Biomechanical Trade-Offs Between Speed and Agility in the Northern Brown Bandicoot
Abstract
1. Introduction
2. Methods
2.1. Field Work
2.2. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Johnson, C.N.; Isaac, J.L. Body mass and extinction risk in Australian marsupials: The ‘Critical Weight Range’ revisited. Austral Ecol. 2009, 34, 35–40. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Legge, S.; Fitzsimons, J.A.; Traill, B.J.; Burbidge, A.A.; Fisher, A.; Firth, R.S.C.; Gordon, I.J.; Griffiths, A.D.; Johnson, C.N.; et al. The disappearing mammal fauna of northern Australia: Context, cause, and response. Conserv. Lett. 2011, 4, 192–201. [Google Scholar] [CrossRef]
- Murphy, B.P.; Davies, H.F. There is a critical weight range for A ustralia’s declining tropical mammals. Glob. Ecol. Biogeogr. 2014, 23, 1058–1061. [Google Scholar] [CrossRef]
- Bytheway, J.P.; Banks, P.B. Overcoming prey naiveté: Free-living marsupials develop recognition and effective behavioral responses to alien predators in Australia. Glob. Change Biol. 2019, 25, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.W. Effect of size on fast-start performance of rainbow trout Salmo gairdneri, and a consideration of piscivorous predator-prey interactions. J. Exp. Biol. 1976, 65, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.P.; Cowan, I.M.; Holling, C.S. Prey capture by African lion. Can. J. Zoology 1977, 55, 1811–1828. [Google Scholar] [CrossRef]
- Huey, R.B.; Hertz, P.E. Effects of body size and slope on acceleration of a lizard (Stellio stellio). J. Exp. Biol. 1984, 110, 113–123. [Google Scholar] [CrossRef]
- Portalier, S.M.J.; Fussmann, G.F.; Loreau, M.; Cherif, M. The mechanics of predator–prey interactions: First principles of physics predict predator–prey size ratios. Funct. Ecol. 2019, 33, 323–334. [Google Scholar] [CrossRef]
- Hirt, M.R.; Tucker, M.; Müller, T.; Rosenbaum, B.; Brose, U. Rethinking trophic niches: Speed and body mass colimit prey space of mammalian predators. Ecol. Evol. 2020, 10, 7094–7105. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.; Clemente, C.J.; Niehaus, A.C.; Fisher, D.O.; Wilson, R.S. Surface friction alters the agility of a small Australian marsupial. J. Exp. Biol. 2018, 221, e172544. [Google Scholar] [CrossRef] [PubMed]
- Garland, T. Physiological correlates of locomotory performance in a lizard: An allometric approach. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 247, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.W. Trade-Offs in Activity Time and Physiological Performance for Thermoregulating Desert Lizards, Sceloporus Merriami. Ecology 1990, 71, 2323–2333. [Google Scholar] [CrossRef]
