Abstract
The intensive global use of glyphosate has led to the evolution of glyphosate resistant (GR) weed species, including the economically damaging horseweed (Conyza sumatrensis). We evaluated the glyphosate resistance mechanisms of C. sumatrensis. While 5-enolpyruvylshikimate-3-phosphate synthase activity was similar between the glyphosate resistant (GR) and nonresistant biotypes, plants from the GR population accumulated lower shikimate levels than susceptible ones, suggesting the absence of target-site resistance mechanisms. Decreases over time in glyphosate concentrations in GR leaves were not accompanied by increases in glyphosate concentrations in their stem and roots, indicating lower glyphosate distribution rates in GR plants. The early appearance of aminomethylphosphonic acid (the main glyphosate metabolite) in leaves, as well as its presence only in the stems and roots of GR plants, suggests faster glyphosate metabolism in GR plants than in susceptible ones. GR plants treated with glyphosate also showed greater antioxidant (ascorbate peroxidase [APX] and catalase [CAT]) and cytochrome P450-enzyme activities, indicating their great capacity to avoid glyphosate-induced oxidative stress. Three non-target mechanisms (reduced glyphosate translocation, increased metabolism, and increased antioxidant activity) therefore confer glyphosate resistance in C. sumatrensis plants. This is the first time that APX, CAT and P450-enzyme activities are related to GR in C. sumatrensis.
1. Introduction
Glyphosate [N-(phosphonomethyl)glycine], the active ingredient in numerous trade formulations used for total vegetation control, is a postemergence, broad-spectrum, non-selective systemic herbicide [1]. After the introduction of glyphosate-resistant (GR) commercial crop plants, glyphosate became the most widely used herbicide in the world [2]. Its herbicidal effect is due to the inhibition of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), inhibiting the biosynthesis of the amino acids phenylalanine, tyrosine, and tryptophan [3].
Glyphosate is intensively used in Brazil for weed control in areas cultivated with GR crops [4] as preemergence herbicides are rarely used in that country and other postemergence treatments increase weed control costs [5]. With the wide use of that herbicide, increased concentrations of glyphosate have been observed in environments and deleterious effects of the harbicie have been observed in non-target organisms, like algae, aquatic insects and plants [6,7,8,9]. Moreover, as a result of its indiscriminate use, GR weed biotypes are emerging very quickly [4]. The annual cost of glyphosate resistance in soybean crops alone in Brazil is estimated to be between $3.7 and 6.0 billon Brazilian Reais, reaching R$9.0 billon when considering the 5% productivity losses due to competition from GR weeds [10]. The first case of weed glyphosate resistance (in Lolium rigidum) was reported in 1996, twenty years after the introduction of the herbicide–which was considered an indicator of the slow development of weed resistance [11]. By 2022, however, the International Survey of Herbicide Resistant Weeds reported 340 cases of glyphosate resistant weeds in 29 countries, involving a total of 51 species, eleven of which occur in Brazil (Amaranthus palmeri, A. hybridus, Chloris elata, Conyza bonariensis, Conyza canadensis, Coniza sumatrensis, Digitaria insularis, Echinochloa crus-galli var. crus-galli, Eleusine indica, Euphorbia heterophylla, and Lolium perene ssp. multiflorum) (www.weedscience.com; access on 22 May 2022)Horseweeds (Conyza spp.) are among the most common weeds found growing among perennial and annual crops [12]. Its presence in soybean crops reduces yields by up to 90% in relation to areas cultivated in the absence of those weeds [13,14]. Horseweed has been mainly controlled in soybean and corn plantations by the use of glyphosate [15], although horseweed control using that herbicide is no longer satisfactory due to the growing resistance of some biotypes [12,15,16]. Conyza weed plants are highly adaptable, extremely competitive, and easily dispersed [17]. Considering the particularly successful acquisition of herbicide resistance by horseweed, and the economic losses associated with its presence among crops, a better understanding the mechanisms involved in the weed resistance to glyphosate has become increasingly important for defining new strategies for its management.
The mechanisms commonly involved in herbicide resistance acquisition by weeds include changes in herbicide absorption, vacuolar herbicide sequestration (and consequent reduced herbicide movement to the target sites), rapid metabolic herbicide detoxification, herbicide target site alterations, and gene amplification [12,16,18,19,20,21]. There is specific evidence that glyphosate induces oxidative stress [1,22,23] and inhibits cytochrome P450 activity (P450) [24,25] in susceptible plants–raising suspicious that GR weeds have high antioxidant capacities and low P450 sensitivities. While antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) may keep reactive oxygen species (ROS) levels under control (avoiding oxidative damages) in plants, P450 activity may contribute to herbicide detoxification [22,26]. Investigations of oxidative metabolism and P450 activity in GR weeds exposed to glyphosate have nonetheless been very scarce.
We therefore examined the non-target site mechanisms associated with glyphosate resistance in a Conyza sumatrensis biotype by evaluating the translocation and metabolism of that herbicide as well as its effects on the shikimate pathway, oxidative metabolism, and cytochrome P450 activity in both resistant and susceptible horseweed biotypes. We aimed to understand the physiological mechanisms related to the plant biotype resistance to glyphosate. We specifically sought to elucidate the roles of antioxidant enzymes and P450 in glyphosate resistance and thus contribute to the development of techniques (such as biology engineering) for horseweed management when glyphosate has lost its efficiency for weed control.
