Jaw Clenching Alters Neuromuscular Coordination in Dynamic Postural Tasks: A Pilot Study on Single-Leg Sit-to-Stand Movements
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Settings
2.3. Experimental Procedures
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Performance Outcomes
3.2. Comparisons of EMG and Kinematic Outcomes Between Control and Clenching Conditions
3.3. Comparisons of EMG and Kinematic Outcomes Between Successful and Gailed Trials Within the Clenching Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMG | electromyography |
| COM | center of mass |
| IMU | inertial measurement unit |
| MVC | maximal voluntary contraction |
| IQR | interquartile range |
| MA | masseter |
| AD | anterior deltoid |
| RA | rectus abdominis |
| ESL | erector spinae longus |
| RF | rectus femoris |
| BF | biceps femoris |
| TA | tibialis anterior |
| MGAS | medial gastrocnemius |
| APAs | anticipatory postural adjustments |
| RPAs | reactive postural adjustments |
References
- Carroll, T.J.; Baldwin, E.R.; Collins, D.F.; Zehr, E.P. Corticospinal excitability is lower during rhythmic arm movement than during tonic contraction. J. Neurophysiol. 2006, 95, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Floeter, M.K.; Danielian, L.E.; Kim, Y.K. Effects of motor skill learning on reciprocal inhibition. Restor. Neurol. Neurosci. 2013, 31, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pienciak-Siewert, A.; Horan, D.P.; Ahmed, A.A. Role of muscle coactivation in adaptation of standing posture during arm reaching. J. Neurophysiol. 2020, 123, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Beck, O.N.; Ting, L.H. Voluntary muscle coactivation in quiet standing elicits reciprocal rather than coactive agonist-antagonist control of reactive balance. J. Neurophysiol. 2023, 129, 1378–1388. [Google Scholar] [CrossRef]
- Mari, S.; Serrao, M.; Casali, C.; Conte, C.; Martino, G.; Ranavolo, A.; Coppola, G.; Draicchio, F.; Padua, L.; Sandrini, G.; et al. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum 2014, 13, 226–236. [Google Scholar] [CrossRef]
