Abstract
Expanding fundamental understanding of the complex and far-reaching impacts of anthropogenic climate change is essential for formulating mitigation strategies. There is abundant evidence of ongoing damage and threat to plant health across both natural and cultivated ecosystems, with potentially immeasurable cost to humanity and the health of the planet. Plant–soil systems are multi-faceted, incorporating key variables that are individually and interactively affected by climatic factors such as rainfall, solar radiation, air temperature, atmospheric CO2, and pollution. This synthesis focuses on climate effects on plant–metal interactions and related plant–soil dynamics. Ecosystems native to metalliferous soils incorporate vegetation well adapted to metal oversupply, yet climate-change is known to induce the oversupply of certain immobile soil metals by altering the chemistry of non-metalliferous soils. The latter is implicated in observed stress in some non-metal-adapted forest trees growing on ‘normal’ non-metalliferous soils. Vegetation native to riverine habitats reliant on flooding is increasingly at risk under drying conditions caused by anthropogenic water removal and climate change that ultimately limit plant access to essential trace-metal nutrients from nutrient poor sandy soils. In agricultural plant systems, it is well known that environmental conditions alter soil chemistries and plant responses to drive plant metal toxicity stress. These aspects are addressed with reference to specific scenarios and studies linking climate to plant–metal interactions, with emphasis on land plants.
1. Introduction
While land plants are crucial to life on Earth, marine and aquatic plants help sustain the health of our planet and its provision of oceanic and freshwater food resources. Research into climatic effects on land plant–metal interactions far outweighs that of the latter; hence, this synthesis prioritises it to examine the implications of climate change for plant health, specifically, metal interactions. Despite an overall scarcity of direct research into how climate change may affect plant–metal interactions, the findings of past studies not originally intended to interrogate this subject can be drawn upon to provide insights in the present context, as will be done here. Plant status has been conceptually represented within the framework of internal and external factors below and above the substrate surface [1,2] (Figure 1), some dynamic and/or mutually interactive. The uptake of mineral nutrients, non-nutrient metals, and other elements is directly determined by specific plant and substrate traits; however, environmental conditions can influence these processes indirectly [2,3,4,5,6,7,8,9,10].
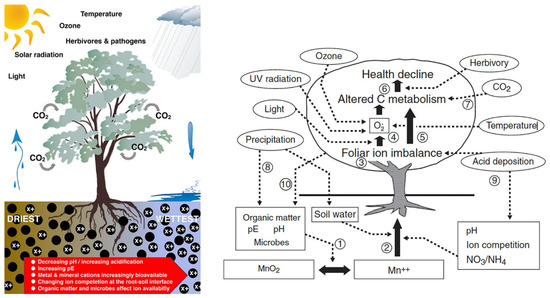
Figure 1.
Examples of plant health conceptual models [1,2]. In the left depiction, X+ denotes solubilised cations in their highly reduced forms [1] (Reprinted with permission from [1]. Copyright 2021 Copyright Department of Ecology in Australia). The right depiction shows soil Mn chemistry and plant Mn toxicity. Mn reduction (1) regulated by many factors. Reduced Mn taken up by roots (2) influenced by multiple factors. Mn toxicity occurs in leaves (3). Antioxidant chemistry impeded by excess Mn in leaves (4). Oxidative stress generated by excess Mn exacerbated by ozone, temperature extremes, and possibly UV radiation (5). Carbon resource depletion over time limits maintenance, growth and defence, which, in combination with pathogen attack and herbivory, leads to health decline (6). Increases in atmospheric CO2 can alter carbon gain with various effects on plant processes including tissue dilution of nutrients (7). Precipitation influences soil pE, soil microbial processes and soil water status, all of which influence Mn availability (8). Acid deposition lowers soil pH and inhibits mycorrhizal associations, which has strong effects on ion acquisition by roots; acid precipitation has also been shown to deplete base cations from foliage by leaching (9). There are important biogeochemical feedbacks of plant function and productivity on soil nutrient availability: root exudation and leaf litter chemistry and quantity affect soil organic matter, litter-derived nutrients, soil redox potential, pH, and microbial composition and function (10). (Reprinted with permission from [2]. Copyright 2004 Copyright Department of Horticulture in USA).
