Abstract
Low-crystalline hydroxyapatite was synthesized from an aqueous solution of calcium chloride (CaCl2), and a mixed-anionic (HPO42− и CO32−) aqueous solution prepared from potassium hydrophosphate trihydrate (K2HPO4·3H2O), and potassium carbonate (K2CO3). The interaction of K2CO3 and K2HPO4 salts during synthesis from a mixed-anionic solution in the reaction zone without additional regulation provided the pH level necessary for the synthesis of hydroxyapatite. For comparison, as references, powders were also synthesized from an aqueous solution of CaCl2 and from aqueous solutions of either K2HPO4 or K2CO3. The phase composition of the powder synthesized from aqueous solutions of CaCl2 and K2HPO4 included brushite (CaHPO4·2H2O). The phase composition of the powder synthesized from aqueous solutions of CaCl2 and K2CO3 included calcite (CaCO3). The phase composition of all synthesized powders contained potassium chloride (sylvine, KCl), as a reaction by-product. After heat treatment at 1000 °C of the powder containing low-crystalline hydroxyapatite and KCl, powder of chlorapatite (Ca10(PO4)6Cl2) was obtained. After heat treatment of a powder containing brushite (CaHPO4·2H2O) and KCl at 800 and 1000 °C, a powder with the phase composition including β-calcium pyrophosphate (β-Ca2P2O7), β-calcium orthophosphate (β-Ca3(PO4)2), and potassium-calcium pyrophosphate (K2CaP2O7) was obtained. Heat treatment of calcite (CaCO3) powder at 800 °C, as expected, led to the formation of calcium oxide (CaO). Synthesized powders, including biocompatible minerals such as hydroxyapatite, chlorapatite, brushite, monetite, calcium pyrophosphate, calcium potassium pyrophosphate, tricalcium phosphate, and calcite, can be used for the creation of biocompatible inorganic materials or composite materials with a biocompatible polymer matrix. The potassium chloride present in the synthesized powders can act as one of the precursors of biocompatible minerals, such as chlorapatite or calcium potassium pyrophosphate, or it can be treated as a removable inorganic porogen.
1. Introduction
Fine powders with a given chemical and phase composition and high uniformity of component distribution are required for the creation of inorganic materials or composite materials with unique functional properties [1,2,3]. The simplest and most obvious is the method of mechanical homogenization of powder mixtures, which is carried out using special equipment, such as a planetary mill [4]. High-temperature solid-phase synthesis from a homogeneous mixture of salts obtained by drying a solution of these salts at subzero temperatures is more difficult for implementation [5]. The maximum level of homogenization is achieved in mixed-anionic compounds, which are produced using high-temperature and low-temperature reactions [6]. High-temperature reactions for the preparation of mixed-anionic compounds can take place during synthesis in the solid phase, in synthesis by interaction of gas and solid phases, and synthesis in conditions using pressure. Low-temperature reactions for the preparation of mixed-anionic compounds can take place in topochemical synthesis, solvothermal synthesis, and synthesis in thin films. Precipitation of hydroxides [7], carbonates, or oxalates is also used to obtain homogeneous precursors of oxide powders [8]. Synthesis of inorganic powders consisting of small particles with a high specific surface area and activity via precipitation from solution is the most convenient for implementation [9].
High uniformity of the distribution of components in a powder intended for the production of biocompatible materials can be achieved using synthesis from both mixed-cationic [10] and mixed-anionic solutions, for example, containing HPO42−/P2O74− [11], HPO42−/CO32− [12,13,14], P2O74−/CO32− [15], HPO42−/SiO32− [16], and HPO42−/SO42− [17]. High uniformity of the distribution of components in powder can be reached in case of similarity of crystal structures of minerals, for example, brushite (CaHPO4·2H2O), gypsum (CaSO4·2H2O), and ardealite (Ca(HPO4)x(SO4)1−x·2H2O) [17]. In previous investigations, it was shown that the simultaneous presence of different anions (HPO42−/CO32− [12], P2O74−/CO32− [15], and HPO42−/SiO32− [16]) in the reaction zone causes the formation of a quasi-amorphous phase. And this phenomenon can be not only a sign of high uniformity of the distribution of components but also a sign of the presence of defects in the crystal lattice of the precipitated minerals, which then can provide high activity of the synthesized powders in sintering or in chemical interaction.
The mineral component of bone tissue is mainly represented by carbonate-substituted hydroxyapatite [18]. Calcium hydroxyapatite (Ca10(PO4)6(OH)2) is a well-known and unique ion exchanger [19,20,21]; therefore, various cations such as Na+, K+, Mg2+, Zn2+, Ba2+, or Sr2+ [22] and anions such as CO32− or SiO44−, F−, or Cl− can be included in the composition of bone tissue [23,24].
Various powders obtained by one of the methods of chemical synthesis are used for the manufacture of bone implants, both based on calcium phosphates [25,26,27,28] and calcium carbonates [29,30,31]. Precipitation from solutions including a hydrophosphate ion, a carbonate ion, and a calcium ion is preferable due to the possibility of obtaining powders similar in chemical and phase composition to natural bone tissue [12,13,14,32]. Powders of calcium phosphates with a molar ratio Ca/P < 1.67 and calcium carbonates are used to create materials for temporary bone implants. At the same time, hydroxyapatite-based materials (molar ratio Ca/P = 1.67) are resistant to dissolution and can be used as implants for long-term substitution [33].
Materials based on calcium carbonates [34,35,36] and calcium phosphates with a molar ratio of Ca/P < 1.67 [37,38] are able to dissolve gradually during implantation, and, therefore, they are used in regenerative methods of bone defect treatment [39]. Synthetic powders of calcium carbonate (CaCO3), and calcium phosphates with a molar ratio of Ca/P < 1.67 can be used as fillers in biocompatible and biodegradable composites with polymer [40] or mineral (obtained as a result of chemical bonding reactions) matrices [41]. In addition, these powders can also be used to produce biocompatible ceramic materials, with the phase composition belonging to oxide systems, including calcium oxide and phosphorus oxide [42].
The aim of the present work consisted of preparing and investigating powders synthesized from an aqueous solution of calcium chloride (CaCl2), and a mixed-anionic solution containing orthophosphate and carbonate ions. Potassium hydroorthophosphate (K2HPO4), was used as the source of orthophosphate ions, and potassium carbonate (K2CO3), was used as the source of carbonate ions. For comparison, as references, powders were also synthesized from an aqueous solution of calcium chloride (CaCl2), and aqueous solutions including either potassium hydrophosphate (K2HPO4), or potassium carbonate (K2CO3). Synthesis from aqua solution of CaCl2 and a mixed-anionic solution containing orthophosphate and carbonate ions can provide the preparation of a powder of high quality as a starting point for the creation of different biocompatible materials and materials with other specific properties. To the best of our knowledge, synthesis from a mixed-anionic solution containing K2HPO4 and K2CO3 and an aqua solution of CaCl2 has not been considered in the scientific literature.
2. Materials and Methods
For the synthesis of powders, calcium chloride (CaCl2) (CAS No. 10043-52-4, analytical pure grade, Rushim, Moscow, Russia), potassium hydrophosphate trihydrate (K2HPO4·3H2O) (CAS No. 16788-57-1, analytical pure grade, Rushim, Moscow, Russia), and potassium carbonate (K2CO3) (CAS No. 584-08-7, chemical pure grade, Rushim, Moscow, Russia), were used.
The following reactions were used to calculate the amounts of starting salts and expected products:
CaCl2 + K2HPO4·3H2O → CaHPO4·2H2O + 2KCl + H2O
CaCl2 + 0.5K2HPO4·3H2O + 0.5K2CO3 → 0.5CaCO3 + 0.5CaHPO4·2H2O + 2KCl + 0.5H2O
CaCl2 + K2CO3 → CaCO3 + 2KCl
The labeling and synthesis conditions of the powders from CaCl2 and K2HPO4 and/or K2CO3 are shown in Table 1.

Table 1.
The labeling and synthesis conditions of the powders.
A total of 500 mL of 0.5 M aqueous solution of K2HPO4 (PO4 powder), 500 mL of 0.5 M aqueous solution of K2CO3 (CO3 powder), 500 mL aqueous solution containing 0.125 M of K2HPO4, and 0.125 M of K2CO3 (PO4_CO3 powder) were added to 500 mL of 0.5 M aqueous CaCl2 solutions. The resulting suspensions were kept on a stirrer (MAG C Hs7 IKA (IKA-Werke GmbH & Co. KG, Staufen, Germany)) for 30 min. The resulting precipitates were separated from the mother liquor using vacuum filtration. The synthesized powders were dried in a thin layer at room temperature for a week. After drying, the powders were crushed using an agate mortar and pestle and then passed through a polyester sieve with a mesh size of 200 microns. For the isolation of salts dissolved in the mother liquors, they were dried at 40 °C for a month. The synthesized powders and isolated reaction by-products were weighed to determine their mass and to estimate the yield of synthesized powders and reaction by-products relative to the theoretically possible amounts. Table 2 shows the initial quantities, expected target products, and reaction by-products calculated in accordance with reactions (1–3).

Table 2.
Quantities of initial reagents, as well as expected target products and reaction by-products.
To study the thermal evolution of the phase composition of the synthesized powders, they were placed in porcelain boats and heated at a heating rate of 5 °C/min, followed by exposure for 2 h at various temperatures in the range of 200–1000 °C. The labeling of the powders after heat treatment used in the figures is shown in Table 3.