2. Results
2.1. Oxidative Stress Markers
Significant interactions between biotypes, glyphosate concentrations, and times of exposure were observed for SOD and APX activities (Table S1). Exposure for 24 h to glyphosate resulted in increased SOD activity in GR plants (R + Gly) in relation to non-resistant plants (Figure 1A). At 48 h, high SOD activity was observed in all plants exposed to glyphosate, regardless of their biotype (S/R + Gly) (Figure 1A). At 72 h, glyphosate exposure increased SOD activity in resistant biotype plants (R + Gly) as compared to GR plants without glyphosate treatment (R–Gly) (Figure 1A). Regardless of the glyphosate treatment (0 or 1080 g ae ha−1), SOD activity at 48 and 72 h was lower in the GR biotype (R −/+ Gly) in relation to 12 and 24 h of exposure (Figure 1A).
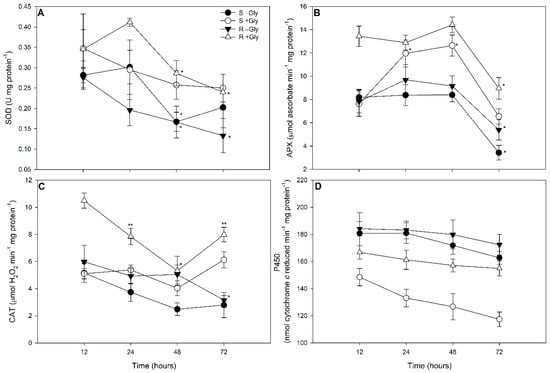
Figure 1.
Activities of superoxide dismutase (A), ascorbate peroxidase (B), catalase (C), and NADPH-cytochrome P450 reductase (D) in glyphosate susceptible (S) and resistant (R) Conyza spp. plants exposed to 0 (-Gly) or 1080 g glyphosate (a.e.) ha−1 (+Gly) for 72 h. Values are represented as the mean ± standard error of five replicates. *, ** indicates significant differences between times with the same biotype and glyphosate concentrations, by the Contrast test (considering p < 0.05).
Regardless of the biotype, glyphosate exposure (S/R + Gly) resulted in increased APX and CAT activities after 24 h of exposure; however, enzyme activity increases were observed earlier (after 12 h) in GR-resistant plants (R + Gly) as compared to the susceptible biotype (S + Gly) (Figure 1B,C). With the exception of susceptible plants exposed to glyphosate (S + Gly; which did not show significantly different APX activities between 12 h and 72 h), APX activity decreased at 72 h regardless of the biotype and glyphosate exposure (Figure 1B). CAT activity was greater at 72 h in relation to 48 h of exposure in plants of both biotypes when exposed to glyphosate (S/R + Gly; Figure 1C).
2.2. NADPH-Cytochrome P450 Reductase Evaluations
Significant interactions between biotypes and glyphosate concentrations were observed in terms of NADPH-cytochrome P450 reductase (P450) activity (Table S1). Regardless of the biotype, P450 activity was greater in plants without any exposure to glyphosate (Figure 1D). When treated with glyphosate, greater P450 activity was observed in GR plants than in the susceptible biotype (Figure 1D).
2.3. Shikimate Concentrations and EPSPS Activities
Significant interactions between biotypes, glyphosate concentrations, and times of evaluation were observed in terms of shikimate concentrations in plant leaves (Table S1). In plants not exposed to glyphosate, leaf shikimate concentrations did not differ between the two biotypes or in terms of experimental times (Figure 2A). Leaf shikimate concentrations increased over time in susceptible plants treated with glyphosate, while GR plants did not demonstrate significantly different shikimate concentrations after 48 or 72 h of glyphosate exposure (Figure 2A). Leaf EPSPS activities did not significantly differ between biotypes, but were greater in plants not exposed to glyphosate treatments in relation to those exposed to that herbicide (Figure 2B).
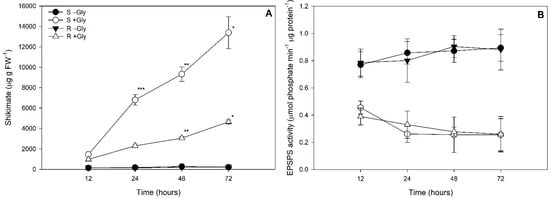
Figure 2.
Shikimate concentrations (A) and 5-enolpyruvylshikimate-3-phosphate synthase activities (B) in glyphosate susceptible (S) and resistant (R) Conyza spp. plants exposed to 0 (-Gly) or 1080 g glyphosate (a.e.) ha−1 (+Gly) for 72 h. Values are represented as the mean ± standard error of five replicates. *, **, *** indicates significant differences between times with the same biotype and glyphosate concentrations, by the Contrast test (considering p < 0.05).
