Abstract
Maintenance of zinc homeostasis is pivotal to the regulation of cell growth, differentiation, apoptosis, and defense mechanisms. In mammalian cells, control of cellular zinc homeostasis is through zinc uptake, zinc secretion, and zinc compartmentalization, mediated by metal transporters of the Zrt-/Irt-like protein (ZIP) family and the Cation Diffusion Facilitators (CDF) or ZnT family. We quantified transcript levels of ZIP and ZnT zinc transporters expressed by non-tumorigenic UROtsa cells and compared with those expressed by UROtsa clones that were experimentally transformed to cancer cells by prolonged exposure to cadmium (Cd). Although expression of the ZIP8 gene in parent UROtsa cells was lower than ZIP14 (0.1 vs. 83 transcripts per 1000 β-actin transcripts), an increased expression of ZIP8 concurrent with a reduction in expression of one or two zinc influx transporters, namely ZIP1, ZIP2, and ZIP3, were seen in six out of seven transformed UROtsa clones. Aberrant expression of the Golgi zinc transporters ZIP7, ZnT5, ZnT6, and ZnT7 were also observed. One transformed clone showed distinctively increased expression of ZIP6, ZIP10, ZIP14, and ZnT1, with a diminished ZIP8 expression. These data suggest intracellular zinc dysregulation and aberrant zinc homeostasis both in the cytosol and in the Golgi in the transformed UROtsa clones. These results provide evidence for zinc dysregulation in transformed UROtsa cells that may contribute in part to their malignancy and/or muscle invasiveness.
Keywords:
bladder cancer; cadmium; gene expression; qRT/PCR; zinc homeostasis; zinc transporters; ZIP; ZnT 1. Introduction
Cadmium (Cd) is one of the environmental toxicants of continuing public health concern worldwide due to its persistence, widespread exposure, and wide-ranging adverse health effects, cancer risk included [1,2,3]. Although Cd can be inhaled in polluted air as Cd oxide, it is acquired primarily from food or tobacco grown in contaminated soil [1,2,3]. Blood Cd levels in cigarette smokers were higher than non-smokers by 2 to 6 fold [2]. The International Agency for Research on Cancer has classified Cd as a cancer causing agent in humans [4]. However, the mechanism underlying the carcinogenicity of Cd remains elusive, as does the mechanism of its entry into a relevant target such as urinary bladder epithelium, where the metal Cd may initiate cancer cell transformation.
Bladder cancer ranks the sixth most common cancer in the U.S., with annual incidence rates of 30 to 33 cases per 100,000. In most cases, cancer originated from the transitional cells of bladder mucosal epithelium, and it is named transitional cell carcinoma (TCC) [5,6,7]. One third of bladder cancer cases manifested as non-papillary tumors with high invasive and metastasis potential, while two thirds manifested as non-invasive, resettable papillary tumors, with recurrence rates between 30% and 70% [5]. Such high recurrence rates necessitate frequent cystoscopy and urine cytology [5], making bladder cancer the fifth highest cancer treatment and the highest care cost per patient in the U.S. [8,9].
In a Spanish study of 1219 newly diagnosed bladder cancer cases and 1271 controls, cigarette smoking accounted for nearly all excess bladder cancer risk in men [10]. In a study of 172 bladder cancer cases and 395 non-cancer controls, the mean blood Cd in bladder cancer cases was 1.6 fold higher than the mean blood Cd in controls of 0.7 µg/L, and the risk of bladder cancer was increased by 5.7 fold as blood Cd rose from the lowest tertile to the highest tertile [11]. The estimated risk was adjusted for gender, age, smoking habits, and workplace exposure [11]. In another study, the median urinary Cd in bladder cancer cases was 2.25 fold higher than controls of 0.8 µg/L [12].
Research to dissect the carcinogenic mechanism of Cd in urothelial cells has been hindered by limited human cell models of bladder cancer. Works in our laboratory have established that the non-tumorigenic UROtsa cell line, derived from the ureter epithelium of a 12-year-old female donor, immortalized with SV40 large T-antigen, could be a suitable experimental model [13]. UROtsa cells display the phenotypic and morphologic characteristics resembling primary transitional epithelial cells of the bladder [14], and we have shown that Cd increased expression of the metal binding protein, metallothionein (MT), in UROtsa cells [15] and that prolonged exposure to Cd as low as 1 µM concentration caused UROtsa cells to undergo cancer cell transformation [16]. In viewing the increasing evidence that cellular zinc homeostasis is critical for regulating cell growth, differentiation, apoptosis, and defense mechanisms, and for preventing cancer development [17,18]; we hypothesized that MT induction by Cd causes shrinkage of the cytosolic labile zinc pool secondary to the sequestration of zinc by apo-MT, and that changes in expression of zinc transporters restore the “labile” zinc pool and zinc homoeostasis.
In mammalian cells, zinc homeostasis is maintained through the regulation of zinc uptake, zinc secretion, and zinc compartmentalization, mediated by metal transporters of the Zrt-/Irt-like protein (ZIP) family and the ZnT or Cation Diffusion Facilitators (CDF) family [19,20,21,22]. ZIP1, ZIP2, and ZIP3 have been shown to be involved in Zn2+ influx [23,24,25,26], while ZIP7, ZnT5, ZnT6, and ZnT7 were responsible for the Golgi Zn2+ transport [27,28,29,30]. ZIP8 and ZIP14 mediated Cd2+ influx, while ZnT1 was involved in Cd2+ efflux [31,32,33,34,35].