- Rinaldi, M.; Ranavolo, A.; Conforto, S.; Martino, G.; Draicchio, F.; Conte, C.; Varrecchia, T.; Bini, F.; Casali, C.; Pierelli, F.; et al. Increased lower limb muscle coactivation reduces gait performance and increases metabolic cost in patients with hereditary spastic paraparesis. Clin. Biomech. 2017, 48, 63–72. [Google Scholar] [CrossRef]
- Boudreau, S.A.; Falla, D. Chronic neck pain alters muscle activation patterns to sudden movements. Exp. Brain Res. 2014, 232, 2011–2020. [Google Scholar] [CrossRef]
- Nagai, K.; Yamada, M.; Uemura, K.; Yamada, Y.; Ichihashi, N.; Tsuboyama, T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch. Gerontol. Geriatr. 2011, 53, 338–343. [Google Scholar] [CrossRef]
- Hellmann, D.; Giannakopoulos, N.N.; Blaser, R.; Eberhard, L.; Schindler, H.J. The effect of various jaw motor tasks on body sway. J. Oral. Rehabil. 2011, 38, 729–736. [Google Scholar] [CrossRef]
- Hellmann, D.; Stein, T.; Potthast, W.; Rammelsberg, P.; Schindler, H.J.; Ringhof, S. The effect of force-controlled biting on human posture control. Hum. Mov. Sci. 2015, 43, 125–137. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshino, N.; Sako, K.; Ono, Y.; Maeda, Y. Occlusal support and postural stability in children: An observational study. Pediatr. Int. 2021, 63, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Tanaka, Y.; Sako, K.; Ono, Y.; Tanaka, M. Effect of jaw clenching on postural adjustments to a predictable external perturbation. J. Electromyogr. Kinesiol. 2021, 57, 102512. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Zafar, H.; Iqbal, Z.A. Effect of three different jaw positions on postural stability during standing. Funct. Neurol. 2015, 30, 53–57. [Google Scholar]
- Ringhof, S.; Leibold, T.; Hellmann, D.; Stein, T. Postural stability and the influence of concurrent muscle activation—Beneficial effects of jaw and fist clenching. Gait Posture 2015, 42, 598–600. [Google Scholar] [CrossRef]