2.4. Glyphosate and AMPA Concentrations
No glyphosate or AMPA were found in plants not exposed to glyphosate treatments. Significant interactions between biotypes and times of evaluation were observed for glyphosate and AMPA concentrations in all plant organs (Table S2). While leaf glyphosate concentrations were greatest after 12 h of exposure, those concentrations were lower after 24 and 72 h in GR plants in relation to susceptible plants (Figure 3A). Leaf glyphosate concentrations decreased over time in both biotypes (Figure 3A). Stem and root concentrations of glyphosate were greater over time in susceptible plants than in GR plants (Figure 3B,C); glyphosate concentrations in the stems and roots of susceptible plants increased after 24 h and 48 h of exposure, respectively, but did not significantly differ after different times of exposure among GR plants (Figure 3B,C).
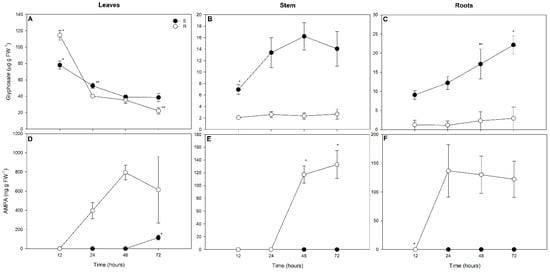
Figure 3.
Glyphosate and AMPA concentrations in the leaves (A,D), stems (B,E), and roots (C,F) of glyphosate susceptible (S) and resistant (R) Conyza spp. plants exposed to 0 (-Gly) or 1080 g glyphosate (a.e.) ha−1 (+Gly) for 72 h. Values are represented as the mean ± standard error of five replicates. *, ** indicates significant differences between times with the same biotype and glyphosate concentrations, by the Contrast test (considering p < 0.05).
AMPA was not detected in either the stems or roots of susceptible plants (Figure 3E,F). AMPA was detected earlier in the leaves of GR plants than in susceptible plants, being detected after 24 h of exposure in GR plants and at 72 h in the susceptible biotype (Figure 3D); at 72 h, leaf AMPA concentrations were greater in GR than in susceptible plants (Figure 3D).
3. Discussion
The glyphosate resistance of the GR horseweed populations examined in the present study was confirmed by the lower shikimate concentrations observed in their leaves in relation to those found in susceptible plants after herbicide exposure (Figure 2A). Shikimate accumulation tests, however, do not reveal which type of mechanism [target-site resistance (TSR) or non-target site resistance (NTSR)] conferred glyphosate resistance [27]. To exclude the involvement of TSR mechanisms (such as EPSPS mutations or EPSPS gene amplification) [28], herbicide target enzymes assays are necessary [29]. The enzymatic activity of EPSPS were similar between the two horseweed biotypes, whether exposed or not to glyphosate (Figure 2B), thus excluding the involvement of TSR mechanisms in glyphosate resistance [28]. We therefore investigated biochemical responses as well as glyphosate distribution and metabolism in plants, aiming to elucidate possible NTSR mechanisms involved in their GR tolerance.
NTSR mechanisms have been related to glyphosate weed resistance [30]. Reduced glyphosate absorption and translocation, as well as its increased metabolism, conferred resistance in saltmarsh aster (Aster squamatus) [28]. In Echinochloa colona, aldo-keto reductase metabolizes glyphosate and confers resistance [31]. The antioxidant system activity of Amaranthus palmeri was found to be related to glyphosate resistance [32]. Glyphosate resistance in C. canadensis was related to impaired glyphosate translocation and its metabolism [33], while lower distribution rates between plant organs assured glyphosate resistance in C. bonariensis [15]. Similarly, the GR C. sumatrensis population investigated here evidenced differential glyphosate translocation and metabolism in relation to the sensitive population. In contrast to susceptible plants, in which glyphosate concentrations decreases over time were followed by increased glyphosate concentrations in the stems and roots (Figure 3), in GR plants, no increases in glyphosate concentration were observed in their stems and roots over time (Figure 3)–indicating that glyphosate translocation differs between the biotypes, being lower in GR plants. One could argue that decreased leaf uptake of that herbicide could result in glyphosate resistance in the GR biotype, although the higher glyphosate concentrations at 12 h observed in GR leaves in relation to susceptible plants eliminates that hypothesis (Figure 3A). Interestingly, the rate of glyphosate disappearance in the leaves of GR plants was not followed by its increased translocation. We therefore became interested in investigating herbicide metabolism. One of the main glyphosate by-products is AMPA, which has been found in the tissues of plants exposed to glyphosate [34]. The early appearance of AMPA in the leaves of GR plants, as well as the presence of that metabolite in their stems and roots, indicate that this biotype can metabolize glyphosate much faster than susceptible plants. Therefore, similar to previous findings [15,31], we observed that impaired glyphosate translocation and heightened glyphosate metabolism are mechanisms related to glyphosate resistance in C. sumatrensis plants.
