Prospect and Challenges for Sustainable Management of Climate Change-Associated Stresses to Soil and Plant Health by Beneficial Rhizobacteria
Abstract
:1. Introduction
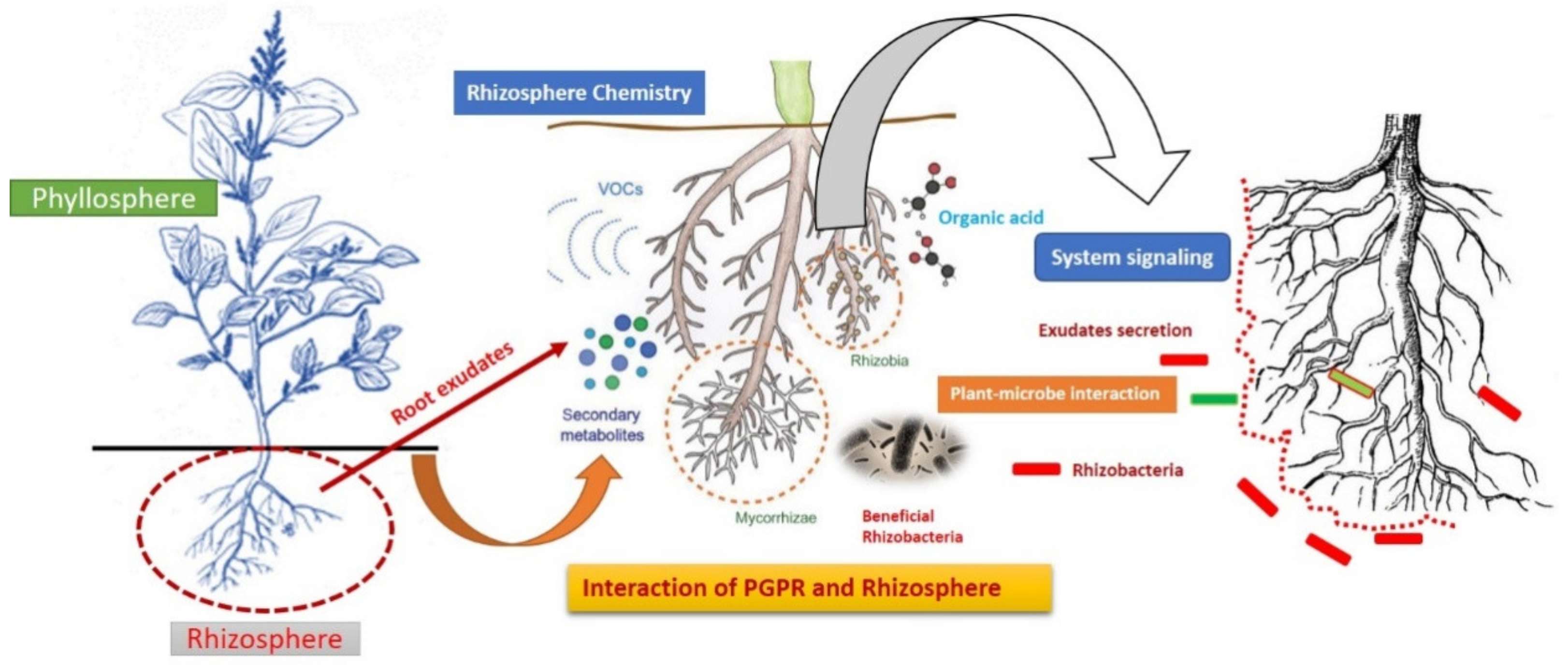
2. Rhizosphere as a Crucial Hotbed for Plant-Beneficial Rhizobacteria Interaction
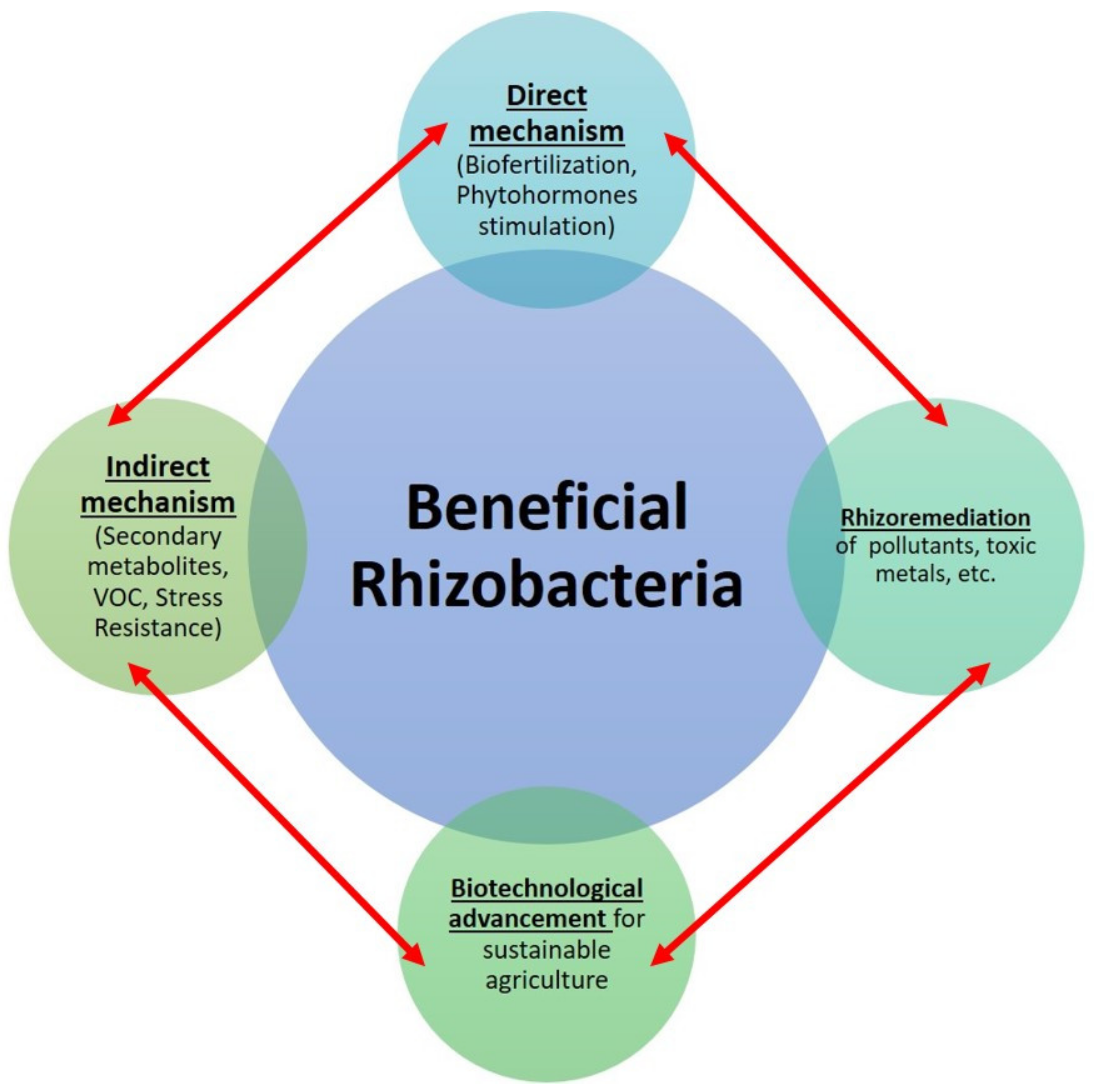
3. Beneficial Role of Rhizobacteria for the Enhancement of Soil and Plant Health
4. Improvement of Plant Health under Stressful Conditions by Beneficial Rhizobacteria
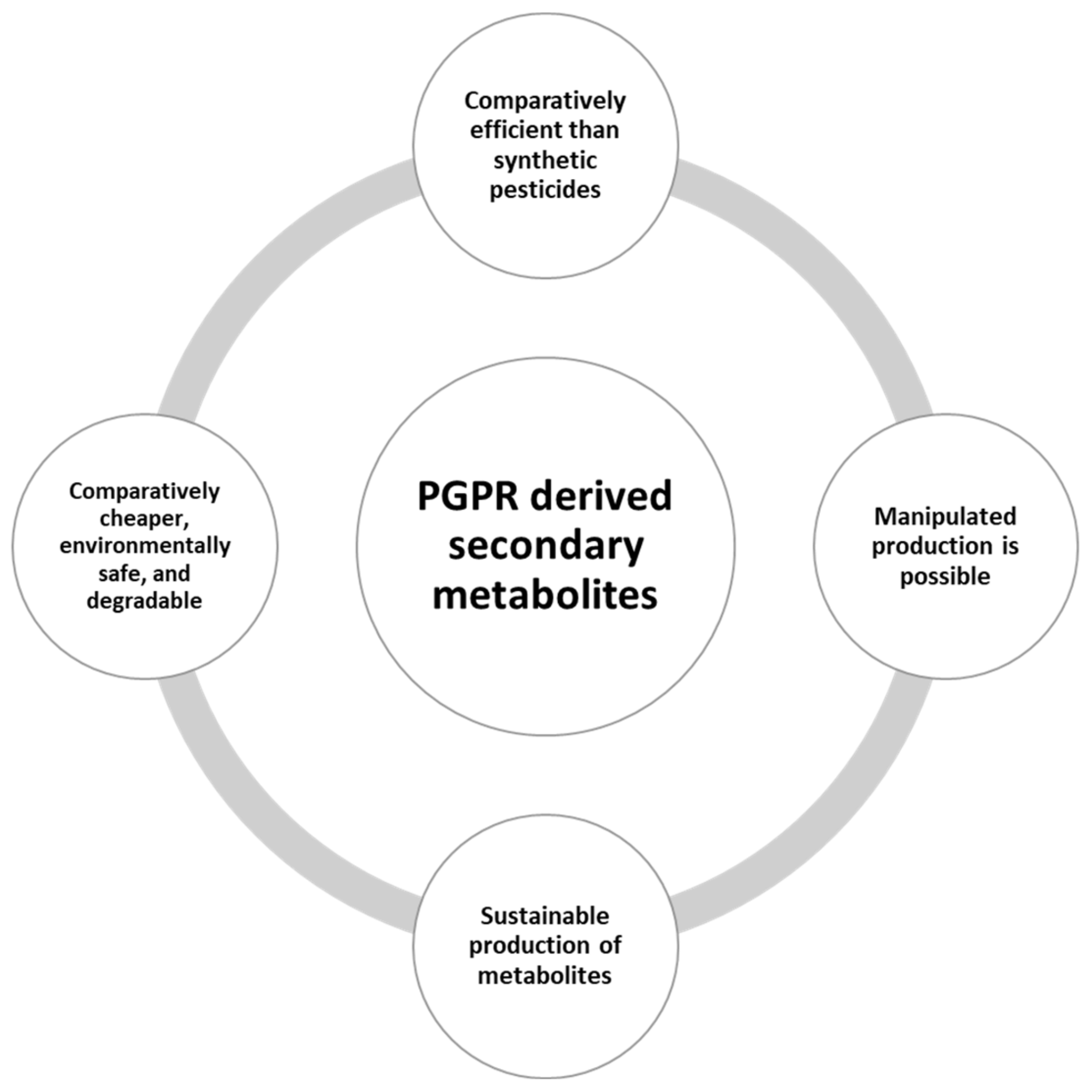
4.1. Production of Secondary Metabolites
4.2. Enhancement of Plant Stress Tolerance
4.3. Mitigation of Biotic Stress
4.4. Mitigation of Abiotic Stress
4.5. Remediation of Plant Stress Caused by Pollutants
4.6. Biocontrol Activities of Beneficial Bacteria
4.7. Production of Antibiotics and Siderophores
4.8. Induced Systemic Resistance
5. Application of PGPR for Sustainable Soil Health and Native Microbial Diversity
6. Industry-Laboratory Research Gap for Commercial Applications
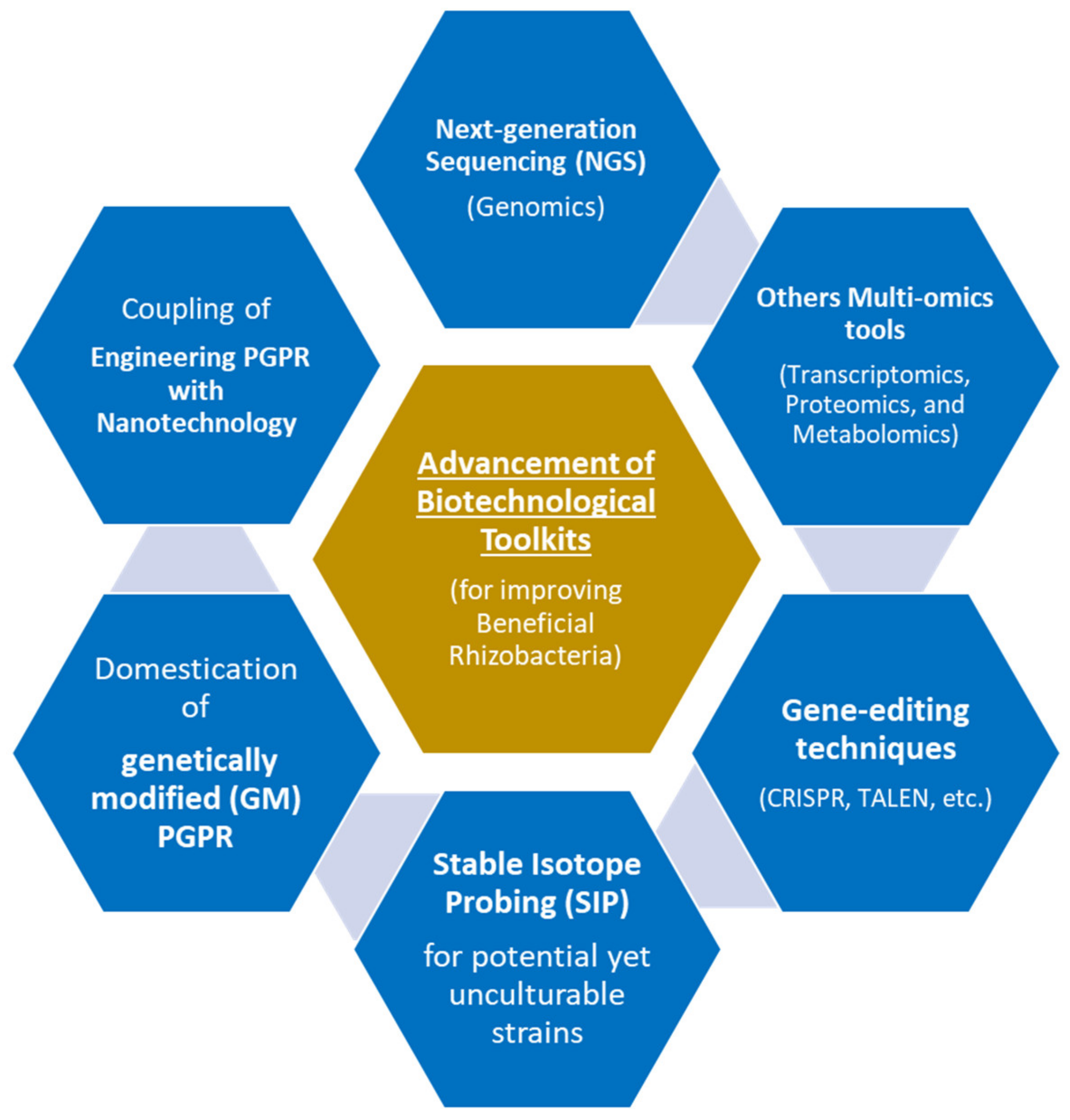
7. Advanced Biotechnological Tools for Improving Beneficial Rhizobacteria
8. Omics-Driven Approaches for Engineering of Beneficial Rhizobacteria
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.M.; Waqas, M.; Khan, A.L.; Lee, I.J. Plant-Growth-Promoting Rhizobacteria: Potential Candidates for Gibberellins Production and Crop Growth Promotion. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, pp. 1–19. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmood, U.; Inam-ul-Haq, M.; Saeed, M.; Altaf, A.; Azam, F.; Hayat, S. A Brief Review on Plant Growth Promoting Rhizobacteria (PGPR): A Key Role in Plant Growth Promotion. Plant Prot. 2018, 2, 77–82. [Google Scholar]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, N.; Yadav, A.N.; Kumar, A.; Meena, V.S.; Singh, B.; Chauhan, V.S.; Dhaliwal, H.S.; Saxena, A.K. Rhizospheric Microbiomes: Biodiversity, Mechanisms of Plant Growth Promotion, and Biotechnological Applications for Sustainable Agriculture. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability; Springer: Singapore, 2019; pp. 19–65. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, M.A. The Root Growth of Wheat Plants, the Water Conservation and Fertility Status of Sandy Soils Influenced by Plant Growth Promoting Rhizobacteria. Symbiosis 2016, 72, 195–205. [Google Scholar] [CrossRef]
- Islam, M.T.; Hashidoko, Y.; Deora, A.; Ito, T.; Tahara, S. Suppression of Damping-off Disease in Host Plants by the Rhizoplane Bacterium Lysobacter sp. Strain SB-K88 Is Linked to Plant Colonization and Antibiosis against Soilborne Peronosporomycetes. Appl. Environ. Microbiol. 2005, 71, 3786–3796. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, M.S.; Gupta, D.R.; Rahman, M.M.; Mahboob, M.G.; et al. Emergence of Wheat Blast in Bangladesh Was Caused by a South American Lineage of Magnaporthe oryzae. BMC Biol. 2016, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Rahman, M.M.; Pandey, P.; Boehme, M.H.; Haesaert, G. Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer International Publishing: New York, NY, USA, 2019; Volume 2. [Google Scholar]
- Verma, D.K.; Pandey, A.K.; Mohapatra, B.; Srivastava, S.; Kumar, V.; Talukdar, D.; Yulianto, R.; Zuan, A.T.K.; Jobanputra, A.H.; Asthir, B. Plant Growth-Promoting Rhizobacteria: An Eco-Friendly Approach for Sustainable Agriculture and Improved Crop Production. In Microbiology for Sustainable Agriculture, Soil Health, and Environmental Protection; Apple Academic Press: Boca Raton, FL, USA, 2019; pp. 3–80. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture: Perspectives and Challenges. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2019; pp. 129–157. [Google Scholar] [CrossRef]
- Gupta, K.; Dubey, N.K.; Singh, S.P.; Kheni, J.K.; Gupta, S.; Varshney, A. Plant Growth-Promoting Rhizobacteria (PGPR): Current and Future Prospects for Crop Improvement. In Current Trends in Microbial Biotechnology for Sustainable Agriculture. Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 203–226. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial Biofertilizers: Bioresources and Eco-Friendly Technologies for Agricultural and Environmental Sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria on Soil Health and the Sustainability of Agricultural Systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Shameer, S.; Prasad, T.N.V.K.V. Plant Growth Promoting Rhizobacteria for Sustainable Agricultural Practices with Special Reference to Biotic and Abiotic Stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant Growth-Promoting Rhizobacteria—Alleviators of Abiotic Stresses in Soil: A Review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Aeron, A.; Khare, E.; Jha, C.K.; Meena, V.S.; Aziz, S.M.A.; Islam, M.T.; Kim, K.; Meena, S.K.; Pattanayak, A.; Rajashekera, H.; et al. Revisiting the Plant Growth-Promoting Rhizobacteria: Lessons from the Past and Objectives for the Future. Arch. Microbiol. 2020, 202, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.N.; Kour, D.; Rana, K.L.; Kumar, V.; Dhaliwa, S.; Verma, P.; Singh, B.; Chauahan, V.S.; Sugitha, T.C.K.; Saxena, A.K. Plant Microbiomes and Its Beneficial Multifunctional Plant Growth Promoting Attributes. Int. J. Environ. Sci. Nat. Resour. 2017, 3, 555601. [Google Scholar] [CrossRef]
- Larsen, J.; Jaramillo-López, P.; Nájera-Rincon, M.; González-Esquivel, C. Biotic Interactions in the Rhizosphere in Relation to Plant and Soil Nutrient Dynamics. J. Soil Sci. Plant Nutr. 2015, 15, 449–463. [Google Scholar] [CrossRef] [Green Version]
- Sharaff, M.M.; Subrahmanyam, G.; Kumar, A.; Yadav, A.N. Mechanistic Understanding of the Root Microbiome Interaction for Sustainable Agriculture in Polluted Soils. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–84. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining Intraspecific Diversity in Plant Secondary Metabolites in an Ecological Context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, A.; Canfora, L.; Nigro, F.; De Vita, P.; Beleggia, R. Relationships between Root Morphology, Root Exudate Compounds and Rhizosphere Microbial Community in Durum Wheat. Appl. Soil Ecol. 2021, 158, 103781. [Google Scholar] [CrossRef]
- Bashey, F. Within-Host Competitive Interactions as a Mechanism for the Maintenance of Parasite Diversity. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [Green Version]
- Dignac, M.F.; Derrien, D.; Barré, P.; Barot, S.; Cécillon, L.; Chenu, C.; Chevallier, T.; Freschet, G.T.; Garnier, P.; Guenet, B.; et al. Increasing Soil Carbon Storage: Mechanisms, Effects of Agricultural Practices and Proxies. A Review. Agron. Sustain. Dev. 2017, 37, 14. [Google Scholar] [CrossRef] [Green Version]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The Role of Microbial Signals in Plant Growth and Development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Du, H.; Zeng, F.; Song, T.; Peng, W. Diminished Rhizosphere and Bulk Soil Microbial Abundance and Diversity across Succession Stages in Karst Area, Southwest China. Appl. Soil Ecol. 2021, 158, 103799. [Google Scholar] [CrossRef]
