Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium
Abstract
1. Introduction
2. Results
2.1. Expression Profiles of Zinc Transporter Genes in Parent UROtsa Cells
2.2. Aberrant Expression of Multiple Zinc Transporter Genes in Transformed UROtsa Cells
2.3. Doubling Time and Tumor Phenotypes
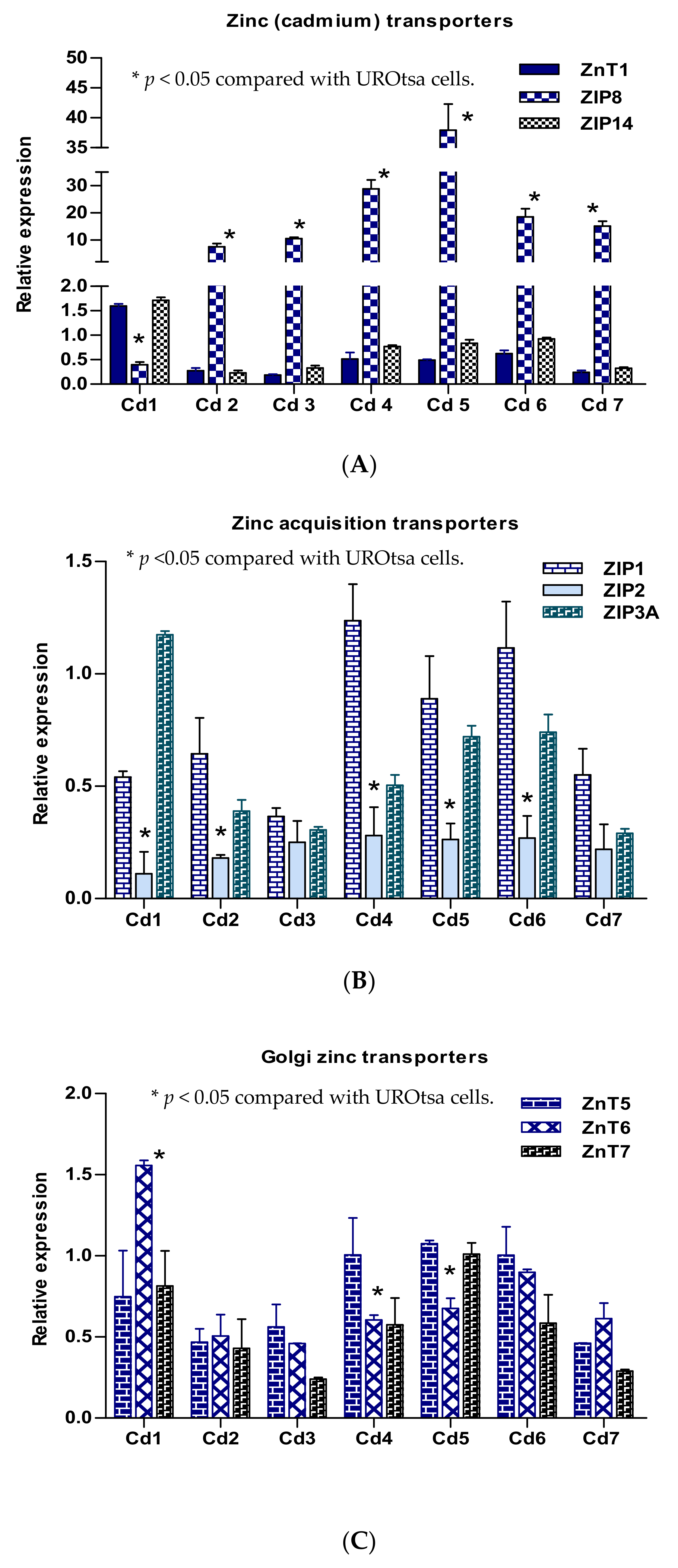
2.4. Expression Profiles of Selected Zinc Transporter Genes in Transformed UROtsa Cells
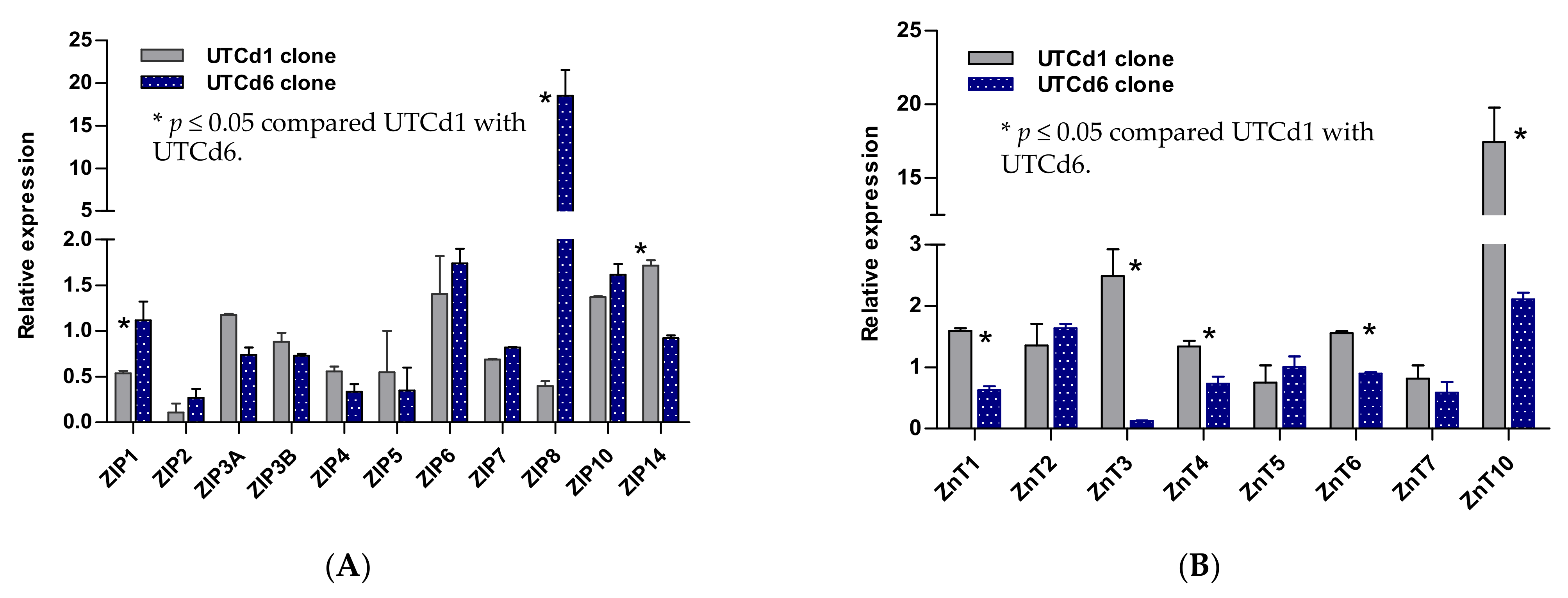
2.5. Zinc Transporter Gene Expression Profiles in UTCd1 vs. UTCd6
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Maintenance
4.2. Transformation of UROtsa Cells by Cadmium
4.3. Soft-Agar Test and Tumor Hetero-Transplantation
4.4. Quantification of mRNA Expression of Zinc Transporter Gene Families
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, Environmental Exposure, and Health Outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current Health Risk Assessment Practice for Dietary Cadmium: Data from Different Countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef]
- Satarug, S.; Phelps, K.R. Cadmium Exposure and Toxicity. In Metal Toxicology Handbook; Bagchi, D., Bagchi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 219–274. [Google Scholar]
- International Agency for Research on Cancer (IARC). Cadmium. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 119–238. [Google Scholar]
- Pasin, E.; Josephson, D.Y.; Mitra, A.P.; Cote, R.J.; Stein, J.P. Superficial Bladder Cancer: An Update on Etiology, Molecular Development, Classification, and Natural History. Rev. Urol. 2008, 10, 31–43. [Google Scholar]
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C. Global Burden of Urologic Cancers, 1990–2013. Eur. Urol. 2017, 71, 437–446. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishiyama, H. Epidemiology of Urothelial Carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.M.; Loughlin, K.R. Economic Impact of Tumor Markers in Bladder Cancer Surveillance. Urology 2008, 71, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Garren, B.; Nielsen, M.E.; Tang, L. Lifestyle and Nutritional Modifiable Factors in the Prevention and Treatment of Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Samanic, C.; Kogevinas, M.; Dosemeci, M.; Malats, N.; Real, F.X.; Garcia-Closas, M.; Real, F.X.; Garcia-Closas, M.; Serra, C.; Carrato, A.; et al. Smoking and Bladder Cancer in Spain: Effects of Tobacco Type, Timing, Environmental Tobacco Smoke, and Gender. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood Cadmium May be Associated with Bladder Carcinogenesis: The Belgian Case-Control Study on Bladder Cancer. Cancer Detect. Prev. 2007, 31, 77–82. [Google Scholar] [CrossRef]