To understand how Cd increases the risk of bladder cancer seen in epidemiological studies discussed above (10–12), we sought to identify the putative Cd transporter(s) in UROtsa cells and to explore the zinc transporter(s) that may contribute to invasiveness/metastasis of bladder cancer. Previously, we have shown that the tumors derived from transformed UROtsa cells displayed gene expression profiles similar to the basal subtype of muscle invasive bladder carcinomas [36,37], while our microarray data using the Affymetrix 133 Plus 2.0 chip indicated that at least 285 genes are up-regulated and 215 genes are down regulated [38]. Indeed, dysregulated intracellular zinc homeostasis and abnormal expression of specific zinc transporters such as ZnT1, ZIP1, ZIP4, ZIP6, ZIP7, and ZIP10 have been observed in various types of cancer, including urinary bladder, prostate, pancreatic, and breast cancers [39,40,41,42,43,44,45,46,47,48].
2. Results
2.1. Expression Profiles of Zinc Transporter Genes in Parent UROtsa Cells
Transcript levels of the ZnT and ZIP zinc transporter genes expressed in UROtsa cells are shown in Table 1. The Golgi zinc transporter ZnT7 was expressed in the highest abundance (734 copies per 1000 β-actin transcripts). ZnT1 and ZnT5 were expressed at 181 and 150 copies, respectively, per 1000 β-actin transcripts, while ZnT4 and ZnT6 were expressed at 16 and 45 copies, respectively, per 10,000 β-actin transcripts. ZnT2, ZnT3, and ZnT10 were expressed at 1 to 4 copies per 100,000 β-actin transcripts.

Table 1.
Expression profiles of zinc transporter genes in parent UROtsa cells.
ZIP12 expression was below the detection limit. ZIP7 and ZIP14 were expressed at the higher levels, estimated to be 121 and 83 copies per 1000 β-actin transcripts, respectively, compared with those of ZIP1, ZIP3A, ZIP6, and ZIP10, which were between 5 and 20 copies per 1000 β-actin transcripts. The ZIP2, ZIP3B, ZIP4, ZIP5, and ZIP8 genes were expressed in lower abundance of between 1 to 50 copies per 100,000 β-actin transcripts.
2.2. Aberrant Expression of Multiple Zinc Transporter Genes in Transformed UROtsa Cells
Expression levels of nineteen zinc transporter genes in seven transformed URotsa clones relative to parent UROtsa cells are provided in Table 2. The average doubling time, and histologic phenotypes of tumors formed in nude mice as reported previously [16,39], are also presented in Table 2.

Table 2.
Changes in expression profiles of zinc transporter genes in seven transformed UROtsa clones relative to non-tumorigenic parent UROtsa cells.
Of seven transformed UROtsa clones examined, expression of the efflux transporter ZnT1 increased in UTCd1 and it fell in all other six clones. ZnT2 expression increased in three clones (UTCd1, 5, 6), decreased in three clones (UTCd2, 3, 7), and there was no change in UTCd4. In parallel to ZnT1, expression of ZnT3 increased in UTCd1 and fell in all other six clones. ZnT4 expression increased in two clones (UTCd1, 5), decreased in four clones (UTCd2, 3, 6, 7), and there was no change in UTCd4. A reduction in expression of one or two or all of the Golgi zinc transporters, ZnT5, ZnT6, and ZnT7 was seen in six clones. Expression of ZIP7, known to mediate Golgi zinc efflux, showed no change in five clones and reduced in UTCd3 and UTCd7, in which expression of ZnT7 was decreased to 25% of the level expressed in parent UROtsa cells.
For the ZIP class, ZIP1 expression was decreased in four clones (UTCd1, 2, 3, 7) and there was no change in three clones (UTCd4, 5, 6). Expression levels of ZIP2 and ZIP4 decreased in all seven clones. ZIP3A expression decreased in four clones (UTCd2, 3, 4, 7) and there was no change in three clones (UTCd1, 5, 6). ZIP3B expression decreased in two clones (UTCd3, 7) and there was no change in all other five clones. Expression of ZIP5 decreased in five clones (UTCd1, 2, 3, 4, 6) and there was no change in two clones (UTCd5, 7). ZIP6 expression varied; it increased in UTCd1 and UTCd6, decreased in UTCd3 and UTCd7, and there was no changes in three other clones. ZIP7 expression decreased in two clones (UTCd3, 7) and there was no change in all other clones. Distinct from other members of the ZIP class, expression of ZIP8 increased in six clones and it decreased only in UTCd1. Similar to ZIP6, expression of ZIP10 varied; it increased in three clones (UTCd1, 5, 6), decreased in UTCd3 and 7, and there was no change in two other clones. Expression of ZIP14 increased in UTCd1, decreased in UTCd2, 3, and 7, and there was no change in the other three clones.
In summary, increased expression of the zinc transporters other than the ZIP8 gene occurred in low frequencies, but the multiplicity of the zinc transporters with deranged expression has produced individual transformed clones with distinctive profiles.
2.3. Doubling Time and Tumor Phenotypes
Average doubling time of UTCd2, 3, 4, 5, and 6 ranged between 16.4 and 20.7 h (Table 2). These doubling times were shorter than those of the parent UROtsa cells, with an average doubling time of 33.2 h. UTCd1 and UTCd7 had average doubling time lengths in the same range as the parental UROtsa cells. By subcutaneous injection, all seven transformed UROtsa clones formed tumors characteristic of TCC in nude mice. Of seven transformed clones, UTCd1 formed tumors in all five nude mice. UTCd3 and UTCd 7 formed tumors in four mice, while UTCd5 formed tumors in three mice, and the remaining clones (UTCd2, 4, 6) formed tumors in two mice. By intraperitoneal injection, only the UTCd1 clone showed the propensity to invade other tissues. Expression profiles of ZnT and ZIP classes in this UTCd1 clone were examined, together with those of UCdT6 that had shorter doubling time but were lacking invasive potential.