- Fadillioglu, C.; Kanus, L.; Möhler, F.; Ringhof, S.; Schindler, H.J.; Stein, T.; Hellmann, D. Influence of controlled masticatory muscle activity on dynamic reactive balance. J. Oral. Rehabil. 2022, 49, 327–336. [Google Scholar] [CrossRef]
- Fadillioglu, C.; Kanus, L.; Möhler, F.; Ringhof, S.; Hellmann, D.; Stein, T. Effects of jaw clenching on dynamic reactive balance task performance after 1-week of jaw clenching training. Front. Neurol. 2013, 14, 1140712. [Google Scholar] [CrossRef]
- Fadillioglu, C.; Kanus, L.; Möhler, F.; Ringhof, S.; Schmitter, M.; Hellmann, D.; Stein, T. Persisting effects of jaw clenching on dynamic steady-state balance. PLoS ONE 2024, 19, e0299050. [Google Scholar] [CrossRef]
- Sako, K.; Tanaka, Y.; Tomita, Y.; Yoshida, T.; Ono, Y.; Kashiwagi, K. Effect of jaw clenching on head acceleration during a predictable load impact. J. Oral. Rehabil. 2021, 48, 1327–1336. [Google Scholar] [CrossRef]
- Ringhof, S.; Stein, T.; Hellmann, D.; Schindler, H.J.; Potthast, W. Effect of jaw clenching on balance recovery: Dynamic stability and lower extremity joint kinematics after forward loss of balance. Front. Psychol. 2016, 7, 291. [Google Scholar] [CrossRef]
- Rainville, J.; Jouve, C.; Finno, M.; Limke, J. Comparison of four tests of quadriceps strength in L3 or L4 radiculopathies. Spine 2003, 28, 2466–2471. [Google Scholar] [CrossRef]
- Suri, P.; Rainville, J.; Katz, J.N.; Jouve, C.; Hartigan, C.; Limke, J.; Pena, E.; Li, L.; Swaim, B.; Hunter, D.J. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine 2011, 36, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hagino, H.; Wada, T.; Kobayashi, E. Locomotive syndrome presents a risk for falls and fractures in the elderly Japanese population. Osteoporos. Sarcopenia 2016, 2, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Sousa, A.S.; Pinheiro, R.; Ferraz, J.; Tavares, J.M.; Santos, R.; Sousa, F. Activation timing of soleus and tibialis anterior muscles during sit-to-stand and stand-to-sit in post-stroke vs. healthy subjects. Somatosens. Mot. Res. 2013, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.; Dong, X.N.; Dalby, A.; Goh, C.H. The influence of smoothness and speed of stand-to-sit movement on joint kinematics, kinetics, and muscle activation patterns. Front. Hum. Neurosci. 2024, 18, 1399179. [Google Scholar] [CrossRef]