In addition to these already described mechanisms, we report here, for the first time, evidence for the involvement of antioxidant and P450 enzymes in glyphosate resistance in C. sumatrensis plants. The higher activities of antioxidant enzymes in GR as opposed to sensitive A. palmeri populations assured lower lipid peroxidation (an oxidative stress burst) and was related to plant glyphosate resistance [31,35]. Similarly, we observed greater APX and CAT activities in GR leaves exposed to glyphosate in relation to susceptible plants. APX and CAT are important antioxidant enzymes involved in H2O2 scavenging, and therefore in the avoidance of oxidative bursts caused by the accumulation of reactive oxygen species. Additionally, the increased activities of those enzymes have been reported in cases of plant tolerance to xenobiotics [36], including glyphosate [9]. In contrast to H2O2 scavenging enzymes (APX and CAT), SOD appears not to be involved in glyphosate resistance, as enzyme activity differed between the glyphosate exposed biotypes only at 24 h (Figure 1A). In addition to antioxidant enzyme activities, the biotypes differed in terms of the responses of P450 to glyphosate (Figure 1D). Glyphosate is a known inhibitor of cytochrome P450 activity (P450) [24,25], but P450 activity in the leaves of GR plants was less sensitive to glyphosate than in the sensitive biotype.
Cytochrome P450 monooxygenase constitutes the largest family of enzymes in plant metabolism [37] and is responsible for detoxifying herbicides that are not chemically similar, such as ACCase- and ALS-inhibitors [30]. In Lolium spp., for instance, resistance to pinoxaden and iodosulfuron-mesosulfuron was related to the insensibility of its P450 enzymes, assuring herbicide degradation in resistant biotypes [38]. Similarly, the GR population examined here showed lower sensitivity to glyphosate, which is a known P450 inhibitor in both plants [24,25] and animals [39,40]. However, in contrast to ACCase- and ALS-inhibitors, there have been no reports implicating P450 in glyphosate metabolism. In addition to C-P lyase (which degrades glyphosate into sarcosine and inorganic phosphate), glyphosate oxidoreductase (GOX) activity (which produces AMPA) has been identified as the main mechanisms of glyphosate degradation in plants [41]. The conversion of glyphosate to AMPA by GR horseweed plants indicates that that biotype possesses a GOX-like enzyme, as do other weeds [41,42,43]. The higher P450 activity observed in GR horseweed biotypes may therefore not be related to herbicide degradation. P450, however, is involved in protection against stress in plants through the biosynthesis and regulation of hormones, fatty acids, sterols, cell wall components, biopolymers, and other defense compounds (such as terpenoids, alkaloids, flavonoids, furanocoumarins, glucosinolates, and allelochemicals) [44]. Additionally, cytochrome P450 has critical roles in maintaining redox homeostasis and protecting the organism from toxic ROS accumulations [45]. Silencing the P450 genes in Apis cerana cerana, for example, resulted in the enhanced activities of peroxidase and CAT. Similarly, the overexpression of P450 genes in tobacco resulted in decreased oxidative bursts (due to decreased ROS accumulations) when plants were submitted to drought conditions [46]. Those findings suggest the role of P450 in cell antioxidant defenses. In this context, in addition to inhibiting amino acid synthesis, the ability of glyphosate to kill plants has been related to its capacity to induce oxidative stress [1,22,47,48], and the maintenance of high antioxidant activities may therefore contribute to herbicide resistance of horseweed plants.
Adverse environmental effects associated with herbicide applications have emerged in the form of increases in resistant weed populations [49]. Changes in light, temperature and precipitation regimes, for instance, may collaborate to select plant species able to deal with ROS stress through the activation of antioxidant systems. In this scenario, climate changes may favor glyphosate resistance in weeds with the natural ability to cope with ROS formation and accumulation. The established relationship between antioxidant capacity and GR in C. sumatrensis therefore suggests avoiding glyphosate applications during warm weather or during periods of high light intensity-when antioxidant activities tend to increase in plant cells [50].
4. Material and Methods
4.1. Greenhouse Experiments
Conyza sumatrensis (Retz.) E.Waker seeds of both the resistant and susceptible biotypes were collected among soybean crops in Paraná State, Brazil. A rapid resistance test was performed to confirm weed susceptibility to glyphosate that involved sowing seeds in polypropylene pots (0.8 L, 14 × 6 cm) containing a sterile substrate (shells of composted pine, vermiculite, peat, and fertilizers [nitrogen, phosphorus and potassium]). The pots were then kept under greenhouse conditions with minimum/maximum temperatures of 25/32 °C and natural light supplemented by sodium vapor lamps to provide a 12 h photoperiod and an average photosynthetic active radiation of 825 µmol photons m−2 s−1. When the seedlings were at the rosette stage (4 to 6 leaves), corresponding to the appropriate stage of herbicide application, the two biotypes were treated with 0 (ultrapure water) or 1080 g ae ha−1 of glyphosate (Roundup Original, 356 g equivalent acid (ea) L−1, Monsanto, Brazil), according to the manufacturer’s recommendations for horseweed plants, and using an experimental backpack sprayer (CO2 pressurized, equipped with two flat-fan nozzles) to apply the equivalent of 200 L of the syrup per hectare. After 72 h of exposure to glyphosate, the susceptible (but not the resistant) plants began to show symptoms of intoxication (chlorosis, necrotic spots, and wilting)–symptoms that were easily noted after 12 days of exposure (Figure 4). The tests were conducted until the resistant biotype produced seeds (110 days); no resprouting was observed in susceptible plants exposed to glyphosate.

Figure 4.