It is noteworthy that deranged expressions of zinc transporters were observed also in tumor heterotransplants (data not shown), but they were not identical to those in their precursor (transformed) clones. The incongruity was most likely due to an adaptation to differences in microenvironment, nutrient levels, and growth factors present or absent in vitro (culture medium) vs. in vivo (nude mice) conditions.
2.4. Expression Profiles of Selected Zinc Transporter Genes in Transformed UROtsa Cells
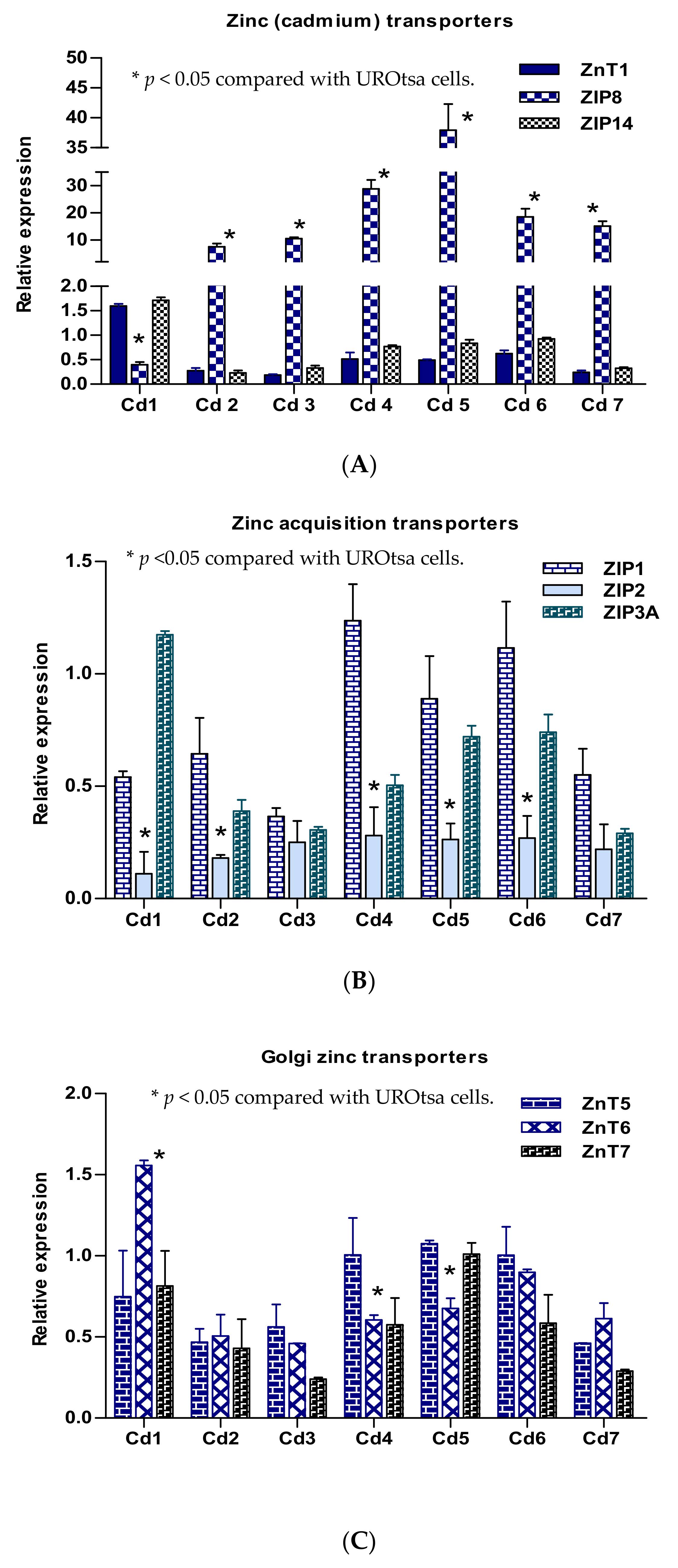
Expression levels of nine zinc transporter genes expressed in seven transformed UROtsa clones are graphically depicted in groups of three transporters in each group (Figure 1). Among those involved in the influx/efflux of Zn2+ and Cd2+, ZIP8 gene expression increased concurrently with a decrease in ZnT1 expression in six clones (Figure 1A). ZIP8 expression was decreased in the UTCd1 clone, only in which expression levels of ZIP14 and ZnT1 were increased. Expression of ZIP1 reduced in five clones: UTCd1, 2, 4, 5, 6 (Figure 2B). Of note, ZIP1 expression was more remarkably down-regulated in UTCd1 compared with the other four clones. With respect to the Golgi zinc transporter group (Figure 1C), expression of ZnT6 increased only in UTCd1.
Figure 1.
Expression profiles of ZIP and ZnT zinc transporter genes in transformed UROtsa cells. Relative expression levels of zinc (cadmium) transporters (A), zinc acquisition transporters (B), and the Golgi zinc transporters (C) in seven transformed UROtsa clones. Each bar indicates mean with standard error of mean (SE) for an expression ratio of an individual zinc transporter. Clone identity is indicated at the bottom. The forefront bar, middle bar, and last bar each represents expression of a transporter whose identity is indicated in a box at the top right corner. The ratio is defined as number of transcripts of a given gene in each clone relative to β–actin/number of transcripts of the same gene in the parent UROtsa cells relative to β–actin. A ratio of 1, less than 1, or greater than 1 indicate no change, a decrease, or an increase in expression levels, respectively, compared with the parent UROtsa cells.