Table 3.
Labeling of powders obtained after heat treatment.
The phase composition of powders after synthesis and after heat treatment was performed by X-ray powder diffraction (XRD) analysis using CuKa radiation (λ = 1.5418 Å, step 2θ—0.02°) using Rigaku D/Max-2500 diffractometers (Rigaku Corporation, Tokyo, Japan) in the angle range 2θ from 2 to 70° or Tongda TD-3700 (Dandong Tongda Science & Technology Co., Ltd., Dandong, China) in the angle range 2θ from 3 to 70°. The X-ray patterns were analyzed using the WinXPOW program (Version 1.06) using the ICDD PDF-2 (https://www.icdd.com/pdf-2/, accessed on 18 August 2025) [43] databases and the Match! program ((Version 3.15), https://www.crystalimpact.com/, accessed on 18 August 2025). The quantitative ratio of the target and related products in the obtained powders was determined using the Match! Program ((Version 3.15), https://www.crystalimpact.com/, accessed on 18 August 2025). The infrared (IR) spectra were collected in the wavelength range 500–4000 cm−1 using the Spectrum Three IR spectrometer (Perkin Elmer, Waltham, MA, USA) in the mode of disturbed total internal reflection using the Universal ATR accessory (crystal diamond/KRS-5). The bands in the spectra were assigned based on the literature data [44]. Synchronous thermal analysis (TA), including thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), was performed on a NETZSCH STA 449 F3 Jupiter thermal analyzer (NETZSCH, Selb, Germany) in air in the temperature range of 40–1000 °C at a heating rate of 10 °C/min, using pre-thermostating at 40 °C for 30 min. The mass of the sample was at least 10 mg. The composition of the gas phase was monitored using a Netzsch QMS 403 Quadro quadrupole mass spectrometer (NETZSCH, Selb, Germany) combined with a NETZSCH STA 449 F3 Jupiter thermal analyzer (NETZSCH, Selb, Germany). Mass spectra (MS) were recorded for m/z = 44 (CO2). The microstructure of the powders was studied by scanning electron microscopy (SEM) using a scanning electron microscope with an auto emission source JEOL JSM-6000PLUS Neoscope II (JEOL Ltd., Tokyo, Japan). For the study, the samples were glued onto a copper substrate using carbon tape, and a layer of gold ~15nm was sprayed. The survey was carried out in vacuum mode. The accelerating voltage of the electron gun was up to 5 kV. The images were obtained in secondary electrons at magnifications up to 1000× and recorded in digitized form on a computer.
3. Results and Discussion
The XRD data of the synthesized powders are shown in Figure 1.
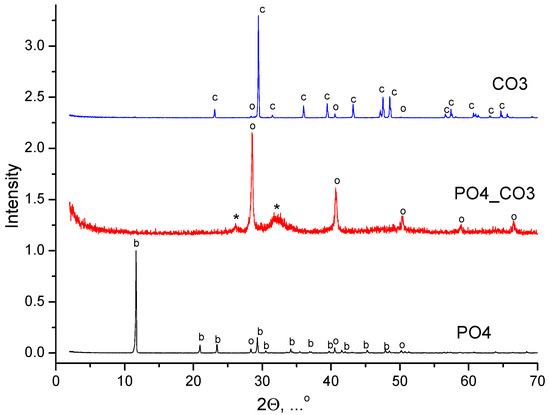
Figure 1.
XRD data of synthesized powders: с—calcium carbonate (calcite, CaCO3) (PDF card No 5-586; No 96-210-0190); о—potassium chloride (sylvine, KCl) (PDF card No 41-1476; No 96-900-8652); *—hydroxyapatite (Ca10(PO4)6(OH)2) (PDF card No 9-432, No 96-900-1234); b—brushite (CaHPO4·2H2O) (PDF card No 9-77; No 96-231-0527).
The phase composition of powder synthesized from water solutions of CaCl2 and K2CO3 included calcite (CaCO3). The phase composition of powder synthesized from water solutions of CaCl2 and K2HPO4 included brushite (CaHPO4·2H2O). The phase composition of powder synthesized from a water solution of CaCl2 and mixed-anionic solution containing K2CO3 and K2HPO4 included a low-crystalline product with the main peaks corresponding to hydroxyapatite (Ca10(PO4)6(OH)2). All synthesized powders included potassium chloride (sylvine, KCl), as a reaction by-product. XRD data of reaction by-products isolated from mother liquors are presented in Figure 2. The phase composition of the by-products isolated from the mother liquors was presented by potassium chloride (sylvine, KCl).
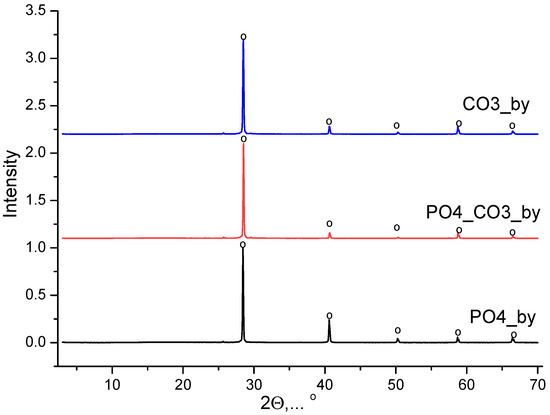
Figure 2.
XRD data of products isolated from mother liquors after synthesis: o—potassium chloride (sylvine, KCl) (PDF card No 41-1476; No 96-900-8652).
It should be noted that in this investigation, the stage of reaction by-product removal from all synthesized powders via precipitate washing was not implemented. The first reason consisted of the necessity to carry out the same synthesis conditions for all powders. The second reason consisted of the intended choice of pairs of resources providing the formation of biocompatible salt (KCl) as a reaction by-product. The third reason consisted of the possibility for KCl to be used as an inorganic porogen when it is present in synthesized powders and which can be removed from created composite materials with inorganic or polymer matrices via washing. To play the role of an inorganic porogen, the substance (inorganic salt) has to be soluble in water when other constituents of materials do not have such a possibility [45,46,47]. KCl as a component of the starting powder mixture used for the calcium phosphate ceramics preparation can be treated as a temporary sintering additive with a low melting point, which can be removed during stage of sintering at temperatures above its melting point [48,49,50].
The formation of brushite (CaHPO4·2H2O), and calcite (CaCO3), can be caused by reactions (1) and (3), respectively. During the preparation of the mixed-anionic aqua solution from K2HPO4 and K2CO3 taken in an equimolar ratio, not only the release of CO2 was observed, but also the formation of potassium orthophosphate (K3PO4), occurred. Reaction (4) indicates that when mixing solutions of these two salts, the presence of potassium carbonate, K2CO3, also persists.
2K2HPO4 + K2CO3 → 2K3PO4 + H2O + CO2
So, due to hydrolysis (reactions 5 and 6), the mixed-anionic solution had an alkaline pH, characteristic of aqua solutions of salts formed by a strong base and a weak acid:
K2CO3 + H2O → KHCO3 + KOH
K3PO4 + H2O → K2HPO4 + KOH
The formation of a specific calcium phosphate is determined by the pH level in the reaction zone. Formation of hydroxyapatite takes place at pH > 6 in the reaction zone. The same phenomenon took place, for example, when the mixed-anionic solution of Na2SiO3 and Na2HPO4, having an alkaline pH, interacted with a water solution of Ca(NO3)2 [51]. The synthesis of hydroxyapatite from mixed-anionic solution of K2HPO4 and K2CO3 can be represented formally as reaction (7), taking into account the molar ratio of the salts used for synthesis, as it was described in Materials and Methods (reaction 2):
12CaCl2 + 6K2HPO4 + 6K2CO3 → Ca10(PO4)6(OH)2 + 2CaCO3 + 24KCl + 2H2O + 4CO2
In this case, the theoretically possible amount of hydroxyapatite (Ca10(PO4)6(OH)2) (0.0208 mol = 20.9 g) can be calculated as 1/6 of the amount of potassium hydrophosphate (K2HPO4) (0.125 mol) used for preparation of a mixed-anionic solution (Table 1). Taking into account the possibility of the formation of CaCO3, the mass of the precipitate can be calculated as the sum of the possible amounts of hydroxyapatite and calcium carbonate. The amount of CaCO3 (0.0417 mol = 4.17 g) can be calculated as 1/6 of the amount of CaCl2 (0.25 mol). Thus, the mass of the precipitate in the synthesis of PO4_CO3 powder can be calculated as 25.07 g. Theoretically calculated (expected) and experimentally obtained masses of target products and by-products, as well as their comparison, are presented in Table 4.

Table 4.
The expected and obtained masses of synthesized powders and reaction by-products.
The data presented in Table 4 indicate that the total yield of synthesis products was close to ~90% in all cases. According to the estimation obtained using the Match! program, the content of the reaction by-product (KCl) was maximal (30.9%) in the powder synthesized from a mixed-anionic solution. The amount of KCl in the PO4 powder was determined as 2.8%, and in the CO3 powder, it was determined as 1.8%. The estimation of the amount of by-product in synthesized powders obtained using the Match! program is consistent with the estimation of the amount of by-product retained in PO4_CO3 and CO3 powders (Table 4). A small particle size and a significant specific surface area may determine the ability of the synthesized PO4_CO3 powder to retain (occlude) mother liquors and the by-product dissolved in it, as it has been shown in other studies [52].
Figure 3 shows the FTIR spectroscopy data of the synthesized powders.
