Abstract
Boron-dipyrromethene (BODIPY) has attracted extensive research attention in recent years due to its excellent photophysical properties, good chemical stability, and structural tunability, demonstrating broad application in fields such as fluorescence imaging, electroluminescence, biosensing and medical diagnostics. Researchers have extensively studied the synthesis, properties, and applications of BODIPY derivatives. This review summarizes five synthetic methods for the BODIPY core, with comparative analysis of their respective advantages, limitations and applicable scopes, aiming to provide valuable references for the future design and synthesis of BODIPY derivatives.
1. Introduction
Boron-dipyrromethene (BODIPY) fluorescent dyes are a class of organic heterocyclic compounds characterized by a boron-chelated structure and extended π-conjugation [1,2]. These compounds have garnered significant and sustained research interest due to their outstanding photophysical properties, which include narrow absorption and emission bands, high molar extinction coefficients (typically ε > 80,000 M−1·cm−1) and high fluorescence quantum yields (commonly ΦF > 0.50). In addition, BODIPYs exhibit remarkable chemical stability, showing negligible sensitivity to changes in solvent polarity and pH. Moreover, the structural versatility of the BODIPY core allows for systematic tuning of its optical and electronic characteristics through rational functionalization (Figure 1) [3,4,5,6].
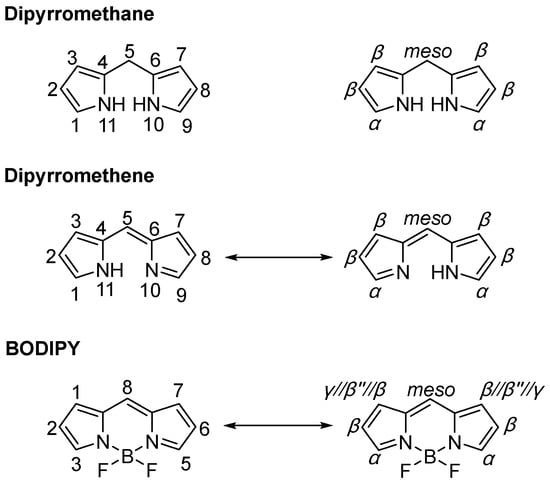
Figure 1.
Nomenclature conventions for BODIPY and two essential precursors (IUPAC left/Numbering right).
Since the pioneering synthesis by Treibs and Kreuzer in 1968 [7], systematic studies by Burgess [8], Ziessel [9,10] and colleagues have advanced the understanding of the fundamental chemical properties of BODIPY derivatives. Yang and coworkers [11] comprehensively reviewed functionalization methods at various reactive sites of BODIPY, whereas Bumagina and colleagues [12] focused on the primary approaches for structural modification of BODIPY derivatives. In recent years, BODIPY derivatives have demonstrated substantial application potential across diverse fields, including optoelectronic devices [13,14], biomedical engineering [15,16] and environmental monitoring [17,18].
The Liu group [19] reported a novel electron acceptor, NBN-2, based on a resonant N–B←N unit. By introducing a pentafluorophenyl group at its terminal, the performance of short-wave infrared organic photodetectors (SWIR OPDs) was significantly enhanced. This acceptor consists of four thiophene-fused BODIPY units. Through lowering the highest occupied molecular orbital energy level and increasing the electrostatic potential, the blend system of NBN-2 with the polymer donor PBDB-T achieved efficient exciton dissociation, with a charge transfer ratio of 85.8%. This study provides a new design strategy for developing high-performance narrow-bandgap organic optoelectronic materials. In a pioneering work, the teams of Wenhai Lin and Shengliang Li [20] developed a novel 808 nm light-responsive system for photoimmunotherapy of “cold” tumors via immunomodulator release. They designed a construct linking imiquimod (R837) to a NIR BODIPY photosensitizer with a reactive oxygen species (ROS) cleavable linker, allowing precise light-controlled drug release within tumors. In 2025, Liu’s research group [21] reported four color-tunable BODIPY-type fluorescent dyes (BDP-G, BDP-Y, BDP-O and BDP-R). By introducing different electron-donating groups into the BODIPY core via Knoevenagel condensation, emission spectra were tuned from green to red (516 nm to 653 nm), with absolute fluorescence quantum yields exceeding 50% for all dyes. This research provides a practical solution for high-contrast, rapid detection of latent fingerprints at crime scenes.
Nevertheless, the optimization of their performance and expansion of applications critically depend on the precision synthesis of the BODIPY core. This review systematically summarizes five synthetic approaches for BODIPY core, providing a comparative analysis of key parameters such as reaction conditions, yields and applicability. The aim is to offer practical guidance for researchers in selecting optimal synthetic strategies tailored to specific application requirements, thereby facilitating the rational design of BODIPY functional materials.