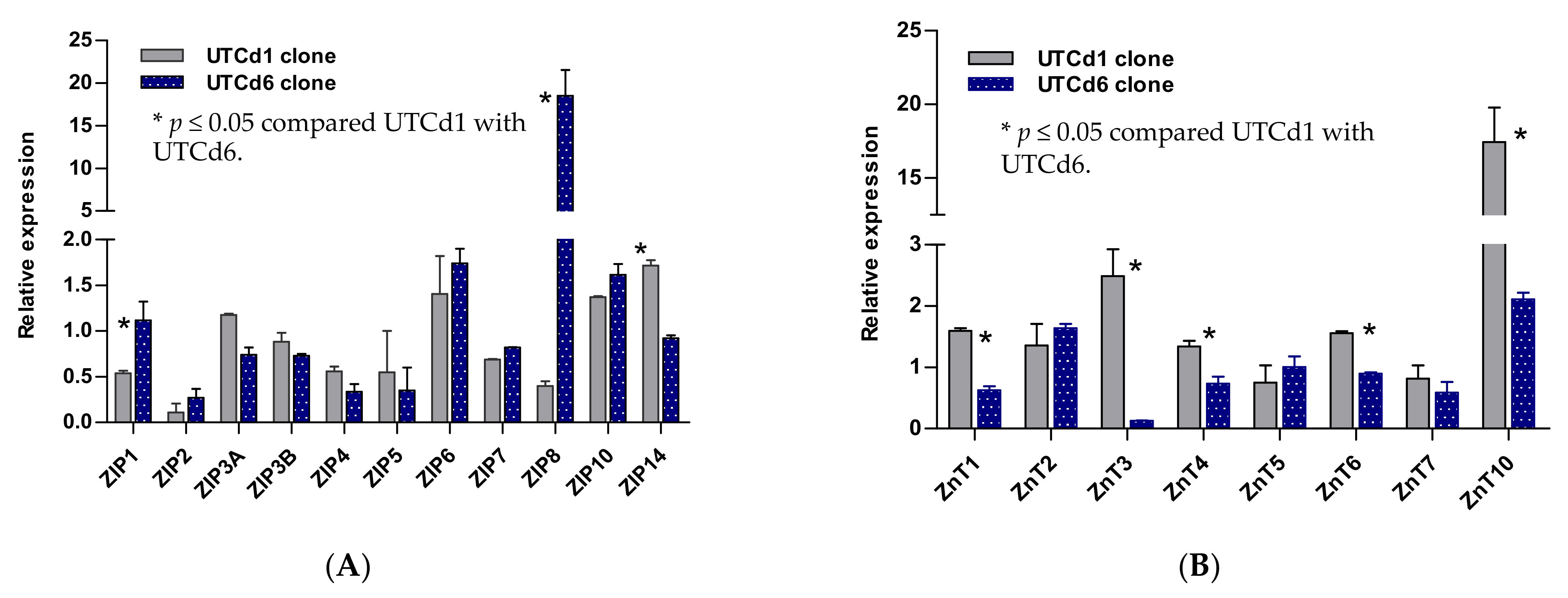
Figure 2.
Expression profiles for zinc transporter genes in UTCd1 vs. UTCd6. Relative expression levels of ZIP zinc transporters (A). Expression levels of ZnT zinc transporters (B). The ratio was defined as number of transcripts of a given gene in each clone relative to β–actin/number of transcripts of the same gene in the parental UROtsa cell line relative to β–actin. A ratio of 1, less than 1, or greater than 1 indicates no change, a decrease, or an increase in expression levels, respectively, compared with the parent UROtsa cells.
2.5. Zinc Transporter Gene Expression Profiles in UTCd1 vs. UTCd6
Figure 2 depicts transcript levels of ZIP and ZnT zinc transporter genes expressed by UTCd1 vs. UTCd6. These two clones formed tumors characteristic of TCC in nude mice, but only the UTCd1 clone appeared to have metastasis potential and high malignancy propensities (Table 2). UTCd1 and UTCd6 both showed a similar decrease in expression of ZIP2, ZIP4, and ZIP5. The UTCd1 clone showed a decrease also in the expression of ZIP1, ZIP8, and ZnT3. In contrast, UTCd6 showed an 18-fold increase in ZIP8 expression together with a 2-fold increase in ZnT3 expression, while showing no change in ZIP1 expression. Both clones showed a modest increase in expression of ZIP6 and ZIP10.
3. Discussion
Expression levels of ZIP8, ZIP14, and ZnT1 genes in parent UROtsa cells were 0.1, 83, and 181 transcripts per 1000 copies of β-actin gene transcripts, respectively (Table 1). Works in other laboratories have demonstrated a role for ZIP8 and ZIP14 in Cd2+ uptake by many other cell types [29,30], while ZnT1 could be responsible for Cd2+ excretion [32]. Lower expression levels of ZIP8 than ZIP14 suggested that ZIP14 and ZnT1 could potentially be involved in Cd accumulation and cytotoxicity of Cd in this cell line. ZIP8 protein expressed by UROtsa cells was detectable by Western blot analysis [37]. Expression levels of ZIP2, ZIP5, ZnT2, and ZnT3 in parent UROtsa cells were beyond an order of magnitude below an estimate of one transcript per cell, assuming a total of 10 pg of RNA per cell. Low expression levels of ZnT3 and ZIP2 genes may reflect cell-specific gene expression because these zinc transporter genes are distinctively expressed in developing keratinocytes [19,20].