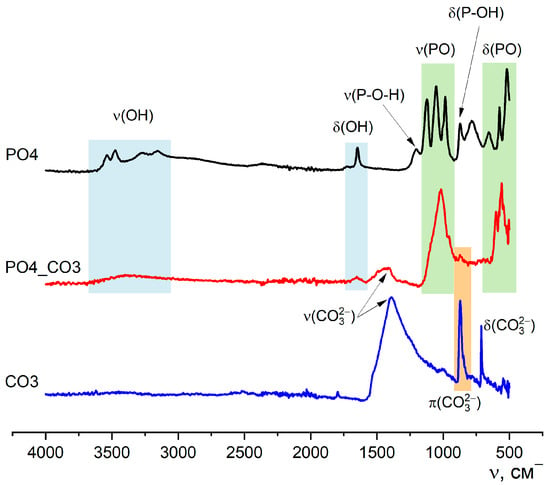
Figure 3.
FTIR spectra of synthesized PO4, PO4_CO3, and CO3 powders.
The FTIR spectroscopy data (Figure 3) is consistent with the X-ray diffraction data, since the appearance of the curves corresponds to the reference and literature data for brushite (SpectraBase Spectrum ID 9u0yn3G2J6i https://spectrabase.com/spectrum/9u0yn3G2J6i (accessed on 23 August 2025) [53,54,55]), calcite (SpectraBase Compound ID YYVCfbcpX1) https://spectrabase.com/compound/YYVCfbcpX1 (accessed on 23 August 2025) [56,57,58]), hydroxyapatite (SpectraBase Spectrum ID BxeLPnr9PTc https://spectrabase.com/spectrum/BxeLPnr9PTc (accessed on 23 August 2025) [59] and carbonate-hydroxyapatite [14,60,61]). On the spectra of PO4 and CO3 powders, there are valence (ν) and deformation (δ) vibrations, which are characteristic of brushite, CaHPO4·2H2O: ν(OH)—3539, 3473, 3270, 3153 см−1; ν(P-O-H)—1204 см−1; ν(PO)—1120, 1052, 980 см−1 δ(OH)—1645 см−1; 980 см−1; δ(P-OH)—871 см−1; and δ(PO)—(656, 575, 519 см−1) and calcite CaCO3: ν(CO32−)—1390 см−1; π(CO32−)—874 см−1; and δ(CO32−)—(710 см−1).
Characteristic vibrations of the phosphate group PO43−, which confirm the formation of weakly crystallized hydroxyapatite, can also be seen on the spectrum of the PO4_CO3 powder. Vibrations of ν(CO32−)—1410 см−1 and π(CO32−)—874 см−1 in the spectrum of PO4_CO3 powder suggest the presence of carbonate groups in the structure of the synthesized low-crystalline hydroxyapatite. The presence of KCl, regardless of its content in the synthesized powders, is not determined due to the absence of absorption bands in the studied region [62,63].
Figure 4 shows micrographs of the synthesized powders.
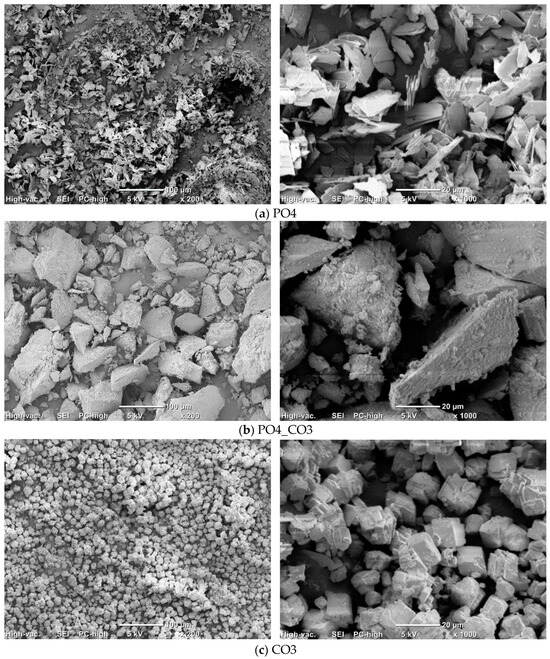
Figure 4.
Micrographs of synthesized PO4 (a), PO4_CO3 (b), and CO3 (c) powders.
The particles of PO4 powder (Figure 4a) with a size of 10–20 microns and a thickness of 1–2 mm have a lamellar morphology characteristic of brushite (CaHPO4·2H2O) [64]. The particles of CO3 powder (Figure 4c) with a size of 10–20 mm have the cubic shapes, which is typical for calcite (CaCO3) [65]. The PO4_CO3 powder (Figure 4b) is composed of large aggregates of 5–60 mm in size, consisting of particles less than 1 mm in size. Hydroxyapatite powders synthesized by precipitation from solutions, as a rule, consist of particles having a size of less than a micron [66]. It was estimated that the mass (33.5 g) of the synthesized PO4_CO3 powder after drying was 8.4 g higher than the expected (25.1 g) mass of the powder (Table 4). The mass of reaction by-product (PO4_CO3_by) isolated from the mother liquor was 11.8 g less than the expected (37.3 g) mass. Thus, after drying and grinding in a mortar, KCl (reaction by-product) retained in the synthesized powder acted as a binder, binding hydroxyapatite particles in large aggregates visible in the micrographs (Figure 4b).
Phase composition of all synthesized powders included biocompatible phases of hydroxyapatite (PO4_CO3 powder), brushite (PO4 powder), and calcite (CO3 powder). For starting, all these powders can be recommended for the creation of composite materials with a polymer [35,67,68,69] or inorganic matrix [70]. Powders with a phase composition including brushite and hydroxyapatite, after removing reaction by-products, can be used for the preparation of ceramics with a phase composition in the CaO-P2O5 presented by β-Ca2P2O7 [71,72] or β-Ca3(PO4)2 [73,74,75], and in the CaO-P2O5-H2O [76,77] oxide systems. Powder with a phase composition presented by calcite can be used for the preparation of biocompatible materials in the form of powder, granulate, or ceramics with phase composition in different oxide systems (for example, as mentioned above) as a precursor of CaO [42,78].
Figure 5 shows the TA data of the synthesized powders. According to the TGA data (Figure 5a), the total weight loss at 1000 °C for PO4 powder was 27.9%. The change in the weight of the PO4 powder is possible due to the removal of physically bound water, dehydration of brushite (CaHPO4·2H2O), and the formation of monetite (CaHPO4) (reaction 8), and the conversion of monetite (CaHPO4) into pyrophosphate (Ca2P2O7) (reaction 9).
CaHPO4·2H2O → CaHPO4 + 2H2O
2CaHPO4 → Ca2P2O7 + H2O
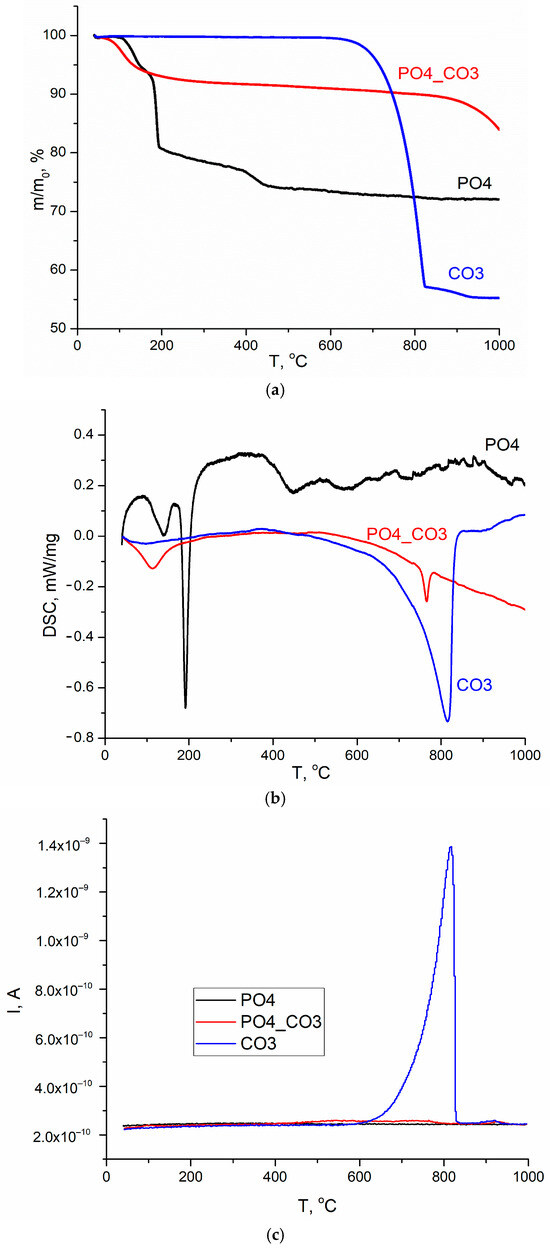
Figure 5.
TA data of synthesized powders: TGA (a), DSC (b), MS for m/z = 44 (CO2) (c).