2. The Synthesis of BODIPY Core
2.1. The Synthesis of BODIPY Core from Pyrrole and Aldehyde Precursors
Dipyrromethene is a planar, fully conjugated, intensely colored, and stable basic bipyrrolic compound [22]. It can be easily obtained by oxidation of dipyrromethane. Since dipyrromethane is a key precursor in the synthesis of porphyrin analogs and BODIPY, growing interest in these compounds over the past few decades has driven the development of diverse synthetic methods for dipyrromethane and its analogs [23]. In particular, under acid-catalyzed condensation, protonation typically occurs at the 2-position of pyrrole, transforming it into an electrophile that can be attacked by another pyrrole molecule, resulting in the formation of “pyrrole trimers” [24]. This coupling process can undergo multiple cycles, generating mixtures of higher oligopyrromethanes. However, the occurrence of side reactions may lead to the formation of insoluble polymeric materials, making the isolation of distinct oligopyrromethane entities nearly impossible.
2.1.1. Method 1—The Synthesis of the BODIPY Core from Pyrrole and Aldehyde Precursors via Acid Catalysis
As early as 1968, Treibs and Kreuzer unexpectedly obtained the BODIPY core substituted with 2-acetyl and 2,6-diacetyl groups by reacting pyrrole with acetic anhydride under Boron trifluoride diethyl etherate (BF3·Et2O) conditions [7]. In 1994, Lindsey and colleagues published a seminal study on the formation of dipyrromethanes from aromatic aldehydes [25]. To enable the direct synthesis of dipyrromethanes while suppressing subsequent oligomerization, the pyrrole-aldehyde condensation was carried out with a substantial excess of pyrrole (approximately 40 equiv). In this optimized protocol, pyrrole fulfills dual roles, acting both as the excess reactant and as the reaction solvent, thereby facilitating the immediate formation of the desired dipyrromethane product. This approach, coupled with chromatographic purification, successfully afforded the target 5-substituted dipyrromethanes 2a–f in 49–76% yields (Figure 2). Their methodology has now become the standard procedure for preparing dipyrromethanes with various functional groups. Two years later, they further developed one of the most prevalent synthetic approaches for constructing the BODIPY core, a method that remains widely used to this day [26]. Lindsey’s method entails the acid-catalyzed condensation of an aldehyde, typically an aromatic aldehyde, with two equiv of pyrrole. The process primarily involves the formation of the corresponding dipyrromethane from α-substituted pyrrole (such as 2-methylpyrrole), followed by oxidation with p-chloranil to produce dipyrromethene. Finally, the desired 3,5-dimethyl-8-aryl-substituted BODIPY is obtained through boron chelation facilitated by amine compounds (Figure 3a). A critical and universal step in the formation of the BODIPY core is the chelation of the dipyrromethene ligand with a boron source. BF3·Et2O is the most commonly employed reagent for this purpose, typically used in the presence of a base (e.g., Et3N or DIPEA) to scavenge the generated HF and drive the reaction to completion [27,28,29,30]. For the synthesis of α-unsubstituted BODIPY compounds, the more potent oxidant 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) must be employed to achieve immediate and complete oxidation of the α-unsubstituted dipyrromethane intermediate (Figure 3b). Subsequently, the researchers enhanced the experimental procedure by excluding column chromatography [31] and adjusting the pyrrole-to-aldehyde ratio to 25:1, resulting in successful synthesis of compounds 2c–h with medium yields (Figure 2). They observed that 2,3′-bipyrrolylmethane 3 and tripyrrolylmethane 4, formed through acid-catalyzed condensation, could be eliminated by recrystallization and distillation, respectively. Trifluoroacetic acid (TFA) was identified as the optimal acid catalyst, streamlining purification and minimizing product loss. However, some dipyrromethanes with large substituents cannot be purified by this method.
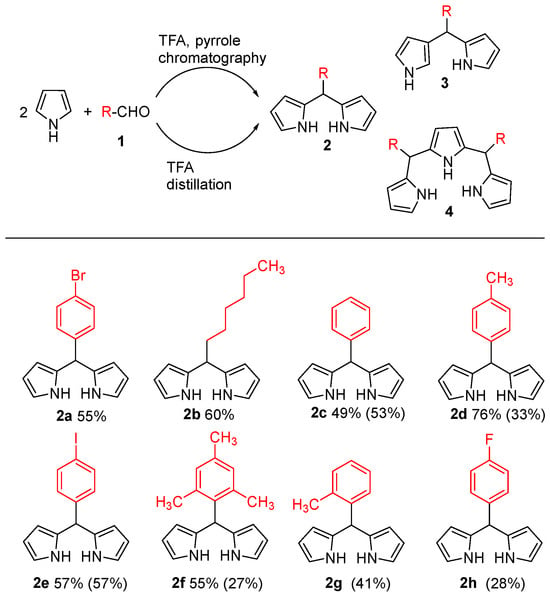
Figure 2.