With an exception for UTCd1, a notable increase in ZIP8 expression was seen in all other six transformed UROtsa clones, together with a decrease in ZnT1 expression (Table 2, Figure 1A). Expression levels of ZIP8 as transcript copy numbers were in line with ZIP8 protein abundance in Western blot analysis [37]. Up-regulation of ZIP8 in these six clones may indicate that they have acquired an increased capacity for zinc uptake with minimal capacity to excrete zinc due to a concurrent reduction in ZnT1 expression. In a study using primary human lung epithelial cells, ZIP8 expression was increased, along with an increase in zinc uptake and the translocation of ZIP8 protein in response to the inflammatory cytokine tumor necrosis factor alpha (TNFα) [31]. Up-regulation of ZIP8 has been noted in different pathological conditions [19,20,21,22]. The increased ZIP8 expression seen in six transformed UROtsa clones in the present study may, in part, reflect a compensatory response to a contraction of the labile zinc pool because expression levels of zinc uptake zinc transporters, namely ZIP1, ZIP2, and ZIP3, were simultaneously elevated (Figure 1B) and/or induction of MT by Cd, as stated in our hypothesis. It may also reflect that expression of ZIP8 and ZnT1 genes are co-regulated. We have observed an increased expression of ZIP8 in all six clones showing low expression levels of ZnT1 (Figure 1A). Conversely, ZIP8 expression was diminished in the transformed UROtsa clone in which ZnT1 expression was elevated (Figure 1A, Table 2). It is conceivable that up-regulation of Zn influx transporters, notably ZIP8, simultaneously with down-regulation of the efflux transporter ZnT1, would be required to prevent excess Zn accumulation and toxicity from overdose. Hence, the maintenance of Zn homeostasis is an integrative function of influx and efflux transporters.
In a study using a rat liver epithelial cell line (TRL 1215), Takiguchi et al. have shown that pre-treatment of cells with cyproterone, a synthetic steroidal antiandrogen with a structure related to progesterone, decreased sensitivity to Cd through a decreased accumulation of Cd rather than through an increase in MT synthesis [35]. Although Takiguchi et al. suggested that such a decrease in Cd accumulation was due likely to up-regulation of the genes associated with zinc efflux, they did not investigate expression of any zinc transporters. Later, Ohana et al. showed that the silencing of ZnT1 expression increased Cd2+ accumulation and enhanced Cd2+ toxicity [34]. In another study, Fujishiro et al. observed a decrease in ZIP8 expression, assessed by ZIP8 mRNA and ZIP8 protein levels, in MT-null cells that were resistant to Cd toxicity [48], and they suggested that down regulation of ZIP8 was a common feature of cells resistant to Cd2+ [49]. Transformed UROtsa cells with distinctively high level of ZnT1 and reduced expression of ZIP8, such as UTCd1, may thus have acquired tolerance to Cd2+ toxicity. A further study is required to show if UTCd1 indeed tolerates higher levels of Cd than other clones in which ZnT1 was expressed in low levels, such as UTCd3 and UTCd8.
UTCd1 had the doubling time similar to parent UROtsa cells and it was the only clone in which ZIP8 expression was reduced. In this clone, a reduction in expression of ZIP8 occurred in conjunction with increased expression of ZIP14 and ZnT1 (Figure 1A). UTCd6 had a doubling time shorter than UTCd1 (16.9 h vs. 27.8 h) but it had lower malignancy potential as this clone formed tumors in only two mice, while UTCd1 formed tumors in all five nude mice (Table 2). Another feature of these clones is that both showed an increase in expression of ZIP6 and ZIP10, known to regulate cell migration [45,46,47], but only UTCd1 exhibited metastasis propensity. These data suggest that changes in additional zinc transporters such as ZIP1 down regulation, together with ZIP14 up-regulation of ZIP14, may also be required to gain the invasive propensities that UTCd1 exhibited.
Of relevance, Zhu et al. have reported that miR-411 may inhibit growth and metastasis of bladder cancer through suppression of ZnT1 expression [41]. Decreased ZIP1 expression and zinc depletion were associated with the onset of prostate cancer, whereas ZIP1 overexpression decreased malignancy potential [36]. Expression of the ZIP6 and ZIP10 genes were increased in breast cancers with highly invasive properties [45], suggesting the function of ZIP6 and ZIP10 to be analogous to their homologous Drosophila fear of intimacy (foi) gene, involved in the control of cell migration [46]. Likewise, increased ZIP6 expression in human cervical cancer cells was associated with invasive properties through the MAPK/Snail-Slug signaling pathway [50]. Increased expression of ZIP4, ZIP11, ZnT1, or ZnT6 has been associated with poor prognosis in patients with pancreatic adenocarcinoma [40].
4. Materials and Methods
4.1. Cells and Culture Maintenance
UROtsa cells were cultured in 25 cm2 tissue culture flasks in Dulbeco’s modified Eagle’s medium supplemented with 5% vol/vol fetal bovine serum [14]. Cells were maintained at 37 °C in humidified incubators with 5% CO2/95% atmospheric air. Cells were fed fresh growth medium every three days. At confluence, cells were sub-cultured at a 1:4 ratio using trypsin-EDTA (0.05%, 0.02%). For experimentation, cells were grown in 6-well plates containing 2 mL growth media per well (35 mm in diameter).
4.2. Transformation of UROtsa Cells by Cadmium
The protocol used to transform UROtsa cells by cadmium has been detailed previously [16]. Briefly, seven independent cultures of UROtsa cells were grown to confluency in 25 cm2 cell culture flasks and, when confluent, they were fed fresh growth media containing 1 μM Cd2+ (CdCl2, Sigma St. Louis, MO, USA). Following Cd2+ exposure, the cells were thereafter fed fresh growth media every three days that contained 1 μM Cd2+. The cultures were observed immediately before and 24 h after each feeding by light microscopy. The strategy was to hold the cultures at confluency with continued feeding until cell death ensued, and then allowing the surviving cells in the culture to proliferate to confluency, and then to continue serial passaging of the cells in the continued presence of Cd2+ until the cells were able to form colonies in soft agar.