Resistant (A) and susceptible (B) Conyza spp. biotypes exposed to 1080 g glyphosate (ea) ha−1 for 12 days.
After the confirmation of glyphosate resistance/susceptibility of the biotypes, new experiments, under the same conditions described above, were performed using a randomized block design with five pots per biotype (corresponding to replicates) per treatment, in a two (herbicide concentrations) x five (times of evaluation) factorial scheme. The plants were harvested 0, 12, 24, 48 and 72 h after the beginning of the glyphosate treatments. Evaluations were halted after 72 h of exposure (as the susceptible biotype plants treated with glyphosate began showing pronounced symptoms of intoxication, including chlorosis and leaf necrotic spots) to allow evaluations of their metabolic conditions prior to the onset of acute cell damage and plant death.
Five plants from each biotype and each glyphosate treatment were harvested at each evaluation time, thoroughly washed with distilled water, and separated into roots, stems, and leaves. Samples of the fifth and sixth nodes (from the shoot apex), corresponding to fully expanded leaves, were selected for the enzyme assays. All plant samples were immediately frozen in liquid nitrogen and stored at −80 °C until assayed.
4.2. Enzymatic Evaluations of the Leaves
To study the antioxidant enzymes, 0.1 g of leaves were macerated in 1 mL of an extraction buffer containing 100 mM potassium buffer (pH 7.8), 100 mM EDTA, 1 mM L-ascorbate, and 2% polivinilpirrolidona (PVP)-40 (m/v). The protein contents of the samples were determined using the Bradford method [51]. Superoxide dismutase (SOD; EC 1.15.1.1) [52], catalase (CAT; EC 1.11.1.6) [53], and ascorbate peroxidase (APX; EC 1.11.1.11) [54] activities were evaluated as oxidative stress markers. The measurements of NADPH-cytochrome P450 reductase were performed following [55]. EPSPS (EC 2.5.1.19) was extracted from the leaves according to [56], and its enzymatic activity measured according to [57], using an EnzCheckQR phosphate assay Kit (Invitrogen, Carlsbad, CA, USA). The specific activities of EPSPS were determined in the absence and in the presence of 100 µM glyphosate (Sigma Aldrich, Brazil), and expressed as phosphate (µmol) liberated per µg of total soluble protein (determined using Bradford method) per minute.
4.3. Glyphosate, AMPA and Shikimate Evaluations
The concentrations of glyphosate, aminomethylphosphonic acid (AMPA), and shikimate in the plants were determined using a LC-MS/MS system composed of a XEVO TQD triple quadrupole (Walters) mass spectrometer equipped with an electrospray (ESI) ionization source coupled to an HPLC Varian SYS-LC-240-E equipped with an autosampler, following Gomes et al. [58]. Glyphosate and AMPA concentrations were measured in the roots, stems, and leaves, while shikimate was evaluated only in the leaves. Extractions were performed with 0.4 g of each plant organ, previously thoroughly washed in ultrapure water, using 50 mL of acidified water (pH 2.5) following Matallo et al. [59]. The plant extracts were filtered through C18 SPR cartridges (500 mg/6 mL; Applied Separations, Allentown, PA, USA) previously conditioned with 15 mL of acidified water (pH 2.5) and 5 mL of methanol. The cartridges containing the samples were washed with 3 mL of 50% methanol in water (v/v). The eluate was then dried in a SpeedVac machine (RC1010, Thermo), and the residues resuspended in the mobile phase A. Before injection, the samples were filtered through nylon syringe filters (13 mm x 0.25 µm, Filtrilo Brazil).
An Ascentis® C18 column (Sigma-Aldrish, São Paulo, Brazil) was used for chromatographic separation, with a mobile phase consisting of 5 mM of ammonium acetate in water (phase A) and 5 mM of ammonium acetate in methanol (phase B), both pH 7.0. The mass spectrometry analyses were performed in a negative ion mode. Analytical-grade glyphosate, AMPA (Pestanal grade, Sigma-Aldrich, São Paulo, Brazil), and shikimate (analytical standard, Sigma-Aldrich, Brazil) were used to prepare the calibration curves. The six-point calibration curves showed good linearity for the analytes (r2 ≥ 0.95; p < 0.0001). For quality control, each sample batch included three blanks, three standards, and three fortified samples. The recovery rates of all of the compounds were greater than 89%.
4.4. Statistical Analyses
The experiments consisted of combinations of the two biotypes (sensitive and resistant) and two glyphosate concentrations (0 and 1080 g ea ha−1) allotted to the main plots, and four evaluation times (0, 12, 24, 48, 72 h) allotted to the subplots and tested in split-plot design with five replications. Statistical analyses were performed using JMP 7.0 software (SAS Institute Inc.). Data were tested for normality (Shapiro–Wilk) and homogeneity (Bartlett), and then statistically evaluated. For the biochemical evaluations, data were submitted to three-way analyses of variance (ANOVA). Interactions between biotypes, glyphosate concentrations, and times of evaluation were added to the model. For glyphosate and AMPA concentrations, data were evaluated by using two-away ANOVA, with interactions between biotypes and times of evaluation being included in the model. When differences were detected by ANOVA, the means were compared using the Contrast test (p < 0.05).