- Milkereit, J.; Geisseler, D.; Lazicki, P.; Settles, M.L.; Durbin-Johnson, B.P.; Hodson, A. Interactions between Nitrogen Availability, Bacterial Communities, and Nematode Indicators of Soil Food Web Function in Response to Organic Amendments. Appl. Soil Ecol. 2021, 157, 103767. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R.; Meena, V.S.; Islam, M.T. Co-Inoculation with Enterobacter and Rhizobacteria on Yield and Nutrient Uptake by Wheat (Triticum aestivum L.) in the Alluvial Soil Under Indo-Gangetic Plain of India. J. Plant Growth Regul. 2017, 36, 608–617. [Google Scholar] [CrossRef]
- Sarker, A.; Talukder, N.M.; Islam, M.T. Phosphate Solubilizing Bacteria Promote Growth and Enhance Nutrient Uptake by Wheat. Plant Sci. Today 2014, 1, 86–93. [Google Scholar] [CrossRef]
- Khanom, A.; Azad, M.A.K.; Ali, M.M.; Ali, M.Y.; Biswas, S.K.; Rahman, M.M. Plants and Microbes’ Responses to the Net Nitrification Rates of Chemical Fertilizers in Vegetable Soils. Appl. Soil Ecol. 2021, 158, 103783. [Google Scholar] [CrossRef]
- Khan, M.A.; Khatun, A.; Islam, T. Promotion of Plant Growth by Phytohormone Producing Bacteria. In Microbes in Action; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–43. [Google Scholar]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, O.C.; Nwadibe, E.C.; Kalu, A.U.; Unah, U.V. Plant Growth Promoting Rhizobacteria (PGPR): A Novel Agent for Sustainable Food Production. Am. J. Agric. Biol. Sci. 2019, 14, 35–54. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.O.; Omena-Garcia, R.P.; Oliveira, F.S.; Silva, W.A.; Hajirezaei, M.R.; Vallarino, J.G.; Ribeiro, D.M.; Fernie, A.R.; Nunes-Nesi, A.; Araújo, W.L. Differential Root and Shoot Responses in the Metabolism of Tomato Plants Exhibiting Reduced Levels of Gibberellin. Environ. Exp. Bot. 2019, 157, 331–343. [Google Scholar] [CrossRef]
- Dinnage, R.; Simonsen, A.K.; Barrett, L.G.; Cardillo, M.; Raisbeck-Brown, N.; Thrall, P.H.; Prober, S.M. Larger Plants Promote a Greater Diversity of Symbiotic Nitrogen-Fixing Soil Bacteria Associated with an Australian Endemic Legume. J. Ecol. 2019, 107, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing Micronutrient Uptake and Yield of Wheat through Bacterial PGPR Consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- Kundan, R.; Pant, G.; Jadon, N.; Agrawal, P.K. Plant Growth Promoting Rhizobacteria: Mechanism and Current Prospective. J. Fertil. Pestic. 2015, 6, 9. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2020, 21, 49–68. [Google Scholar] [CrossRef]
- Heydari, M.M.; Brook, R.M.; Jones, D.L. The Role of Phosphorus Sources on Root Diameter, Root Length and Root Dry Matter of Barley (Hordeum vulgare L.). J. Plant Nutr. 2018, 42, 1–15. [Google Scholar] [CrossRef]
- Tao, G.C.; Tian, S.J.; Cai, M.Y.; Xie, G.H. Phosphate-Solubilizing and -Mineralizing Abilities of Bacteria Isolated from Soils. Pedosphere 2008, 18, 515–523. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ahmad, H. Plant Growth Promoting Bacteria: Role in Soil Improvement, Abiotic and Biotic Stress Management of Crops. Plant Cell Rep. 2018, 37, 1599–1609. [Google Scholar] [CrossRef]
- Kafle, A.; Cope, K.R.; Raths, R.; Yakha, J.K.; Subramanian, S.; Bücking, H.; Garcia, K. Harnessing Soil Microbes to Improve Plant Phosphate Efficiency in Cropping Systems. Agronomy 2019, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects—A Review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium Solubilizing Rhizobacteria (KSR): Isolation, Identification, and K-Release Dynamics from Waste Mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Potassium-Solubilizing Microorganisms: Mechanism and Their Role in Potassium Solubilization and Uptake. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 203–219. [Google Scholar] [CrossRef]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial Siderophores Efficiently Provide Iron to Iron-Starved Tomato Plants in Hydroponics Culture. Antonie Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on Iron Availability in Soil: Interaction of Fe Minerals, Plants, and Microbes. J. Soils Sediments 2013, 14, 538–548. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic Bacteria and Their Potential to Enhance Heavy Metal Phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Kim, A.Y.; Lee, S.U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 Tolerates Oxidative and Nitrosative Stress and Promotes the Growth of Soybean by Modulating the Production of Phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [Green Version]
- Naz, I.; Bano, A.; Ul-Hassan, T. Isolation of Phytohormones Producing Plant Growth Promoting Rhizobacteria from Weeds Growing in Khewra Salt Range, Pakistan and Their Implication in Providing Salt Tolerance to Glycine max L. Afr. J. Biotechnol. 2011, 8, 5762–5766. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Rana, A.; Kabi, S.R.; Verma, S.; Adak, A.; Pal, M.; Shivay, Y.S.; Prasanna, R.; Nain, L. Prospecting Plant Growth Promoting Bacteria and Cyanobacteria as Options for Enrichment of Macro- and Micronutrients in Grains in Rice–Wheat Cropping Sequence. Cogent Food Agric. 2015, 1, 1037379. [Google Scholar] [CrossRef]
- Singh, D.; Geat, N.; Rajawat, M.V.S.; Mahajan, M.M.; Prasanna, R.; Singh, S.; Kaushik, R.; Singh, R.N.; Kumar, K.; Saxena, A.K. Deciphering the Mechanisms of Endophyte-Mediated Biofortification of Fe and Zn in Wheat. J. Plant Growth Regul. 2017, 37, 174–182. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Ansary, W.R.; Prince, F.R.K.; Haque, E.; Sultana, F.; West, H.M.; Rahman, M.; Mondol, A.M.; Akanda, A.M.; Rahman, M.; Clarke, M.L.; et al. Endophytic Bacillus spp. from Medicinal Plants Inhibit Mycelial Growth of Sclerotinia sclerotiorum and Promote Plant Growth. Z. Naturforsch. 2018, 73, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Mahmud, N.U.; Ullah, C.; Rahman, M.; Islam, T. Biological and Biorational Management of Blast Diseases in Cereals Caused by Magnaporthe oryzae. Crit. Rev. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Babalola, O.O. Microbial Inoculants for Improving Crop Quality and Human Health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.A.; Haque, E.; Paul, N.C.; Khaleque, M.A.; Al-Garni, S.M.S.; Rahman, M.; Islam, M.T. Enhancement of Growth and Grain Yield of Rice in Nutrient Deficient Soils by Rice Probiotic Bacteria. Rice Sci. 2017, 24, 264–273. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, T.; Jett, L.; Kotcon, J. Biocontrol Agent, Biofumigation, and Grafting with Resistant Rootstock Suppress Soil-Borne Disease and Improve Yield of Tomato in West Virginia. Crop Prot. 2021, 145, 105630. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic Nitrogen Fixation by Rhizobia—The Roots of a Success Story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Halder, S.K.; Yadav, A.N.; Mondal, K.C. Microbial Consortium with Multifunctional Plant Growth-Promoting Attributes: Future Perspective in Agriculture. In Advances in Plant Microbiome and Sustainable Agriculture. Microorganisms for Sustainability; Springer: Singapore, 2020; Volume 20, pp. 219–258. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, M.; Anand, K.; Saurabh, S.; Kaur, T.; Kour, D.; Yadav, A.N.; Kumar, M. Role and Potential Applications of Plant Growth-Promoting Rhizobacteria for Sustainable Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–60. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A Multidisciplinary Approach to Clean up Heavy Metal Contaminated Soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-Mediated Drought Tolerance: Current and Future Prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Paque, S.; Weijers, D. Q&A: Auxin: The Plant Molecule That Influences Almost Anything. BMC Biol. 2016, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Fatima, T.; Arora, N.K. Role of Secondary Metabolites from Plant Growth-Promoting Rhizobacteria in Combating Salinity Stress. In Plant Microbiome: Stress Response. Microorganisms for Sustainability; Springer: Singapore, 2018; Volume 5, pp. 127–163. [Google Scholar] [CrossRef]