- Robson, M.A.; Miles, D.B. Locomotor performance and dominance in male Tree Lizards, Urosaurus ornatus. Funct. Ecol. 2000, 14, 338–344. [Google Scholar] [CrossRef]
- Adolph, S.C.; Pickeringt, T. Estimating maximum performance: Effects of intraindividual variation. J. Exp. Biol. 2008, 211, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Howland, H.C. Optimal strategies for predator avoidance: The relative importance of speed and manoeuvrability. J. Theor. Biol. 1974, 47, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Lowe, J.C.; Roskilly, K.; Hudson, P.E.; Golabek, K.A.; McNutt, J.W. Locomotion dynamics of hunting in wild cheetahs. Nature 2013, 498, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Combes, S.; Rundle, D.E.; Iwasaki, J.M.; Crall, J.D. Linking biomechanics and ecology through predator-prey interactions: Flight performance of dragonflies and their prey. J. Exp. Biol. 2012, 215, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.P.; Griffiths, I.W.; Mills, M.G.L.; Carbone, C.; Wilson, J.W.; Scantlebury, D.M. Mass enhances speed but diminishes turn capacity in terrestrial pursuit predators. ELife 2015, 4, e06487. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.J.; Wilson, R.S. Balancing Biomechanical Constraints: Optimal Escape Speeds When There Is a Trade-off between Speed and Maneuverability. Integr. Comp. Biol. 2015, 55, 1142–1154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haagensen, T.; Gaschk, J.L.; Schultz, J.T.; Clemente, C.J. Exploring the limits to turning performance with size and shape variation in dogs. J. Exp. Biol. 2022, 225, jeb244435. [Google Scholar] [CrossRef] [PubMed]
- Wynn, M.L.; Clemente, C.; Nasir, A.F.A.A.; Wilson, R.S. Running faster causes disaster: Trade-offs between speed, manoeuvrability and motor control when running around corners in northern quolls (Dasyurus hallucatus). J. Exp. Biol. 2015, 218, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.S.; Schilling, N.; Schmidt, M.; Haarhaus, D.; Witte, H. Basic limb kinematics of small therian mammals. J. Exp. Biol. 2002, 205, 1315–1338. [Google Scholar] [CrossRef] [PubMed]
