1. Introduction
The growing demand for efficient, reliable, and high-power energy storage technologies has positioned supercapacitors (SCs) as essential solutions, owing to their rapid charging capabilities, outstanding power delivery, and extended operational lifespan [
1]. We classify supercapacitor devices into three main categories: electric double-layer capacitors (EDLCs), pseudo capacitors (also known as faradaic SCs), and hybrid supercapacitors [
2]. In the case of EDLCs configuration, charges are stored electrostatically at the electrode–electrolyte interface, enabling rapid charge–discharge cycles; however, their energy storage capacity is limited due to the absence of faradaic reactions [
3]. In contrast, pseudo capacitors leverage rapid and reversible surface faradaic reactions to achieve significantly enhanced energy densities. However, their cyclic stability is often undermined by material degradation processes, including lattice distortions, phase transformations, and dissolution of active components, which arise from prolonged redox cycling [
4,
5]. Meanwhile, hybrid supercapacitors integrate the advantageous characteristics of both EDLCs and pseudo capacitors, delivering high power output alongside enhanced energy density [
6]. However, achieving optimal performance critically depends on precise control over the composition, morphology, and structural connectivity of the electrode material, as these factors directly dictate ionic diffusion, electrical conductivity, and overall stability [
7,
8,
9,
10].
In parallel with advances in materials design, the emergence of printed electronics has opened new frontiers for the scalable and cost-effective fabrication of energy storage devices [
11]. Among various printing techniques, direct ink writing (DIW) has garnered particular attention for enabling precise patterning of complex geometries using functional inks under ambient conditions. This additive manufacturing approach not only minimizes material waste and enables device customization but also facilitates seamless integration of energy storage components with flexible substrates and wearable platforms [
12]. The compatibility of advanced materials with printing technologies is thus pivotal in bridging the gap between laboratory-scale innovations and real-world applications, making printed electronics a key enabler for next-generation, decentralized, and structurally integrated power solutions.
Among promising electrode candidates, Metal-Organic Frameworks (MOFs) have drawn considerable research interest due to their high surface areas, customizable porous structures, and abundant redox-active sites [
13,
14]. Despite these advantages, their practical application is frequently limited by low intrinsic electrical conductivity, particle agglomeration, and difficulties in large-scale fabrication processes [
15]. To overcome these challenges, recent research has explored MOF-based hybrid nanocomposites integrated with conductive carbon supports such as reduced graphene oxide, carbon fibers, and carbon nanotubes, among others [
16].
In the approach presented herein, GO nanoflakes dispersed in the solution function as nucleation sites for the continuous formation of MOF structures, which are in turn driven by laser ablation of the metal target. After mild thermal reduction at 250°, the resulting Co/ZIF-67-rGO hybrids exhibit complementary pseudocapacitive and double-layer electrochemical behavior. Optimized synthesis conditions, specifically 10 min of laser ablation at 330 mJ/pulse, yield nanocomposites that achieve exceptional capacitance values of 1152 F/g. Moreover, the incorporation of these materials into fully 3D-printed devices through direct ink writing (DIW) demonstrates practical feasibility and scalability, offering promising capabilities for innovative energy storage devices. Comprehensive structural and electrochemical evaluations confirm the maintenance of crystallinity, stable interfacial bonding, and consistent electrochemical performance, underscoring the effectiveness of the LASiS method as a scalable route to advanced supercapacitor electrode materials.
2. Materials and Methods
The following procedure details the synthesis of Co/ZIF-67-rGO hybrid nanocomposites, optimized for integration into SC electrodes. Their electrochemical performance was subsequently evaluated, and the materials were ultimately incorporated into 3D-printed energy storage devices, as outlined in detail below.