The theoretically possible mass loss during reactions 8 and 9 is 26.16%, which is close to the value of the total mass loss for PO4 powder determined by the TGA method. All the processes indicated for the PO4 powder, leading to weight loss, occur with heat absorption in the ranges 87–168 °C, 168–243 °C, and 375–477 °C (Figure 5b). It can be assumed that the presence of KCl in the PO4 powder, as well as the stage of thermostating the sample at 40 °C for 30 min before heating at a set rate (10 °C/min), causes decomposition of the metastable brushite at lower temperatures in the range of 87–168 °C (reaction 8). In [79], the authors conclude that two-stage conversion of brushite into monetite was possible with the formation of an amorphous phase at the first stage. The range 168–243 °C should be considered as characteristic of the monetite (CaHPO4) formation from brushite (CaHPO4·2H2O) [80], and the range 375–477 °C should be considered as characteristic of the calcium pyrophosphate (Ca2P2O7) from monetite (CaHPO4) formation [81,82]. In the range of 477–840 °C, the smooth change in mass was 3%, while it is difficult to identify the intervals for processes that occur with heat absorption or release.
According to the TGA data (Figure 5a), the total weight loss at 1000 °C for the CO3 powder was 44.9%. The theoretically possible mass change for calcite during thermal decomposition in accordance with reaction (10) is 44%. The change in the mass of the CO3 powder occurred with heat absorption in the range 622–844 °C, amounted to 42.9% due to the decomposition of calcite and was accompanied by the release of CO2 (reaction 10).
CaCO3 → CaO + CO2
The graph of the mass change of the CO3 powder (Figure 5a) and the dependence of the ion current on temperature (Figure 5c) have the forms characteristic of calcite CaCO3 [83]. In Figure 5c, for the CO3 powder, one can see a graph of the dependence of the ion current on temperature for m/z = 44 (CO2), typical for CaCO3. It should be noted that in the range 844–949 °C, a loss (2%) of mass is observed, which is most likely associated with both the removal of CO2 and the removal of that insignificant amount of KCl (Table 4), which could be captured by the synthesized CO3 powder, above the melting point of KCl (776 °C [84] or 769 ± 2 °C (1042 ± 2 K) [85]).
According to the TGA data (Figure 5a), the total weight loss at 1000 °C for PO4_CO3 powder was 16.2%. Three sections can be distinguished on the curve of mass versus temperature for PO4_CO3 powder: removal of physically bound water (7.5% mass loss), which proceeds with heat absorption in the range of 43–240 °C; a section of smooth weight reduction (2.6%) in the range of 240–829 °C; and a section of mass loss (6.1%), apparently related to the removal of KCl, in the range of 829–1000 °C. The endothermic peak at 765 °C, clearly seen on the DSC curve (Figure 5b), can be attributed to the melting of KCl, since according to the estimation (Table 4), the mass of the retained reaction by-product (KCl) in the PO4_CO3 powder was the maximum. No endothermic peaks that could be attributed to the KCl melting process were found in the DSC graphs for PO4 and CO3 powders synthesized for comparison. Significantly lower values of the ion current can be seen on the curve m/z = 44 (CO2) for PO4-CO3 powder (Figure 5c) in wide ranges of 460–800 °C and 900–940 °C than for CO3 powder.
Figure 6 shows the XRD of the CO3 powder synthesized from aqueous solutions of CaCl2 and K2CO3 after heat treatment at various temperatures.
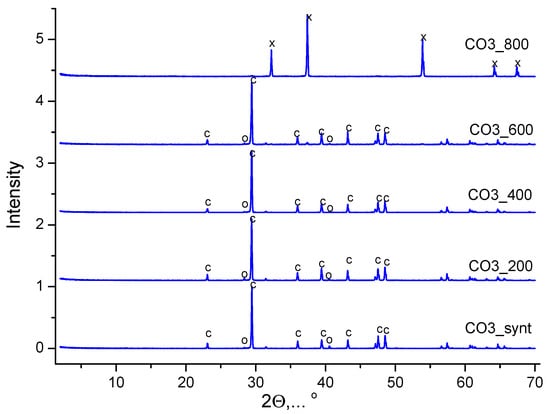
Figure 6.
XRD data of CO3 powder after synthesis and after heat treatment at different temperatures. с—calcium carbonate (calcite, CaCO3) (PDF card No 5-586; No 96-210-0190); о—potassium chloride (sylvine, KCl) (PDF No 41-1476; No 96-900-8652); х—calcium oxide (CaO) (PDF No 37-1497, No 96-101-1096).
According to the XRD data, the phase composition of the CO3 powder synthesized from aqueous solutions of CaCl2 and K2CO3, after heat treatment at various temperatures in the range of 200–600 °C for 2 h, was represented by calcite (CaCO3). And after heat treatment at 800 °C, the phase composition of the CO3 powder was represented by calcium oxide (CaO) (reaction 10).
Figure 7 shows micrographs of CO3 powders after heat treatment for 2 h at various temperatures in the range of 200–600 °C. The particle size and shape of the powder particles after heat treatment did not significantly differ from the particle size and shape of the synthesized powder. It is difficult to detect particles with dimensions less than 2 mm and more than 20 mm in the micrographs.
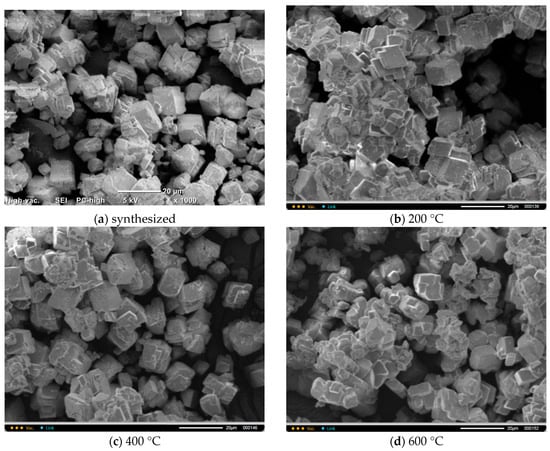
Figure 7.
Microphotographs of CO3 powder after synthesis (a) and after heat treatment at different temperatures: 200 °C (b), 400 °C (c), 600 °C (d).
Figure 8 shows the XRD of PO4 powder synthesized from aqueous solutions of CaCl2 and K2HPO4 after heat treatment at various temperatures. After heat treatment at 200 °C for 2 h, the phase composition of the PO4_200 powder was represented by monetite CaHPO4 (reaction 9). The phase composition of the PO4_400 and PO4_600 powders was represented by γ-calcium pyrophosphate (γ-Ca2P2O7) (reaction 10) after heat treatment at 400 °C and at 600 °C for 2 h. The phase composition of the PO4_800 and PO4_1000 powders included β-tricalcium phosphate (β-Ca3(PO4)2), β-calcium pyrophosphate (β-Ca2P2O7), and calcium potassium pyrophosphate (K2CaP2O7) after heat treatment at 800 °C and at 1000 °C for 2 h. According to data from Match! Software, after heat treatment at 1000 °C, PO4_1000 powder consisted of 43.7% of β-Ca3(PO4)2, 41.7% of β-Ca2P2O7, and 14.6% of K2CaP2O7.
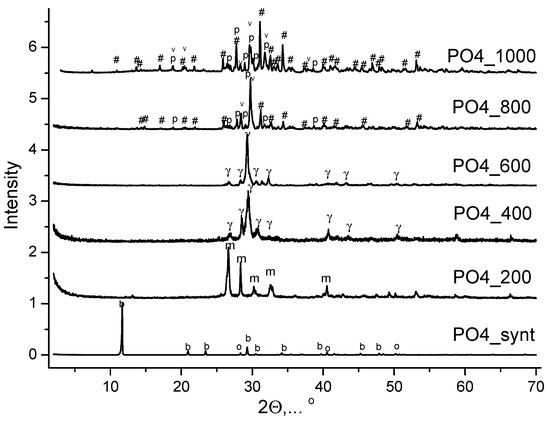
Figure 8.
XRD data of PO4 powder after synthesis and after heat treatment at different temperatures. о—potassium chloride (sylvine, KCl) (PDF No 41-1476; No 96-900-8652); b—brushite (CaHPO4·2H2O) (PDF card No 9-77; No 96-231-0527); m—monetite (CaHPO4) (PDF card No 9-80; 96-210-6185); γ—γ-calcium pyrophosphate (γ-Ca2P2O7) (PDF card No 17-499); p—β-calcium pyrophosphate (β-Ca2P2O7) (PDF card 9-346; No 96-100-1557); #—β-tricalcium phosphate (β-Ca3(PO4)2) (PDF card No 9-169; No 96-151-7239); v—K2CaP2O7 (PDF card No 22-805; No 96-220-2941).
Reaction (11) may reflect the formation of β-calcium orthophosphate (β-Ca3(PO4)2) and calcium potassium pyrophosphate (K2CaP2O7):
2Ca2P2O7 + 2KCl + H2O → Ca3(PO4)2 + K2CaP2O7 + 2HCl
Figure 9 shows micrographs of PO4 powders after thermal treatment for 2 h at various temperatures in the range of 200–1000 °C. The particle size and shape of the powder particles after heat treatment at 200 and 400 °C practically do not differ from the particle size and shape of the synthesized powder. After heat treatment in the range of 600–1000 °C, as the temperature increases, the powder particles lose their lamellar morphology more and more. And after heat treatment at 1000 °C, the PO4_1000 powder is composed of conglomerates 5–20 microns in size, consisting of particles 1–3 microns in size, sintered together.