- Iriarte-Díaz, J. Differential scaling of locomotor performance in small and large terrestrial mammals. J. Exp. Biol. 2002, 205, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.C.; Cornette, R.; Goswami, A.; Peigné, S. Do constraints associated with the locomotor habitat drive the evolution of forelimb shape? A case study in musteloid carnivorans. J. Anat. 2015, 226, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.E.; McGinley, S.; Rowe, M.P. Meek males and fighting females: Sexually dimorphic antipredator behavior and locomotor performance is explained by morphology in bark scorpions (Centruroides vittatus). PLoS ONE 2014, 9, e97648. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.C.; Husak, J.F. Locomotor Performance and Sexual Selection: Individual Variation in Sprint Speed of Collared Lizards (Crotaphytus collaris). Copeia 2006, 2006, 216–224. [Google Scholar] [CrossRef]
- Schmitt, D.; Lemelin, P. Origins of primate locomotion: Gait mechanics of the woolly opossum. Am. J. Phys. Anthropol. 2002, 118, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.B.; Polk, J.D.; Schmitt, D. Forelimb and hindlimb forces in walking and galloping primates. Am. J. Phys. Anthropol. 2006, 130, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Botton-Divet, L.; Houssaye, A.; Herrel, A.; Fabre, A.C.; Cornette, R. Swimmers, Diggers, Climbers and More, a Study of Integration Across the Mustelids’ Locomotor Apparatus (Carnivora: Mustelidae). Evol. Biol. 2018, 45, 182–195. [Google Scholar] [CrossRef]
- Warburton, N.M.; Malric, A.; Yakovleff, M.; Leonard, V.; Cailleau, C. Hind limb myology of the southern brown bandicoot (Isoodon obesulus) and greater bilby (Macrotis lagotis). Aust. J. Zool. 2015, 63, 147–162. [Google Scholar] [CrossRef]
- Cuff, A.R.; Sparkes, E.L.; Randau, M.; Pierce, S.E.; Kitchener, A.C.; Goswami, A.; Hutchinson, J.R. The scaling of postcranial muscles in cats (Felidae) II: Hindlimb and lumbosacral muscles. J. Anat. 2016, 229, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, B.M.; Hoffman, L.C. Scale effects between body size and limb design in quadrupedal mammals. PLoS ONE 2013, 8, e78392. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.U.; Pyne, D.B.; Telford, R.D.; Hawley, J.A. Factors affecting running economy in trained distance runners. Sports Med. 2004, 34, 465–485. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E. Mechanical and physiological responses to lower extremity loading during running. Med. Sci. Sports Exerc. 1985, 17, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.; Pavlic, T.P.; Levy, O.; Wilson, R.S. Habitat features and performance interact to determine the outcomes of terrestrial predator–prey pursuits. J. Anim. Ecol. 2020, 89, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Carthey, A.J.R.; Banks, P.B. When does an alien become a native species? a vulnerable native mammal recognizes and responds to its long-term alien predator. PLoS ONE 2012, 7, e31804. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.S.K.; Carthey, A.J.R.; Banks, P.B. Does historical coexistence with dingoes explain current avoidance of domestic dogs? Island bandicoots are naïve to dogs, unlike their mainland counterparts. PLoS ONE 2016, 11, e0161447. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Coetsee, A.L.; Doyle, R.E.; Sutherland, D.R.; Parrott, M.L. Sniffing out danger: Rapid antipredator training of an endangered marsupial. Aust. Mammal. 2022, 44, 109–116. [Google Scholar] [CrossRef]
- Garland, K.; Marcy, A.; Sherratt, E.; Weisbecker, V. Out on a limb: Bandicoot limb co-variation suggests complex impacts of development and adaptation on marsupial forelimb evolution. Evol. Dev. 2017, 19, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Warburton, N.M.; Grégoire, L.; Jacques, S.; Flandrin, C. Adaptations for digging in the forelimb muscle anatomy of the southern brown bandicoot (Isoodon obesulus) and bilby (Macrotis lagotis). Aust. J. Zool. 2013, 61, 402–419. [Google Scholar] [CrossRef]
- Gordon, G.; Hulbert, A.J. Peramelidae. In Fauna of Australia. Mammalia; Richardson, B.J., Walton, D.W., Eds.; Australian Government Publishing Service: Canberra, NSW, Australian, 1989; Volume 1B, pp. 603–624. [Google Scholar]
- Braithwaite, R.W. Southern Brown Bandicoot Isoodon Obesulus: The Mammals of Australia; Reed Books Sydney: Sydney, NSW, Australia, 1995; pp. 176–177. [Google Scholar]
- Pashchenko, D.I. A New Interpretation of the Crocodile Forelimb Morphological Features as Adaptation to Parasagittal Quadrupedal Locomotion on the Ground. Dokl. Biol. Sci. 2018, 483, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Voges, D.; Fischer, M.S. Shoulder movements during quadrupedal locomotion in arboreal primates. Z. Morphol. Anthropol. 2002, 83, 235–242. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Matthews, P.G.D.; Seymour, R.S. Balancing the competing requirements of saltatorial and fossorial specialisation: Burrowing costs in the spinifex hopping mouse, Notomys alexis. J. Exp. Biol. 2006, 209, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.J.; Cooper, C.E.; Withers, P.C.; Freakley, C.; Singh, S.; Terrill, P. The private life of echidnas: Using accelerometry and GPS to examine field biomechanics and assess the ecological impact of a widespread, semi-fossorial monotreme. J. Exp. Biol. 2016, 219, 3271–3283. [Google Scholar] [CrossRef] [PubMed]