- Takahashi, M.; Bando, Y.; Fukui, T.; Sugita, M. Light Clenching Differentially Affects Balance Ability According to Occlusal Contact Stability. Appl. Sci. 2024, 14, 10314. [Google Scholar] [CrossRef]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89, S9–S16. [Google Scholar] [CrossRef]
- Gonzalez, Y.M.; Schiffman, E.; Gordon, S.M.; Seago, B.; Truelove, E.L.; Slade, G.; Ohrbach, R. Development of a brief and effective temporomandibular disorder pain screening questionnaire: Reliability and validity. J. Am. Dent. Assoc. 2011, 142, 1183–1191. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Tsukiboshi, T.; Sato, H.; Tanaka, Y.; Saito, M.; Toyoda, H.; Morimoto, T.; Türker, K.S.; Maeda, Y.; Kang, Y. Illusion caused by vibration of muscle spindles reveals an involvement of muscle spindle inputs in regulating isometric contraction of masseter muscles. J. Neurophysiol. 2012, 108, 2524–2533. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, H.; Toyoda, H.; Saito, M.; Maeda, Y.; Kang, Y. The mechanism for regulating the isometric contraction of masseter muscles is involved in determining the vertical dimension of occlusion. J. Neurophysiol. 2023, 129, 211–219. [Google Scholar] [CrossRef]
- Miyahara, T.; Hagiya, N.; Ohyama, T.; Nakamura, Y. Modulation of human soleus H reflex in association with voluntary clenching of the teeth. J. Neurophysiol. 1996, 76, 2033–2041. [Google Scholar] [CrossRef]
- Waldhelm, A.; Gubler, C.; Sullivan, K.; Witte, C.; Buchheister, D.; Bartz-Broussard, J. Inter-rater and test-retest reliability of two new single leg sit-to-stand tests. Int. J. Sports Phys. Ther. 2020, 15, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Miyahara, T.; Tanaka, T.; Ohyama, T.; Nakamura, Y. Modulation of H reflex of pretibial muscles and reciprocal Ia inhibition of soleus muscle during voluntary teeth clenching in humans. J. Neurophysiol. 