- Kumar, P.; Dey, S.R.; Dwivedi, P. Plant- and Microbes-Mediated Secondary Metabolites: Remunerative Venture for Discovery and Development. In Current Trends in Microbial Biotechnology for Sustainable Agriculture. Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 353–385. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Mathivanan, N. Management of Plant Diseases by Microbial Metabolites. In Bacteria in Agrobiology: Plant Nutrient Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–265. [Google Scholar] [CrossRef]
- Borriss, R. Phytostimulation and Biocontrol by the Plant-Associated Bacillus amyloliquefaciens FZB42: An Update. In Bacilli and Agrobiotechnology; Springer: Cham, Switzerland, 2016; pp. 163–184. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus Velezensis AK-0 as a Biocontrol Agent against Apple Bitter Rot Caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus Amyloliquefaciens GA1 as a Source of Potent Antibiotics and Other Secondary Metabolites for Biocontrol of Plant Pathogens. Microb. Cell Factories 2009, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How Reactive Oxygen Species and Proline Face Stress Together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M.N.V. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer Science & Business Media: London, UK, 2011. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Das Mohapatra, P.K.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current Scenario and Future Prospects of Plant Growth-Promoting Rhizobacteria: An Economic Valuable Resource for the Agriculture Revival under Stressful Conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of Microorganisms in Adaptation of Agriculture Crops to Abiotic Stresses. World J. Microbiol. Biotechnol. 2010, 27, 1231–1240. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S. Pseudomonas Putida Attunes Morphophysiological, Biochemical and Molecular Responses in Cicer arietinum L. during Drought Stress and Recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Gulati, S.; Sharma, D.; Singh, R.N.; Rajawat, M.V.S.; Kumar, R.; Dey, R.; Pal, K.K.; Kaushik, R.; Saxena, A.K. Seasonal Variations in Culturable Archaea and Their Plant Growth Promoting Attributes to Predict Their Role in Establishment of Vegetation in Rann of Kutch. Biologia 2019, 74, 1031–1043. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Akhgar, A.R.; Arzanlou, M.; Bakker, P.A.H.M.; Hamidpour, M. Characterization of 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase-Containing Pseudomonas spp. in the Rhizosphere of Salt-Stressed Canola. Pedosphere 2014, 24, 461–468. [Google Scholar] [CrossRef]
- Yadav, A.N.; Yadav, N. Stress-Adaptive Microbes for Plant Growth Promotion and Alleviation of Drought Stress in Plants. Acta Sci. Agric. 2018, 2, 2–6. [Google Scholar]
- Dame, Z.T.; Rahman, M.; Islam, T. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green Chem. Lett. Rev. 2021, 14, 245–270. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mahmud, N.U.; Muzahid, A.N.M.; Rabby, S.M.F.; Islam, T. Oligomycins Inhibit Magnaporthe oryzae Triticum and Suppress Wheat Blast Disease. PLoS ONE 2020, 15, e0233665. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Mahmud, N.U.; Gupta, D.R.; Tareq, F.S.; Shin, H.J.; Islam, T. Inhibitory Effects of Linear Lipopeptides From a Marine Bacillus subtilis on the Wheat Blast Fungus Magnaporthe oryzae Triticum. Front. Microbiol. 2020, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Potentials for Biological Control of Plant Diseases by Lysobacter spp., with Special Reference to Strain SB-K88. In Bacteria in Agrobiology: Plant Growth Responses; Springer: Berlin/Heidelberg, Germany, 2011; pp. 335–363. [Google Scholar] [CrossRef]
- Islam, M.T.; Hossain, M.M. Biological Control of Peronosporomycete Phytopathogen by Bacterial Antagonist. In Bacteria in Agrobiology: Disease Management; Springer: Berlin/Heidelberg, Germany, 2013; pp. 167–218. [Google Scholar] [CrossRef]
- Islam, M.T. Disruption of Ultrastructure and Cytoskeletal Network Is Involved with Biocontrol of Damping-off Pathogen Aphanomyces cochlioides by Lysobacter sp. Strain SB-K88. Biol. Control. 2008, 46, 312–321. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Haggag, W.M.; Abouziena, H.F.; Abd-El-Kreem, F.; El Habbasha, S. Agriculture Biotechnology for Management of Multiple Biotic and Abiotic Environmental Stress in Crops. J. Chem. Pharm. Res. 2015, 7, 882–889. [Google Scholar]
- Jing, Y.; He, Z.; Yang, X. Role of Soil Rhizobacteria in Phytoremediation of Heavy Metal Contaminated Soils. J. Zhejiang Univ. Sci. B 2007, 8, 192–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and Identification of Plant Growth Promoting Rhizobacteria from Cucumber Rhizosphere and Their Effect on Plant Growth Promotion and Disease Suppression. Front. Microbiol. 2016, 6, 1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant Microbial Diversity Is Suggested as the Key to Future Biocontrol and Health Trends. FEMS Microbiol. Ecol. 2017, 93, 50. [Google Scholar] [CrossRef] [PubMed]