Plants access soil metal cations in their highly reduced oxidation states as determined by soil chemical dynamics, to which root exudates contribute within the rhizosphere [11]. Roots are also capable of releasing low molecular weight organic acids that bind to soluble metals to inhibit plant uptake as an exclusion strategy [12,13,14]. The mediation of metals by plants from uptake at the root-soil interface, through translocation to above-ground parts is well researched for many metals, with transporters linked to specific metal cations, as well as various counter-anions including organic acids implicated in xylem transport and vacuolar storage [15,16,17,18,19,20,21,22].
Soil metals are mobilized by reduction (solubilisation) arising from acidification, redox conditions, waterlogging, and microbial activity [3,8,19,23]. Plant response to metal oversupply variously ranges between the extremities of tolerance and sensitivity, which are genetic [24,25]. At one end is plant metal hyperaccumulation, an intrinsic ability to safely accumulate extraordinarily high metal concentrations via this rare and extreme affinity for metals evolved on metalliferous soils [24,25,26,27]. Metal sensitivity manifests in non-adapted plants growing on substrates metal enriched by natural processes, environmental pollution, and climatic factors that mobilise normally immobile substrate metals [28,29]. Conversely, trace-metal nutrient deficiency arises when plants cannot access essential elements for reasons including altered environmental conditions that limit soil bioavailability and natural soil conditions of trace metal immobility or scarcity [1,30,31]. The acidification of water bodies from streams to oceans is having wide-ranging impacts such as coral bleaching [32]; however, understanding about the direct effects of climate change on water plant–metal interactions is limited [10,33] notwithstanding the issue of metal pollution not addressed here. The most tangible effects of environmental change on plant–metal interactions arise primarily from alterations to the chemistries of the substrates in which they grow and the ambient conditions. This synthesis interrogates specific case studies and scenarios relating to plant–metal interactions to inform discussion around broader trends linked to climate change.
2. Metal Hyperaccumulating Plant Ecosystems
The unusual occurrence of Alyssum bertolonii (Brassicaceae) on bare rock in the Upper Tibre Valley was noted four centuries ago [34] (Figure 2). Much later, this was classified as a Ni-hyperaccumulator on serpentine or ultramafic rock, [35], the very first characterisation of plant metal hyperaccumulation. The rare trait manifests in extraordinarily high metal concentrations in plant aearial tissues, commonly foliage as is currently documented in around 0.24% of all angiosperms on Earth [36,37].

Figure 2.
Alyssum bertolonii growing on serpentine rock, Upper Tiber Valley, Tuscany, Italy (Photo: D. Fernando 2006).
Hyperaccumulators are edaphic specialists found primarily on naturally metalliferous soils [27,38], the majority serpentine with several of the following properties: high Fe, high Mg:Ca ratios, low N, P, K and phytotoxic Ni levels, and other heavy metals such as Pb, Cd, Zn, Mn, Co [27,35,39]. These nutritionally depauperate metal-rich soils have shaped specialised floras, often with high degrees of endemism in habitats recognized for their rich biodiversity and major conservation value [40,41]. They vary from sparse vegetation on shallow soils, to large-stature rainforest on deep soils overlaying ultramafic bedrock [39]. Depending on the metal, sequestration in hyperaccumulator shoots occurs in a variety of tissues, both non-photosynthetic and photsynthetic, with evidence of carboxylate and other counter anion associations, along with mediation via several classes of metal transporter proteins as implicated in these extreme and complex detoxification strategies [17,42,43]. While such mechanisms are of ongoing interest, there have been no studies into how climate change may impact them. Such novel systems are invaluable for expanding fundamental knowledge across multiple scientific disciplines, yet mining and land-clearing pose anthropogenic threats, with little yet known about potential climate change impacts. Laterite, another naturally metalliferous soil type for metal hyperaccumulators forms from deep weathering and leaching in the tropics where rainfall is high, or on geologically unchanged land where soils are relict [44,45]. For example, on the eastern seaboard of Australia, laterites are Al-, Fe- and Mn-rich, with smaller disjunct Ni-rich areas over Ni ore bodies. Bedrocks of ore bodies or igneous rock such as basalt or serpentine can give rise to high metal content in early soil horizons [45]. Oxidation of the parent rock gives rise to new minerals that get flushed out or concentrated, depending on their solubilities [46,47]. Whether the increasing frequency of major rain events attributable to climate change [48] will accelerate soil laterisation is yet to be determined.