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated Metallothionein-Bound Cadmium Concentrations in Urine from Bladder Carcinoma Patients, Investigated by Size Exclusion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2009, 631, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, L.; Leigh, I.M.; Duffy, P.G.; Sexton, C.; Masters, R.W. Immortalisation of Human Urothelial Cells. Urol. Res. 1995, 23, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.R.; Masters, J.R.W.; Park, S.; Todd, J.H.; Garrett, S.H.; Sens, M.A.; Somji, S.; Nath, J.; Sens, D.A. The Immortalized UROtsa Cell Line as a Potential Cell Culture Model of Human Urothelium. Environ. Health Perspect. 2001, 109, 801–808. [Google Scholar] [CrossRef]
- Sens, D.; Rossi, M.; Park, S.; Gurel, V.; Nath, J.; Garrett, S.; Sens, M.A.; Somji, S. Metallothionein Isoform 1 and 2 Gene Expression in a Human Urothelial Cell Line (UROtsa) Exposed to CdCl2 and NaAsO2. J. Toxicol. Environ. Health A 2003, 66, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Sens, D.A.; Park, S.; Gurel, V.; Sens, M.A.; Garrett, S.H.; Somji, S. Inorganic Cadmium-and Arsenite-Induced Malignant Transformation of Human Bladder Urothelial Cells. Toxicol. Sci. 2004, 79, 56–63. [Google Scholar] [CrossRef]
- Bafaro, B.; Liu, Y.; Xu, Y.; Dempski, R.E. The Emerging Role of Zinc Transporters in Cellular Homeostasis and Cancer. Signal Transduct. Target. Ther. 2017, 2, e17029. [Google Scholar] [CrossRef]
- Takatani-Nakase, T. Zinc Transporters and the Progression of Breast Cancers. Biol. Pharm. Bull. 2018, 41, 1517–1522. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Zinc Transporter 1 (ZNT1) Expression on the Cell Surface is Elaborately Controlled by Cellular Zinc Levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc Transporters and Their Functional Integration in Mammalian Cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. The Human ZIP1 Transporter Mediates Zinc Uptake in Human K562 Erythroleukemia Cells. J. Biol. Chem. 2001, 276, 22258–22264. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Functional Expression of the Human hZIP2 Zinc Transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human ZIP1 is a Major Zinc Uptake Transporter for the Accumulation of Zinc in Prostate Cells. J. Inorg. Biochem. 