5. Conclusions
In addition to the reduced translocation and increased metabolism of glyphosate already described for C. sumatrensis, increased APX, CAT, and P450 enzyme activities constitute non-target mechanisms endowing glyphosate resistance. The activities of antioxidant systems and P450 were shown for the first time here to be related to glyphosate resistance in C. sumatrensis plants. Our findings open new avenue for studies, establishing cell antioxidant mechanisms as targets for investigating glyphosate resistance in weeds.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/stresses3010005/s1, Table S1: ANOVA for the interactive effects of biotypes (susceptible and resistant), glyphosate concentrations (0 or 1080 g glyphosate (ae ha−1), and times of exposure (12, 24, 48, and 72 hours), Table S2: ANOVA for the interactive effects of biotypes (susceptible and resistant) and times of exposure (12, 24, 48, and 72 hours).
Author Contributions
G.C.K., R.Z.M. and R.S.A.K. methodology, investigation, formal analysis, review and editing; A.A.M.B. conceptualization, funding acquisition, preparing the review and editing; P.J. and M.P.G. conceptualization, methodology, investigation, formal analysis, preparing the original draft, reviewing & editing, supervision, funding acquisition, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)–Finance Code 001, and the Universidade Federal do Paraná (Edital de Apoio à Pesquisa 02/2020). M.P. Gomes and A.A.M. Barroso received research productivity grants from CNPq.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid (AMPA), an overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Siehl, D. Inhibitors of EPSPS synthase, glutamine synthetase and histidine synthesis. In Herbicide Activity: Toxicology, Biochemistry and Molecular Biology; Roe, R., Burton, J., Kuhr, R., Eds.; IOS Press: Amsterdam, The Netherlands, 1997; pp. 37–67. [Google Scholar]
- Ferreira Mendes, K.; Nogueira de Sousa, R.; Flávia Souza Laube, A.; Current Approaches to Pesticide Use and Glyphosate-Resistant Weeds in Brazilian Agriculture. Multifunctionality and Impacts of Organic and Conventional Agriculture IntechOpen. 2020. Available online: https://www.intechopen.com/books/multifunctionality-and-impacts-of-organic-and-conventional-agriculture/current-approaches-to-pesticide-use-and-glyphosate-resistant-weeds-in-brazilian-agriculture (accessed on 3 September 2022).
- Alcántara-de la Cruz, R.; Domínguez-Martínez, P.A.; da Silveira, H.M.; Cruz-Hipólito, H.E.; Palma-Bautista, C.; Vázquez-García, J.G.; Dominguez-Valenzuela, J.A.; de Prado, R. Management of glyphosate-resistant weeds in Mexican citrus groves: Chemical alternatives and economic viability. Plants 2019, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Smedbol, É.; Lucotte, M.; Labrecque, M.; Lepage, L.; Juneau, P. Phytoplankton growth and PSII efficiency sensitivity to a glyphosate-based herbicide (Factor 540®). Ecotoxicol. Environ. Saf. 2017, 192, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Smedbol, E.; Gomes, M.P.; Paquet, S.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Effects of low concentrations of glyphosate-based herbicide Factor 540® on an agricultural stream freshwater phytoplankton community. Chemosphere 2018, 192, 133–141. [Google Scholar] [CrossRef]
- Gomes, M.P.; dos Santos, M.P.; de Freitas, P.L.; Schafaschek, A.M.; de Barros, E.N.; Kitamura, R.S.A.; Paulete, V.; Navarro-Silva, M.A. The aquatic macrophyte Salvinia molesta mitigates herbicides (glyphosate and aminomethylphosphonic acid) effects to aquatic invertebrates. Environ. Sci. Pollut. Res. 2022, 1–14. Available online: https://link.springer.com/10.1007/s11356-022-23012-w (accessed on 16 September 2022). [CrossRef]
- Mendes, E.J.; Malage, L.; Rocha, D.C.; Kitamura, R.S.A.; Gomes, S.M.A.; Navarro-Silva, M.A.; Gomes, M.P. Isolated and combined effects of glyphosate and its by-product aminomethylphosphonic acid on the physiology and water remediation capacity of Salvinia molesta. J. Hazard. Mater. 2021, 417, 125694. [Google Scholar] [CrossRef]
- EMBRAPA. Resistência de Plantas Daninhas a Herbicidas Preocupa Agricultores. 2018. Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/37661812/resistencia-de-plantas-daninhas-a-herbicidas-preocupa-agricultores (accessed on 2 May 2019).
