.; Evecen, M. Morphological Defects in Turkey Semen. Turk. J. Vet. Anim. Sci. 2002, 26, 1087–1092. [Google Scholar]
- Jamieson, B.G.M. Reproductive Biology and Phylogeny of Birds, Part A: Phylogeny, Morphology, Hormones and Fertilization; CRC Press: Oxford, UK, 2011. [Google Scholar]
- Omprakash, A.V.; Venkatesh, G. Effect of Vaginal Douching and Different Semen Extenders on Bacterial Load and Fertility in Turkeys. Br. Poult. Sci. 2006, 47, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.; Vaid, R.K.; Phulia, S.K.; Singh, P. Assessment of bacterial diversity in fresh bubaline semen. Indian J. Anim. Sci. 2012, 82, 596–598. [Google Scholar]
- Farsimadan, M.; Motamedifar, M. Bacterial infection of the male reproductive system causing infertility. J. Reprod. Immunol. 2020, 142, 103183. [Google Scholar] [CrossRef]
- Quiñones-Pérez, C.; Martínez, A.; Ortiz, I.; Crespo, F.; Vega-Pla, J.L. The Semen Microbiome and Semen Parameters in Healthy Stallions. Animals 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Belić, L.; Tokárová, K.; Žiarovská, J.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Syst. Biol. Reprod. Med. 2021, 67, 438–449. [Google Scholar] [CrossRef]
- Lenický, M.; Slanina, T.; Kačániová, M.; Galovičová, L.; Petrovičová, M.; Ďuračka, M.; Benko, F.; Kováč, J.; Tvrdá, E. Identification of Bacterial Profiles and Their Interactions with Selected Quality, Oxidative, and Immunological Parameters of Turkey Semen. Animals 2021, 11, 1771. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kačániová, M.; Baláži, A.; Vašíček, J.; Vozaf, J.; Jurčík, R.; Ďuračka, M.; Žiarovská, J.; Kováč, J.; Chrenek, P. The Impact of Bacteriocenoses on Sperm Vitality, Immunological and Oxidative Characteristics of Ram Ejaculates: Does the Breed Play a Role? Animals 2022, 12, 54. [Google Scholar] [CrossRef]
- Gączarzewicz, D.; Udała, J.; Piasecka, M.; Błaszczyk, B.; Stankiewicz, T. Bacterial Contamination of Boar Semen and its Relationship to Sperm Quality Preserved in Commercial Extender Containing Gentamicin Sulfate. Polish J. Vet. Sci. 2016, 19, 451–459. [Google Scholar] [CrossRef]
- Agarwal, J.; Srivastava, S.; Singh, M. Pathogenomics of uropathogenic Escherichia coli. Indian J. Med. Microbiol. 2012, 30, 141–149. [Google Scholar] [CrossRef]
- Tvrdá, E.; Petrovičová, M.; Benko, F.; Ďuračka, M.; Kováč, J.; Slanina, T.; Galovičová, L.; Žiarovská, J.; Kačániová, M. Seminal Bacterioflora of Two Rooster Lines: Characterization, Antibiotic Resistance Patterns and Possible Impact on Semen Quality. Antibiotics 2023, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Reiber, M.A.; McInroy, J.A.; Conner, D.E. Enumeration and identification of bacteria in chicken semen. Poult. Sci. 1995, 74, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.A.; Stern, N.J.; Wilson, J.L.; Musgrove, M.T.; Buhr, R.J.; Hiett, K.L. Isolation of Campylobacter spp. from semen samples of commercial broiler breeder roosters. Avian Dis. 2002, 46, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Haines, M.D.; Parker, H.M.; McDaniel, C.D.; Kiess, A.S. Impact of 6 different intestinal bacteria on broiler breeder sperm motility in vitro. Poult. Sci. 2013, 92, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Althouse, G.C. Sanitary procedures for the production of extended semen. Reprod. Domest. Anim. 2008, 43, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future After the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef]
- Akpan, U.E.; Ofongo-Abule, R.T.S. Preliminary results on sources of bacteria of economic importance from three broiler chicken farms in Uyo metropolis of Akwa Ibom State. Niger. J. Anim. Sci. 2019, 21, 99–105. [Google Scholar]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil Steril 2007, 88, 1076–1085. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Arnett, W.P.; Foussias, G.; Pappas, H.; Ghandi, S.; Melegos, D.N.; Mullen, B.; Yu, H.; Srigley, J.; Jarvi, K. Seminal plasma biochemical markers and their association with semen analysis findings. Urology 1999, 53, 596–603. [Google Scholar] [CrossRef]