2.1. Synthesis and Characterization of the Co/ZIF-67-rGO Hybrid Nanocomposites
Initially, the LASiS conditions were optimized to produce uniform ZIF-67 structures by varying laser energies (110 and 330 mJ/pulse) and ablation durations (5, 10, 15, and 20 min), as detailed in the
Supplementary Information (Figure S1). Graphene oxide (GO) nanosheets were synthesized from commercial graphite flakes using the modified Hummers method [
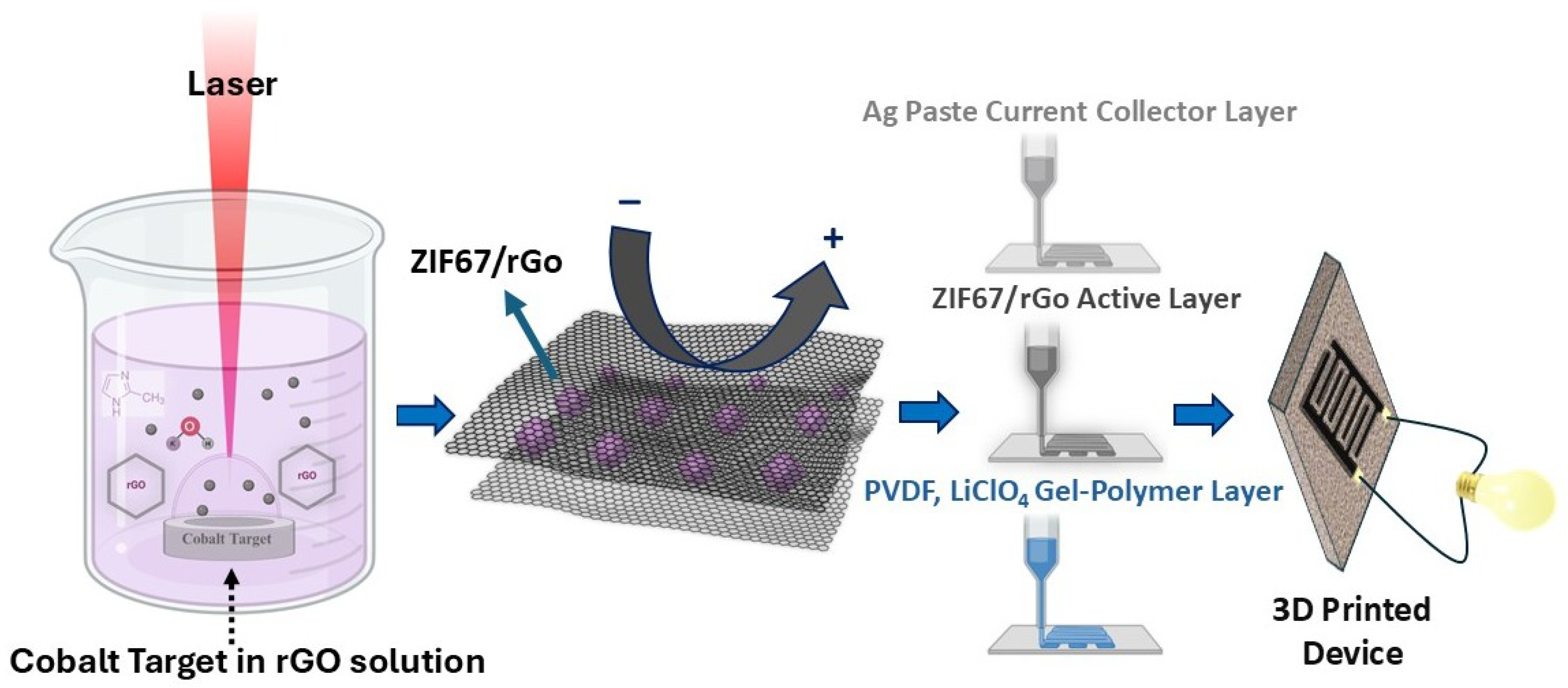
17]. The prepared GO suspension at a concentration of 20 mg/L was ultrasonicated in deionized water for approximately one hour to achieve complete exfoliation and homogeneity. The suspension pH was then adjusted to 13 using potassium hydroxide (KOH, purity 99%). Subsequently, 1.8 g of 2-methylimidazole (Hmim), the crucial organic ligand for crystallization of ZIF-67, was dissolved in 35 mL of the alkaline GO dispersion, forming the precursor solution, which was then placed in the LASiS reaction vessel. LASiS is a fast and green technique that utilizes a pulsed laser to ablate a metallic target submerged in a liquid solution. The process creates a high-energy plasma plume that initiates reactions between metal ions and precursors in the solution phase, enabling the formation of nanostructured materials with controlled morphology and composition [
11,
18,
19,
20,
21]. A high-purity cobalt pellet (99.5%) served as the metal source, submerged in the GO precursor solution. Laser ablation was conducted for 10 min with a pulsed Nd:YAG laser (wavelength 1064 nm, energy output 330 mJ per pulse, repetition rate 10 Hz Quantel Laser, Bozeman, MT, USA). Ablation conditions facilitated the simultaneous nucleation and controlled growth of ZIF-67 nanoparticles directly onto GO sheets, forming well-dispersed Co/ZIF-67-GO hybrid nanocomposites. After stabilization, the resulting solid was collected by centrifugation for 20 min at 4700 rpm. The precipitate was subjected to several cycles of thorough washing with deionized water, and subsequently with methanol, to eliminate any residual impurities and unreacted components. To generate the final reduced form (Co/ZIF-67-rGO), the just-washed nanocomposites were dried under vacuum for 24 h, then exposed to gentle heat treatment at 250 °C under vacuum for an estimated 4 h, converting GO to reduced graphene oxide (rGO).