Empirical studies into the direct effects of climatic changes on hyperaccumulator plant–metal interactions are extremely scarce, although several meta-analyses have attempted to assess impacts on specialised metallohphytic ecosystems [49,50,51]. It is plausible that shifts in climatic conditions detrimental to the long-term physiological health of hyperaccumulator plants may ultimately indirectly impair their intrinsic ability to mediate excess metals. Further enhancement of soil metal bioavailability through altered climatic conditions is unlikely to adversely impact plants already well adapted to metal oversupply provided they remain healthy [50]. Interestingly, controlled experiments on metal hyperaccumulators have been able to demonstrate metal over-accumulation to elicit stress thus far not reported in natural systems [52,53,54]. A meta-study modeling assessment of potential climate change effects on serpentine ecosystems cautiously suggests these plants may be less sensitive than non-serpentine plants, while stressing the need for a multidimensional comparative approach incorporating floras on many soil types, from ‘normal’ to ‘special’ such as metalliferous soils [51].
3. Agricultural and Other Land–Plant Systems
Nutrient metal imbalance in agricultural plants is well researched [55], useful for interrogating plant–metal interactions in a changing climate. By far, Mn is the most important example of a soil metal whose interaction with plants is climate-associated [3]. It is soil abundant and nutritionally essential in trace amounts only; yet its bioavailability is easily enhanced by environmental fluctuations, which in turn can drive Mn phytotoxicity, a common agricultural problem in regions such as eastern Australia on naturally Mn-rich soils [3,5,23,56,57,58,59]. It is noteworthy that Mn is commonly tolerated by plants in foliar concentrations above their critical nutritional needs, and this is unlike most other heavy metals that have clearly defined phytotoxicity thresholds [55]. While Mn oversupply and accumulation can induce dark leaf-spotting under experimental conditions [60], Mn phytotoxicity damage as observed in the field and further supported by controlled studies occurs as photobleaching by solar radiation, and oxidative stress due to ion antagonism between excess Mn(II) and metal cations [4,28,61]. Photobleaching manifests as leaf ‘bleaching’ (Figure 3) when photosynthetic apparatus are damaged by reactive oxygen species [28]. The presence of excess metal cations such as Mn(II) can lead to oxidative stress when they outcompete trace-metal-cation cofactors essential to the activities of mitigating enzymes such as superoxide dismutase [28]. These have been demonstrated in common bean, sugar maple (Figure 3) [28], wheat, soybean, and canola plants exposed to combined treatments of Mn and light exposure, and also observed in field canola and wheat crops exhibiting seasonal Mn toxicity stress (Figure 4) [4,30,61].
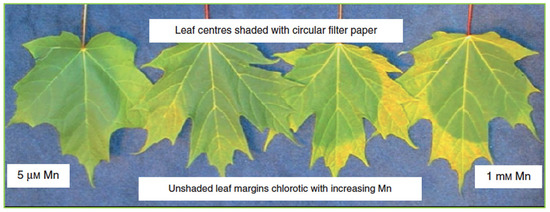
Figure 3.
Mn toxicity-induced photobleaching of Mn-treated maple leaves exposed to sunlight [60].

Figure 4.
Seasonal Mn toxicity in canola and wheat field-crops in southeastern Australia (Photo: D. Eksteen 2017).
It is now well established that solar radiation triggers Mn phytotoxicity-induced photobleaching damage to photosynthetic apparatus when leaf-Mn concentrations are elevated [3]. Increasingly lengthy periods of sunshine due to longer hotter summers and/or increased intensity due to ozone depletion have implications for plants normally capable of tolerating above-normal foliar Mn levels, as is common among certain crop cultivars [3]. Commercially valuable sugar maple forest trees known to overaccumulate foliar Mn due to anthropogenic soil acidification [30] have been documented as declining in parts of the Allegheny Plateau in Pennsylvania (Figure 5), and shown experimentally to be susceptible to Mn toxicity-induced oxidative stress and photobleaching [28] (Figure 3). Other climatic effects known to enhance Mn bioavailability to induce Mn overaccumulation and stress include soil waterlogging, soil acidification, extreme soil wetting and drying cycles, and increased ambient temperatures [3,5,23,56,57,58,59].

Figure 5.
Sugar maple trees showing signs of stress, Allegheny Plateau, Pennsylvania (Photo: D. Fernando 2013).