Synthesis of dipyrromethanes condensed with pyrrole and aldehyde precursors developed by Lindsey et al. (Ref. [26] in parenthesized yields).
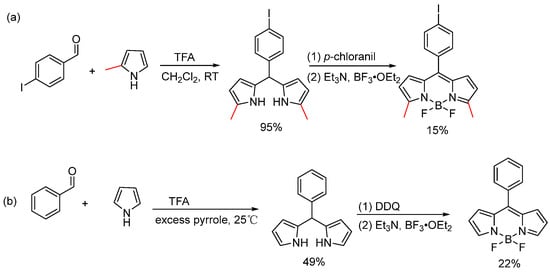
Figure 3.
Synthesis of (a) α-substituted and (b) α-unsubstituted BODIPY core.
With continuous efforts by researchers, the conditions and scope of acid-catalyzed synthesis of BODIPY cores have been progressively developed and expanded. Knut Rurack et al. [32] synthesized β,β-phenanthrene-fused BODIPYs using a one-pot method under acid-catalyzed conditions, enhancing their π-conjugated system (Figure 4a). Despite a propeller-like distortion in the crystal lattice, these dyes possess strong absorption (log ε ≥ 5), high fluorescence efficiency (Φf ≥ 0.8), and can be easily converted into light-up probes. Their incorporation into latex beads produces highly bright and photostable single-dye and Förster Resonance Energy Transfer (FRET) particles for advanced microscopy (Figure 4b). Expanding on this, in 2015, Lijuan Jiao’s team [33] proposed that the cyclohexadiene moiety in compound 9 could be oxidized, leading to the formation of α,β-benzene-fused BODIPY dimers 10a and 10b with the use of an appropriate oxidant. By employing HBr as a catalyst at 40 °C, they obtained α,β-cyclohexadiene-fused BODIPY dimers 9a and 9b, which were further converted to the final products using DDQ as the oxidant. Interestingly, at a higher complexation temperature of 90 °C, compound 7 reacted with compounds 8a and 8b in toluene to directly produce α,β-benzene-fused BODIPY dimers without the need for additional oxidation steps (Figure 5).
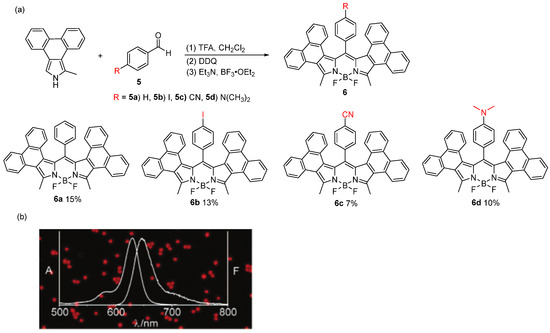
Figure 4.
(a) Synthesis of β,β-phenanthrene-fused BODIPYs. (b) FRET particles for advanced microscopy. Reprinted from Ref. [32] with permission; Copyright American Chemical Society 2008.
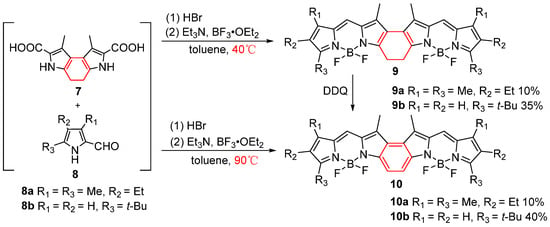
Figure 5.
Synthesis of α,β-benzene-fused BODIPY dimers.
Under acid-catalyzed conditions, not only symmetric BODIPY cores can be synthesized, but asymmetric BODIPY derivatives also can be obtained through the condensation reaction between carbonyl-containing pyrroles and α-unsubstituted pyrrole molecules catalyzed by phosphorus oxychloride (POCl3) [10,34]. Subsequently, coordination with BF3·Et2O in the presence of triethylamine yields the asymmetric BODIPY (Figure 6).