- Escallón, G.; Becker, M.H.; Walke, J.B.; Jensen, R.V.; Cormier, G.; Belden, L.K.; Moore, I.T. Testosterone levels are positively correlated with cloacal bacterial diversity and the relative abundance of Chlamydiae in breeding male rufous-collared sparrows. Funct. Ecol. 2017, 31, 192–203. [Google Scholar] [CrossRef]
- Ďuračka, M.; Husarčíková, K.; Jančov, M.; Galovičová, L.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content. Animals 2021, 11, 3309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, S.; Tang, X.; Zhou, G.; Yuan, J.; Li, Q.; Chen, Y.; Xu, X.; Sun, X.; Zhu, D.; et al. Chlamydia trachomatis infection in the genital tract is associated with inflammation and hypospermia in the infertile male of China. Asian J. Androl. 2022, 24, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Eini, F.; Kutenaei, M.A.; Zareei, F.; Dastjerdi, Z.S.; Shirzeyli, M.H.; Salehi, E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with Leukocytospermia. BMC Mol. Cell. Biol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Zeyad, A.; Hamad, M.; Amor, H.; Hammadeh, M.E. Relationships between bacteriospermia, DNA integrity, nuclear protamine alteration, sperm quality and ICSI outcome. Reprod. Biol. 2018, 18, 115–121. [Google Scholar] [CrossRef]
- Moretti, E.; Capitani, S.; Figura, N.; Pammolli, A.; Federico, M.G.; Giannerini, V.; Collodel, G. The presence of bacteria species in semen and sperm quality. J. Assist. Reprod. Genet. 2009, 26, 47–56. [Google Scholar] [CrossRef]
- Ďuračka, M.; Kováčik, A.; Bučko, O.; Galovičová, L.; Kačániová, M.; Lukáč, N.; Tvrdá, E. The dependence of sperm progressive motility and seminal plasma biochemical composition on bacterial load in duroc boar semen. J. Microbiol. Biotechnol. Food Sci. 2021, 12, e3284. [Google Scholar]
- Stones, D.H.; Krachler, A.M. Against the tide: The role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 2016, 44, 1571–1580. [Google Scholar] [CrossRef]
- Monga, M.; Roberts, J.A. Spermagglutination by bacteria: Receptor-specific interactions. J. Androl. 1994, 15, 151–156. [Google Scholar]
- Tvrdá, E.; Ďuračka, M.; Benko, F.; Lukáč, N. Bacteriospermia—A formidable player in male subfertility. Open Life Sci. 2022, 17, 1001–1029. [Google Scholar] [CrossRef]
- McCue, P. Alkaline Phosphatase: A marker for Ejaculation. In Equine Reproductive Procedures, 2nd ed.; Dascanio, J., McCue, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 523–524. [Google Scholar]
- Henricks, D.M.; Kouba, A.J.; Lackey, B.R.; Boone, W.R.; Gray, S.L. Identification of insulin-like growth factor I in bovine seminal plasma and its receptor on spermatozoa: Influence on sperm motility. Biol. Reprod. 1998, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Muiño-Blanco, T.; Pérez-Pé, R.; Cebrián-Pérez, J.A. Seminal plasma proteins and sperm resistance to stress. Reprod. Domest. Anim. 2008, 43, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, F.; Hammami, M.B.; Omar, S.; Aribia, H.B.; Sanhaji, H.; Feki, M. Semen Creatine and Creatine Kinase Activity as an Indicator of Sperm Quality. Clin. Lab. 2020, 66, 9. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stea, T.H.; Engeset, D. Creatine as a Promising Component of Paternal Preconception Diet. Nutrients 2022, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Role of Uric Acid in Semen. Biomolecules 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Pandey, V.; Nigam, R.; Saxena, A.; Swain, D.K.; Yadav, B. Association of oxidative status and semen characteristics with seminal plasma proteins of buffalo semen. Iran J. Vet. Res. 2016, 17, 226–230. [Google Scholar]
- Janiszewska, E.; Kokot, I.; Kmieciak, A.; Gilowska, I.; Faundez, R.; Kratz, E.M. Are There Associations between Seminal Plasma Advanced Oxidation Protein Products and Selected Redox-Associated Biochemical Parameters in Infertile Male Patients? A Preliminary Report. Cells 2022, 11, 3667. [Google Scholar] [CrossRef]
- Collodel, G.; Nerucci, F.; Signorini, C.; Iacoponi, F.; Moretti, E. Associations between biochemical components of human semen with seminal conditions. Syst. Biol. Reprod. Med. 2019, 65, 155–163. [Google Scholar] [CrossRef]
- The Streak Plate Protocol. Available online: https://asm.org/ASM/media/Protocol-Images/The-Streak-Plate-Protocol.pdf?ext=.pdf (accessed on 23 March 2023).