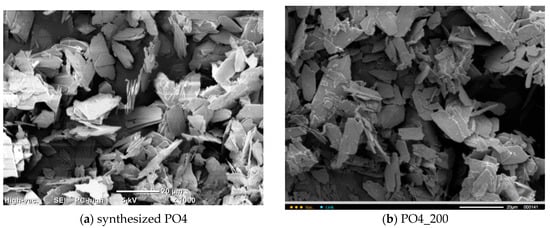
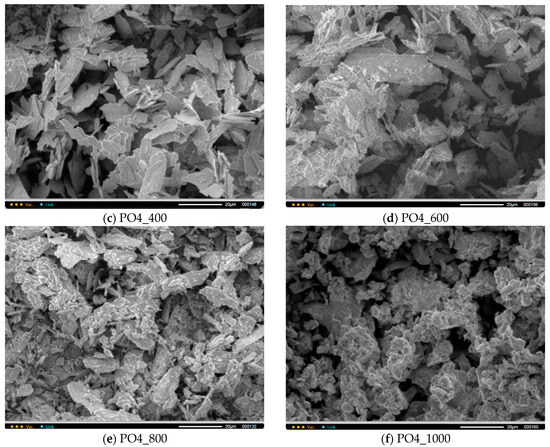
Figure 9.
Micrographs of PO4 powder after synthesis (a) and after heat treatment at various temperatures: 200 (b), 400 (c), 600 (d), 800 (e), 1000 (f).
Figure 10 shows the FTIR data for PO4 and PO4_1000 powders, along with the XRD and SEM data, confirming the transformation of the synthesized powder after heat treatment at 1000 °C. After heat treatment of the PO4 powder at 1000 °C, according to the FTIR data, the presence of PO43− groups remains and a δ(POP) 720 cm−1 vibration appears, which confirms the presence of the pyrophosphate ion P2O74− in both calcium pyrophosphate formed from brushite (reactions 8 and 9) and calcium potassium pyrophosphate (reaction 11).
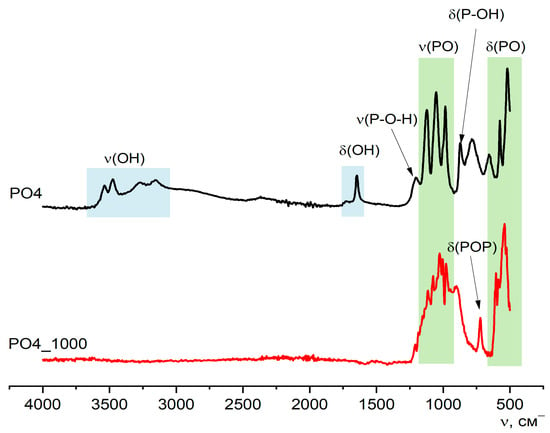
Figure 10.
FTIR spectra of synthesized PO4 powder and PO4_1000 powder after heat treatment at 1000 °C.
Figure 11 shows the XRD of the PO4_CO3 powder synthesized from aqueous solutions of CaCl2, KHPO4, and K2CO3 after heat treatment at various temperatures. The phase composition of PO4_CO3 powders after thermal treatment in the range of 200–800 °C is represented by weakly crystallized hydroxyapatite and potassium chloride (sylvine, KCl). After heat treatment at 1000 °C, the phase composition of the PO4_CO3_1000 powder is represented by chlorapatite (Ca10(PO4)6Cl2). The formation of chlorapatite can be reflected by the reaction (12):
Ca10(PO4)6(OH)2 + 2KCl → Ca10(PO4)6Cl2 + 2KOH
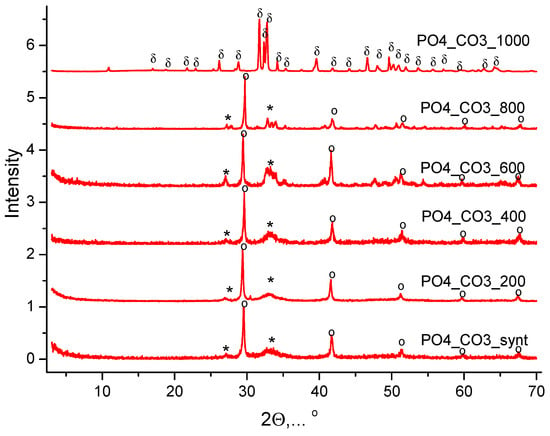
Figure 11.
XRD data of PO4_CO3 powder after synthesis and after heat treatment at different temperatures. *—hydroxyapatite (Ca10(PO4)6(OH)2) (PDF No 96-901-4314); o—potassium chloride (sylvine, KCl) (PDF No 41-1476; No 96-900-8652); δ—chlorapatite (Ca10(PO4)6Cl2) (PDF No 73-1728; No 96-101-0917).
A similar formation of chlorapatite was observed at 1000 °C from weakly crystalline hydroxyapatite of natural origin and sodium chloride (NaCl) [86], or in powder consisting of CaHPO4·2H2O, CaHPO4, and NaCl when heated at a range of 800–1100 °C [17].
Micrographs of PO4_CO3 powder after synthesis and after heat treatment at various temperatures are presented in Figure 12. The microstructure of PO4_CO3 powders, after thermal treatment in the range of 200–800 °C, does not significantly differ from the microstructure of the powder after synthesis and drying. Powders consist of sufficiently large agglomerates (up to 100–200 mm) consisting of particles of weakly crystallized hydroxyapatite bonded with reaction by-product KCl. The microstructure of the PO4_CO3_1000 powder after heat treatment at 1000 °C differs significantly from the microstructure of powders after heat treatment in the range of 200–800 °C. The PO4_CO3_1000 powder after heat treatment at 1000 °C consists of particles with a prismatic shape 5–20 mm long and a transverse dimension of 2–5 mm. In the micrograph (Figure 12e, left side) with a lower magnification, one can see loose aggregates up to 100 microns in size, consisting of the prismatic particles mentioned above.
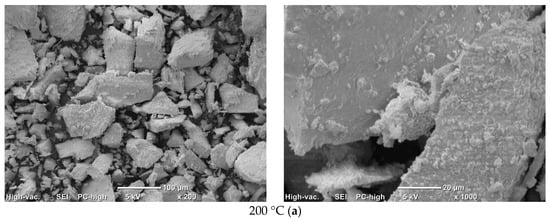
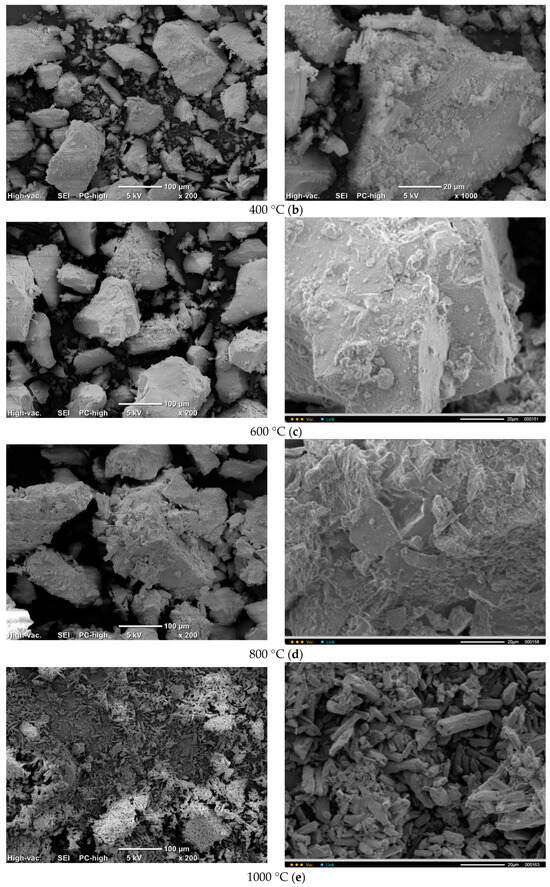
Figure 12.
Micrographs of PO4_CO3 powder after heat treatment at various temperatures: 200 (a), 400 (b), 600 (c), 800 (d), 1000 (e).
Figure 13 shows the FTIR spectroscopy data for PO4_CO3 powder after synthesis and after heat treatment at 1000 °C. In the spectrum of the PO4_CO3_1000 powder, there are no peaks that could be attributed to ν(OH), ν(CO32−), or π(CO32−). The FTIR spectrum for the PO4_CO3_1000 powder corresponds to the reference data for chlorapatite (SpectraBase Compound ID KKRjtSFM3Pp, https://spectrabase.com/spectrum/KKRjtSFM3Pp (accessed on 23 August 2025)).
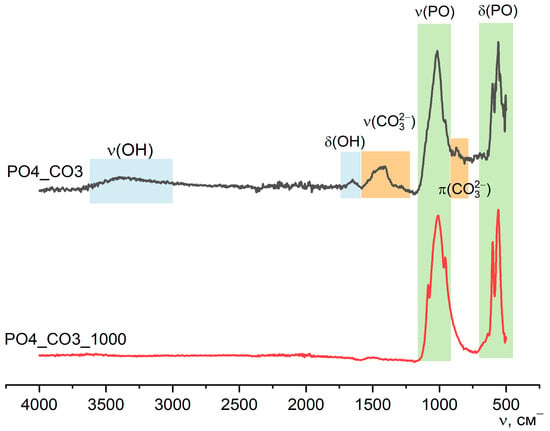
Figure 13.
FTIR spectra of PO4_CO3 powder after synthesis and after heat treatment at 1000 °C.