2000, 83, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Goulart, F.R.; Valls-Solé, J. Patterned electromyographic activity in the sit-to-stand movement. Clin. Neurophysiol. 1999, 110, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.; Edelhoff, D.; Schubert, O.; Erdelt, K.J.; Pho Duc, J.M. Effect of treatment with a full-occlusion biofeedback splint on sleep bruxism and TMD pain: A randomized controlled clinical trial. Clin. Oral. Investig. 2020, 24, 4005–4018. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mikami, S.; Maeda, M.; Saito, T.; Nakajima, T.; Yachida, W.; Gotouda, A. Portable and wearable electromyographic devices for the assessment of sleep bruxism and awake bruxism: A literature review. Cranio 2023, 41, 69–77. [Google Scholar] [CrossRef]
- Colonna, A.; Lobbezoo, F.; Bracci, A.; Ferrari, M.; Val, M.; Manfredini, D. Long-term study on the fluctuation of self-reported awake bruxism in a cohort of healthy young adults. J. Oral. Rehabil. 2025, 52, 37–42. [Google Scholar] [CrossRef]
- Miró, A.; Buscà, B.; Arboix-Alió, J.; Huertas, P.; Aguilera-Castells, J. Acute effects of jaw clenching while wearing a customized bite-aligning mouthguard on muscle activity and force production during maximal upper body isometric strength. J. Exerc. Sci. Fit. 2023, 21, 157–164. [Google Scholar] [CrossRef]
- Okubo, M.; Fujinami, Y.; Minakuchi, S. Effect of complete dentures on body balance during standing and walking in elderly people. J. Prosthodont. Res. 2010, 54, 42–47. [Google Scholar] [CrossRef]
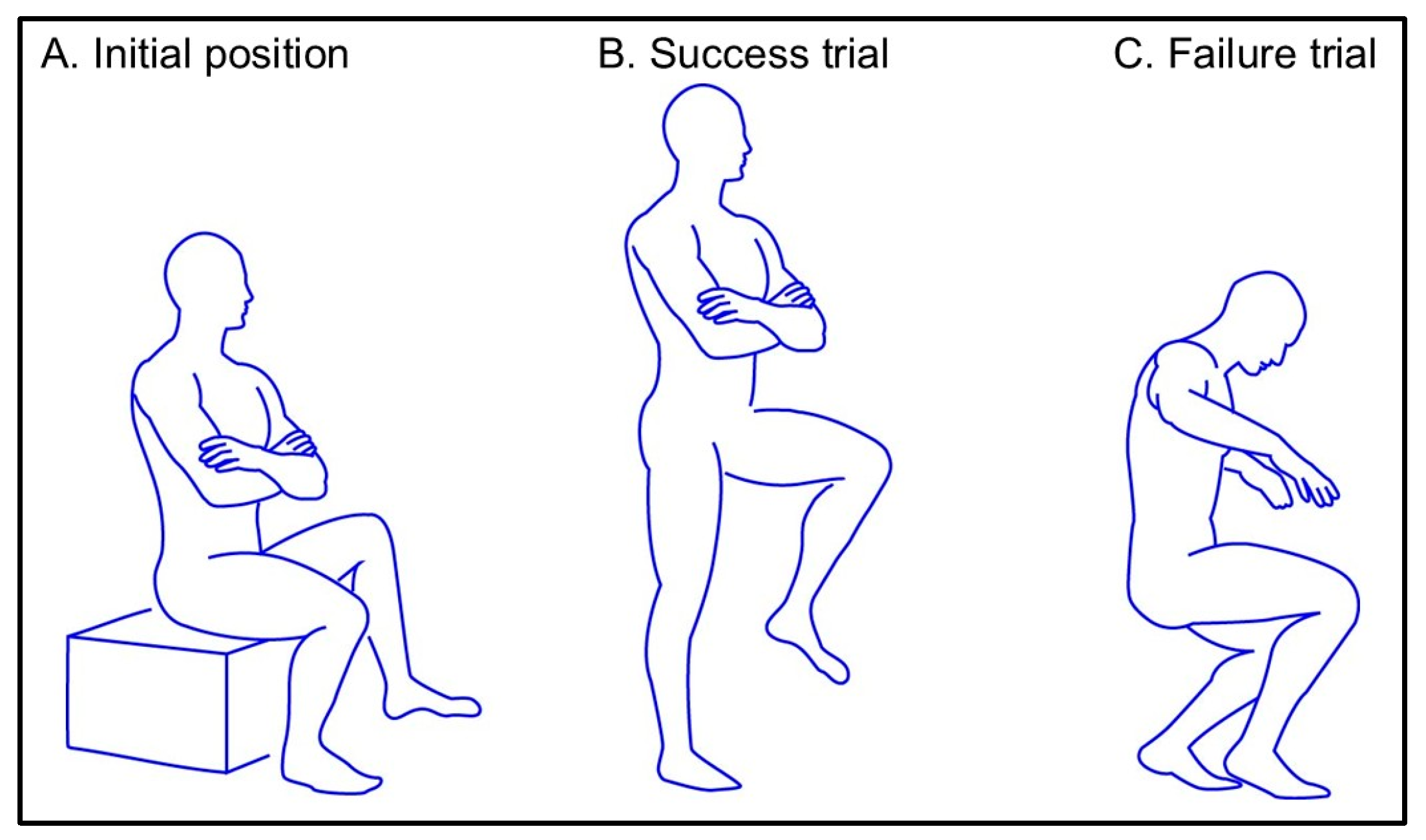
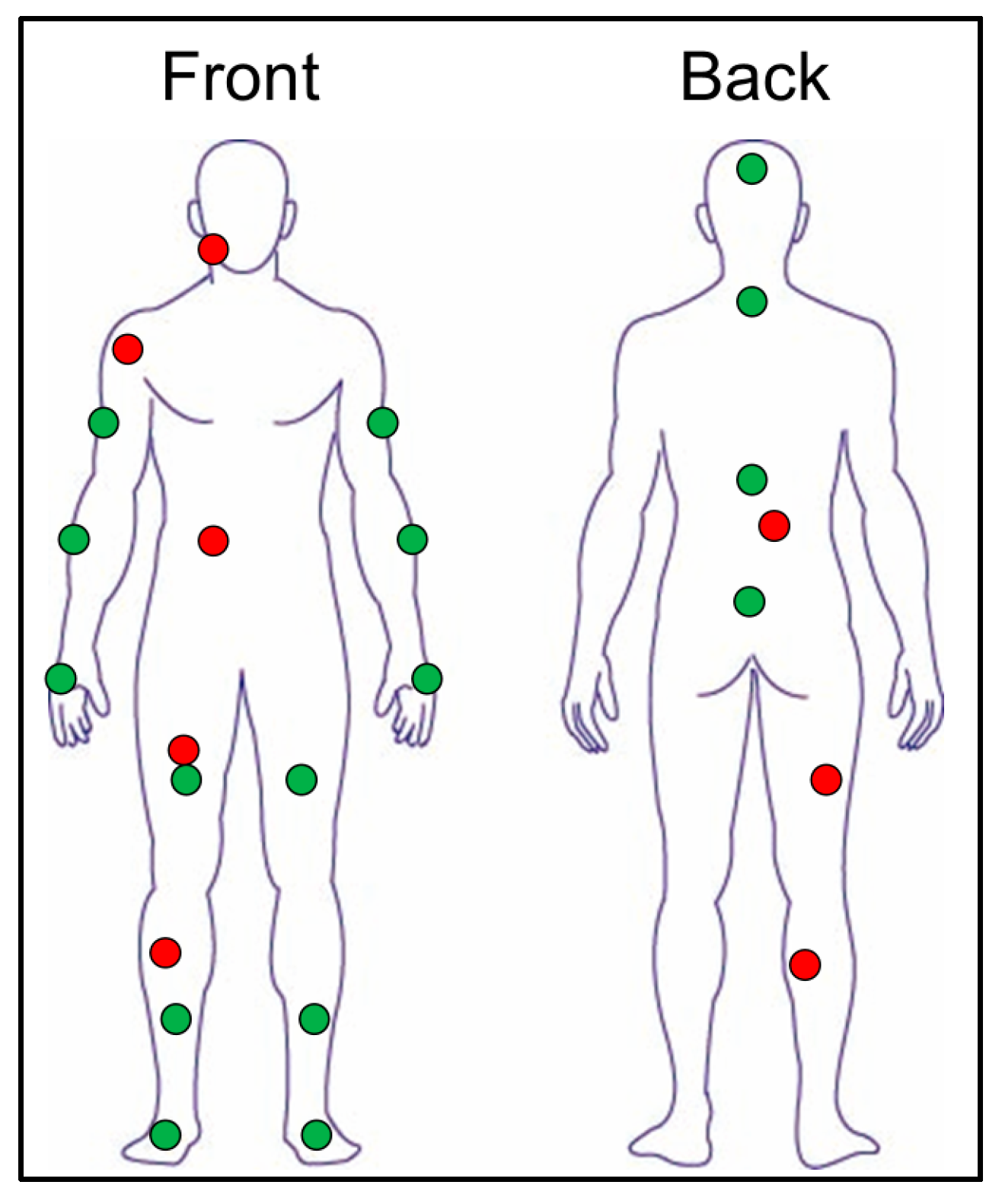
| Age, years | 21.2 ± 0.4 |
| Sex, male/female | 6:5 |
| Height, mm | 167.9 ± 9.6 |
| Body weight, kg | 59.7 ± 8.1 |
| Dominant leg, right/left | 11:0 |
| Control | Clenching | ES | p-Value | |
|---|---|---|---|---|
| Total attempts | 9.82 ± 3.52 | 9.91 ± 4.32 | 0.021 | 0.878 |
| Success count | 9.45 ± 3.80 | 8.18 ± 4.87 | 0.228 | 0.031 * |
| Success rate | 0.96 ± 0.13 | 0.78 ± 0.29 | 0.694 | 0.047 * |
| Control | Clenching | ES | p-Value | |
|---|---|---|---|---|
| EMG activity, %MVC | ||||