- Khatun, A.; Farhana, T.; Sabir, A.A.; Islam, S.M.N.; West, H.M.; Rahman, M.; Islam, T. Pseudomonas and Burkholderia Inhibit Growth and Asexual Development of Phytophthora Capsici. Z. Nat. 2018, 73, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaturvedi, A.K.; Yadav, K.; Arunkumar, K.P.; Malyan, S.K.; Raja, P.; Kumar, R.; Khan, S.A.; Yadav, K.K.; Rana, K.L.; et al. Fungal Phytoremediation of Heavy Metal-Contaminated Resources: Current Scenario and Future Prospects. In Recent Advancement in White Biotechnology through Fungi; Springer: Cham, Switzerland, 2019; pp. 437–461. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-Mediated Induction of Systemic Resistance and Physiochemical Alterations in Plants against the Pathogens: Current Perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, R.Z.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; Enshasy, H.E. Siderophore Production in Groundnut Rhizosphere Isolate, Achromobacter sp. RZS2 Influenced by Physicochemical Factors and Metal Ions. Environ. Sustain. 2019, 2, 117–124. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2011, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Ranjan Patra, R.; Lawrence, R. Characterization of Plant Growth Promoting Rhizobacteria Associated with Chickpea (Cicer arietinum L.). Int. J. Plant Prod. 2012, 1, 141–152. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Navathe, S.; Chand, R.; Pandey, S.P. A Halotolerant Growth Promoting Rhizobacteria Triggers Induced Systemic Resistance in Plants and Defends against Fungal Infection. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Zhou, M.; Li, P.; Wu, S.; Zhao, P.; Gao, H. Bacillus Subtilis CF-3 Volatile Organic Compounds Inhibit Monilinia fructicola Growth in Peach Fruit. Front. Microbiol. 2019, 10, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Hu, H.; Peng, H.; Wang, W.; Zhang, X. Comparative Genomic Analysis of Four Representative Plant Growth-Promoting Rhizobacteria in Pseudomonas. BMC Genom. 2013, 14, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, D.; Défago, G. Biological Control of Soil-Borne Pathogens by Fluorescent Pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic Colonization of Vitis vinifera L. by Plant Growth-Promoting Bacterium Burkholderia sp. Strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadfi, N.; Chérif, M.; Fliss, I.; Boudabbous, A.; Antoun, H. Evaluation of Bacterial Isolates from Salty Soils and Bacillus thuringiensis Strains for the Biocontrol of Fusarium Dry Rot of Potato Tubers. J. Plant Pathol. 2001, 83, 101–118. [Google Scholar] [CrossRef]
- Kanjanasopa, D.; Aiedhet, W.; Thitithanakul, S.; Paungfoo-Lonhienne, C. Plant Growth Promoting Rhizobacteria as Biological Control Agent in Rice. Agric. Sci. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. From Interaction to Gene Induction: An Eco-Friendly Mechanism of PGPR-Mediated Stress Management in the Plant. In Plant Microbiome: Stress Response; Springer: Singapore, 2018; pp. 217–232. [Google Scholar] [CrossRef]
- Mathiyazhagan, S.; Kavitha, K.; Nakkeeran, S.; Chandrasekar, G.; Manian, K.; Renukadevi, P.; Krishnamoorthy, A.; Fernando, W. PGPR Mediated Management of Stem Blight of Phyllanthus amarus (Schum and Thonn) Caused by Corynespora cassiicola (Berk and Curt) Wei. Arch. Phytopath. Plant Prot. 2011, 37, 183–199. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernández, M.G.; Ibarra-Laclette, E.; Délano-Frier, J.P. Inoculation of Tomato Plants (Solanum Lycopersicum) with Growth-Promoting Bacillus subtilis Retards Whitefly Bemisia tabaci Development. Planta 2009, 231, 397–410. [Google Scholar] [CrossRef]
- Agami, R.; Medani, R.A.; Abd El-Mola, I.A.; Taha, R.S. Exogenous Application with Plant Growth Promoting Rhizobacteria (PGPR) or Proline Induces Stress Tolerance in Basil Plants (Ocimum basilicum L.) Exposed to Water Stress. Int. J. Environ. Agric. Res. 2016, 2, 78–92. [Google Scholar]
- Santoyo, G.; Equihua, A.; Flores, A.; Sepulveda, E.; Valencia-Cantero, E.; Sanchez-Yañez, J.M.; Morales, L.R.; Govindappa, M.; de los Santos-Villalobos, S. Plant Growth Promotion by ACC Deaminase-Producing Bacilli under Salt Stress Conditions. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol. Bacilli in Climate Resilient Agriculture and Bioprospecting; Springer: Cham, Switzerland, 2019; pp. 81–95. [Google Scholar] [CrossRef]
- Grover, M.; Madhubala, R.; Ali, S.Z.; Yadav, S.K.; Venkateswarlu, B. Influence of Bacillus spp. Strains on Seedling Growth and Physiological Parameters of Sorghum under Moisture Stress Conditions. J. Basic Microbiol. 2014, 54, 951–961. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of Plant Growth-Promoting Rhizobacteria and Their Exopolysaccharide in Drought Tolerance of Maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought Stress Amelioration in Wheat through Inoculation with Burkholderia phytofirmans Strain PsJN. Plant Growth Regul. 2013, 73, 121–131. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense Ameliorates the Response of Arabidopsis Thaliana to Drought Mainly via Enhancement of ABA Levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; El Enshasy, H.A.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides Producing Bacteria for the Amelioration of Drought Stress in Wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Saharan, B.S.; Nehra, V. Plant Growth Promoting Rhizobacteria: A Critical Review. Life Sci. Med. Res. 2011, 2011, 21. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2014, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant Growth Promoting Activity of an Auxin and Siderophore Producing Isolate of Streptomyces under Saline Soil Conditions. World J. Microbiol. Biotechnol. 2011, 28, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal Changes in Relation to Biomass Partitioning and Shoot Growth Impairment in Salinized Tomato (Solanum lycopersicum L.) Plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]
- Rabhi, N.E.H.; Silini, A.; Cherif-Silini, H.; Yahiaoui, B.; Lekired, A.; Robineau, M.; Esmaeel, Q.; Jacquard, C.; Vaillant-Gaveau, N.; Clément, C.; et al. Pseudomonas knackmussii MLR6, a Rhizospheric Strain Isolated from Halophyte, Enhances Salt Tolerance in Arabidopsis thaliana. J. Appl. Microbiol. 2018, 125, 1836–1851. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth Promotion and Protection against Salt Stress by Pseudomonas putida Rs-198 on Cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-Resistant Plant Growth Promoting Rhizobacteria Ameliorates Sodium Chloride Stress on Tomato Plants. J. Plant Interact. 2009, 5, 51–58. [Google Scholar] [CrossRef]
- Hamdia, M.A.E.-S.; Shaddad, M.A.K.; Doaa, M.M. Mechanisms of Salt Tolerance and Interactive Effects of Azospirillum brasilense Inoculation on Maize Cultivars Grown under Salt Stress Conditions. Plant Growth Regul. 2004, 44, 165–174. [Google Scholar] [CrossRef]
- El-Azeem, S.A.M.A.; Elwan, M.W.M.; Sung, J.K.; Ok, Y.S. Alleviation of Salt Stress in Eggplant (Solanum melongena L.) by Plant-Growth-Promoting Rhizobacteria. Comm. Soil Sci. Plant Anal. 2012, 43, 1303–1315. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Kavino, M.; Raguchander, T.; Subbian, P.; Samiyappan, R. Plant Growth Promoting Bacteria Enhance Water Stress Resistance in Green Gram Plants. Acta Physiol. Plant. 2010, 33, 203–209. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201. [Google Scholar] [CrossRef]
- Castillo, P.; Escalante, M.; Gallardo, M.; Alemano, S.; Abdala, G. Effects of Bacterial Single Inoculation and Co-Inoculation on Growth and Phytohormone Production of Sunflower Seedlings under Water Stress. Acta Physiol. Plant. 2013, 35, 2299–2309. [Google Scholar] [CrossRef]
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [Green Version]
- Sarma, R.K.; Saikia, R. Alleviation of Drought Stress in Mung Bean by Strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2013, 377, 111–126. [Google Scholar] [CrossRef]