Powder with the phase composition in the K2O-CaO-P2O5 system, including β-calcium pyrophosphate (β-Ca2P2O7), β-calcium orthophosphate (β-Ca3(PO4)2), and potassium calcium pyrophosphate (K2CaP2O7) can be prepared from synthesized powder PO4 consisting of brushite and KCl. Phase composition after heat treatment at temperatures 800 and 1000 °C for PO4 powder included such biocompatible phases as β-calcium pyrophosphate (β-Ca2P2O7) [62] and β-calcium orthophosphate (β-Ca3(PO4)2) [87]. Minerals in the K2O-CaO-P2O5 system are under consideration as biocompatible [88,89]. Nevertheless, potassium calcium pyrophosphate (K2CaP2O7), according to the scientific literature, is known as a matrix for luminescent materials [90], or it was mentioned as the most promising fertilizer material [91]. Taking into account the possibility for K2CaP2O7 to be used as fertilizer material and the relatively low (14.6%) content of K2CaP2O7 in the PO4_1000 powder, it is possible to expect that materials that can be created based on PO4_1000 powder will be treated as biocompatible after ordinary tests in vitro and in vivo. Powder of chlorapatite (Ca10(PO4)6Cl2) can be prepared from synthesized PO4_CO3 powder consisting of hydroxyapatite (Ca10(PO4)6(OH)2) and KCl at 1000 °C. Chlorapatite (Ca10(PO4)6Cl2) can also be used both as a matrix for the creation of luminescent materials [92] and as a component of biocompatible materials [93].
4. Conclusions
Powders with the phase composition including target products such as brushite (CaHPO4·2H2O), and calcium carbonate (calcite, CaCO3), as well as potassium chloride (sylvine, KCl), as a reaction by-product, were synthesized from aqueous solutions of calcium chloride (CaCl2), potassium hydrophosphate (K2HPO4), and potassium carbonate (K2CO3). The interaction of an aqueous mixed-anionic solution including HPO42− and CO32− anions and an aqueous solution of calcium chloride (CaCl2), made it possible to obtain a powder that combined weakly crystallized hydroxyapatite and a significant amount (estimated to be up to 30–35% by weight) of potassium chloride (sylvine, KCl), in its phase composition. The XRD, SEM, and FTIR data confirmed the possibility of synthesizing chlorapatite (Ca10(PO4)6Cl2) from this powder via heat treatment at 1000 °C for 2 h.
After heat treatment of the synthesized powder containing brushite (CaHPO4·2H2O), and potassium chloride (sylvine, KCl), at 800 and 1000 °C, powders with the phase composition including β-calcium pyrophosphate (β-Ca2P2O7), β-calcium orthophosphate (β-Ca3(PO4)2), and potassium calcium pyrophosphate (K2CaP2O7) were obtained. Heat treatment of calcite (CaCO3) powder at 800 °C, as was expected, led to the formation of calcium oxide (CaO).
Powders including phases such as hydroxyapatite, chlorapatite, brushite, monetite, calcium pyrophosphate, calcium potassium pyrophosphate, tricalcium phosphate, and calcite can be used for the creation of biocompatible inorganic materials or composite materials with a biocompatible polymer matrix. Powders of chlorapatite can be used as a matrix for the creation of luminescent materials. Potassium chloride (sylvine, KCl), present in synthesized powders can act as one of the precursors of chlorapatite or calcium potassium pyrophosphate, or it can act as a removable inorganic porogen.
Author Contributions
Conceptualization, T.V.S.; methodology, T.V.S.; investigation, T.V.S., H.M.N.L., T.B.S., A.M.M., T.V.F., E.A.M., D.M.T., D.O.G., O.V.B. and A.V.K.; resources, T.V.S., T.B.S., D.M.T. and O.V.B.; writing—original draft preparation, T.V.S. and H.M.N.L.; writing—review and editing, T.V.S.; visualization, T.V.S., H.M.N.L., T.B.S., A.M.M., T.V.F., D.M.T., D.O.G., O.V.B. and A.V.K.; supervision, T.V.S.; project administration, T.V.S.; funding acquisition, T.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of the State assignment of the Lomonosov Moscow State University: AAAA-A21-121011590082-2.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was carried out using the equipment of the MSU Shared Research Equipment Center “Technologies for obtaining new nanostructured materials and their complex study” and was purchased by MSU within the framework of the Equipment Renovation Program (National Project “Science”) and within the framework of the MSU Program of Development.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andrianov, N.T.; Balkevich, V.L.; Belyakov, A.V.; Vlasov, A.S.; Guzman, I.Y.; Lukin, E.S.; Mosin, Y.M.; Skidan, B.S. Chemical technology of ceramics: Textbook. In Handbook for Universities; Guzmanm, I.Y., Ed.; Rif Stroymaterialy LLC: Moscow, Russia, 2012; 496p, ISBN 978-5-94026-01966. (In Russian) [Google Scholar]
- Butt, Y.M.; Sychev, M.M.; Timashev, V.V. Chemical technology of binder materials. In Textbook for Universities on Spec. “Chemical Technology of Binding Materials”; Timashev, V.V., Ed.; Higher School: Moscow, Russia, 1980; 472p. ill., 22. (In Russian) [Google Scholar]
- Lee, B.; Komarneni, S. (Eds.) Chemical Processing of Ceramics; CRC Press: Boca Raton, FL, USA, 2005; 756p, ISBN 978-0-42911-963-7. [Google Scholar] [CrossRef]
- Gerasimov, M.D.; Latyshev, S.S.; Bogdanov, N.E.; Loktionov, I.O. Review of constructive solutions in the field of grinding mills. In Energy-Saving Technological Complexes and Equipment for the Production of Building Materials: An Interuniversity Collection of Articles; Bogdanov, V.S., Ed.; Volume Issue XVII; Belgorod State Technological University named after V.G. Shukhov: Belgorod, Russia, 2018; pp. 132–146. Available online: https://www.elibrary.ru/item.asp?id=42997967 (accessed on 23 August 2025). (In Russian)
- Shlyakhtin, O.A.; Tretyakov, Y.D. Recent progress in cryochemical synthesis of oxide materials. J. Mater. Chem. 1999, 9, 19–24. [Google Scholar] [CrossRef]
- Kageyama, H.; Ogino, H. (Eds.) Mixed-Anion Compounds; Special Collection: 2024 eBook Collection; Royal Society of Chemistry RSC: Cambridge, UK, 024; 276p, ISBN 978-1-83916-512-2. [CrossRef]
- Lukin, E.S.; Makarov, N.A.; Kozlov, A.I.; Popova, N.A.; Anufrieva, E.V.; Vartanyan, M.A.; Kozlov, I.A.; Safina, M.N.; Lemeshev, D.O.; Gorelik, E.I. Oxide ceramics of the new generation and areas of application. Glass Ceram. 2008, 65, 348–352. [Google Scholar] [CrossRef]
- Lukin, E.S. Modern high-density oxide ceramics with controlled microstructure. Part I. Effect of aggregation of oxide powders on the sintering and microstructure of ceramics. Refractories 1996, 37, 6–14. [Google Scholar] [CrossRef]
- Ring, T.A. Fundamentals of Ceramic Powder Processing and Synthesis, 1st ed.; Elsevier: Amsterdam, The Netherlands; Academic Press, Elsevier: Cambridge, MA, USA, 30 April 1996; ISBN 9780080532196. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Filippov, Y.Y.; Shatalova, T.B.; Fatin, D.S. Ceramics Based on Brushite Powder Synthesized from Calcium Nitrate and Disodium and Dipotassium Hydrogen Phosphates. Inorg. Mater. 2018, 54, 195–207. [Google Scholar] [CrossRef]
- Safronova, T.V.; Knot’ko, A.V.; Shatalova, T.B.; Evdokimov, P.V.; Putlyaev, V.I.; Kostin, M.S. Calcium phosphate ceramic based on powder synthesized from a mixed-anionic solution. Glass Ceram. 2016, 73, 25–31. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Filippov, Y.Y.; Knot’ko, A.V.; Klimashina, E.S.; Peranidze, K.K.; Evdokimov, P.V.; Vladimirova, S.A. Powders synthesized from calcium acetate and mixed-anionic solutions, containing orthophosphate and carbonate ions, for obtaining bioceramic. Glass Ceram. 2018, 75, 118–123. [Google Scholar] [CrossRef]
- Song, Y.; Hahn, H.H.; Hoffmann, E. The effect of carbonate on the precipitation of calcium phosphate. Environ. Technol. 2002, 23, 207–215. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.; Kol’tsov, A.; Kuz’mina, M.; Zorina, M.; Poritskaya, L. Ion substitutions and non-stoichiometry of carbonated apatite-(CaOH) synthesised by precipitation and hydrothermal methods. J. Mol. Struct. 2011, 992, 9–18. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Filippov, Y.; Kazakova, G.; Shatalova, T.; Rau, J.V. Powders Based on Ca2P2O7-CaCO3-H2O System as Model Objects for the Development of Bioceramics. Ceramics 2022, 5, 423–434. [Google Scholar] [CrossRef]