There is now a strong body of field and experimental evidence pointing to future scenarios of large-scale Mn phytotoxicity induced by changing climatic conditions in agricultural plants and in natural systems unadapted to metal oversupply, particularly where soils are Mn-rich. In areas where soil-Mn is insufficiently available to crop plants requiring Mn supplements, it is possible that such changing climatic conditions may boost soil-Mn bioavailability; however, there are no studies to support this other than a newspaper report in Western Australia of a first-time observation of Mn toxicity in canola crops that historically required Mn addition. Although the soil bioavailabilities of metals other than Mn can also be affected by soil chemical changes such as acidification leading to phytotoxicity, interaction between their bioavailabilities and environmental fluctuations are comparatively less well defined [3,55]. Increasing atmospheric CO2 levels, the main driver soil acidification will leave unadapted plants vulnerable to metal stress [20,29].
4. Plants Associated with Marine and Aquatic Ecosystems
Fresh- and salt-water ecosystems support diverse plant communities variously reliant on these water-bodies, from total continuous submersence to periodic flooding, for example, in riverine riparian–floodplain zones [1,31] (Figure 6). Changing climatic influences on these plant–metal interactions has attracted far less attention than land-only plants; however, a few disjunct studies collectively provide preliminary insights into environmental shifts including atmospheric CO2 emissions and the increasingly warming and drying conditions in many regions of the world [48]. Among the most damaging to sensitive oceanic ecosystems is acidification due to emissions, the driver of coral bleaching, which, combined with rising water temperatures, is driving down biodiversity [32].

Figure 6.
Essential flooding on a sandy riverine floodplain lake system assists native trees to access trace-metal nutrients [1] (Reprinted with permission from [1]. Copyright 2021 Copyright Department of Ecology in Australia).
Although overwhelming evidence exists for large-scale soil acidification from pH 6.5 to well below that, levels conducive to land (only) plant metal uptake beyond phytotoxicity thresholds depending on the metal [55], there is no evidence that water acidification has a similar direct effect in enhancing metal uptake by rooted water-submersed or water-emergent plants. While metal accumulation in rooted macrophytes has been shown to correlate with available metal content in their host substrates [62], understanding about how climate interacts with these underwater plant–substrate relationships is scarce. One study reports a direct relationship between temperature increase and metal uptake in two common rooted aquatic species without toxicity symptoms [33]. Another on seagrass response to seawater-pH manipulation from 8.36 to 8.06 showed no increased metal uptake nor detectable stress [10]. Whether this apparent lack of a ‘pH effect’ is due to the generally far higher pH levels in watery habitats compared to land soils, regardless of climatic water acidification, is unclear. How environmental changes may affect the well-known ability of many aquatic species to safely overaccumulate heavy metals, a trait regarded as useful for remediating metal-contaminated waters [63,64,65] is unknown. The aforementioned study by Fritoff et al. [33] of a single species warrants investigation of others to examine whether their metal-loading capacities are enhanced by temperature elevation.
Flooding in freshwater ecosystems is known to facilitate the soil bioavailability of essential trace-metal nutrients such as Mn, Zn, Fe, and Cu, particularly important for outer-floodplain species at the far reaches of natural flows on nutrient-poor, well-drained sandy soils [1]. There is preliminary evidence [1,66] that even short pulses of water as generated by very infrequent flooding provides necessary access to trace nutrients. A warming, drying climate coupled with water abstraction for agriculture and other purposes is increasingly impacting riverine vegetation in regions such as eastern Australia, where large stature trees in the agriculturally and ecologically important Murray Darling River Basin system are showing marked stress and decline in some parts [67] (Figure 7). While these stresses are not solely nutritional, interrelationships between multiple variables (Figure 1) can trigger a gradual net cascade of overall decline ultimately noticeable in advanced irreversible stages, scenarios playing out in some remote areas of natural habitats that escape attention until decline becomes noticeable.

Figure 7.
Native tree dieback in Hattah Kulkyne National Park, in the Murray Darling Basin, southeastern Australia (Photo: D. Fernando 2012).