- Fan, X.; Hu, H.; Huang, G.; Huang, F.; Li, Y.; Palta, J. Soil Inoculation with Burkholderia sp. LD-11 Has Positive Effect on Water-Use Efficiency in Inbred Lines of Maize. Plant Soil 2015, 390, 337–349. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant Growth Promoting Rhizobacteria Dietzia natronolimnaea Modulates the Expression of Stress Responsive Genes Providing Protection of Wheat from Salinity Stress. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant Growth-Promoting Rhizobacteria Enhance Wheat Salt and Drought Stress Tolerance by Altering Endogenous Phytohormone Levels and TaCTR1/TaDREB2 Expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Rima, F.S.; Biswas, S.; Sarker, P.K.; Islam, M.R.; Seraj, Z.I. Bacteria Endemic to Saline Coastal Belt and Their Ability to Mitigate the Effects of Salt Stress on Rice Growth and Yields. Ann. Microbiol. 2018, 68, 525–535. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt Stress Alleviation in Citrus Plants by Plant Growth-Promoting Rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Vimal, S.R.; Patel, V.K.; Singh, J.S. Plant Growth Promoting Curtobacterium albidum Strain SRV4: An Agriculturally Important Microbe to Alleviate Salinity Stress in Paddy Plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- El-Akhdar, I.; Elsakhawy, T.; Abo-Koura, H.A. Alleviation of Salt Stress on Wheat (Triticum aestivum L.) by Plant Growth Promoting Bacteria Strains Bacillus halotolerans MSR-H4 and Lelliottia amnigena MSR-M49. J. Adv. Microbiol. 2020, 44–58. [Google Scholar] [CrossRef] [Green Version]
- El-Nahrawy, S.; Yassin, M. Response of Different Cultivars of Wheat Plants (Triticum sestivum L.) to Inoculation by Azotobacter sp. under Salinity Stress Conditions. J. Adv. Microbiol. 2020, 59–79. [Google Scholar] [CrossRef] [Green Version]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Plant Growth-Promoting Rhizobacteria Ameliorates Salinity Stress in Pea (Pisum sativum). J. Plant Growth Regul. 2021, 1–10. [Google Scholar] [CrossRef]
- Chitara, M.K.; Chauhan, S.; Singh, R.P. Bioremediation of Polluted Soil by Using Plant Growth–Promoting Rhizobacteria. In Microbial Rejuvenation of Polluted Environment; Springer: Singapore, 2021; pp. 203–226. [Google Scholar] [CrossRef]
- Kumar, M.; Saxena, R.; Rai, P.K.; Tomar, R.S.; Yadav, N.; Rana, K.L.; Kour, D.; Yadav, A.N. Genetic Diversity of Methylotrophic Yeast and Their Impact on Environments. In Recent Advancement in White Biotechnology Through Fungi; Springer: Cham, Switzerland, 2019; pp. 53–71. [Google Scholar] [CrossRef]
- Kumar, G.P.; Desai, S.; Moerschbacher, B.M.; Gueddari, N.E.E. Seed Treatment with Chitosan Synergizes Plant Growth Promoting Ability of Pseudomonas aeruginosa-P17 in Sorghum (Sorhum bicolor L.). bioRxiv 2019, 601328. [Google Scholar] [CrossRef] [Green Version]
- Malyan, S.K.; Kumar, A.; Baram, S.; Kumar, J.; Singh, S.; Kumar, S.S.; Yadav, A.N. Role of Fungi in Climate Change Abatement Through Carbon Sequestration. In Recent Advancement in White Biotechnology through Fungi; Springer: Cham, Switzerland, 2019; pp. 283–295. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.K.; Joner, E. Utilising the Synergy between Plants and Rhizosphere Microorganisms to Enhance Breakdown of Organic Pollutants in the Environment. Environ. Sci. Pollut. Res. 2004, 12, 34–48. [Google Scholar] [CrossRef]
- Yancheshmeh, J.B. Evaluation of Inoculation of Plant Growth-Promoting Rhizobacteria on Cadmium and Lead Uptake by Canola and Barley. Afr. J. Microbiol. Res. 2011, 5, 128–132. [Google Scholar] [CrossRef]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in Bioremediation and Plant Growth Promotion in Heavy Metal Contaminated Soil. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar]
- Kotoky, R.; Das, S.; Singha, L.P.; Pandey, P.; Singha, K.M. Biodegradation of Benzo(a)pyrene by Biofilm Forming and Plant Growth Promoting Acinetobacter sp. Strain PDB4. Environ. Technol. Innov. 2017, 8, 256–268. [Google Scholar] [CrossRef]
- Kour, D.; Kaur, T.; Devi, R.; Rana, K.L.; Yadav, N.; Rastegari, A.A.; Yadav, A.N. Biotechnological Applications of Beneficial Microbiomes for Evergreen Agriculture and Human Health. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 255–279. [Google Scholar] [CrossRef]
- Singh, A.; Kumari, R.; Yadav, A.N.; Mishra, S.; Sachan, A.; Sachan, S.G. Tiny Microbes, Big Yields: Microorganisms for Enhancing Food Crop Production for Sustainable Development. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Ulloa-Ogaz, A.; Munoz-Castellanos, L.; Nevarez-Moorillon, G. Biocontrol of Phytopathogens: Antibiotic Production as Mechanism of Control. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Formatex Research Center: Badajoz, Spain, 2015; pp. 305–309. [Google Scholar]
- Shilev, S. Soil Rhizobacteria Regulating the Uptake of Nutrients and Undesirable Elements by Plants. In Rhizobacteria Regulating the Uptake of Nutrients and Undesirable Elements by Plants; Springer: New Delhi, India, 2013; pp. 147–167. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Nakkeeran, S.; Zhang, Y. Biosynthesis of Antibiotics by PGPR and Its Relation in Biocontrol of Plant Diseases. In PGPR Biocontrol Biofertilization; Springer: Dordrecht, The Netherlands, 2005; pp. 67–109. [Google Scholar] [CrossRef]
- Malfanova, N.; Franzil, L.; Lugtenberg, B.; Chebotar, V.; Ongena, M. Cyclic Lipopeptide Profile of the Plant-Beneficial Endophytic Bacterium Bacillus subtilis HC8. Arch. Microbiol. 2012, 194, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Milner, J.L.; Silo-Suh, L.; Lee, J.C.; Haiyin, H.E.; Clardy, J.; Handelsman, J.O. Production of Kanosamine by Bacillus cereus UW85. Appl. Environ. Microbiol. 1996, 62, 3061–3065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, B.; Nielsen, T.H.; Sørensen, D.; Andersen, J.B.; Christophersen, C.; Molin, S.; Givskov, M.; Sørensen, J.; Nybroe, O. Lipopeptide Production in Pseudomonas sp. Strain DSS73 Is Regulated by Components of Sugar Beet Seed Exudate via the Gac Two-Component Regulatory System. Appl. Environ. Microbiol. 2002, 68, 4509–4516. [Google Scholar] [CrossRef] [Green Version]
- Loper, J.E.; Gross, H. Genomic Analysis of Antifungal Metabolite Production by Pseudomonas fluorescens Pf-5. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer: Dordrecht, The Netherlands, 2007; pp. 265–278. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial Interactions and Biocontrol in the Rhizosphere. J. Exp. Bot. 2001, 52 (Suppl. S1), 487–511. [Google Scholar] [CrossRef]
- Gupta, A.; Gopal, M. Siderophore Production by Plant Growth Promoting Rhizobacteria. Indian J. Agric. Res. 2008, 42, 153–156. [Google Scholar]
- Rastegari, A.A.; Yadav, A.N.; Yadav, N. New and Future Developments in Microbial Biotechnology and Bioengineering: Trends of Microbial Biotechnology for Sustainable Agriculture and Biomedicine Systems: Perspectives for Human Health; Elsevier: Amsterdam, The Netherlands, 2020; p. 299. [Google Scholar]
- Kamal, R.; Gusain, Y.S.; Kumar, V. Interaction and Symbiosis of AM Fungi, Actinomycetes and Plant Growth Promoting Rhizobacteria with Plants: Strategies for the Improvement of Plants Health and Defense System. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 564–585. [Google Scholar]