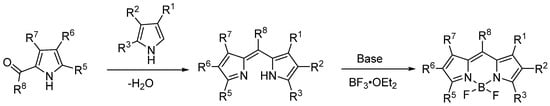
Figure 6.
Synthesis of asymmetric BODIPY core.
Based on previous studies, we suggest that although an excess of pyrrole can suppress the formation of byproducts and improve the yield of the target product, an additional column chromatography purification step may still reduce the final yield and complicate the experimental procedure. Therefore, the optimized general method for the acid-catalyzed synthesis of the BODIPY core involves reducing the relative amount of pyrrole, employing TFA as the acid catalyst, and utilizing recrystallization and distillation for purification. In this protocol, only one column chromatography step is required, thereby enhancing the overall yield (Figure 7).
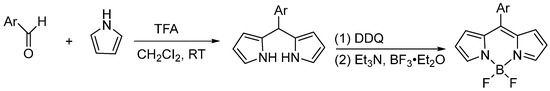
Figure 7.
Acid-catalyzed BODIPY parent synthesis of aldehydes and pyrroles.
2.1.2. Method 2—The Synthesis of the BODIPY Core from Pyrrole and Aldehyde Precursors in Water Solvent
Due to the issue of excessive pyrrole usage in many reported synthetic methods for the BODIPY core, further development of BODIPY core synthesis has been stimulated. Sobral et al. were the first research group to achieve efficient synthesis of dipyrromethanes in aqueous media [35]. By employing an appropriate ratio of pyrrole and aldehyde in the presence of hydrochloric acid, the corresponding high-purity dipyrromethane could be prepared in good yield through a one-step procedure. The efficacy of this approach hinges on the reaction between pyrrole and carbonyl compounds taking place at the interface of pyrrole and an acidic aqueous solution of aldehyde (or ketone). The ensuing dipyrromethane formation within the aqueous layer leads to its subsequent removal from this phase, thereby facilitating the completion of the reaction and safeguarding the product from additional reactions. Consequently, this mechanism minimizes the generation of byproducts like tripyrromethane. The remarkable success of this concept proved highly compelling, prompting Král and his colleagues to further elaborate on this methodology [36]. Using water as the solvent, they investigated the acid-catalyzed condensation of aldehydes or ketones with unsubstituted pyrrole. Their studies revealed that the reaction typically yielded a mixture of dipyrromethane and tripyrromethane, with the selectivity between these pyrrole oligomers being dependent on the nature of the carbonyl compound. Ultimately, they optimized the process by reducing the reaction temperature to 25 °C while increasing the pyrrole-to-aldehyde ratio to 6:1, thereby achieving both high yield and reasonable purity of the target dipyrromethane product. Building upon the experimental insights from these two preceding approaches, Dehaen and colleagues subsequently proposed novel reaction conditions [37]. By reducing the pyrrole-to-aldehyde ratio to 3:1 while simultaneously increasing the concentrations of both aldehyde and acid, they achieved remarkably selective formation of dipyrromethanes with surprising yields and high purity through precise control of reaction time (Figure 8 and Table 1). In 2009, Cozzi and Zoli proposed that aldehydes undergo oxidation to form carboxylic acids at 80 °C, which then catalyze the condensation reaction, followed by final purification through chromatography [38]. Consequently, they recommended using a substantial excess of pyrrole (25 equiv) while eliminating the need for inorganic acids. Under these optimized conditions, the reaction demonstrated broad substrate scope, accommodating various aromatic, heterocyclic, electron-deficient aldehydes as well as electron-rich and aliphatic aldehydes, all of which afforded the corresponding dipyrromethanes in moderate to good yields.
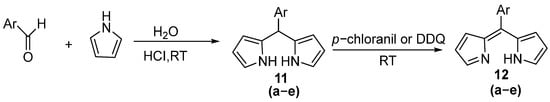
Figure 8.
Synthesis of dipyrromethanes using water as solvent by Dehaen et al.

Table 1.
Synthesis of aryldipyrromethenes.
In recent years, researchers have investigated the direct complexation of one half of the porphyrin macrocycle with BF3·Et2O to produce a π-extended BODIPY derivative (Ex-BODIPY). In 2021, Jiao and colleagues [39] successfully synthesized compound 14a with 10% yield by directly complexing BF3·Et2O under hydrochloric acid catalysis. This synthesis utilized minimal amounts of aldehyde and pyrrole in a mixed solvent system of H2O: MeOH (3:1) without the need for an oxidation step. Expanding on this research, the group substituted the aldehyde with 2,6-dichlorobenzaldehyde and carried out the reaction with pyrrole in a H2O/THF mixed solvent system, leading to the one-pot synthesis of compound 14b post-complexation (Figure 9). These π-extended BODIPYs were developed as fluorescent probes for the rapid and selective detection of GSH and successfully applied in live-cell imaging.