Multiple characterization techniques were used to thoroughly assess the structural, compositional, and morphological properties of the synthesized nanocomposites. Specifically, the crystal structure of each sample was analyzed by X-ray diffraction (XRD, Phillips X’Pert-Pro, Co K radiation at = 0.1541 nm, operating conditions: 45 kV, 40 mA, Anton Paar, Ashland, WV, USA). Functional groups and chemical bonds were identified through Fourier transform infrared (FTIR) spectra with a Nicolet iS50 spectrometer (spectral range: 5000–400 cm−1, Thermo Fisher Scientific, Waltham, MA, USA). Morphological and elemental mapping analyzes were performed using a scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (EDX, Philips XL-30E, Thermo Fisher Scientific, USA), and high-resolution imaging was achieved with a dual-beam focused ion beam SEM (Helios 5UX FIB/SEM, Thermo Fisher Scientific, USA). Further structural analysis at the nanoscale was carried out using transmission electron microscopy (TEM) with JEOL JEM 1400-Flash (JEOL, USA) and Thermo Fisher Tecnai Osiris S/TEM (Thermo Fisher Scientific, US) instruments, both operated at an accelerating voltage of 120 kV.
Rationale for selecting LASiS: The Laser Ablation Synthesis in Solution (LASiS) technique was chosen as it enables a surfactant-free, single-step route for synthesizing highly pure and uniform nanostructures without the need for chemical reducing agents or post-synthetic purification. The transient plasma plume generated from the metal target supplies reactive Co species that nucleate ZIF-67 directly in the ligand-rich solution. Additionally, dispersed GO nanosheets act as anchoring and nucleation sites, promoting uniform ZIF-67 deposition and minimizing agglomeration. Compared to conventional solvothermal and hydrothermal methods, LASiS provides rapid synthesis at room temperature with tunable crystallinity and particle size, while being environmentally benign and compatible with scalable, additive manufacturing processes.
2.2. Electrochemical Characterizations
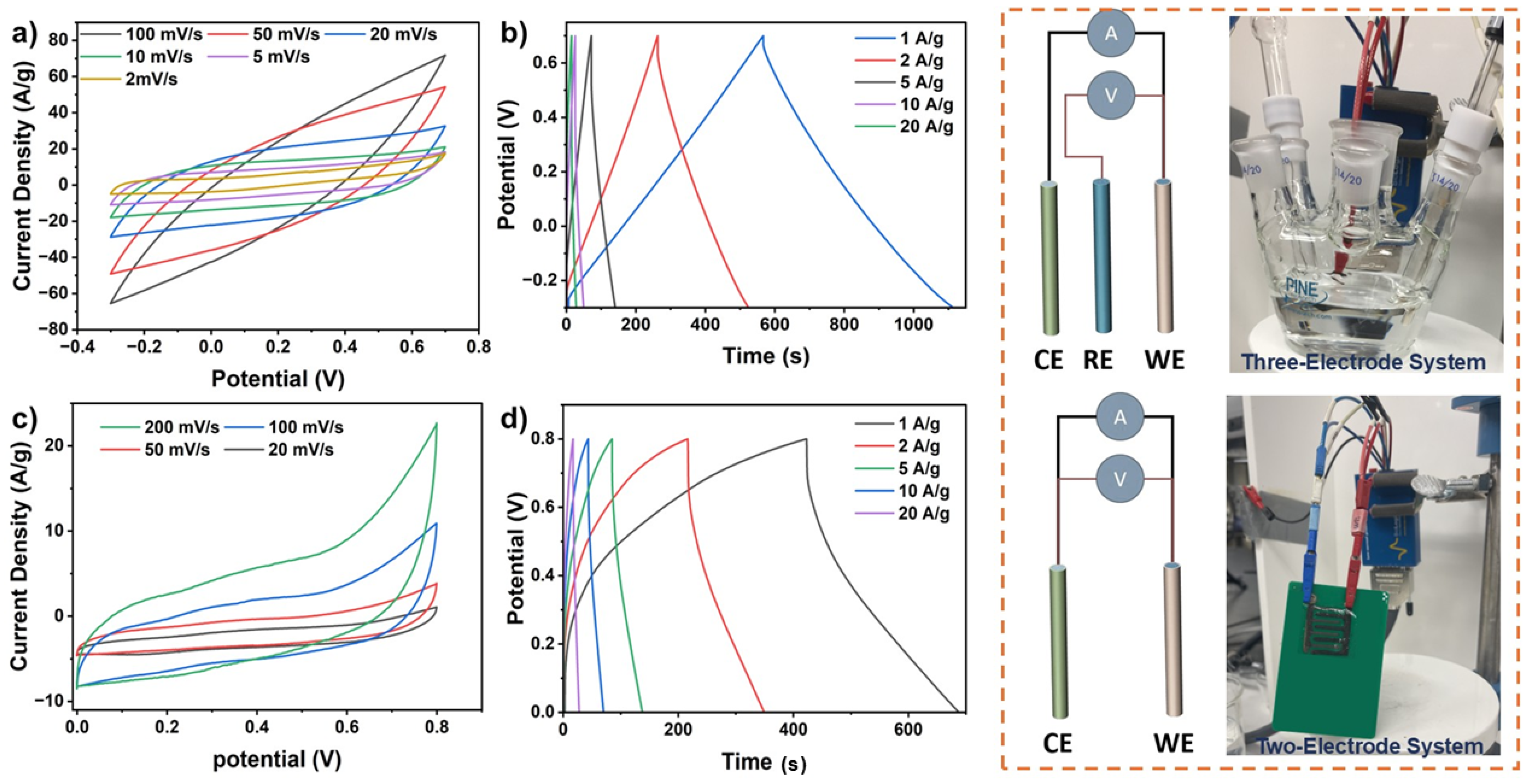
Electrochemical analyses were conducted using a conventional three-compartment electrochemical cell (Pine Instruments). A saturated Ag/AgCl double junction electrode was employed as the reference electrode, while a platinum wire functioned as the counter electrode. Cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) tests were conducted to comprehensively characterize the electrochemical performance. The synthesized nanocomposites were evaluated using a three-electrode configuration, while fully assembled printed devices were characterized using a two-electrode setup.