2003, 96, 435–442. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Huang, Z.L.; Geiser, J.; Xu, W.; Andrews, G.K. Mouse ZIP1 and ZIP3 Genes Together are Essential for Adaptation to Dietary Zinc Deficiency during Pregnancy. Genesis 2006, 44, 239–251. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.J.; Zhang, Y.; Yu, Y.Y. The ZIP7 Gene (Slc39a7) Encodes a Zinc Transporter Involved in Zinc Homeostasis of the Golgi Apparatus. J. Biol. Chem. 2005, 280, 15456–15463. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional Characterization of a Novel Mammalian Zinc Transporter, ZnT6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Matsuura, W.; Kohda, A.; Okumura, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc Transporters, ZnT5 and ZnT7, are Required for the Activation of Alkaline Phosphatases, Zinc-Requiring Enzymes that are Glycosylphosphatidylinositol-Anchored to the Cytoplasmic Membrane. J. Biol. Chem. 2005, 280, 637–643. [Google Scholar] [CrossRef]
- Kirschke, C.P.; Huang, L. ZnT7, A Novel Mammalian Zinc Transporter, Accumulates Zinc in the Golgi Apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 Gene Encodes ZIP14, a Metal/Bicarbonate Symporter: Similarities to the ZIP8 Transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Soleimani, M.; Girijashanker, K.; Reed, J.M.; He, L.; Dalton, T.P.; Nebert, D.W. Cd2+ versus Zn2+ Uptake by the ZIP8 HCO3--Dependent Symporter: Kinetics, Electrogenicity and Trafficking. Biochem. Biophys. Res. Commun. 2008, 365, 814–820. [Google Scholar] [CrossRef]
- Besecker, B.; Bao, S.; Bohacova, B.; Papp, A.; Sadee, W.; Knoell, D.L. The Human Zinc Transporter SLC39A8 (Zip8) is Critical in Zinc-Mediated Cytoprotection in Lung Epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1127–L1136. [Google Scholar] [CrossRef] [PubMed]
- Ohana, E.; Sekler, I.; Kaisman, T.; Kahn, N.; Cove, J.; Silverman, W.F.; Amsterdam, A.; Hershfinkel, M. Silencing of ZnT-1 Expression Enhances Heavy Metal Influx and Toxicity. J. Mol. Med. 2006, 84, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Cherrington, N.J.; Hartley, D.P.; Klaassen, C.D.; Waalkes, M.P. Cyproterone Acetate Induces a Cellular Tolerance to Cadmium in Rat Liver Epithelial Cells Involving Reduced Cadmium Accumulation. Toxicology 2001, 165, 13–25. [Google Scholar] [CrossRef]