- Golubchikov, D.; Safronova, T.V.; Nemygina, E.; Shatalova, T.B.; Tikhomirova, I.N.; Roslyakov, I.V.; Khayrutdinova, D.; Platonov, V.; Boytsova, O.; Kaimonov, M.; et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings 2023, 13, 374. [Google Scholar] [CrossRef]
- Safronova, T.V.; Khantimirov, A.S.; Shatalova, T.B.; Filippov, Y.Y.; Kolesnik, I.V.; Knotko, A.V. Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production. Ceramics 2023, 6, 561–583. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Costantino, U.; Alberti, G.; Howe, A.T.; Ruvarac, A. Inorganic Ion Exchange Materials; Clearfield, A., Ed.; CRC Press: Boca Raton, FL, USA, 2018; 297p, ISBN 0-8493-5930-9. [Google Scholar]
- Ivanets, A.I.; Shashkova, I.L.; Kitikova, N.V.; Radkevich, A.V.; Davydov, Y.P. Recovery of strontium ions with calcium and magnesium phosphates from aqueous solutions against the background of CaCl2. Radiochemistry 2015, 57, 610–615. [Google Scholar] [CrossRef]
- Berlyand, A.S.; Snyakin, A.P.; Prokopov, A.A. Adsorption capacity of hydroxyapatite for several amino acids and heavy metal ions. Pharm. Chem. J. 2012, 46, 292. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Paramonova, E.V.; Filippov, S.V.; Likhachev, I.V.; Bystrova, A.V.; Avakyan, L.A.; Kovrigina, S.A.; Makarova, S.V.; Bulina, N.V. Features of the Structure and Properties of Hydroxypapatite with Various Cationic Substitutions. In Proceedings of the International Conference “Mathematical Biology and Bioinformatics”; Lakhno, V.D., Ed.; IMPB RAS: Pushchino, Russia, 2024; Volume 10, Paper No. e11. (In Russian) [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted hydroxyapatite: A recent development. Mater. Technol. 2020, 35, 785–796. [Google Scholar] [CrossRef]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef]
- Alam, M.K.; Hossain, M.S.; Kawsar, M.; Bahadur, N.M.; Ahmed, S. Synthesis of nano-hydroxyapatite using emulsion, pyrolysis, combustion, and sonochemical methods and biogenic sources: A review. RSC Adv. 2024, 14, 3548–3559. [Google Scholar] [CrossRef]
- Sezanova, K.; Tuparova, Y.; Shestakova, P.; Markov, P.; Kovacheva, D.; Rabadjieva, D. Calcium Phosphate Ceramic Powders Prepared from Mechanochemically Activated Precursors. Inorganics 2025, 13, 313. [Google Scholar] [CrossRef]
- Balbuena, O.B.F.; Paiva, L.F.S.; Ribeiro, A.A.; Monteiro, M.M.; de Oliveira, M.V.; Pereira, L.C. Sintering parameters study of a biphasic calcium phosphate bioceramic synthesized by alcoholic sol-gel technique. Ceram. Int. 2021, 47, 32979–32987. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Ariffin, K.S.; Hussin, H.B.; Temitope, A.E. Synthesis of precipitated calcium carbonate: A review. Carbonates Evaporites 2018, 33, 331–346. [Google Scholar] [CrossRef]
- Cai, J.; Lu, M.; Huang, Q.; Bai, F.; Zhao, D.; Jiang, H.; Chen, J. A Review of Nano-Calcium Carbonate and Its Applications: Preparation, Necessities, Biomedicine, and Environment. Part. Part. Syst. Charact. 2025, e00093. [Google Scholar] [CrossRef]
- Parushev, I.; Gerova-Vatsova, T. Methods for obtaining synthetic carbonate apatite for bone regeneration: A review. Scr. Sci. Med. Dent. 2025, 11, 7–17. Available online: https://journals.mu-varna.bg/index.php/ssmd/article/view/9879/8863 (accessed on 23 August 2025).
- Kapolos, J.; Koutsoukos, P.G. Formation of calcium phosphates in aqueous solutions in the presence of carbonate ions. Langmuir 1999, 15, 6557–6562. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Pikos, M.A.; Nakamura, T.; Imafuji, T.; Zhang, Y.; Shinohara, Y.; Sculean, A.; Shirakata, Y. The development of non-resorbable bone allografts: Biological background and clinical perspectives. Periodontol. 2000 2024, 94, 161–179. [Google Scholar] [CrossRef]
- He, F.; Zhang, J.; Tian, X.; Wu, S.; Chen, X. A facile magnesium-containing calcium carbonate biomaterial as potential bone graft. Colloid. Surf. B 2015, 136, 845–852. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, L.; Parakhonskiy, B.V.; Skirtach, A.G. Hard, Soft, and Hard-and-Soft Drug Delivery Carriers Based on CaCO3 and Alginate Biomaterials: Synthesis, Properties, Pharmaceutical Applications. Pharmaceutics 2022, 14, 909. [Google Scholar] [CrossRef]
- Liu, H.; Wen, Z.; Liu, Z.; Yang, Y.; Wang, H.; Xia, X.; Ye, J.; Liu, Y. Unlocking the potential of amorphous calcium carbonate: A star ascending in the realm of biomedical application. Acta Pharm. Sin. B 2024, 14, 602–622. [Google Scholar] [CrossRef]
- Liu, D.; Cui, C.; Chen, W.; Shi, J.; Li, B.; Chen, S. Biodegradable Cements for Bone Regeneration. J. Funct. Biomater. 2023, 14, 134. [Google Scholar] [CrossRef]
- Bohner, M. Bioresorbable ceramics. In Degradation Rate of Bioresorbable Materials; Buchanan, F., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 95–114. ISBN 978-1-84569-329-9. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Kim, K.H.; Seo, J.-H.; Pack, S.P. Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration. Biomimetics 2024, 9, 511. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielo, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Lunetta, E.; Messina, M.; Cacciotti, I. Doped hydroxyapatite bioceramic from food wastes for orthopedic applications. J. Am. Ceram. Soc. 2025, 108, e70002. [Google Scholar] [CrossRef]
- ICDD. PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010; Available online: http://www.icdd.com/pdf-2/ (accessed on 23 August 2025).
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley: New York, NY, USA, 1986; pp. 156–159. [Google Scholar]
- Lakshmi, D.S.; K. S., R.; Castro-Muñoz, R.; Tańczyk, M. Emerging Trends in Porogens toward Material Fabrication: Recent Progresses and Challenges. Polymers 2022, 14, 5209. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shatalova, T.B.; Filippov, Y.Y.; Toshev, O.U.; Knot’ko, A.V.; Vaimugin, L.A.; Savchenkova, D.V. Na2O–CaO–SO3 Ceramics as Promising Inorganic Porogens. Glass Ceram. 2022, 79, 88–94. [Google Scholar] [CrossRef]
- Şahin, E.; Çiftçioğlu, M. Compositional, microstructural and mechanical effects of NaCl porogens in brushite cement scaffolds. J. Mech. Behav. Biomed. Mater. 2021, 116, 104363. [Google Scholar] [CrossRef]
- Golubko, N.V.; Kaleva, G.M.; Mosunov, A.V.; Politova, E.D.; Sadovskaya, N.V.; Stefanovich, S.Y.; Segalla, A.H. Effects of KCl/LiF additives on the structure, phase transitions and dielectric properties of BSPT ceramics. Ferroelectrics 2015, 485, 95–100. [Google Scholar] [CrossRef]
- Nzihou, A.; Adhikari, B.; Pfeffer, R. Effect of metal chlorides on the sintering and densification of hydroxyapatite adsorbent. Ind. Eng. Chem. Res. 2005, 44, 1787–1794. [Google Scholar] [CrossRef]
- Diaz, J.C.C.A.; Muccillo, E.N.d.S.; Muccillo, R. Porous 8YSZ Ceramics Prepared with Alkali Halide Sacrificial Additives. Materials 2023, 16, 3509. [Google Scholar] [CrossRef]
- Golubchikov, D.O.; Safronova, T.V.; Podlyagin, V.A.; Shatalova, T.B.; Kolesnik, I.V.; Putlayev, V.I. Silicate-substituted hydroxyapatite bioceramics fabrication from the amorphous powder precursor obtained from the silicate-containing solutions. Mendeleev Commun. 2024, 34, 847–849. [Google Scholar] [CrossRef]
- Safronova, T.V.; Sterlikov, G.S.; Kaimonov, M.R.; Shatalova, T.B.; Filippov, Y.Y.; Toshev, O.U.; Roslyakov, I.V.; Kozlov, D.A.; Tikhomirova, I.N.; Akhmedov, M.R. Composite Powders Synthesized from the Water Solutions of Sodium Silicate and Different Calcium Salts (Nitrate, Chloride, and Acetate). J. Compos. Sci. 2023, 7, 408. [Google Scholar] [CrossRef]
- Casciani, F.; Condrate, R.A., Sr. The vibrational spectra of brushite, CaHPO4·2H2O. Spectrosc. Lett. 1979, 12, 699–713. [Google Scholar] [CrossRef]
- Berry, E.E.; Baddiel, C.B. The infra-red spectrum of dicalcium phosphate dihydrate (brushite). Spectrochim. Acta A-M. 1967, 23, 2089–2097. [Google Scholar] [CrossRef]