Electrodes were prepared to evaluate the supercapacitance properties of the LASiS-derived Co/ZIF-67–rGO hybrid nanocomposites (HNCs) as follows. A homogeneous slurry was first prepared by mixing 80 wt% synthesized HNCs, 10 wt% in-house-synthesized porous carbon [
20], and 10 wt% PVDF binder in 1 mL of ethanol solution, followed by manual stirring until a viscous, uniform solution was obtained. Approximately 7–9 mg of the slurry was then applied evenly onto nickel foam substrates (area: ∼1 cm
2), dried under vacuum at 80 °C for 8 h, and subsequently compressed mechanically at 10 MPa for 4 s to enhance electrode integrity and electrolyte accessibility. Electrochemical measurements were conducted in 0.5 M
aqueous electrolyte (pH∼7.2). We performed Cyclic voltammetry (CV) experiments at a scan rate of 20 mV/s within a potential window of
to
V, while we carried out galvanostatic charge–discharge (GCD) tests at a constant current density of
A/g. We computed the specific capacitance (
C, in F/g) directly from the charge–discharge curves using the following relationship:
Here, C (F/g) is the specific capacitance, (V) is the potential drop, (A/g) is the current density during the discharge process, and m (g) is the mass of active material.
This methodology enabled a comprehensive evaluation of the supercapacitive properties of Co/ZIF-67–rGO HNCs, providing insight into the electrochemical performance of hierarchical structures composed of rGO flakes decorated with LASiS-derived Co/ZIF-67. This approach allowed a systematic assessment of the hybrid nanocomposites’ supercapacitive behavior, revealing the synergistic effects of GO incorporation and LASiS processing on their electrochemical properties.
2.3. 3D-Printing of Integrated Supercapacitor Architectures
The integration of Co/ZIF-67–rGO hierarchical nanocomposites (HNCs) into fully printed supercapacitors enables rapid prototyping, cost-efficient fabrication, and enhanced mechanical flexibility. The electrode architecture, designed to maximize porosity and surface area, promotes efficient electrode–electrolyte interactions, thereby substantially improving electrochemical performance.