- Jindrich, D.L.; Full, R.J. Many-legged maneuverability: Dynamics of turning in hexapods. J. Exp. Biol. 1999, 202, 1603–1623. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.M. Kinematics of 90° running turns in wild mice. J. Exp. Biol. 2003, 206, 1739–1749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samuels, J.X.; Meachen, J.A.; Sakai, S.A. Postcranial morphology and the locomotor habits of living and extinct carnivorans. J. Morphol. 2013, 274, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Tomita, D.; Suga, T.; Tanaka, T.; Ueno, H.; Miyake, Y.; Otsuka, M.; Nagano, A.; Isaka, T. A pilot study on the importance of forefoot bone length in male 400-m sprinters: Is there a key morphological factor for superior long sprint performance? BMC Res. Notes 2018, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Suga, T.; Otsuka, M.; Misaki, J.; Miyake, Y.; Kudo, S.; Nagano, A.; Isaka, T. Relationship between the length of the forefoot bones and performance in male sprinters. Scand. J. Med. Sci. Sports 2017, 27, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.R.; Novack, T.A.; Van Werkhoven, H.; Pennell, D.R.; Piazza, S.J. Ankle joint mechanics and foot proportions differ between human sprinters and non-sprinters. Proc. R. Soc. B Biol. Sci. 2012, 279, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Misu, S.; Doi, T.; Asai, T.; Sawa, R.; Tsutsumimoto, K.; Nakakubo, S.; Yamada, M.; Ono, R. Association between toe flexor strength and spatiotemporal gait parameters in community-dwelling older people. J. Neuroeng. Rehabil. 2014, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Werkhoven, H.; Piazza, S.J. Foot structure is correlated with performance in a single-joint jumping task. J. Biomech. 2017, 57, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.M.; Piazza, S.J. Built for speed: Musculoskeletal structure and sprinting ability. J. Exp. Biol. 2009, 212, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.F.; Wynn, M.L.; Wilson, R.S. Sex-specific trade-offs and compensatory mechanisms: Bite force and sprint speed pose conflicting demands on the design of geckos (Hemidactylus frenatus). J. Exp. Biol. 2013, 216, 3781–3789. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.B.; Garden, J.G. locomotion and gaits of the northern brown bandicoot, isoodon macrourus, (Marsupalia: Peramelidae). J. Mammal. 2004, 85, 296–301. [Google Scholar] [CrossRef]
- Fisher, D.O.; Johnson, C.N.; Lawes, M.J.; Fritz, S.A.; McCallum, H.; Blomberg, S.P.; Vanderwal, J.; Abbott, B.; Frank, A.; Legge, S.; et al. The current decline of tropical marsupials in Australia: Is history repeating? Glob. Ecol. Biogeogr. 2014, 23, 181–190. [Google Scholar] [CrossRef]