- Hoggarth, Z.E.; Osowski, D.B.; Freeberg, B.A.; Garrett, S.H.; Sens, D.A.; Sens, M.A.; Zhou, X.D.; Zhang, K.K.; Somji, S. The Urothelial Cell Line UROtsa Transformed by Arsenite and Cadmium Display Basal Characteristics Associated with Muscle Invasive Urothelial Cancers. PLoS ONE 2018, 13, e0207877. [Google Scholar] [CrossRef]
- Ajjimaporn, A.; Botsford, T.; Garrett, S.H.; Sens, M.A.; Zhou, X.D.; Dunlevy, J.R.; Sens, D.A.; Somji, S. ZIP8 Expression in Human Proximal Tubule Cells, Human Urothelial Cells Transformed by Cd+2 and As+3 and in Specimens of Normal Human Urothelium and Urothelial Cancer. Cancer Cell Int. 2012, 12, 16. [Google Scholar] [CrossRef]
- Garrett, S.H.; Somji, S.; Sens, D.A.; Zhang, K.K. Prediction of the Number of Activated Genes in Multiple Independent Cd+2 and As+3-Induced Malignant Transformations of Human Urothelial Cells (UROtsa). PLoS ONE 2014, 9, e85614. [Google Scholar] [CrossRef]
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 Expression and Function Lead to Impaired Intracellular Zinc Homeostasis in Cancer. Cell Death Discov. 2019, 5, 144. [Google Scholar] [CrossRef]
- Zhu, B.; Huo, R.; Zhi, Q.; Zhan, M.; Chen, X.; Hua, Z.C. Increased Expression of Zinc Transporter ZIP4, ZIP11, ZnT1, and ZnT6 Predicts Poor Prognosis in Pancreatic Cancer. J. Trace Elem. Med. Biol. 2021, 65, 126734. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Jin, H.; Yin, L.; Yu, H.; Bi, J. MiR-411 Suppresses the Development of Bladder Cancer by Regulating ZnT1. OncoTargets Ther. 2018, 11, 8695–8704. [Google Scholar] [CrossRef]
- Golovine, K.; Makhov, P.; Uzzo, R.G.; Shaw, T.; Kunkle, D.; Kolenko, V.M. Overexpression of the Zinc Uptake Transporter hZIP1 Inhibits Nuclear Factor-KappaB and Reduces the Malignant Potential of Prostate Cancer Cells in Vitro and in Vivo. Clin. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.M.; Cousins, R.J.; et al. Aberrant Expression of Zinc Transporter ZIP4 (SLC39A4) Significantly Contributes to Human Pancreatic Cancer Pathogenesis and Progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Vichova, P.; Jordan, N.; Hiscox, S.; Hendley, R.; Nicholson, R.I. ZIP7 Mediated Intracellular Zinc Transport Contributes to Aberrant Growth Factor Signaling in Antihormone-Resistant Breast Cancer Cells. Endocrinology 2008, 149, 4912–4920. [Google Scholar] [CrossRef]