| Parameter | Arithmetic Mean (n = 27) | HQ (PRO ≥ 56%) (n = 9) | IQ (43% ≤ PRO < 56%) (n = 9) | LQ (PRO < 43%) (n = 9) |
|---|---|---|---|---|
| CFU [log10 CFU/mL] | 12.78 ± 2.36 | 6.81 ± 0.63 | 13.35 ± 0.75 * HQ | 18.18 ± 0.94 ** HQ, * IQ |
| PRO [%] | 44.37 ± 3.15 | 62.11 ± 2.31 | 45.56 ± 1.31 ** HQ | 25.44 ± 2.46 *** HQ, ** IQ |
| CON [M/mL] | 11,789.00 ± 837.10 | 9840.00 ± 765.00 | 11,299.00 ± 591.70 | 14,226.00 ± 1456.00 * HQ |
| ALP [U/L] | 1.36 ± 0.78 | 75.33 ± 4.19 | 53.33 ± 2.12 ** HQ | 57.89 ± 2.84 ** HQ |
| ALT [U/L] | 1.81 ± 0.67 | 21.94 ± 1.49 | 18.41 ± 1.32 | 18.34 ± 1.19 |
| Ca [mmol/L] | 0.45 ± 0.17 | 1.66 ± 0.26 | 1.17 ± 0.34 * HQ | 1.07 ± 0.34 ** HQ |
| Mg [mmol/L] | 0.17 ± 0.02 | 1.99 ± 0.39 | 1.86 ± 0.27 | 1.56 ± 0.24 * HQ |
| Phos [mmol/L] | 0.57 ± 0.03 | 0.49 ± 0.05 | 0.45 ± 0.06 | 0.41 ± 0.06 |
| Chol [mmol/L] | 12.72 ± 0.53 | 0.64 ± 0.05 | 0.49 ± 0.07 * HQ | 0.55 ± 0.05 |
| Creat [µmol/L] | 62.19 ± 2.56 | 143.50 ± 12.75 | 124.80 ± 14.56 | 113.20 ± 13.56 * HQ |
| BILI [µmol/L] | 19.57 ± 0.79 | 0.80 ± 0.17 | 0.61 ± 0.20 | 0.75 ± 0.15 |
| Trigs [mmol/L] | 0.72 ± 0.09 | 0.18 ± 0.02 | 0.16 ± 0.02 | 0.16 ± 0.03 |
| UA [mmol/L] | 0.62 ± 0.04 | 0.75 ± 0.06 | 0.63 ± 0.04 | 0.46 ± 0.05 ** HQ |
| Urea [mmol/L] | 1.89 ± 0.06 | 2.08 ± 0.28 | 1.84 ± 0.15 | 1.74 ± 0.20 |
| TP [g/L] | 4.46 ± 1.20 | 5.00 ± 0.55 | 4.41 ± 0.27 * HQ | 3.95 ± 0.51 *** HQ |
| ALB [g/L] | 2.84 ± 1.36 | 3.01 ± 0.31 | 2.87 ± 0.18 | 2.65 ± 0.23 |
| Identified bacterial species | ||||
| Staphylococcus warneri, Citrobacter braakii, Corynebacterium xerosis, Enterococcus avium, Pseudomonas pseudoalcaligenes, Corynebacterium glutamicum | Enterococcus avium, Ochrobactrum anthropi, Escherichia coli, Alcaligenes faecalis, Enterococcus casseliflavus, Pseudomonas composti | Staphylococcus aureus, Enterococcus avium, Escherichia coli, Enterococcus feacalis, Pseudomonas aeruginosa, Serratia liquefaciens, Rothia terrae, Pantoea agglomerans, Pseudomonas putida | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ďuračka, M.; Petrovičová, M.; Benko, F.; Kováčik, A.; Lukáč, N.; Kačániová, M.; Tvrdá, E. Lohmann Brown Rooster Semen: Intrinsic Bacteria and Their Impact on Sperm Progressive Motility and Seminal Biochemical Parameters—A Preliminary Study. Stresses 2023, 3, 424-433. https://doi.org/10.3390/stresses3020031
Ďuračka M, Petrovičová M, Benko F, Kováčik A, Lukáč N, Kačániová M, Tvrdá E. Lohmann Brown Rooster Semen: Intrinsic Bacteria and Their Impact on Sperm Progressive Motility and Seminal Biochemical Parameters—A Preliminary Study. Stresses. 2023; 3(2):424-433. https://doi.org/10.3390/stresses3020031
Chicago/Turabian StyleĎuračka, Michal, Michaela Petrovičová, Filip Benko, Anton Kováčik, Norbert Lukáč, Miroslava Kačániová, and Eva Tvrdá. 2023. "Lohmann Brown Rooster Semen: Intrinsic Bacteria and Their Impact on Sperm Progressive Motility and Seminal Biochemical Parameters—A Preliminary Study" Stresses 3, no. 2: 424-433. https://doi.org/10.3390/stresses3020031
APA StyleĎuračka, M., Petrovičová, M., Benko, F., Kováčik, A., Lukáč, N., Kačániová, M., & Tvrdá, E. (2023). Lohmann Brown Rooster Semen: Intrinsic Bacteria and Their Impact on Sperm Progressive Motility and Seminal Biochemical Parameters—A Preliminary Study. Stresses, 3(2), 424-433. https://doi.org/10.3390/stresses3020031