4.3. Soft-Agar Test and Tumor Hetero-Transplantation
Cell cultures were tested for the ability to form colonies in soft agar using a published protocol [51] with some modification. In brief, an agar layer was formed with DMEM containing 0.5% agar and 5% fetal calf serum, overlaid with 2 × 104 or 2 × 105 tested cells in DMEM containing 0.25% agar and 5% fetal calf serum. Microscopic examination was done 24 h later to verify the absence of initial large cell clumps. Subsequent examination was undertaken weekly for over three weeks to detect colony formation. The soft-agar test positive cultures were named as UTCd1, UTCd2, UTCd3, UTCd4, UTCd5, UTCd6, and UTCd7. These cadmium-transformed UROtsa clones were further tested for their ability to form tumors in vivo by injection into groups of 5 to 6 nude mice (NCr-nu/nu) each via subcutaneous and intraperitoneal routes at a dose level of 1 × 106 cells each. Mice were examined weekly for tumor appearance and sacrificed 10 weeks later or when conditions dictated. Tumors were collected in several aliquots for later analysis and for histological characterization [36,37].
4.4. Quantification of mRNA Expression of Zinc Transporter Gene Families
Total RNA was extracted from UROTsa cells, transformed UROtsa clones, and tumor heterotransplants using TRI Reagent® (Molecular Research Center, Inc., Cincinnati, OH, USA). RNA content and purity assessment were based on UV absorption properties. Aliquots of RNA samples with 20 ng/µL concentration were prepared, and 2 μL of each sample (40 ng total RNA) was subjected to cDNA synthesis using the iScript™cDNA synthesis Kit (Bio-Rad, Hercules, CA, USA) in a 20 μL total volume. The resultant cDNA was stored at −20 °C for later analysis. Real-time PCR was performed in triplicate with iQ™SYBR® Green Supermix (Bio-Rad) in the iCycler Real-Time Detection System (Bio-Rad), using 2 μL of cDNA in a 20 μL final volume containing 0.2 μM each of the primers. Primer pairs and PCR conditions have been detailed previously [52]. Target amplification was ascertained with post-run melt curve analysis. Quantification was achieved with a standard curve, constructed for each specific gene amplimer. In evaluation of mRNA expression, a ratio was calculated for each gene, normalized to β-actin. The ratio was defined as number of transcripts of a given gene in each transformed clone relative to β–actin/number of transcripts of the same gene in the UROtsa relative to β–actin.
4.5. Statistical Analysis
Statistical tests were done with the SPSS package version 17 (SPSS Inc., Chicago, IL, USA). Differences in zinc transporter gene expression between parent UROtsa cells vs. cadmium-transformed UROtsa cells were determined with the Mann–Whitney test. Variation in expression of various zinc transporter genes among seven clones were determined with the Kruskal–Wallis test with Dunn’s post hoc tests. The p values of 0.05 or less were considered to identify statistical significance.
5. Conclusions
Higher expression levels of ZIP14 than ZIP8 suggest that influx transporter ZIP14 may contribute to Cd accumulation in UROtsa cells, together with the efflux transporter ZnT1. Transformed UROtsa cells exhibited aberrant expression of multiple zinc uptake zinc transporters, namely ZIP1, ZIP6, ZIP8, and ZIP10, along with those of the Golgi zinc transporters, including ZnT5, ZnT6, and ZnT7. Expression levels of ZIP6, ZIP10, ZIP14, and ZnT1 were increased, while ZIP8 expression was diminished in only one transformed clone. These results provide evidence for zinc dysregulation in transformed UROtsa cells that may contribute in part to their malignancy and/or muscle invasiveness.
Author Contributions
Conceptualization, S.S. (Soisungwan Satarug), S.H.G., and S.S. (Seema Somji); methodology, S.S. (Soisungwan Satarug), S.H.G., and S.S. (Seema Somji); formal analysis, S.S. (Soisungwan Satarug); investigation, S.S. (Soisungwan Satarug); resources, M.A.S.; original draft preparation, S.S. (Soisungwan Satarug); review and editing, S.H.G., S.S. (Seema Somji), and M.A.S.; project administration, M.A.S. and D.A.S.; funding acquisition, D.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grant number R01 ES015100 from the National Institute of Environmental Health Sciences (NIEHS), NIH, USA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The Commission for Higher Education, Thailand Ministry of Education in partnership with the National Research Centre for Environmental Toxicology, The University of Queensland provided S.S. overseas travel support. Opinions expressed in this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, Environmental Exposure, and Health Outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current Health Risk Assessment Practice for Dietary Cadmium: Data from Different Countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef]
- Satarug, S.; Phelps, K.R. Cadmium Exposure and Toxicity. In Metal Toxicology Handbook; Bagchi, D., Bagchi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 219–274. [Google Scholar]
- International Agency for Research on Cancer (IARC). Cadmium. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 58, 119–238. [Google Scholar]