| Masseter | 3.3 ± 5.8 | 10.8 ± 7.0 | 1.323 | <0.001 * |
| Anterior deltoid | 9.3 ± 14.2 | 8.3 ± 11.6 | 0.028 | 0.845 |
| Rectus abdominis | 7.2 ± 7.8 | 6.7 ± 7.3 | 0.007 | 0.961 |
| Erector spinae | 39.5 ± 16.9 | 41.9 ± 18.1 | 0.125 | 0.375 |
| Rectus femoris | 57.9 ± 15.1 | 53.3 ± 13.7 | 0.163 | 0.246 |
| Biceps femoris | 40.3 ± 18.9 | 40.5 ± 18.7 | 0.029 | 0.839 |
| Tibialis anterior | 51.5 ± 18.2 | 44.3 ± 17.3 | 0.146 | 0.303 |
| Medial gastrocnemius | 15.3 ± 8.0 | 16.6 ± 8.4 | 0.217 | 0.126 |
| Coactivation index | ||||
| ES-RA | 0.84 ± 0.15 | 0.85 ± 0.14 | 0.107 | 0.448 |
| RF-BF | 0.39 ± 0.11 | 0.41 ± 0.12 | 0.194 | 0.169 |
| MG-TA | 0.24 ± 0.13 | 0.26 ± 0.14 | 0.088 | 0.535 |
| Control | Clenching | ES | p-Value | |
|---|---|---|---|---|
| Joint angle, degree | ||||
| Trunk pitch | 15.4 ± 17.2 | 15.6 ± 17.8 | 0.076 | 0.892 |
| Trunk yaw | 2.3 ± 9.5 | 3.2 ± 7.6 | 0.006 | 0.969 |
| Trunk roll | 1.9 ± 5.6 | 6.3 ± 5.1 | 1.020 | <0.001 * |
| Hip flexion | 101.7 ± 20.4 | 105.8 ± 15.9 | 0.698 | <0.001 * |
| Hip abduction | −20.6 ± 10.2 | −21.0 ± 10.8 | 0.549 | <0.001 * |
| Hip internal rotation | 13.0 ± 15.4 | 17.5 ± 16.5 | 0.631 | <0.001 * |
| Knee flexion | 109.7 ± 14.1 | 113.1 ± 15.1 | 0.816 | <0.001 * |
| Ankle dorsiflexion | 16.9 ± 7.8 | 17.8 ± 7.9 | 0.211 | 0.145 |
| Hip angular velocity | 3.6 ± 8.5 | 6.0 ± 11.7 | 0.258 | 0.068 |
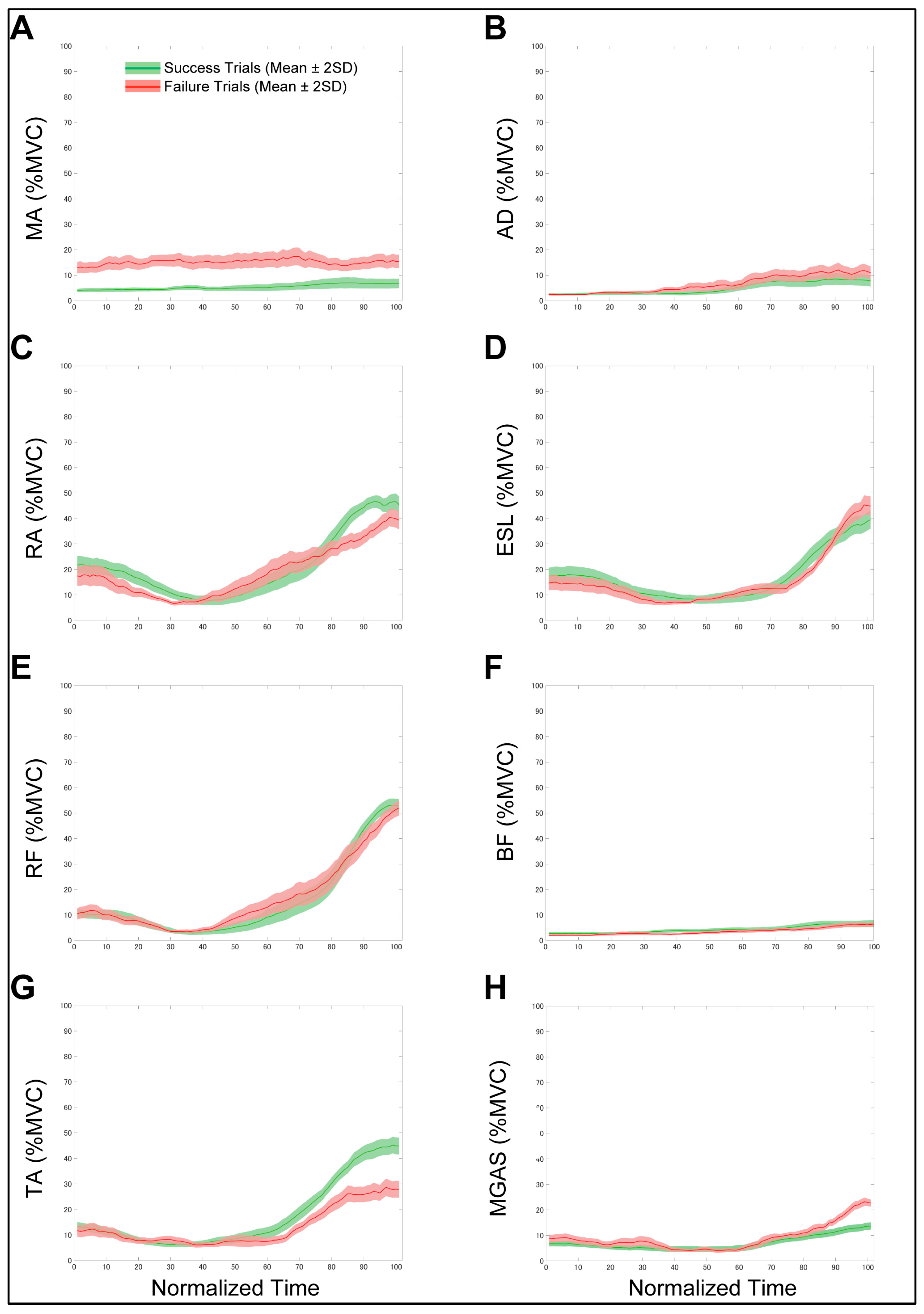
| Success (n = 90) | Failure (n = 19) | ES | p-Value | |
|---|---|---|---|---|
| EMG activity, %MVC | ||||
| Masseter | 6.8 ± 9.71 | 15.5 ± 13.4 | 0.831 | <0.001 * |
| Anterior deltoid | 7.7 ± 11.3 | 11.0 ± 12.9 | 0.559 | 0.022 * |
| Rectus abdominis | 6.8 ± 4.7 | 6.3 ± 4.7 | 0.100 | 0.768 |
| Erector spinae | 44.9 ± 17.0 | 27.9 ± 17.0 | 1.007 | <0.001 * |
| Rectus femoris | 53.7 ± 13.5 | 52.0 ± 15.2 | 0.147 | 0.639 |
| Biceps femoris | 39.6 ± 18.5 | 44.9 ± 19.4 | 0.305 | 0.260 |
| Tibialis anterior | 45.4 ± 17.0 | 39.34 ± 18.3 | 0.387 | 0.170 |
| Medial gastrocnemius | 13.7 ± 7.3 | 22.6 ± 7.3 | 1.200 | <0.001 * |
| Coactivation index | ||||
| ES-RA | 0.88 ± 0.11± | 0.74 ± 0.22 | 1.042 | <0.001 * |