- Kagara, N.; Tanaka, N.; Noguchi, S.; Hirano, T. Zinc and Its Transporter ZIP10 are Involved in Invasive Behavior of Breast Cancer Cells. Cancer Sci. 2007, 98, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Mathews, W.R.; Wang, F.; Eide, D.J.; Doren, M.V. Drosophila Fear of Intimacy Encodes a Zrt/IRT-Like Protein (ZIP) Family Zinc Transporter Functionally Related to Mammalian ZIP Proteins. J. Biol. Chem. 2005, 280, 787–795. [Google Scholar] [CrossRef]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc Transporter ZIP10 Forms a Heteromer with ZIP6 which Regulates Embryonic Development and Cell Migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Fujishiro, H.; Okugaki, S.; Yasumitsu, S.; Enomoto, S.; Himeno, S. Involvement of DNA Hypermethylation in Down-Regulation of the Zinc Transporter ZIP8 in Cadmium-Resistant Metallothionein-Null Cells. Toxicol. Appl. Pharmacol. 2009, 241, 195–201. [Google Scholar] [CrossRef]
- Fujishiro, H.; Ohashi, T.; Takuma, M.; Himeno, S. Suppression of ZIP8 Expression is a Common Feature of Cadmium-Resistant and Manganese-Resistant RBL-2H3 Cells. Metallomics 2013, 5, 437–444. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, W.; Taylor, K.M.; Cai, B.; Li, X. LIV-1 Suppression Inhibits HeLa Cell Invasion by Targeting ERK1/2-Snail/Slug Pathway. Biochem. Biophys. Res. Commun. 2007, 363, 82–88. [Google Scholar] [CrossRef]
- San, R.H.C.; Laspia, M.F.; Soiefer, A.I.; Maslansky, C.J.; Rice, J.M.; Williams, G.M. A Survey of Growth in Soft Agar and Cell Surface Properties as Markers for Transformation in Adult Rat Liver Epithelial-Like Cell Cultures. Cancer Res. 1979, 39, 1026–1034. [Google Scholar] [PubMed]
- Albrecht, A.L.; Somji, S.; Sens, M.A.; Sens, D.A.; Garrett, S.H. Zinc Transporter mRNA Expression in the RWPE-1 Human Prostate Epithelial Cell Line. Biometals 2008, 21, 405–416. [Google Scholar] [CrossRef] [PubMed]
| Zinc Transporter Genes | Transcript Levels | |
|---|---|---|
| Copies in 4 ng Total RNA | Copies in 1000 β-Actin | |
| SLC30A (ZnT)gene family (CDF) | ||
| ZnT1 | 42,650 ± 5450 | 181 ± 23 |
| ZnT2 | 1.6 ± 0.3 | 0.01 ± 0.001 |
| ZnT3 | 6.7 ± 1.9 | 0.03 ± 0.007 |
| ZnT4 | 381 ± 65 | 1.6 ± 0.26 |
| ZnT5 | 34,859 ± 3527 | 150 ± 19 |
| ZnT6 | 1049 ± 69 | 4.5 ± 0.15 |
| ZnT7 | 173,250 ± 11,981 | 734 ± 28 |
| ZnT10 | 10.3 ± 2.5 | 0.04 ± 0.005 |
| SLC39A (ZIP) gene family | ||
| ZIP1 | 3512 ± 549 | 19.5 ± 2.0 |
| ZIP2 | 7 ± 1.3 | 0.02 ± 0.004 |
| ZIP3A | 2410 ± 89 | 9.1 ± 0.4 |
| ZIP3B | 112 ± 15 | 0.48 ± 0.05 |
| ZIP4 | 42 ± 8 | 0.18 ± 0.04 |
| ZIP5 | 2.7 ± 0.7 | 0.01 ± 0.003 |
| ZIP6 (LIV-1) | 4300 ± 613 | 18.3 ± 2.6 |
| ZIP7 (KE4) | 28725 ± 3076 | 121 ± 9.5 |
| ZIP8 (BIG103) | 22 ± 3 | 0.09 ± 0.01 |
| ZIP10 | 1190 ± 113 | 5 ± 0.3 |
| ZIP12 | Undetectable | − |
| ZIP14 | 19525 ± 2703 | 83.4 ± 10.5 |