5. Concluding Comments
Substantial knowledge gaps need addressing in order to formulate actionable evidence-based hypotheses and predictions about the wider implications of climate change for plant–metal interactions across agricultural, non-agricultural, and water-associated systems. As discussed here, there is substantial direct and indirect evidence pointing to large-scale environmental imbalances caused by climatic change that are capable of driving plant stress from metal toxicity to nutrient-metal deficiency. Decades of research into plant–metal interactions of land-only plant communities variously point to dire predictions for a changing climate, where soil acidification, elevated atomospheric temperatures, greater exposure to solar radiation, reduced water availability in many regions, increasing frequencies of drastic weather events such as flooding, stand to detrimentally alter the healthy equilibria of ‘normal’ plant–metal interactions to elicit metal toxicity or trace-metal deficiency. There is early indication that plants highly adapted and evolved to tolerate metal oversupply may be less vulnerable to climate-induced metal toxicity, although more basic data-gathering is necessary for forecasting their long-term health and persistence under changing conditions. Very limited understanding of the potential direct impacts of climatic changes on plant–metal interactions associated with water bodies preliminarily suggests warming-enhanced metal accumulation for water plants, and reduced trace-metal nutrient bioavailability on some river floodplains. A global approach incorporating currently disparate evidence of the potential long term destabilising effects of climate change on ‘normal’ healthy plant–metal interactions would assist future data-gathering and predictive modeling to address possible scenarios of broad-scale damage.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fernando, D.R.; Fernando, A.E.; Koerber, G.R.; Doody, T.M. Tree-soil interactions through water release to a floodplain ecosystem: A case study of Black Box (Eucalyptus largiflorens) on loamy sands. Wetlands 2021, 41, 17–35. [Google Scholar] [CrossRef]
- Lynch, J.P.; Clair, S.B.S. Mineral stress: The missing link in understanding how global climate change will affect plants in real world soils. Fields Crops Res. 2004, 90, 101–115. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.R.; Moroni, S.J.; Scott, B.J.; Conyers, M.K.; Lynch, J.P.; Marshall, A.T. Temperature and light drive manganese accumulation and stress in crops across three major plant families. Environ. Exp. Bot. 2016, 132, 66–79. [Google Scholar] [CrossRef]
- Heenan, D.P.; Carter, O.G. Influence of temperature on the expression of manganese toxicity by two soybean varieties. Plant Soil 1977, 47, 219–227. [Google Scholar] [CrossRef]
- Rajkumar, M.; Narasimha, M.; Prasad, V.; Swaminathan, S.; Freitas, H. Climate change driven plant–metal–microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Clair, S.B.S.; Lynch, J.P. The opening of Pandora’s Box: Climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 2010, 335, 101–115. [Google Scholar] [CrossRef]
- White, R.E. Principles and Practices of Soil Science—The Soil as a Natural Resource; Blackwell Science: Melbourne, Australia, 1997. [Google Scholar]
- Grobelak, A.; Kowalska, A. Heavy metal mobility in soil under futuristic climatic conditions. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 437–451. [Google Scholar]
- de los Santosa, C.B.; Arenasa, F.; Neupartha, T.; Santosa, M. Interaction of short-term copper pollution and ocean acidification in seagrass ecosystems: Toxicity, bioconcentration and dietary transfer. Mar. Pollut. Bull. 2019, 142, 155–163. [Google Scholar] [CrossRef]
- Luo, C.L.; Shen, Z.G.; Li, X.D. Root exudates increase metal accumulation in mixed cultures: Implications for naturally enhanced phytoextraction. Water Air Soil Pollut. 2008, 193, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Wang, Y. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef]