- Johnson, C. Australia’s Mammal Extinctions: A 50,000 Year History; Cambridge University Press: Port Melbourne, VIC, Australia, 2006. [Google Scholar]
- McGregor, H.W.; Legge, S.; Jones, M.E.; Johnson, C.N. Landscape Management of Fire and Grazing Regimes Alters the Fine-Scale Habitat Utilisation by Feral Cats. PLoS ONE 2014, 9, e109097. [Google Scholar] [CrossRef] [PubMed]
- Hohnen, R.; Tuft, K.; Legge, S.; Walters, N.; Johanson, L.; Carver, S.; Radford, I.J.; Johnson, C.N. The significance of topographic complexity in habitat selection and persistence of a declining marsupial in the Kimberley region of Western Australia. Aust. J. Zool. 2016, 64, 198–216. [Google Scholar] [CrossRef]
- Radford, J.Q.; Woinarski, J.C.Z.; Legge, S.; Baseler, M.; Bentley, J.; Burbidge, A.A.; Bode, M.; Copley, P.; Dexter, N.; Dickman, C.R.; et al. Degrees of population-level susceptibility of Australian mammal species to predation by the introduced red fox Vulpes vulpes and feral cat Felis catus. Wildl. Res. 2018, 45, 645–657. [Google Scholar] [CrossRef]
- Irschick, D.J. Measuring Performance in Nature: Implications for Studies of Fitness Within Populations. Integr. Comp. Biol. 2003, 43, 396–407. [Google Scholar] [CrossRef] [PubMed]
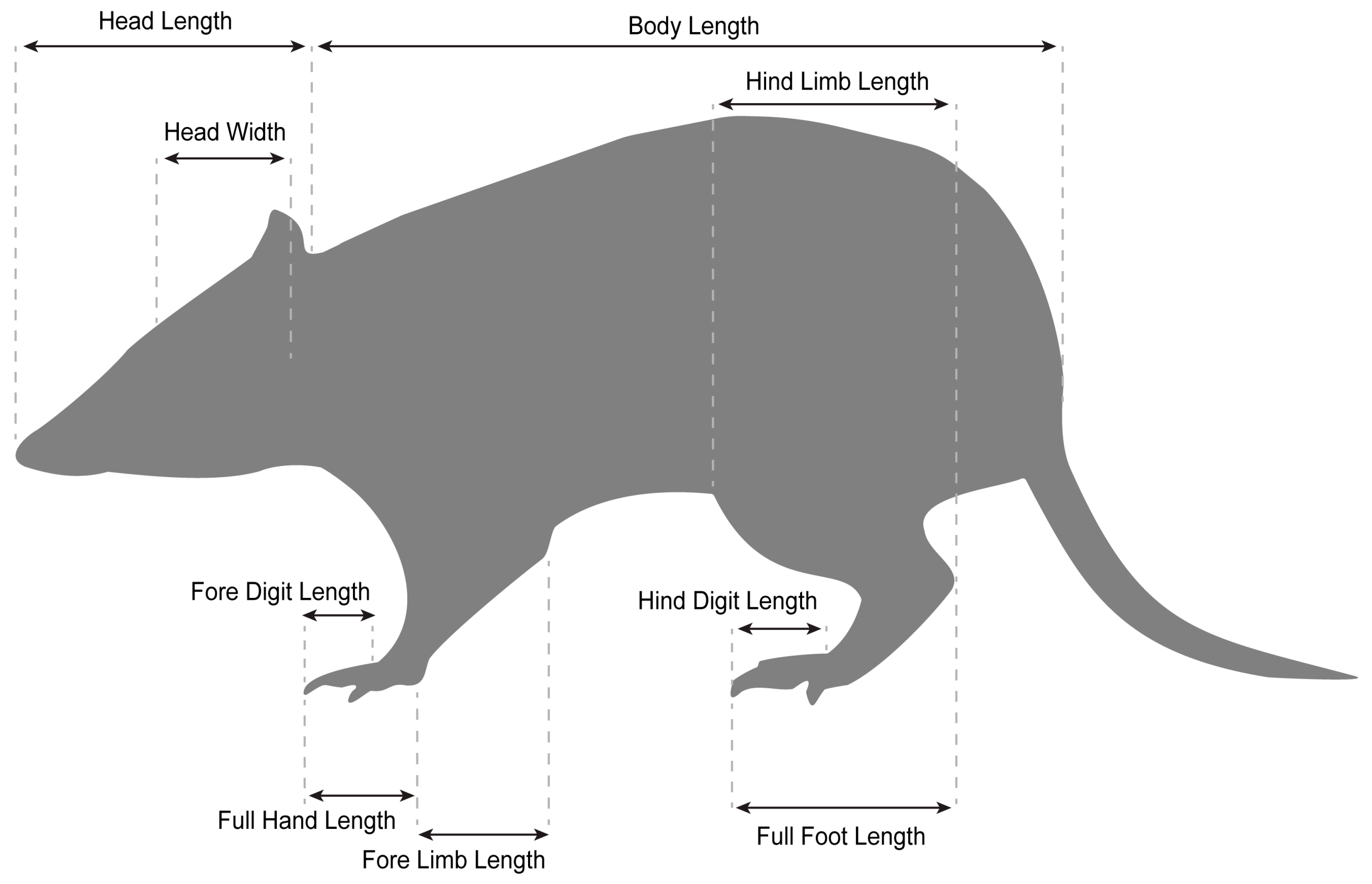
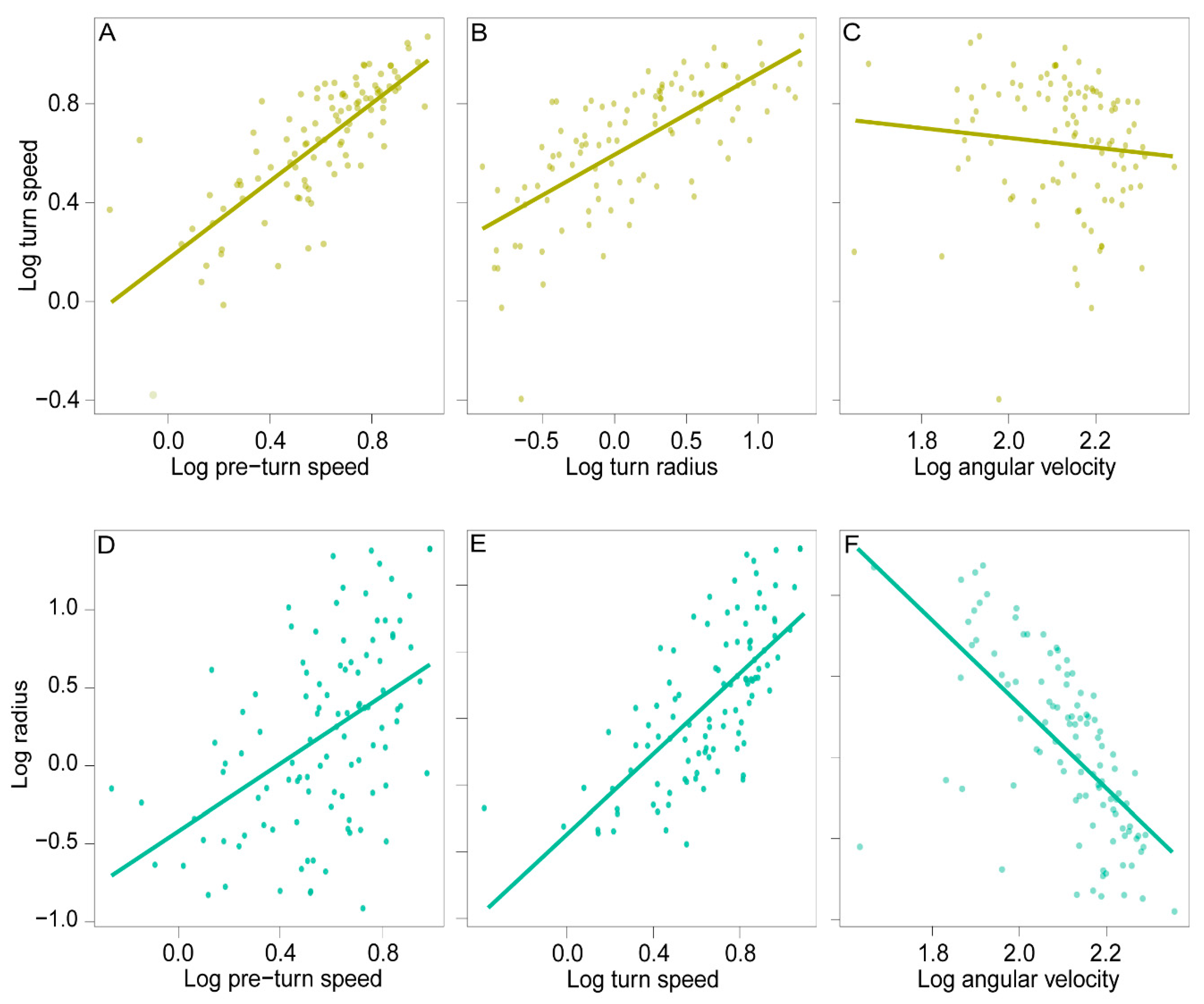

| Sum Sq | Mean Sq | Num Df | Den DF | F | p | |
|---|---|---|---|---|---|---|
| Radius | 1.159 | 1.159 | 1 | 98.877 | 130.345 | <0.001 |
| Angular velocity | 0.392 | 0.392 | 1 | 98.701 | 34.966 | <0.001 |
| Pre-turn speed | 0.234 | 0.534 | 1 | 81.320 | 47.657 | <0.001 |
| Sum Sq | Mean Sq | Num Df | Den DF | F | p | |
|---|---|---|---|---|---|---|
| Angular velocity | 0.370 | 0.370 | 1 | 92.375 | 7.569 | 0.007 ** |
| Turn speed | 0.774 | 0.774 | 1 | 96.126 | 15.833 | <0.001 *** |
| Pre-turn speed | 0.283 | 0.283 | 1 | 78.444 | 5.788 | 0.018 * |
| Angular velocity + turn speed | 0.450 | 0.450 | 1 | 96.040 | 9.216 | 0.003 ** |
| Turn speed + pre-turn speed | 0.250 | 0.250 | 1 | 90.967 | 5.120 | 0.026 * |
| Sum Sq | Mean Sq | Num Df | Den DF | F | p | |
|---|---|---|---|---|---|---|
| Radius | 0.901 | 0.901 | 1 | 99.178 | 169.676 | <0.001 |
| Turn speed | 0.312 | 0.312 | 1 | 84.834 | 58.781 | <0.001 |
| Body Measurement | Sex | Slope (95% CI) | Intercept (95% CI) | R2 | p | Slope Comparison (Likelihood Ratio) | p | Elevation Comparison (Wald’s Statistic) | p |
|---|---|---|---|---|---|---|---|---|---|
| Body Length | Male | 0.34 (0.30, 0.38) | 3.16 (2.88, 3.44) | 0.97 | <0.0001 | 0.7951 | 0.3735 | 0.0794 | 0.7781 |
| Female | 0.40 (0.28, 5.61) | 2.77 (1.84, 3.70) | 0.86 | <0.0001 | |||||