- Berendsen, R.L.; van Verk, M.C.; Stringlis, I.A.; Zamioudis, C.; Tommassen, J.; Pieterse, C.M.J.; Bakker, P.A.H.M. Unearthing the Genomes of Plant-Beneficial Pseudomonas Model Strains WCS358, WCS374 and WCS417. BMC Genom. 2015, 16, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Ramadan, E.M.; AbdelHafez, A.A.; Hassan, E.A.; Saber, F.M. Plant Growth Promoting Rhizobacteria and Their Potential for Biocontrol of Phytopathogens. Afr. J. Microbiol. Res. 2016, 10, 486–504. [Google Scholar] [CrossRef]
- Di Salvo, L.P.; Cellucci, G.C.; Carlino, M.E.; García de Salamone, I.E. Plant Growth-Promoting Rhizobacteria Inoculation and Nitrogen Fertilization Increase Maize (Zea mays L.) Grain Yield and Modified Rhizosphere Microbial Communities. Appl. Soil Ecol. 2018, 126, 113–120. [Google Scholar] [CrossRef]
- Hayat, R.; Ahmed, I.; Sheirdil, R.A. An Overview of Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. In Crop Production for Agricultural Improvement; Springer: Dordrecht, The Netherlands, 2012; pp. 557–579. [Google Scholar] [CrossRef]
- Sarker, A.; Nandi, R.; Kim, J.E.; Islam, T. Remediation of chemical pesticides from contaminated sites through potential microorganisms and their functional enzymes: Prospects and challenges. Environ. Technol. Innov. 2021, 23, 101777. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A Comprehensive Review on Enzymatic Degradation of the Organophosphate Pesticide Malathion in the Environment. J. Environ. Sci. Health Part C 2019, 37, 288–329. [Google Scholar] [CrossRef]
- Malyan, S.K.; Singh, R.; Rawat, M.; Kumar, M.; Pugazhendhi, A.; Kumar, A.; Kumar, V.; Kumar, S.S. An Overview of Carcinogenic Pollutants in Groundwater of India. Biocatal. Agric. Biotechnol. 2019, 21, 101288. [Google Scholar] [CrossRef]
- Parewa, H.P.; Yadav, J.; Rakshit, A.; Meena, V.S.; Karthikeyan, N. Plant growth promoting rhizobacteria enhance growth and nutrient uptake of crops. Agric. Sustain. Dev. 2014, 2, 101–116. [Google Scholar]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P.; et al. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Khan, A.; Sayyed, R.Z.; Seifi, S. Rhizobacteria: Legendary Soil Guards in Abiotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 327–343. [Google Scholar]
- Zahedi, H. Toward the mitigation of biotic and abiotic stresses through plant growth promoting rhizobacteria. In Advances in Organic Farming; Woodhead Publishing: Sawston, UK, 2021; pp. 161–172. [Google Scholar]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 682–1694. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Li, D.M.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Minkina, T.; Singh, R.K.; Singh, P.; Li, Y.R. Interactive role of silicon and plant–rhizobacteria mitigating abiotic stresses: A new approach for sustainable agriculture and climate change. Plants 2020, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Khan, M.Y.; Rai, P.; Prasad, R.; Tripathi, D.K.; Sharma, S. Exploring plant rhizobacteria synergy to mitigate abiotic stress: A new dimension toward sustainable agriculture. In Plant Life under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 861–882. [Google Scholar]
- Mantelin, S.; Touraine, B. Plant Growth-promoting Bacteria and Nitrate Availability: Impacts on Root Development and Nitrate Uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Ahemad, M.; Oves, M. Functional Diversity among Plant Growth-Promoting Rhizobacteria: Current Status. In Microbial Strategies for Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2009; pp. 105–132. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Chromium-Reducing and Plant Growth-Promoting Mesorhizobium Improves Chickpea Growth in Chromium-Amended Soil. Biotechnol. Lett. 2007, 30, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kumar, M.; Varma, A. Role of PGPR in Soil Fertility and Plant Health. In Role of PGPR in Soil Fertility and Plant Health; Springer: Cham, Switzerland, 2015; pp. 247–260. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial Siderophores in Community and Host Interactions. Nat. Rev. Microbiol. 2019, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of Plant Growth-Promoting Bacteria in Sustainable Agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Rani, U.; Sharma, S.; Kumar, V. Bacillus Species: A Potential Plant Growth Regulator. In Bacilli in Climate Resilient Agriculture and Bioprospecting; Springer: Cham, Switzerland, 2019; pp. 29–47. [Google Scholar]
- Lynch, J.M.; de Leij, F. Rhizosphere; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Biswas, S.; Kundu, D.; Mazumdar, S.; Saha, A.; Majumdar, B.; Ghorai, A.K.; Ghosh, D.; Yadav, A.N.; Saxena, A.K. Study on the activity and diversity of bacteria in a New Gangetic alluvial soil (Eutrocrept) under rice-wheat-jute cropping system. J. Environ. Biol. 2018, 39, 379–386. [Google Scholar] [CrossRef]
- Plant Growth and Health Promoting Bacteria; Maheshwari, D.K. (Ed.) Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Humaira, Y.; Asghari, B. Screening of PGPR isolates from semi-arid region and their implication to alleviate drought stress. Pak. J. Bot. 2013, 45, 51–58. [Google Scholar]
- Zahedi, A.M.; Fazeli, I.; Zavareh, M.; Dorry, H.; Gerayeli, N. Evaluation of the Sensitive Components in Seedling Growth of Common Bean (Phaseolus vulgaris L.) Affected by Salinity. Asian J. Crop Sci. 2012, 4, 159–164. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Rathore, A.P.; Sharma, S. Effect of Halotolerant Plant Growth Promoting Rhizobacteria Inoculation on Soil Microbial Community Structure and Nutrients. Appl. Soil Ecol. 2020, 150, 103461. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; Kloepper, J.W.; Reddy, M.S. Plant Growth-Promoting Rhizobacteria as Transplant Amendments and Their Effects on Indigenous Rhizosphere Microorganisms. Appl. Soil Ecol. 2006, 31, 91–100. [Google Scholar] [CrossRef]
- Jamal, Q.; Lee, Y.S.; Jeon, H.D.; Kim, K.Y. Effect of Plant Growth-Promoting Bacteria Bacillus amyloliquefaciens Y1 on Soil Properties, Pepper Seedling Growth, Rhizosphere Bacterial Flora and Soil Enzymes. Plant Prot. Sci. 2018, 54, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ma, J.; Mark Ibekwe, A.; Wang, Q.; Yang, C.H. Influence of Bacillus Subtilis B068150 on Cucumber Rhizosphere Microbial Composition as a Plant Protective Agent. Plant Soil 2018, 429, 519–531. [Google Scholar] [CrossRef]
- Kari, A.; Nagymáté, Z.; Romsics, C.; Vajna, B.; Kutasi, J.; Puspán, I.; Kárpáti, É.; Kovács, R.; Márialigeti, K. Monitoring of Soil Microbial Inoculants and Their Impact on Maize (Zea mays L.) Rhizosphere Using T-RFLP Molecular Fingerprint Method. Appl. Soil Ecol. 2019, 138, 233–244. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher-Jenull, J.; Ceccherini, M.T.; Pietramellara, G.; Renella, G.; Schloter, M. Beyond Microbial Diversity for Predicting Soil Functions: A Mini Review. Pedosphere 2020, 30, 5–17. [Google Scholar] [CrossRef]
- Gusain, P.; Bhandari, B.S. Rhizosphere Associated PGPR Functioning. J. Pharmacogn. Phytochem. 2019, 8, 1181–1191. [Google Scholar]