| House-keeping genes | ||
| β-actin | 235,000 ± 16,289 | 1000 |
| GAPDH | 3,682,500 ± 253,192 | 15,670 ± 1077 |
| 18S rRNA | 253,000,000 ± 18,995,610 | 1,076,596 ± 80832 |
| Gene Expression/Phenotypes | Cadmium-Transformed UROtsa Clones | ||||||
|---|---|---|---|---|---|---|---|
| UTCd1 | UTCd2 | UTCd3 | UTCd4 | UTCd5 | UTCd6 | UTCd7 | |
| SLC30A gene family | |||||||
| ZnT1 | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| ZnT2 | ↑ | ↓ | ↓ | ↔ | ↑ | ↑ | ↓ |
| ZnT3 | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| ZnT4 | ↑ | ↓ | ↓ | ↔ | ↑ | ↓ | ↓ |
| ZnT5 | ⟷ | ↓ | ↓ | ↔ | ↔ | ↔ | ↓ |
| ZnT6 | ↑ | ↓ | ↓ | ↓ | ↓ | ↔ | ↓ |
| ZnT7 | ⟷ | ↓ | ↓ | ↓ | ↔ | ↓ | ↓ |
| ZnT10 | ↑ | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ |
| SLC39A gene family | |||||||
| ZIP1 | ↓ | ↓ | ↓ | ↔ | ↔ | ↔ | ↓ |
| ZIP2 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| ZIP3A | ↔ | ↓ | ↓ | ↓ | ↔ | ↔ | ↓ |
| ZIP3B | ↔ | ↔ | ↓ | ↔ | ↔ | ↔ | ↓ |
| ZIP4 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| ZIP5 | ↓ | ↓ | ↓ | ↓ | ↔ | ↓ | ↔ |
| ZIP6 | ↑ | ↔ | ↓ | ↔ | ↔ | ↑ | ↓ |
| ZIP7 | ↔ | ↔ | ↓ | ↔ | ↔ | ↔ | ↓ |
| ZIP8 | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ZIP10 | ↑ | ↔ | ↓ | ↔ | ↑ | ↑ | ↓ |
| ZIP14 | ↑ | ↓ | ↓ | ↔ | ↔ | ↔ | ↓ |
| Phenotypes | |||||||
| Doubling time (h) | 27.8 | 18.8 * | 16.4 * | 20.7 * | 18.2 * | 16.9 * | 27.1 |
| § Heterotransplant (sc) | 5/5 | 2/5 | 4/5 | 2/5 | 3/5 | 2/5 | 4/5 |
| Histology | TCC | TCC | TCC | TCC | TCC | TCC | TCC |
| § Heterotransplant (ip) | + | − | − | − | − | − | − |
| Histology | TCC | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S.; Garrett, S.H.; Somji, S.; Sens, M.A.; Sens, D.A. Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium. Stresses 2021, 1, 78-89. https://doi.org/10.3390/stresses1020007
Satarug S, Garrett SH, Somji S, Sens MA, Sens DA. Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium. Stresses. 2021; 1(2):78-89. https://doi.org/10.3390/stresses1020007
Chicago/Turabian StyleSatarug, Soisungwan, Scott H. Garrett, Seema Somji, Mary Ann Sens, and Donald A. Sens. 2021. "Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium" Stresses 1, no. 2: 78-89. https://doi.org/10.3390/stresses1020007
APA StyleSatarug, S., Garrett, S. H., Somji, S., Sens, M. A., & Sens, D. A. (2021). Aberrant Expression of ZIP and ZnT Zinc Transporters in UROtsa Cells Transformed to Malignant Cells by Cadmium. Stresses, 1(2), 78-89. https://doi.org/10.3390/stresses1020007