- Powles, S.B. Evolved glyphosate-resistant weeds around the world: Lessons to be learnt. Am. J. Manag. Care. 2013, 19, 871–880. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Stasinopoulou, P.; Kanatas, P.; Travlos, I. Differences in metabolism of three Conyza species to herbicides glyphosate and triclopyr revealed by LC-MS/MS. Child. J. Agric. Res. 2020, 80, 100–107. [Google Scholar] [CrossRef]
- Agostinetto, D.; Silva DRO da Vargas, L. Soybean yield loss and economic thresholds due to glyphosate resistant hairy fleabane interference. Arq. Inst. Biológico 2018, 84. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1808-16572017000100230&lng=en&tlng=en (accessed on 2 September 2022). [CrossRef]
- Gazziero, D.L.P.; Adegas, F.S.; Voll, E.; Vargas, L.; Karam, D.; Matallo, M.B. Interferência da buva em áreas cultivadas com soja. In Proceedings of the XXVII Congresso Brasileiro da Ciência das Plantas Daninhas, Ribeirão Preto, Brazil, 19–23 July 2010; Sociedade Brasileira de Plantas Daninhas: São Paulo, SP, Brazil; pp. 1555–1558. [Google Scholar]
- Ferreira, E.; Galon, L.; Aspiazú, I.; Silva, A.; Concenço, G.; Silva, A.; Oliveira, J.; Vargas, L. Glyphosate translocation in hairy fleabane (Conyza bonariensis) biotypes. Planta Daninha 2008, 26, 637–643. [Google Scholar] [CrossRef][Green Version]
- Vargas, L.; Silva, D.; Agostinetto, D.; Matallo, M.; Santos, F.; Almeida, S.; Chavarria, G. Glyphosate influence on the physiological parameters of Conyza bonariensis biotypes. Planta Daninha 2014, 32, 151–159. [Google Scholar] [CrossRef][Green Version]
- Hao, J.-H.; Qiang, S.; Liu, Q.-Q.; Cao, F. Reproductive traits associated with invasiveness in Conyza sumatrensis. J. Syst. Evol. 2009, 47, 245–254. [Google Scholar] [CrossRef]
- De Prado, J.L.; Osuna, M.D.; Heredia, A.A.; De Prado, R. Lolium rigidum, a Pool of Resistance Mechanisms to ACCase Inhibitor Herbicides. J. Agric. Food Chem. 2005, 53, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Huang, S.; Powles, S. Direct measurement of paraquat in leaf protoplasts indicates vacuolar paraquat sequestration as a resistance mechanism in Lolium rigidum. Pestic. Biochem. Physiol. 2010, 98, 104–109. [Google Scholar] [CrossRef]
- Busi, R.; Vila-Aiub, M.M.; Powles, S.B. Genetic control of a cytochrome P450 metabolism-based herbicide resistance mechanism in Lolium rigidum. Heredity 2011, 106, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’H, S.G.; Moingt, M.; Smedbol, E.; Paquet, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Impact of phosphate on glyphosate uptake and toxicity in willow. J. Hazard. Mater. 2016, 304, 269–279. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Maccario, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic. Biochem. Physiol. 2016, 130, 65–70. [Google Scholar] [CrossRef]
- Lamb, D.C.; Kelly, D.E.; Hanley, S.Z.; Mehmood, Z.; Kelly, S.L. Glyphosate is an inhibitor of plant cytochrome P450: Functional expression of Thlaspi arvensae cytochrome P45071B1/reductase fusion protein in Escherichia coli. Biochem. Biophys. Res. Commun. 1998, 244, 110–114. [Google Scholar] [CrossRef]
- Gomes, M.P.; Tavares, D.S.; Richardi, V.S.; Marques, R.Z.; Wistuba, N.; Moreira de Brito, J.C.; Soffiatti, P.; Sant’Anne-Santos, B.F.; de Silva, M.A.N.; Juneau, P. Enrofloxacin and Roundup® interactive effects on the aquatic macrophyte Elodea canadensis physiology. Environ. Pollut. 2019, 249, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yu, Q.; Beffa, R.; González, S.; Maiwald, F.; Wang, J.; Powles, S.B. Cytochrome P450 CYP81A10v7 in Lolium rigidum confers metabolic resistance to herbicides across at least five modes of action. Plant J. 2020, 105, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant. Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Valenzuela, J.A.; Alcántara-de la Cruz, R.; Palma-Bautista, C.; Vázquez-García, J.G.; Cruz-Hipolito, H.E.; De Prado, R. Non-Target Site Mechanisms Endow Resistance to Glyphosate in Saltmarsh Aster (Aster squamatus). Plants 2021, 10, 1970. [Google Scholar] [CrossRef]
- Bracamonte, E.; Silveira HM da Alcántara-de la Cruz, R.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; De Prado, R. From tolerance to resistance: Mechanisms governing the differential response to glyphosate in Chloris barbata. Pest. Manag. Sci. 2018, 74, 1118–1124. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Q.; Han, H.; Mao, L.; Nyporko, A.; Fan, L.; Bai, L.; Powles, S. Aldo-keto Reductase Metabolizes Glyphosate and Confers Glyphosate Resistance in Echinochloa colona. Plant Physiol. 2019, 181, 1519–1534. [Google Scholar] [CrossRef]
- Maroli, A.S.; Nandula, V.K.; Dayan, F.E.; Duke, S.O.; Gerard, P.; Tharayil, N. Metabolic Profiling and Enzyme Analyses Indicate a Potential Role of Antioxidant Systems in Complementing Glyphosate Resistance in an Amaranthus palmeri Biotype. J. Agric. Food Chem. 2015, 63, 9199–9209. [Google Scholar] [CrossRef]