| Hind Limb length | Male | 0.32 (0.29, 0.34) | 2.36 (2.20, 2.52) | 0.99 | <0.0001 | 4.673 | 0.0306 | 1.6450 | 0.1996 |
| Female | 0.43 (0.33, 0.58) | 1.55 (0.73, 2.38) | 0.88 | <0.0001 | |||||
| Fore Limb Length | Male | 0.30 (0.27, 0.34) | 1.97 (1.7, 2.19) | 0.98 | <0.0001 | 8.677 | 0.0032 | 0.0054 | 0.9413 |
| Female | 0.51 (0.37, 0.70) | 0.60 (−0.48, 1.67) | 0.79 | 0.0002 | |||||
| Foot Length | Male | 0.20 (0.17, 0.25) | 2.45 (2.14, 2.76) | 0.97 | <0.0001 | 5.505 | 0.0189 | 1.574 | 0.2096 |
| Female | 0.34 (0.24, 0.37) | 1.58 (0.82, 2.35) | 0.91 | <0.0001 | |||||
| Hind digit Length | Male | 0.30 (0.22, 0.40) | 0.30 (−0.34, 0.95) | 0.87 | <0.0001 | 4.083 | 0.0433 | 3.932 | 0.0473 |
| Female | −0.56 (−0.97, −0.33) | 6.03 (3.93, 8.14) | 0.57 | 0.0374 | |||||
| Palm Length | Male | 0.24 (0.18, 0.33) | 1.18 (0.66, 1.70) | 0.92 | <0.0001 | 8.906 | 0.0028 | 0.0086 | 0.9261 |
| Female | 058 (0.36, 0.94) | −1.08 (−2.99, 0.83) | 0.54 | 0.0289 | |||||
| Fore digit Length | Male | 0.37 (0.27, 0.51) | 0.28 (−0.55, 1.11) | 0.79 | <0.0001 | 1.427 | 0.2322 | 5.018 | 0.0025 |
| Female | 0.53 (0.31, 0.86) | −0.67 (−2.44, 1.10) | 0.61 | 0.0119 | |||||
| Head Width | Male | 0.26 (0.23, 0.30) | 1.72 (1.49, 1.94) | 098 | <0.0001 | 0.4086 | 0.5228 | 0.779 | 0.3774 |
| Female | 0.24 (0.17, 0.33) | 1.91 (1.38, 2.44) | 0.96 | <0.0001 | |||||
| Head Length | Male | 0.22 (0.19, 0.24) | 2.95 (2.77, 3.14) | 0.98 | <0.0001 | 4.552 | 0.0328 | 0.6690 | 0.4133 |
| Female | 0.31 (0.23, 0.43) | 2.31 (1.66, 2.98) | 0.93 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Simone, K.; Cameron, S.F.; Clemente, C.J.; Dick, T.J.M.; Wilson, R.S. Biomechanical Trade-Offs Between Speed and Agility in the Northern Brown Bandicoot. Biomechanics 2025, 5, 52. https://doi.org/10.3390/biomechanics5030052
Del Simone K, Cameron SF, Clemente CJ, Dick TJM, Wilson RS. Biomechanical Trade-Offs Between Speed and Agility in the Northern Brown Bandicoot. Biomechanics. 2025; 5(3):52. https://doi.org/10.3390/biomechanics5030052
Chicago/Turabian StyleDel Simone, Kaylah, Skye F. Cameron, Christofer J. Clemente, Taylor J. M. Dick, and Robbie S. Wilson. 2025. "Biomechanical Trade-Offs Between Speed and Agility in the Northern Brown Bandicoot" Biomechanics 5, no. 3: 52. https://doi.org/10.3390/biomechanics5030052
APA StyleDel Simone, K., Cameron, S. F., Clemente, C. J., Dick, T. J. M., & Wilson, R. S. (2025). Biomechanical Trade-Offs Between Speed and Agility in the Northern Brown Bandicoot. Biomechanics, 5(3), 52. https://doi.org/10.3390/biomechanics5030052