| RF-BF | 0.41 ± 0.11 | 0.45 ± 0.15 | 0.420 | 0.149 |
| MG-TA | 0.23 ± 0.11 | 0.39 ± 0.19 | 1.284 | <0.001 * |
| Success (n = 90) | Failure (n = 19) | 95% Confidence Interval (Lower Limit, Upper Limit) | ES | p-Value | |
|---|---|---|---|---|---|
| Joint angle, degree | |||||
| Trunk pitch | 18.15 ± 15.59 | 12.76 ± 26.66 | −3.584, 14.371 | 0.361 | 0.236 |
| Trunk yaw | −0.39 ± 6.72 | 5.70 ± 9.19 | 2.421, 8.969 | 0.713 | 0.001 * |
| Trunk roll | 1.45 ± 6.60 | 8.83 ± 5.69 | −10.611, −4.145 | 0.958 | <0.001 * |
| Hip flexion | 104.68 ± 14.71 | 110.93 ± 20.38 | −1.662, 14.158 | 0.479 | 0.120 |
| Hip abduction | −20.69 ± 11.38 | −22.33 ± 7.11 | 0.763, 17.547 | 0.164 | 0.549 |
| Hip internal rotation | 15.80 ± 16.74 | 24.96 ± 16.90 | 0.763, 17.547 | 0.518 | 0.033 * |
| Knee flexion | 111.21 ± 13.78 | 122.11 ± 17.79 | 3.629, 18.178 | 0.637 | 0.004 * |
| Ankle dorsiflexion | 17.53 ± 6.56 | 21.88 ± 10.74 | 0.629, 8.065 | 0.556 | 0.022 * |
| Hip angular velocity | 5.86 ± 11.83 | 8.38 ± 12.74 | −3.477, 8.518 | 0.206 | 0.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Ono, Y.; Tomita, Y. Jaw Clenching Alters Neuromuscular Coordination in Dynamic Postural Tasks: A Pilot Study on Single-Leg Sit-to-Stand Movements. Biomechanics 2025, 5, 89. https://doi.org/10.3390/biomechanics5040089
Tanaka Y, Ono Y, Tomita Y. Jaw Clenching Alters Neuromuscular Coordination in Dynamic Postural Tasks: A Pilot Study on Single-Leg Sit-to-Stand Movements. Biomechanics. 2025; 5(4):89. https://doi.org/10.3390/biomechanics5040089
Chicago/Turabian StyleTanaka, Yuto, Yoshiaki Ono, and Yosuke Tomita. 2025. "Jaw Clenching Alters Neuromuscular Coordination in Dynamic Postural Tasks: A Pilot Study on Single-Leg Sit-to-Stand Movements" Biomechanics 5, no. 4: 89. https://doi.org/10.3390/biomechanics5040089
APA StyleTanaka, Y., Ono, Y., & Tomita, Y. (2025). Jaw Clenching Alters Neuromuscular Coordination in Dynamic Postural Tasks: A Pilot Study on Single-Leg Sit-to-Stand Movements. Biomechanics, 5(4), 89. https://doi.org/10.3390/biomechanics5040089