- Oburger, E.; Gruber, B.; Schindlegger, Y.; Schenkeveld, W.D.C.; Hann, S.; Kraemer, S.M.; Wenzel, W.W.; Puschenreite, M. Root exudation of phytosiderophores from soil-grown wheat. New Phytol. 2014, 203, 1161–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel-Rozas, M.M.; Madejon, E.; Madejon, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecht-Christoffers, M.M.; Braun, H.P.; Lemaitre-Guillier, C.; Van Dorsselaer, A.; Horst, W.J. Effect of manganese toxicity on the proteome of the leaf apoplast in cowpea. Plant Physiol. 2003, 133, 1935–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, D.R.; Mizuno, T.; Woodrow, I.E.; Baker, A.J.M.; Collins, R.N. Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol. 2010, 188, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.; Cotter-Howells, J.D.; Charnock, J.M.; Baker, A.J.M.; Smith, J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996, 379, 635–638. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. M-neuvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komal, T.; Mustafa, M.; Ali, Z.; Kazi, A.G. Heavy metal uptake and transport in plants. In Heavy Metal Contamination of Soils; Springer: Berlin/Heidelberg, Germany, 2015; pp. 181–194. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 68, 1707–1719. [Google Scholar] [CrossRef]
- White, M.C.; Decker, A.M.; Chaney, R.L. Metal complexation in xylem fluid. Plant Physiol. 1981, 67, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Saraswat, S.; Rai, J.P.N. Complexation and detoxification of Zn and Cd in metal accumulating plants. Rev. Environ. Sci. Bio/Technol. 2011, 10, 327–339. [Google Scholar] [CrossRef]
- Graham, R.D.; Hannam, R.J.; Uren, N.C. Manganese in soils and plants. In Proceedings of the Presented at the International Symposium on Manganese in Soils and Plants, Glen Osmond, Australia, 22–26 August 1988. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders—Strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A. The genetic basis of metal hyperaccumulation in plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Whiting, S.N. In search of the Holy Grail—A further step in understanding metal hyperaccumulation? New Phytol. 2002, 155, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.R. Plants That Hyperaccumulate Heavy Metals; CAB International: Oxford, UK; New York, NY, USA, 1998. [Google Scholar]
- Clair, S.B.S.; Lynch, J.P. Photosynthetic and antioxidative enzyme responses of sugar maple and red maple seedlings to excess manganese in contrasting light environments. Funct. Plant Biol. 2004, 31, 1005–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, G.H. Acidic deposition, nutrient leaching and forest growth. Biogeochemistry 2003, 65, 51–81. [Google Scholar] [CrossRef]
- St Clair, S.S.; Sharp, W.E.; Lynch, J. Key interactions between nutrient limitation and climatic factors in temperate forests: A synthesis of sugar maple literature. Can. J. For. Res. 2008, 38, 404–414. [Google Scholar] [CrossRef]
- Moreno-Jimenez, E.; Cesar, P.; Hugo, S.; Manzano, R.; Flagmeier, M.; Fernando, T.M. Aridity and reduced soil micronutrient availability in global drylands. Nat. Sustain. 2019, 2, 371–377. [Google Scholar] [CrossRef]
- Fabricus, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muellehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Fritoff, A.; Kautsky, L.; Greger, M. Influence of temperature and salinity on heavy metal uptake by submersed plants. Environ. Pollut. 2004, 133, 265–274. [Google Scholar] [CrossRef]
- Cesalpino, A. De Plantis Libri1583, 16(Florentiae), 369.
- Coleman, R.G.; Jove, C. Geological origin of Serpentinites. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept: Andover, UK, 1992; pp. 1–17. [Google Scholar]
- Lange, B.; van der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M.P. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef]
- van der Ent, A.; Jaffre, T.; Huillier, L.; Gibson, N.; Reeves, R. The flora of ultramafic soils in the Australia–Pacific Region: State of knowledge and research priorities. Plant Soil 2015, 63, 173–190. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M. Metal-accumulating plants. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley and Sons: New York, NY, USA, 2000; pp. 193–221. [Google Scholar]
- Proctor, J.; Nagy, L. Ultramafic rocks and their vegetation: An overview. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept: Andover, UK, 1992; pp. 469–493. [Google Scholar]
- Baker, A.J.M.; Proctor, J.; Reeves, R.D. (Eds.) The Vegetation of Ultramafic (Serpentine) Soils; Intercept: Andover, UK, 1992. [Google Scholar]
- Whiting, S.N.; Reeves, R.D.; Richards, D.; Johnson, M.S.; Cooke, J.A.; Malisse, F.; Paton, A.; Smith, J.A.C.; Angle, J.S.; Chaney, R.L.; et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor. Ecol. 2004, 12, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Fernando, D.R.; Marshall, A.; Baker, A.J.M.; Mizuno, T. Microbeam methodologies as powerful tools in manganese hyperaccumulation research: Present status and future directions. Front. Plant Sci. 2013, 4, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M. Lateritic Soils. Sci. Am. 1964, 211, 96–106. [Google Scholar] [CrossRef]
- CSIRO. Soils: An Australian Viewpoint; Academic Press: London, UK, 1983.