- Hirsch, A.; Azuri, I.; Addadi, L.; Weiner, S.; Yang, K.; Curtarolo, S.; Kronik, L. Infrared absorption spectrum of brushite from first principles. Chem. Mater. 2014, 26, 2934–2942. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Ye, Y.; Wang, C.; Liu, D.; Shi, X.; Wang, S.; Zhu, X. In-situ High-Temperature XRD and FTIR for Calcite, Dolomite and Magnesite: Anharmonic Contribution to the Thermodynamic Properties. J. Earth Sci. 2019, 30, 964–976. [Google Scholar] [CrossRef]
- Bosch Reig, F.; Gimeno Adelantado, J.V.; Moya Moreno, M.C.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Čadež, V.; Šegota, S.; Sondi, I.; Lyons, D.M.; Saha, P.; Saha, N.; Sikirić, M.D. Calcium phosphate and calcium carbonate mineralization of bioinspired hydrogels based on β-chitin isolated from biomineral of the common cuttlefish (Sepia officinalis, L.). J. Polym. Res. 2018, 25, 226. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, S. FTIR spectrum analysis to predict the crystalline and amorphous phases of hydroxyapatite: A comparison of vibrational motion to reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef]
- Lee, I.H.; Lee, J.A.; Lee, J.H.; Heo, Y.W.; Kim, J.J. Effects of pH and reaction temperature on hydroxyapatite powders synthesized by precipitation. J. Korean Ceram. Soc. 2020, 57, 56–64. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Carbonate apatite type A synthesized at high pressure: New space group (P3) and orientation of channel carbonate ion. J. Solid State Chem. 2003, 174, 412–417. [Google Scholar] [CrossRef]
- Shiehpour, M.; Solgi, S.; Tafreshi, M.J.; Ghamsari, M.S. ZnO-doped KCl single crystal with enhanced UV emission lines. Appl. Phys. A 2019, 125, 531. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Zelek-Pogudz, S.; Solecka, U.; Solecki, M.L.; Szostak, E.; Zborowski, K.K.; Zając, M. Use of the Far Infrared Spectroscopy for NaCl and KCl Minerals Characterization—A Case Study of Halides from Kłodawa in Poland. Minerals 2022, 12, 1561. [Google Scholar] [CrossRef]
- Toshima, T.; Hamai, R.; Tafu, M.; Takemura, Y.; Fujita, S.; Chohji, T.; Tanda, S.; Li, S.; Qin, G.W. Morphology control of brushite prepared by aqueous solution synthesis. J. Asian Ceram. Soc. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Liu, J.H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef] [PubMed]
- Yelten-Yilmaz, A.; Yilmaz, S. Wet chemical precipitation synthesis of hydroxyapatite (HA) powders. Ceram. Int. 2018, 44, 9703–9710. [Google Scholar] [CrossRef]
- Radulescu, D.-E.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers 2022, 14, 899. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Deyneko, D.V.; Knotko, A.V.; Olkhov, A.A.; Slukin, P.V.; Davydova, G.A.; Trubitsyna, T.A.; Preobrazhenskiy, I.I.; Gosteva, A.N.; Antoniac, I.V.; et al. Antibacterial Composite Material Based on Polyhydroxybutyrate and Zn-Doped Brushite Cement. Polymers 2023, 15, 2106. [Google Scholar] [CrossRef]
- del-Mazo-Barbara, L.; Gomez-Cuyas, J.; Martinez-Orozco, L.; Perez, O.S.; Bou-Petit, E.; Ginebra, M.P. In vitro degradation of 3D-printed polycaprolactone\biomimetic hydroxyapatite scaffolds: Impact of the sterilization method. Polym. Test. 2024, 139, 108566. [Google Scholar] [CrossRef]
- Mirkiani, S.; Mesgar, A.S.; Mohammadi, Z.; Matinfar, M. Synergetic reinforcement of brushite cements by monetite/apatite whisker-like fibers and carboxymethylcellulose. Materialia 2022, 21, 101329. [Google Scholar] [CrossRef]
- Safronova, T.; Kiselev, A.; Selezneva, I.; Shatalova, T.; Lukina, Y.; Filippov, Y.; Toshev, O.; Tikhonova, S.; Antonova, O.; Knotko, A. Bioceramics Based on β-Calcium Pyrophosphate. Materials 2022, 15, 3105. [Google Scholar] [CrossRef]
- Bolarinwa, A.; Gbureck, U.; Purnell, P.; Bold, M.; Grover, L.M. Cement casting of calcium pyrophosphate based bioceramics. Adv. Appl. Ceram. 2010, 109, 291–295. [Google Scholar] [CrossRef]
- Saxena, A.; Vignesh, R.; Roy, S.; Sahai, S.; Bhattacharjee, D.; Basu, B.; Mukherjee, S. Synthesis and properties of tailored β-tricalcium phosphate for bone filler applications. Int. J. Appl. Ceram. Technol. 2025, 22, e14957. [Google Scholar] [CrossRef]
- Safronova, T.; Grigorev, G.; Shatalova, T.; Roslyakov, I.; Platonov, V.; Khayrutdinova, D. Microporous Ceramics Based on β-Tricalcium Phosphate. Ceramics 2022, 5, 1269–1285. [Google Scholar] [CrossRef]
- Toshev, O.U.; Safronova, T.V.; Mironova, Y.S.; Matveeva, A.S.; Shatalova, T.B.; Filippov, Y.Y.; Knotko, A.V.; Akhmedov, M.R.; Kukueva, E.V.; Lukina, Y.S. Ultraporous submicron-grained β-Ca3(PO4)2-based ceramics. Inorg. Mater. 2022, 58, 1208–1219. [Google Scholar] [CrossRef]
- Martin, R.I.; Brown, P.W. Phase equilibria among acid calcium phosphates. J. Am. Ceram. Soc. 1997, 80, 1263–1266. [Google Scholar] [CrossRef]
- Rasouli, M.; Darghiasi, S.F.; Naghib, S.M.; Rahmanian, M. Multifunctional hydroxyapatite-based nanoparticles for biomedicine: Recent progress in drug delivery and local controlled release. Curr. Mech. Adv. Mater. 2021, 1, 3–16. [Google Scholar] [CrossRef]
- Ismail, R.; Cionita, T.; Shing, W.L.; Fitriyana, D.F.; Siregar, J.P.; Bayuseno, A.P.; Nugraha, F.W.; Muhamadin, R.C.; Junid, R.; Endot, N.A. Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate. Materials 2022, 15, 5712. [Google Scholar] [CrossRef] [PubMed]
- Dosen, A.; Giese, R.F. Thermal decomposition of brushite, CaHPO4·2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Mineral. 2011, 96, 368–373. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Griesiute, D.; Gaidukevic, J.; Zarkov, A.; Kareiva, A. Synthesis of β-Ca2P2O7 as an Adsorbent for the Removal of Heavy Metals from Water. Sustainability 2021, 13, 7859. [Google Scholar] [CrossRef]
- El Hazzat, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Complex evolution of phase during the thermal investigation of Brushite-type calcium phosphate CaHPO4.2H2O. Materialia 2021, 16, 101055. [Google Scholar] [CrossRef]
- Babou-Kammoe, R.; Hamoudi, S.; Larachi, F.; Belkacemi, K. Synthesis of CaCO3 nanoparticles by controlled precipitation of saturated carbonate and calcium nitrate aqueous solutions. Can. J. Chem. Eng. 2012, 90, 26–33. [Google Scholar] [CrossRef]
- Rabinovich, V.A.; Khavin, Z.Y. A Short Chemical Reference Book; Chemistry: Leningrad, Russia, 1978; 392p. (In Russian) [Google Scholar]
- Zhou, D.; Dong, J.; Si, Y.; Zhu, F.; Li, J. Melting Curve of Potassium Chloride from in situ Ionic Conduction Measurements. Minerals 2020, 10, 250. [Google Scholar] [CrossRef]
- Cavalcante, L.d.A.; Ribeiro, L.S.; Takeno, M.L.; Aum, P.T.P.; Aum, Y.K.P.G.; Andrade, J.C.S. Chlorapatite Derived from Fish Scales. Materials 2020, 13, 1129. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Putlyaev, V.I.; Evdokimov, P.V.; Safronova, T.V.; Klimashina, E.S.; Orlov, N.K. Fabrication of osteoconductive Ca3–x M2x (PO4)2 (M = Na, K) calcium phosphate bioceramics by stereolithographic 3D printing. Inorg. Mater. 2017, 53, 529–535. [Google Scholar] [CrossRef]
- Alaoui, Y.; El Moudane, M.; Er-rafai, A.; Khachani, M.; Ghanimi, A.; Sabbar, A.; Tabyaoui, M.; Guenbour, A.; Bellaouchou, A. Structural study, thermal and physical properties of K2O-CaO-P2O5 phosphate glasses. Moroc. J. Chem. 2021, 9, 454–463. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, Y.J.; Geng, X.J.; Yang, Y.; Li, Z.Q.; Zuo, X.Y. Effect of heavy-doping Eu3+ and charge compensation on crystalline phase and luminescence properties of K2CaP2O7 phosphors emitting orange-red light. J. Chem. Sci. 2024, 136, 16. [Google Scholar] [CrossRef]
- Brown, E.H.; Lehr, J.R.; Frazier, A.W.; Smith, J.P. Fertilizer materials, calcium ammonium and calcium potassium pyrophosphate systems. J. Agric. Food Chem. 1964, 12, 70–73. [Google Scholar] [CrossRef]
- Xu, S.; Duan, J.; Dong, P.; Wang, M.; Zhang, Y.; Yu, J. Highly dispersed Eu2+ activated Ca10(PO4)6Cl2 phosphor with enhanced blue emitting through deposition-precipitation process. Opt. Mater. 2020, 110, 110529. [Google Scholar] [CrossRef]
- Kannan, S.; Rebelo, A.; Lemos, A.F.; Barba, A.; Ferreira, J.M.F. Synthesis and mechanical behaviour of chlorapatite and chlorapatite/β-TCP composites. J. Eur. Ceram. Soc. 2007, 27, 2287–2294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).