- Pasin, E.; Josephson, D.Y.; Mitra, A.P.; Cote, R.J.; Stein, J.P. Superficial Bladder Cancer: An Update on Etiology, Molecular Development, Classification, and Natural History. Rev. Urol. 2008, 10, 31–43. [Google Scholar]
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C. Global Burden of Urologic Cancers, 1990–2013. Eur. Urol. 2017, 71, 437–446. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishiyama, H. Epidemiology of Urothelial Carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.M.; Loughlin, K.R. Economic Impact of Tumor Markers in Bladder Cancer Surveillance. Urology 2008, 71, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Garren, B.; Nielsen, M.E.; Tang, L. Lifestyle and Nutritional Modifiable Factors in the Prevention and Treatment of Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Samanic, C.; Kogevinas, M.; Dosemeci, M.; Malats, N.; Real, F.X.; Garcia-Closas, M.; Real, F.X.; Garcia-Closas, M.; Serra, C.; Carrato, A.; et al. Smoking and Bladder Cancer in Spain: Effects of Tobacco Type, Timing, Environmental Tobacco Smoke, and Gender. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood Cadmium May be Associated with Bladder Carcinogenesis: The Belgian Case-Control Study on Bladder Cancer. Cancer Detect. Prev. 2007, 31, 77–82. [Google Scholar] [CrossRef]
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated Metallothionein-Bound Cadmium Concentrations in Urine from Bladder Carcinoma Patients, Investigated by Size Exclusion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal. Chim. Acta 2009, 631, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, L.; Leigh, I.M.; Duffy, P.G.; Sexton, C.; Masters, R.W. Immortalisation of Human Urothelial Cells. Urol. Res. 1995, 23, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.R.; Masters, J.R.W.; Park, S.; Todd, J.H.; Garrett, S.H.; Sens, M.A.; Somji, S.; Nath, J.; Sens, D.A. The Immortalized UROtsa Cell Line as a Potential Cell Culture Model of Human Urothelium. Environ. Health Perspect. 2001, 109, 801–808. [Google Scholar] [CrossRef]
- Sens, D.; Rossi, M.; Park, S.; Gurel, V.; Nath, J.; Garrett, S.; Sens, M.A.; Somji, S. Metallothionein Isoform 1 and 2 Gene Expression in a Human Urothelial Cell Line (UROtsa) Exposed to CdCl2 and NaAsO2. J. Toxicol. Environ. Health A 2003, 66, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Sens, D.A.; Park, S.; Gurel, V.; Sens, M.A.; Garrett, S.H.; Somji, S. Inorganic Cadmium-and Arsenite-Induced Malignant Transformation of Human Bladder Urothelial Cells. Toxicol. Sci. 2004, 79, 56–63. [Google Scholar] [CrossRef]
- Bafaro, B.; Liu, Y.; Xu, Y.; Dempski, R.E. The Emerging Role of Zinc Transporters in Cellular Homeostasis and Cancer. Signal Transduct. Target. Ther. 2017, 2, e17029. [Google Scholar] [CrossRef]
- Takatani-Nakase, T. Zinc Transporters and the Progression of Breast Cancers. Biol. Pharm. Bull. 2018, 41, 1517–1522. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian Zinc Transporters: Nutritional and Physiologic Regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Zinc Transporter 1 (ZNT1) Expression on the Cell Surface is Elaborately Controlled by Cellular Zinc Levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc Transporters and Their Functional Integration in Mammalian Cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. The Human ZIP1 Transporter Mediates Zinc Uptake in Human K562 Erythroleukemia Cells. J. Biol. Chem. 2001, 276, 22258–22264. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Functional Expression of the Human hZIP2 Zinc Transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human ZIP1 is a Major Zinc Uptake Transporter for the Accumulation of Zinc in Prostate Cells. J. Inorg. Biochem. 2003, 96, 435–442. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Huang, Z.L.; Geiser, J.; Xu, W.; Andrews, G.K. Mouse ZIP1 and ZIP3 Genes Together are Essential for Adaptation to Dietary Zinc Deficiency during Pregnancy. Genesis 2006, 44, 239–251. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.J.; Zhang, Y.; Yu, Y.Y. The ZIP7 Gene (Slc39a7) Encodes a Zinc Transporter Involved in Zinc Homeostasis of the Golgi Apparatus. J. Biol. Chem. 2005, 280, 15456–15463. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional Characterization of a Novel Mammalian Zinc Transporter, ZnT6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Matsuura, W.; Kohda, A.; Okumura, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc Transporters, ZnT5 and ZnT7, are Required for the Activation of Alkaline Phosphatases, Zinc-Requiring Enzymes that are Glycosylphosphatidylinositol-Anchored to the Cytoplasmic Membrane. J. Biol. Chem. 2005, 280, 637–643. [Google Scholar] [CrossRef]
- Kirschke, C.P.; Huang, L. ZnT7, A Novel Mammalian Zinc Transporter, Accumulates Zinc in the Golgi Apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 Gene Encodes ZIP14, a Metal/Bicarbonate Symporter: Similarities to the ZIP8 Transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Soleimani, M.; Girijashanker, K.; Reed, J.M.; He, L.; Dalton, T.P.; Nebert, D.W. Cd2+ versus Zn2+ Uptake by the ZIP8 HCO3--Dependent Symporter: Kinetics, Electrogenicity and Trafficking. Biochem. Biophys. Res. Commun. 2008, 365, 814–820. [Google Scholar] [CrossRef]