- Gagné, S.; Dehbi, L.; Le Quéré, D.; Cayer, F.; Morin, J.L.; Lemay, R.; Fournier, N. Increase of Greenhouse Tomato Fruit Yields by Plant Growth-Promoting Rhizobacteria (PGPR) Inoculated into the Peat-Based Growing Media. Soil Biol. Biochem. 1993, 25, 269–272. [Google Scholar] [CrossRef]
- Murphy, J.F.; Zehnder, G.W.; Schuster, D.J.; Sikora, E.J.; Polston, J.E.; Kloepper, J.W. Plant Growth-Promoting Rhizobacterial Mediated Protection in Tomato Against Tomato Mottle Virus. Plant Dis. 2007, 84, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Von Der Weid, I.; Paiva, E.; Nóbrega, A.; Dirk Van Elsas, J.; Seldin, L. Diversity of Paenibacillus polymyxa Strains Isolated from the Rhizosphere of Maize Planted in Cerrado Soil. Res. Microbiol. 2000, 151, 369–381. [Google Scholar] [CrossRef]
- Zandi, P.; Basu, S.K. Role of Plant Growth-Promoting Rhizobacteria (PGPR) as BioFertilizers in Stabilizing Agricultural Ecosystems. In Organic Farming for Sustainable Agriculture; Springer: Cham, Switzerland, 2016; pp. 71–87. [Google Scholar] [CrossRef]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant Growth Promoting Rhizobacteria: Constraints in Bioformulation, Commercialization, and Future Strategies. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–116. [Google Scholar] [CrossRef]
- Mahajan, A.; Gupta, R.D. Integrated Nutrient Management (INM) in a Sustainable Rice-Wheat Cropping System; Springer Science & Business Media: London, UK, 2009. [Google Scholar]
- Kumari, B.; Mallick, M.A.; Solanki, M.K.; Solanki, A.C.; Hora, A.; Guo, W. Plant Growth Promoting Rhizobacteria (PGPR): Modern Prospects for Sustainable Agriculture. In Plant Health under Biotic Stress; Springer: Singapore, 2019; pp. 109–127. [Google Scholar] [CrossRef]
- Riaz, U.; Murtaza, G.; Anum, W.; Samreen, T.; Sarfraz, M.; Nazir, M.Z. Plant Growth-Promoting Rhizobacteria (PGPR) as Biofertilizers and Biopesticides. In Microbiota and Biofertilizers; Springer: Cham, Switzerland, 2021; pp. 181–196. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2013, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Kohler, C.; Lourenço, R.F.; Bernhardt, J.; Albrecht, D.; Schüler, J.; Hecker, M.; Gomes, S.L. A Comprehensive Genomic, Transcriptomic and Proteomic Analysis of a Hyperosmotic Stress Sensitive α-Proteobacterium. BMC Microbiol. 2015, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Köberl, M.; White, R.A.; Erschen, S.; El-Arabi, T.F.; Jansson, J.K.; Berg, G. Draft Genome Sequence of Paenibacillus polymyxa Strain Mc5Re-14, an Antagonistic Root Endophyte of Matricaria Chamomilla. Genome Announc. 2015, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of Genes Contributing to Plant-Beneficial Functions in Plant Growth-Promoting Rhizobacteria and Related Proteobacteria. Sci. Rep. 2014, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, S.; Niazi, A.; Bejai, S.; Meijer, J.; Bongcam-Rudloff, E. Genome Sequence of a Plant-Associated Bacterium, Bacillus amyloliquefaciens Strain UCMB5036. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, D.; Dineshkumar, N.; Nair, S. Proteomics of a Plant Growth-Promoting Rhizobacterium, Pseudomonas fluorescens MSP-393, Subjected to Salt Shock. World J. Microbiol. Biotechnol. 2006, 22, 369–374. [Google Scholar] [CrossRef]
- Palazzotto, E.; Weber, T. Omics and Multi-Omics Approaches to Study the Biosynthesis of Secondary Metabolites in Microorganisms. Curr. Opin. Microbiol. 2018, 45, 109–116. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of Secondary Metabolism in Actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Schenk, P.M.; Carvalhais, L.C.; Kazan, K. Unraveling Plant–Microbe Interactions: Can Multi-Species Transcriptomics Help? Trends Biotechnol. 2012, 30, 177–184. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Nagaraju, G.P.; Kariali, E. Metagenomic Approaches in Understanding the Mechanism and Function of PGPRs: Perspectives for Sustainable Agriculture. In Agriculturally Important Microbes for Sustainable Agriculture; Springer: Singapore, 2017; Volume 1, pp. 163–182. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, V. Role of Metagenomics in Deciphering the Microbial Communities Associated with Rhizosphere of Economically Important Plants. In Current Trends in Microbial Biotechnology for Sustainable Agriculture. Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 79–94. [Google Scholar] [CrossRef]
- Keswani, C.; Prakash, O.; Bharti, N.; Vílchez, J.I.; Sansinenea, E.; Lally, R.D.; Borriss, R.; Singh, S.P.; Gupta, V.K.; Fraceto, L.F.; et al. Re-Addressing the Biosafety Issues of Plant Growth Promoting Rhizobacteria. Sci. Total Environ. 2019, 690, 841–852. [Google Scholar] [CrossRef]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering Rhizobacteria for Sustainable Agriculture. ISME J. 2020, 15, 949–964. [Google Scholar] [CrossRef]
- Setten, L.; Soto, G.; Mozzicafreddo, M.; Fox, A.R.; Lisi, C.; Cuccioloni, M.; Angeletti, M.; Pagano, E.; Díaz-Paleo, A.; Ayub, N.D. Engineering Pseudomonas Protegens Pf-5 for Nitrogen Fixation and Its Application to Improve Plant Growth under Nitrogen-Deficient Conditions. PLoS ONE 2013, 8, e63666. [Google Scholar] [CrossRef]
- Shulse, C.N.; Chovatia, M.; Agosto, C.; Yoshikuni, G.Y.; Hamilton, M.; Deutsch, S.; Yoshikuni, Y.; Blow, M.J. Engineered Root Bacteria Release Plant-Available Phosphate from Phytate. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Shweta, S.; Prasad, M.; Lata, C. Genome-Wide Investigation of GRAM-Domain Containing Genes in Rice Reveals Their Role in Plant-Rhizobacteria Interactions and Abiotic Stress Responses. Int. J. Biol. Macromol. 2020, 156, 1243–1257. [Google Scholar] [CrossRef]
- Basu, S.; Rabara, R.C.; Negi, S.; Shukla, P. Engineering PGPMOs through Gene Editing and Systems Biology: A Solution for Phytoremediation? Trends Biotechnol. 2018, 36, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Khatun, A.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.M.; Mahmud, N.U.; Emes, R.D.; Warry, A.; West, H.M.; Clarke, M.L.; et al. Whole-Genome Sequence of a Plant Growth-Promoting Strain, Serratia marcescens BTL07, Isolated from the Rhizoplane of Capsicum annuum L. Microbiol. Resour. Announc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Surovy, M.Z.; Gupta, D.R.; Mahmud, N.U.; Dame, Z.T.; Roy, P.K.; Islam, M.T. Genomics and Post-Genomics Approaches for Elucidating Molecular Mechanisms of Plant Growth-Promoting Bacilli. In Bacilli in Climate Resilient Agriculture and Bioprospecting; Springer International Publishing: Cham, Switzerland, 2019; pp. 161–200. [Google Scholar]
- Ramachandran, G.; Bikard, D. Editing the Microbiome the CRISPR Way. Philos. Trans. R. Soc. B 2019, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gregorio, P.R.; Michavila, G.; Ricciardi Muller, L.; de Souza Borges, C.; Pomares, M.F.; Saccol de Sá, E.L.; Pereira, C.; Vincent, P.A. Beneficial Rhizobacteria Immobilized in Nanofibers for Potential Application as Soybean Seed Bioinoculants. PLoS ONE 2017, 12, e0176930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Status of Commercialized Biotech/GM Crops; Brief No. 54; ISAAA: Berlin/Heidelberg, Germany, 2018.