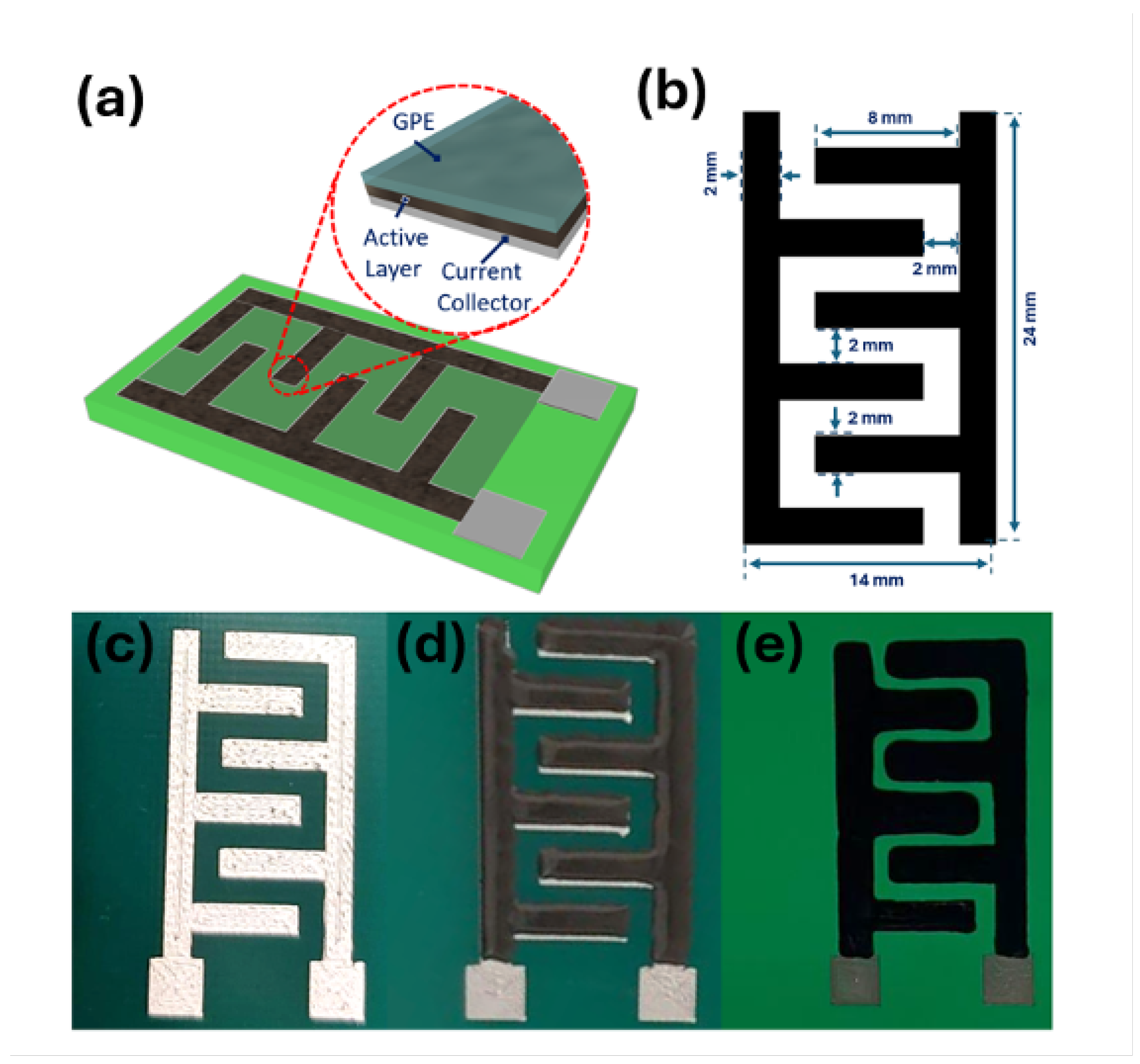
A sequential additive manufacturing strategy was employed using a Voltera V-One desktop PCB printer. The deposition sequence comprised the current collector, followed by the active layer, and finally the gel polymer electrolyte, yielding the multilayer configuration illustrated in
Figure 1a. Interdigitated electrode patterns were digitally designed using Autodesk software (Inventor 2024), exported in Gerber format, and subsequently printed. The digital layouts of the interdigitated current collector and the active layer incorporating LASiS-derived HNCs are presented in
Figure S2 and
Figure 1b, respectively.