- González-Torralva, F.; Rojano-Delgado, A.M.; Luque de Castro, M.D.; Mülleder, N.; De Prado, R. Two non-target mechanisms are involved in glyphosate-resistant horseweed (Conyza canadensis L. Cronq.) biotypes. J. Plant Physiol. 2012, 169, 1673–1679. [Google Scholar] [CrossRef]
- Smedbol, É.; Lucotte, M.; Maccario, S.; Gomes, M.P.M.P.; Paquet, S.; Moingt, M.; Mercier, L.L.C.; Sobarzo, M.R.P.; Blouin, M.-A. Glyphosate and aminomethylphosphonic acid content in glyphosate-resistant soybean leaves, stems and roots and associated phytotoxicity following a single glyphosate-based herbicide application. J. Agric. Food Chem. 2019, 67, 6133–6142. [Google Scholar] [CrossRef]
- Eceiza, M.V.; Gil-Monreal, M.; Barco-Antoñanzas, M.; Zabalza, A.; Royuela, M. The moderate oxidative stress induced by glyphosate is not detected in Amaranthus palmeri plants overexpressing EPSPS. J. Plant Physiol. 2022, 274, 153720. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Kitamura, R.S.A.; Marques, R.Z.; Barbato, M.L.; Zámocký, M. The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation. Antioxidants 2022, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Yanniccari, M.; Gigón, R.; Larsen, A. Cytochrome P450 Herbicide Metabolism as the Main Mechanism of Cross-Resistance to ACCase- and ALS-Inhibitors in Lolium spp. Populations from Argentina: A Molecular Approach in Characterization and Detection. Front. Plant Sci. 2020, 11, 600301. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.A.; Han, G.; Kang, R.; Shen, D.; Shen, J.; Li, C. Disruption of cytochrome P450 enzymes in the liver and small intestine in chicken embryos in ovo exposed to glyphosate. Environ. Sci. Pollut. Res. 2020, 27, 16865–16875. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases. Entropy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate degradation in glyphosate-resistant and -susceptible crops and weeds. J. Agric. Food Chem. 2011, 59, 5835–5841. [Google Scholar] [CrossRef]
- Coupland, D. The effect of temperature on the activity and metabolism of glyphosate applied to rhizome fragments of elymus repens (Agropyron repens). Pestic. Sci. 1984, 15, 226–234. [Google Scholar] [CrossRef]
- Eberbach, P.L.; Bowmer, K. Conversion of 14C-glyphosate to carbon dioxide by alligator weed. J. Aquat. Plant Manag. 1995, 33, 27–29. [Google Scholar]
- Xu, J.; Wang, X.; Guo, W. The cytochrome P450 superfamily: Key players in plant development and defense. J. Integr. Agric. 2015, 14, 1673–1686. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, W.; Li, Z.; Ma, L.; Yu, J.; Wang, H.; Liu, Z.; Xu, B. Identification and Characterization of Three New Cytochrome P450 Genes and the Use of RNA Interference to Evaluate Their Roles in Antioxidant Defense in Apis cerana cerana Fabricius. Front. Physiol. 2018, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Ding, J.; Lee, D.; Lu, X.; Feng, Y.; Song, W. Overexpression of SoCYP85A1, a Spinach Cytochrome p450 Gene in Transgenic Tobacco Enhances Root Development and Drought Stress Tolerance. Front. Plant Sci. 2017, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Maccario, S.; Lucotte, M.; Labrecque, M.; Juneau, P. Consequences of phosphate application on glyphosate uptake by roots: Impacts for environmental management practices. Sci. Total Environ. 2015, 537, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Richardi, V.S.; Bicalho, E.M.; da Rocha, D.C.; Navarro-Silva, M.A.; Soffiatti, P.; Gracia, Q.S.; Sant’Anna-Santos, B.F. Effects of Ciprofloxacin and Roundup on seed germination and root development of maize. Sci. Total Environ. 2019, 651, 2671–2678. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; Mendes, K.F.; de la Cruz, R.A.; Torra, J.; De Prado, R. Non-Target-Site Resistance Mechanisms Endow Multiple Herbicide Resistance to Five Mechanisms of Action in Conyza bonariensis. J. Agric. Food Chem. 2021, 69, 14792–14801. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Temperature and light modulation of herbicide toxicity on algal and cyanobacterial physiology. Front. Environ. Sci. 2017, 5, 50. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Bio. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q.; Gasser, M.; Zhan, Q. Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 2013, 4, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest. Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Owens, D.K.; Corniani, N.; Silva, F.M.L.; Watson, S.B.; Howell, J.; Shaner, D.L. Biochemical Markers and Enzyme Assays for Herbicide Mode of Action and Resistance Studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Gomes, G.L.G.C.; Carbonari, C.A.; Velini, E.D.; Trindade, M.L.B.; Silva, J.R.M. Extraction and Simultaneous Determination of Glyphosate, AMPA and Compounds of the Shikimic Acid Pathway in Plants. Planta Daninha 2015, 33, 295–304. [Google Scholar] [CrossRef]
- Matallo, M.B.; Almeida, S.D.B.; Franco, D.A.S.; Cerdeira, A.L.; Gazzeiro, D.L.P. Glyphosate as a tool to produce shikimic acid in plants. Planta Daninha 2014, 32, 601–608. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).