| Functional Group of Rhizobacteria | Salient Feature | Mode of Action | Reference |
|---|---|---|---|
| N2 fixer (Symbiotic and Non-symbiotic) (e.g., Rhizobium, Bradyrhizobium, Sinorhizobium, Azotobacter, Azospirillum, Gluconacetobacter, Brevibacterium, etc.) | Nitrogen fixation and cycling can be regulated by the nitrogen fixers either by symbiotic or non-symbiotic interaction. The nodules of legumes may act as a harbor for symbiotic N2 fixers, while the rhizosphere may trigger the colonization of non-symbiotic N2 fixers. | Biological Nitrogen Fixation (BNF), Symbiotic and Non-symbiotic interaction for nitrogen fixation, Auxin (IAA) production, ACC-deaminase, Siderophore, HCN, and ammonia production | [2,13,15,33,34,35,36,37,38,39] |
| P-solubilizer (e.g., Pseudomonas, Bacillus, Enterobacter, Burkholderia, Klebsiella, etc.) | P-solubilizers may mineralize/solubilize the fixed or sequestrated phosphate in the soil due to acidic/alkaline pH conditions. The prime mechanisms comprise either enzymatic mineralization or low-molecular-weight acid secretion by the P-solubilizers. In addition, P-solubilizer may enhance the growth of plants through PGP activities by hormones/bioactive molecules | Organic/Inorganic acid production, Proton extrusion, Ammonia, and H2S production, Siderophore, Direct oxidation, Enzymatic mineralization (Particularly, by phosphatases, and phytases) | [32,40,41,42,43,44,45] |
| K-solubilizer (e.g., Pseudomonas, Enterobacter, Acidithiobacillus, Burkholderia, Paenibacillus, etc.) | Potassium solubilizing bacteria (KSB) are the saprophytic bacteria that can play a vital role in K cycling in soil nutrition. The actual mechanism of K solubilization is not yet confirmed, however, several predicted mechanisms (e.g., acid hydrolysis) may be the reason behind the solubilization or mobilization of insoluble K in the soil. | Acid hydrolysis of potassium from the K-minerals, Chelation of K, Undefined solubilization of potassium (Not depicted) | [16,46,47,48] |
| Fe chelating bacteria (e.g., Chryseobacterium, Arthrobacter, or other siderophore secreting bacteria) | Fe chelation and availability of soluble Fe3+ can be mediated by the siderophore-producing bacteria. These rhizobacteria can be useful to mitigate plant stress conditions. | Siderophore production, Catechol, or other phenolic secretion | [40,49,50] |
| Hormone-producing rhizobacteria (e.g., Bacillus, Pseudomonas, Enterobacter, Azospirillum, Staphylococcus, etc.) | Hormone-producing rhizobacteria are acting direct role to control plant growth. The rhizobacteria-mediated hormones are auxin (IAA) and its derivatives, gibberellic acid (GA), cytokinin, etc. | Hormone secretion either under normal, or stress conditions, helps in the plant metabolism directly. | [51,52,53,54,55] |
| Stress | PGPR Strain | Mechanism of Mitigation of Abiotic Stress | Beneficial Host | Reference |
|---|---|---|---|---|
| Drought | P. fluorescens (Pf1) B. subtilis (EPB5, EPB22, and EPB 31) | Proline accumulation, Activation of enzyme systems | Green gram (Vigna radiata) | [134] |
| Drought | B. licheniformis (K11) | Activation of stress-proteins and upregulation of stress-related genes (e.g., Cadhn, VA, sHSP, and CaPR-10) | Capsicum annuum | [135] |
| Drought | Achromobacter xylosoxidans (SF2) B. pumilis (SF3) and SF4) | Secretion and regulation of phytohormones. | Helianthus annuus | [136] |
| Drought | Bacillus spp. (KB122, KB129, KB133, and KB142) | Enhanced relative water content, improved plant physiology, retain soil moisture content, and proline contents | Sorghum bicolor | [83] |
| Drought | B. thuringiensis (AZP2) P. polymyxa (B) | Production of volatiles (e.g., benzaldehyde, β-pinene, and geranyl acetone) | Triticum aestivum | [137] |
| Drought stress | P. aeruginosa (GGRJ21) | Increased the levels of antioxidants, improved cell osmolytes, and upregulation of stress-responsive genes. | V. radiata | [138] |
| Drought | Burkholderia sp. (LD-11) | Enhanced plant physiology and growth regulators in the plants | Zea mays | [139] |
| Drought | A. brasilense | Alteration of physiological and biochemical changes including modulation of photosynthetic pigments, ABA, proline, and lipid peroxidation | A. thaliana | [122] |
| Salinity | Dietzia natronolimnaea (STR1) | ABA signaling, SOS pathway, ion transporters and antioxidant machinery | T. aestivum | [140] |
| Drought and Salinity | B. subtilis A. protophormiae (SA3) D. natronolimnaea (STR1) | IAA production, regulation of abscisic acid/ACC deaminase level and modulating expression of genes encoding for CTR1/ DREB2 proteins | T. aestivum | [141] |
| Salinity | Sphingomonas sp. (LK11) | Regulation of endogenous phytohormones (abscisic acid, salicylic acid and jasmonic acid) | Solanum pimpinellifolium | [61] |
| Salinity | Halobacillus dabanensis (SB-26) Halobacillus sp. (GSP 34) | Osmotic regulation and physiological modulation | Oryza sativa | [142] |
| Salinity | P. putida Novosphingobium sp. | Reduction of the level of ABA and SA, inhibit the proline and chloride accumulation of root | Citrus | [143] |
| Salinity | Curtobacterium albidum (SRV4) | Inducing systemic tolerance | O. sativa | [144] |
| B. halotolerans Lelliottia amnigena | Judicious manipulation of K+ and Na+ uptake in roots and shoots | T. aestivum | [145] | |
| Salinity | Azotobacter sp. (Az2 and Az6) | Improvement of physiological attributes and enhanced growth dynamics | T. aestivum | [146] |
| Salinity | Acinetobacter bereziniae (IG 2) Enterobacter ludwigii (IG 10), Alcaligenes faecalis (IG 27) | Modulation of chlorophyll, proline contents, total soluble sugar (TSS, electrolyte leakage, and activities of antioxidant enzymes | Pisum sativum | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, A.; Ansary, M.W.R.; Hossain, M.N.; Islam, T. Prospect and Challenges for Sustainable Management of Climate Change-Associated Stresses to Soil and Plant Health by Beneficial Rhizobacteria. Stresses 2021, 1, 200-222. https://doi.org/10.3390/stresses1040015
Sarker A, Ansary MWR, Hossain MN, Islam T. Prospect and Challenges for Sustainable Management of Climate Change-Associated Stresses to Soil and Plant Health by Beneficial Rhizobacteria. Stresses. 2021; 1(4):200-222. https://doi.org/10.3390/stresses1040015
Chicago/Turabian StyleSarker, Aniruddha, Most. Waheda Rahman Ansary, Mohammad Nabil Hossain, and Tofazzal Islam. 2021. "Prospect and Challenges for Sustainable Management of Climate Change-Associated Stresses to Soil and Plant Health by Beneficial Rhizobacteria" Stresses 1, no. 4: 200-222. https://doi.org/10.3390/stresses1040015
APA StyleSarker, A., Ansary, M. W. R., Hossain, M. N., & Islam, T. (2021). Prospect and Challenges for Sustainable Management of Climate Change-Associated Stresses to Soil and Plant Health by Beneficial Rhizobacteria. Stresses, 1(4), 200-222. https://doi.org/10.3390/stresses1040015