Each printed supercapacitor featured six interdigitated electrodes, evenly divided into anodic and cathodic regions. For the current collector, a silver nanoparticle conductive ink was utilized to print the pattern shown in
Figure S2 onto a PCB, which was then annealed at 120 °C for 2 h to induce the coalescence of the Ag particles, thereby favoring the formation of a continuous conductive layer. The resulting printed current collector layer after the thermal treatment is depicted in
Figure 1c.
The active electrode ink was prepared by dispersing graphene nanoflakes (0.9 g), carbon nanofibers (0.1 g), and Co/ZIF-67–rGO HNCs (0.2 g) in 40 mL of methanol, followed by bath sonication for 1 h. After solvent removal by vacuum evaporation at 40 °C for 12 h, the resulting powder was redispersed in 1.5 mL of terpineol with manual stirring. Ethyl cellulose (0.5 g) was subsequently added as a binder and mixed for 5 min. The resulting functional ink was printed onto the current collector layer (
Figure 1d) and annealed at 80 °C for 1 h, yielding the electrode structures shown in
Figure 1e.
The gel polymer electrolyte (GPE) was prepared by dissolving lithium perchlorate (LiClO4) and polyvinylidene difluoride (PVDF) in propylene carbonate at 140 °C, producing a 0.5 M LiClO4 solution with a fixed PVDF:LiClO4 ratio of 3:1. The GPE was subsequently printed onto the assembled electrodes and air-dried at ambient temperature.
This sequential printing protocol highlights the practical viability of MOF-graphene-based HNCs in scalable, high-performance electrochemical energy storage devices. The complete fabrication workflow is schematically summarized in
Figure 2.
3. Results and Discussion
The effectiveness of ZIF-67-based hybrid nanocomposites for supercapacitor electrodes was carefully assessed by systematically investigating the impact of specific LASiS synthesis parameters, particularly laser pulse energy and exposure time, on the morphology, structural uniformity, and spatial composition of the produced samples. As indicated by our optimization experiments and the
Supplementary results (Figure S1), even minor variations in these parameters markedly influenced particle aggregation, porosity, and structural uniformity. Optimal LASiS conditions were identified at an ablation duration of 10 min and a pulse energy of 330 mJ. Under these conditions, the energy input provided sufficient localized heating and rapid cooling cycles to facilitate homogeneous nucleation and growth, producing highly uniform ZIF-67 nanostructures with minimal aggregation. Lower energies or shorter durations resulted in incomplete ZIF-67 formation, while excessive energies led to undesirable particle agglomeration. During LASiS, pulsed laser irradiation of the cobalt pellet surface generates a liquid-confined plasma plume of cobalt species, which rapidly collapses and releases these species into the surrounding solution. The alkaline medium, enriched with dissolved 2-methylimidazole, subsequently drives the nucleation and growth of ZIF-67 frameworks, as detailed in our previous work [
22].
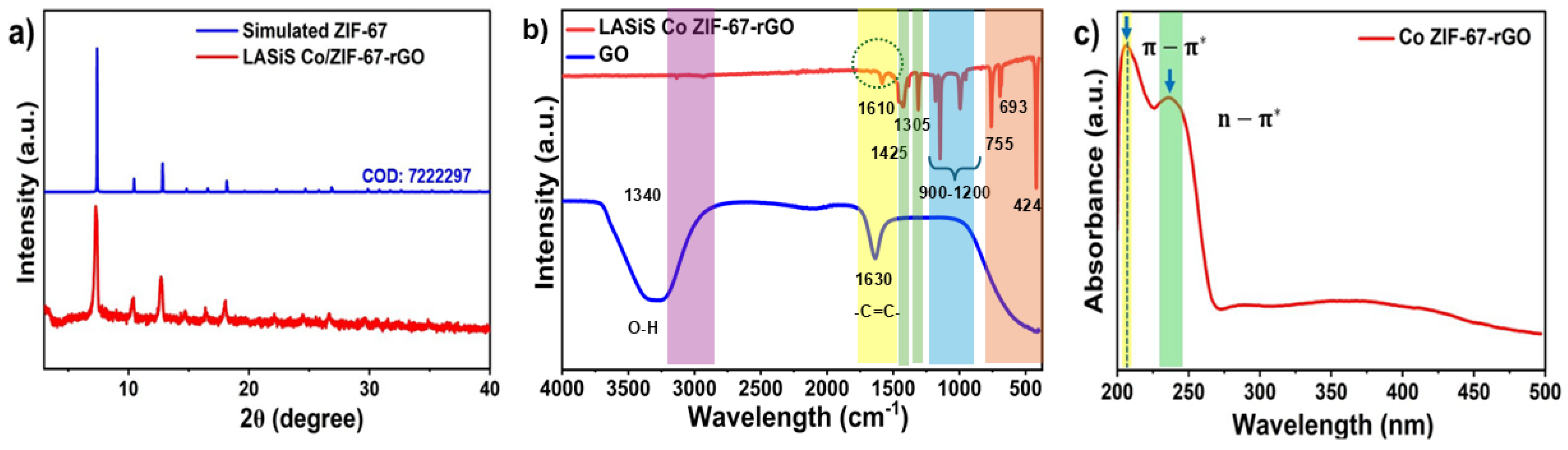
XRD and UV–visible analyses (
Figure 3a,c) confirm the structural and electronic features of the synthesized HNCs. XRD patterns show that the diffraction peaks closely match those of simulated ZIF-67, indicating that the material preserves its crystalline structure upon integration into the graphene oxide (GO) matrix. Retention of crystallinity ensures structural stability and high porosity, which are essential for efficient ion transport and enhanced electrochemical performance. UV–visible absorption spectra reveal pronounced
-
transitions within the rGO layers and n-