- Besecker, B.; Bao, S.; Bohacova, B.; Papp, A.; Sadee, W.; Knoell, D.L. The Human Zinc Transporter SLC39A8 (Zip8) is Critical in Zinc-Mediated Cytoprotection in Lung Epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1127–L1136. [Google Scholar] [CrossRef] [PubMed]
- Ohana, E.; Sekler, I.; Kaisman, T.; Kahn, N.; Cove, J.; Silverman, W.F.; Amsterdam, A.; Hershfinkel, M. Silencing of ZnT-1 Expression Enhances Heavy Metal Influx and Toxicity. J. Mol. Med. 2006, 84, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Cherrington, N.J.; Hartley, D.P.; Klaassen, C.D.; Waalkes, M.P. Cyproterone Acetate Induces a Cellular Tolerance to Cadmium in Rat Liver Epithelial Cells Involving Reduced Cadmium Accumulation. Toxicology 2001, 165, 13–25. [Google Scholar] [CrossRef]
- Hoggarth, Z.E.; Osowski, D.B.; Freeberg, B.A.; Garrett, S.H.; Sens, D.A.; Sens, M.A.; Zhou, X.D.; Zhang, K.K.; Somji, S. The Urothelial Cell Line UROtsa Transformed by Arsenite and Cadmium Display Basal Characteristics Associated with Muscle Invasive Urothelial Cancers. PLoS ONE 2018, 13, e0207877. [Google Scholar] [CrossRef]
- Ajjimaporn, A.; Botsford, T.; Garrett, S.H.; Sens, M.A.; Zhou, X.D.; Dunlevy, J.R.; Sens, D.A.; Somji, S. ZIP8 Expression in Human Proximal Tubule Cells, Human Urothelial Cells Transformed by Cd+2 and As+3 and in Specimens of Normal Human Urothelium and Urothelial Cancer. Cancer Cell Int. 2012, 12, 16. [Google Scholar] [CrossRef]
- Garrett, S.H.; Somji, S.; Sens, D.A.; Zhang, K.K. Prediction of the Number of Activated Genes in Multiple Independent Cd+2 and As+3-Induced Malignant Transformations of Human Urothelial Cells (UROtsa). PLoS ONE 2014, 9, e85614. [Google Scholar] [CrossRef]
- Lehvy, A.I.; Horev, G.; Golan, Y.; Glaser, F.; Shammai, Y.; Assaraf, Y.G. Alterations in ZnT1 Expression and Function Lead to Impaired Intracellular Zinc Homeostasis in Cancer. Cell Death Discov. 2019, 5, 144. [Google Scholar] [CrossRef]
- Zhu, B.; Huo, R.; Zhi, Q.; Zhan, M.; Chen, X.; Hua, Z.C. Increased Expression of Zinc Transporter ZIP4, ZIP11, ZnT1, and ZnT6 Predicts Poor Prognosis in Pancreatic Cancer. J. Trace Elem. Med. Biol. 2021, 65, 126734. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Jin, H.; Yin, L.; Yu, H.; Bi, J. MiR-411 Suppresses the Development of Bladder Cancer by Regulating ZnT1. OncoTargets Ther. 2018, 11, 8695–8704. [Google Scholar] [CrossRef]
- Golovine, K.; Makhov, P.; Uzzo, R.G.; Shaw, T.; Kunkle, D.; Kolenko, V.M. Overexpression of the Zinc Uptake Transporter hZIP1 Inhibits Nuclear Factor-KappaB and Reduces the Malignant Potential of Prostate Cancer Cells in Vitro and in Vivo. Clin. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.M.; Cousins, R.J.; et al. Aberrant Expression of Zinc Transporter ZIP4 (SLC39A4) Significantly Contributes to Human Pancreatic Cancer Pathogenesis and Progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Vichova, P.; Jordan, N.; Hiscox, S.; Hendley, R.; Nicholson, R.I. ZIP7 Mediated Intracellular Zinc Transport Contributes to Aberrant Growth Factor Signaling in Antihormone-Resistant Breast Cancer Cells. Endocrinology 2008, 149, 4912–4920. [Google Scholar] [CrossRef]
- Kagara, N.; Tanaka, N.; Noguchi, S.; Hirano, T. Zinc and Its Transporter ZIP10 are Involved in Invasive Behavior of Breast Cancer Cells. Cancer Sci. 2007, 98, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Mathews, W.R.; Wang, F.; Eide, D.J.; Doren, M.V. Drosophila Fear of Intimacy Encodes a Zrt/IRT-Like Protein (ZIP) Family Zinc Transporter Functionally Related to Mammalian ZIP Proteins. J. Biol. Chem. 2005, 280, 787–795. [Google Scholar] [CrossRef]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc Transporter ZIP10 Forms a Heteromer with ZIP6 which Regulates Embryonic Development and Cell Migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Fujishiro, H.; Okugaki, S.; Yasumitsu, S.; Enomoto, S.; Himeno, S. Involvement of DNA Hypermethylation in Down-Regulation of the Zinc Transporter ZIP8 in Cadmium-Resistant Metallothionein-Null Cells. Toxicol. Appl. Pharmacol. 2009, 241, 195–201. [Google Scholar] [CrossRef]
- Fujishiro, H.; Ohashi, T.; Takuma, M.; Himeno, S. Suppression of ZIP8 Expression is a Common Feature of Cadmium-Resistant and Manganese-Resistant RBL-2H3 Cells. Metallomics 2013, 5, 437–444. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, W.; Taylor, K.M.; Cai, B.; Li, X. LIV-1 Suppression Inhibits HeLa Cell Invasion by Targeting ERK1/2-Snail/Slug Pathway. Biochem. Biophys. Res. Commun. 2007, 363, 82–88. [Google Scholar] [CrossRef]
- San, R.H.C.; Laspia, M.F.; Soiefer, A.I.; Maslansky, C.J.; Rice, J.M.; Williams, G.M. A Survey of Growth in Soft Agar and Cell Surface Properties as Markers for Transformation in Adult Rat Liver Epithelial-Like Cell Cultures. Cancer Res. 1979, 39, 1026–1034. [Google Scholar] [PubMed]
- Albrecht, A.L.; Somji, S.; Sens, M.A.; Sens, D.A.; Garrett, S.H. Zinc Transporter mRNA Expression in the RWPE-1 Human Prostate Epithelial Cell Line. Biometals 2008, 21, 405–416. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).