- Burger, P.A. The Greenvale nickel laterite orebody. In Proceedings of the International Laterite Symposium, New Orleans, LA, USA, July 1979. [Google Scholar]
- Golightly, J.P. Nickeliferous laterities: A general description. In Proceedings of the International Laterite Symposium, New Orleans, LA, USA, 1979. [Google Scholar]
- IPCC. Summary for Policymakers; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Corlett, R.T.; Tomlinson, K.W. Climate change and edaphic specialists: Irresistible force meets immovable object? Environ. Pollut. 2019, 255, 113169. [Google Scholar] [CrossRef]
- Luo, J.; Yang, G.; Igalavithana, A.D.; He, W.; Gaoc, B.; Tsang, D.C.W.; Sik Ok, Y. Effects of elevated CO2 on the phytoremediation efficiency of Noccaea caerulescens. Environ. Pollut. 2020, 35, 367–376. [Google Scholar] [CrossRef]
- Damschen, E.I.; Harrison, S.; Ackerly, D.D.; Fernandez-Going, B.M.; Anacker, B.L. Endemic plant communities on special soils: Early victims or hardy survivors of climate change? J. Ecol. 2012, 100, 1122–1130. [Google Scholar] [CrossRef]
- Wójcika, M.; Vangronsveld, J.; Tukiendorfa, A. Cadmium tolerance in Thlaspi caerulescens: I. Growth parameters, metal accumulation and phytochelatin synthesis in response to cadmium. Environ. Exp. Bot. 2005, 53, 151–161. [Google Scholar] [CrossRef]
- Mesnoua, M.; Mateos-Naranjo, E.; Barcia-Piedras, J.M.; Pérez-Romero, J.A.; Lotmani, B.; Redondo-Gómez, S. Physiological and biochemical mechanisms preventing Cd-toxicity in the hyperaccumulator Atriplex halimus L. Plant Physiol. Biochem. 2016, 106, 30–38. [Google Scholar] [CrossRef]
- Magri, E.; Kieras-Gugelmin, E.; Grabarski, F.A.P.; Barbosa, J.Z.; Auler, A.C.; Wendlinge, I.; Priorf, S.A.; Valdugag, A.T.; Mottad, A.C.V. Manganese hyperaccumulation capacity of Ilex paraguariensis A. St. Hil. and occurrence of interveinal chlorosis induced by transient toxicity. Ecotoxicol. Environ. Saf. 2020, 203, 111010. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2002. [Google Scholar]
- El-Jaoual, T.; Cox, D.A. Manganese toxicity in plants. J. Plant Nutr. 1998, 21, 353–386. [Google Scholar] [CrossRef]
- Elamin, O.M.; Wilcox, G.E. Manganese toxicity development in muskmelons as influenced by nitrogen form. J. Am. Hortic. Soc. 1986, 111, 323–327. [Google Scholar]
- Moraghan, J.T. Manganese nutrition of flax as affected by FeEDDHA and soil air drying. Soil Sci. Soc. Am. 1985, 49, 668–671. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Uren, N.C. Manganese oxidation and reduction in soils: Effects of temperature, water potential, pH and their interactions. Soil Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]
- Horst, W.J.; Marschner, H. Symptome von mangan-uberschuB bei bohnen (Phaseolus vulgaris). Z. Pflanz. Bodenkd 1978, 141, 129–142. [Google Scholar] [CrossRef]
- González, A.; Steffen, K.L.; Lynch, J.P. Light and excess manganese. Plant Physiol. 1998, 118, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.J. Paradigms of metal accumulation in rooted aquatic vascular plants. Sci. Total Environ. 1998, 219, 223–231. [Google Scholar] [CrossRef]
- Soltan, M.E.; Rashed, M.N. Laboratory study on the survival of water hyacinth under several conditions of heavy metal concentrations. Adv. Environ. Res. 2003, 7, 321–334. [Google Scholar] [CrossRef]
- Van Steveninck, R.F.M.; Van Steveninck, M.E.; Fernando, D.R. Heavy-metal (Zn, Cd) tolerance in selected clones of Duck Weed (Lemna minor). Plant Soil 1992, 146, 271–280. [Google Scholar] [CrossRef]
- Vesk, P.A.; Nockolds, C.E.; Allaway, W.G. Metal localization in water hyacinth roots from an urban wetland. Plant Cell Environ. 1999, 22, 149–158. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P.; Reichman, S.; Clark, G.; Miller, R.; Doody, T. Inundation of a floodplain lake woodlands system: Nutritional profiling and benefit to mature Eucalyptus largiflorens (Black Box) trees. Wetl. Ecol. Manag. 2018, 26, 961–975. [Google Scholar] [CrossRef]
- Kingsford, R.T. Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol. 2000, 25, 109–127. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).