transitions associated with the nitrogen functionalities of the ZIF-67 framework, confirming the coexistence of both components. FTIR spectra (
Figure 3b) corroborate these interactions, exhibiting notable shifts and decreased intensity of the aromatic C=C stretching vibrations (1630 cm
−1) in the HNCs relative to pristine GO. Additional absorption bands corresponding to C–N vibrations from the imidazolate linker, Co–N coordination modes, and prominent C=C vibrations from the carbon skeleton further confirm the coexistence of ZIF-67 and GO. The absence of the hydroxyl (–OH) band, originally prominent in pristine GO, after thermal treatment at 250 °C, indicates successful reduction and enhanced conductivity of the HNCs.
Electron microscopy images (
Figure 4) reveal a hierarchical architecture with a quasi-symmetrical configuration, where uniformly anchored ZIF-67 structures are uniformly anchored onto rGO nanosheets. The observed homogeneous dispersion of ZIF-67 nanocrystals on GO sheets can be attributed to the LASiS process, which facilitates rapid nucleation and uniform growth under a surfactant-free environment. Unlike hydrothermal synthesis, where uncontrolled crystal growth often leads to aggregation, the laser-induced plasma in LASiS generates a controlled flux of reactive Co ions that nucleate directly on GO flakes. This process not only minimizes particle agglomeration but also enhances the electrical connectivity and surface accessibility of the hybrid composite, contributing to improved electrochemical performance. TEM and SEM micrographs of
Figure 4a,b display the morphology of LASiS-synthesized ZIF-67 frameworks, while
Figure 4c,d displays the structure and morphology of Co/ZIF-67–rGO HNC with rolling planes of rGO due to strong
-
interactions. The inset of
Figure 4c provides a detailed view of the spatial arrangement resulting from the interfacing of ZIF-67 with rGO, suggesting that dispersed GO nanosheets function as nucleation sites during laser-driven MOF formation, facilitating growth and stabilizing the resulting architectures to suppress agglomeration. We attribute this strong association to robust
-
interactions arising from the sp
2-hybridized carbon centers in both GO and the 2-methylimidazole ligands. Therefore, the observed interfacial bonding between ZIF-67 crystallites and GO nanosheets plays a crucial role in defining the structural and electrochemical stability of the hybrid framework. The strong interfacial bonding arises from both
-
stacking interactions between the aromatic linkers of ZIF-67 and the sp
2 carbon domains of GO, as well as coordination between Co–N active sites and oxygen-containing functional groups on GO. This dual interaction mechanism promotes robust mechanical adhesion of the MOF particles to the conductive carbon matrix and enhances charge transfer efficiency across the hybrid interface. Consequently, the resulting GO/ZIF-67 composite exhibits improved electronic connectivity and mechanical integrity compared to pristine ZIF-67, providing a stable conductive network favorable for redox-active cobalt centers during cycling.
High-angle annular dark-field (HAADF) micrographs and elemental mapping (
Figure 4d) reveal a uniform distribution of cobalt, nitrogen, and carbon throughout the structure. Oxygen species are also detected, attributable to residual cobalt ions electrostatically adsorbed on ZIF-67 surfaces, which subsequently underwent partial oxidation, resulting in the observed oxygen signal. Collectively, these analyses confirm the formation of a well-defined, stable hierarchical Co/ZIF-67–rGO nanocomposite with preserved crystallinity, uniform elemental distribution, and tailored electronic and structural properties.
The electrochemical properties of the synthesized Co/ZIF-67–rGO HNCs and their integration into fully printed supercapacitors were evaluated via cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) measurements (
Figure 5). CV curves of Co/ZIF-67–rGO electrodes at varying scan rates (
Figure 5a) exhibit nearly ideal rectangular shapes, indicative of high capacitive behavior, rapid ion mobility, and efficient electron transport enabled by the interconnected porous architecture and conductive rGO networks. This performance is strongly influenced by the hierarchical morphology of the nanocomposites, in which porous ZIF-67 nanocrystals are uniformly anchored onto rGO sheets, forming an open, accessible structure. SEM and TEM images ((
Figure 4) confirm this architecture, which facilitates ion diffusion and reduces internal resistance by exposing additional electroactive sites.
GCD analysis of the electrodes (
Figure 5b) shows nearly symmetrical and linear charge-discharge profiles across multiple current densities, demonstrating excellent reversibility and electrochemical stability. In a three-electrode configuration at 1 A.g
−1, the HNCs exhibit a gravimetric capacitance of 1152 F.g
−1 and a power density of 500 W.kg
−1. As expected for hybrid nanostructured materials with interconnected channels, we observe slightly shorter discharge durations at higher current densities, due to limited ion penetration, which is consistent with rate-dependent behavior. These results are consistent with previously reported pseudocapacitive behaviors in Co-based hybrid and MOF-derived systems, where both diffusion-limited ion transport and surface redox contributions govern the charge storage process [
23,
24,
25].
For the 3D-printed supercapacitor devices, CV measurements at varying scan rates (
Figure 5c) display broad pseudocapacitive profiles, reflecting efficient ion transport and enhanced electrode-electrolyte interactions afforded by the printed multilayer architecture. The layered design provides mechanically robust, low-resistance pathways for ion transport, complemented by intimate contact between the rGO framework and embedded ZIF-67 structures. GCD curves (
Figure 5d) remain nearly linear and stable across all tested current densities, exhibiting minimal IR drops and confirming efficient charge transfer, high device stability, and low internal resistance. The printed devices demonstrate a gravimetric capacitance of 875 F.g
−1 and a power density of 399.6 W.kg
−1 at 1 A.g
−1. The capacitance-retention profile of
Figure S5 shows that the supercapacitor maintains excellent stability over 200 charge–discharge cycles. After a brief activation period during the first ∼20 cycles, where retention rises slightly above the initial value, the device stabilizes around 100.5–101% retention with only minor fluctuations. This small increase above 100% is commonly observed and can be attributed to electrode wetting or improved ionic accessibility during early cycling. Overall, the data indicate highly stable electrochemical performance with no signs of degradation throughout the tested cycle range, suggesting robust long-term reliability of the device.
The electrochemical impedance spectroscopy (EIS) results of the printed supercapacitor device exhibit the expected characteristics of high-performance capacitive systems, as shown in the Nyquist plots in
Figure S4. In the high-frequency region (
Figure S4a), the intercept on the real impedance axis reveals an equivalent series resistance (ESR) of approximately 3.3
. The small, depressed semicircle corresponds to a low charge-transfer resistance (RCT), indicating efficient ion transport at the electrode–electrolyte interface and minimal diffusional limitations—both hallmarks of ideal capacitive behavior. The complete Nyquist plot over a broader frequency range (
Figure S4b) further underscores the low ESR and transitions into a steep, nearly vertical line in the low-frequency region, characteristic of efficient double-layer charge storage. Collectively, these features confirm the excellent electrochemical performance of the printed supercapacitor.
The outstanding electrochemical performance of both isolated electrodes and fully printed devices arises from a synergistic combination of mechanisms. Pseudocapacitive cobalt centers supply abundant redox-active sites for faradaic charge storage, while the conductive rGO matrix enhances double-layer capacitance. The abundant presence of ZIF-67 on the rGO surface increases surface polarity, electrolyte wettability, and introduces additional redox-active edge sites, further boosting pseudocapacitive contributions and ion–surface interactions. Additionally, strong - interactions between ZIF-67 and rGO nanosheets enhance mechanical integrity and electronic conductivity, facilitating rapid electron transport and structural durability during cycling. Collectively, these effects increase total capacitance and power performance, underscoring the promise of Co/ZIF-67–rGO composites as scalable, high-performance electrode materials for advanced energy storage applications.