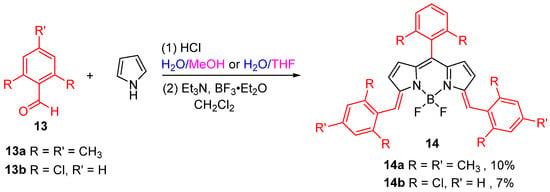
Figure 9.
Synthesis of Ex-BODIPYs.
Inspired by these experimental findings, water has been recognized as an ideal green solvent for organic reactions due to its non-toxicity, low cost and environmental friendliness. This aqueous-phase methodology not only effectively circumvents the issue of pyrrole excess but also affords target products in high yield and excellent purity, representing remarkably advantageous reaction conditions. Moreover, the incorporation of minimal amounts of co-solvents into the aqueous system enables one-pot synthesis of BODIPYs with extended π-conjugated systems. Although this approach demonstrates a broad substrate scope for various aldehydes, the current yields remain suboptimal when employing α-substituted pyrroles as starting materials. The aqueous-phase synthetic procedure for the BODIPY core is summarized in Figure 10. This synthetic approach involves the formation of a dipyrromethane via the condensation of an aldehyde with pyrrole in aqueous media, followed by its subsequent oxidation to the dipyrromethene using DDQ, and final chelation with boron in the presence of an amine to afford the target BODIPY derivative.
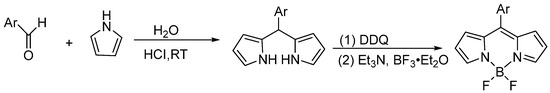
Figure 10.
The aqueous-phase synthetic procedure for the BODIPY core.
2.2. Method 3—The Synthesis of BODIPY Core Structures from Pyrrole and Thiophosgene
Symmetric BODIPY cores can be synthesized by condensing two pyrrole molecules with one thiophosgene molecule, resulting in a dipyrromethanethione intermediate. This intermediate is then alkylated and subjected to standard boron chelation to yield the corresponding 8-methylthio substituted BODIPY core. Biellmann et al. [40] first described this method, demonstrating the reaction of unsubstituted and 2-methyl/ethyl substituted pyrroles with thiophosgene to form thione derivatives without generating additional condensation byproducts. Subsequent methylation with iodomethane under standard conditions, followed by boron chelation, produced the 8-methylthio BODIPY derivatives in high yields (Figure 11).
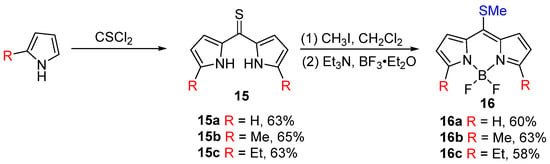
Figure 11.
Synthesis of 8-thiomethyl-substituted BODIPY developed by Biellmann.
Subsequently, some studies have revealed that these 8-methylthio substituted BODIPY cores serve as versatile synthetic intermediates. These cores can be modified into diverse BODIPY derivatives through nucleophilic substitution of the methylthio group and Liebeskind-Srogl cross-coupling (LSCC) reactions. The LSCC reaction represents a transition metal-catalyzed carbon–carbon bond-forming process, typically involving the coupling of π-deficient heterocyclic thioethers or thioesters with boronic acids in the presence of palladium catalysts and copper(I) salts [41,42]. The pioneering work by Peña-Cabrera et al. [43] showcased the successful utilization of LSCC reactions to synthesize functionalized BODIPY cores with high yields. Next, they further expanded on this methodology by demonstrating that treating 8-methylthio BODIPY with three equiv of boronic acid in the presence of tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3), tris(2-furyl)phosphine (TFP) and three equiv of copper(I) thiophene-2-carboxylate yielded the desired products in high yields [44,45]. Their systematic investigation confirmed that the electronic properties of arylboronic acids do not significantly affect either reaction time or product yield. The reaction proceeded efficiently with various electron-donating and electron-withdrawing substituents, including bulky groups, consistently producing the desired products in satisfactory yields. Notably, arylboronic acids containing reactive functional groups underwent smooth transformation without the need for protective group strategies (Figure 12a). Furthermore, they found that 8-methylthio-substituted BODIPY serves both in Pd-catalyzed LSCC reactions and as an effective leaving group in SNAr-like reactions. It reacts efficiently with amines, alcohols, and phenols to afford SNAr-like products, which often exhibit very high fluorescence quantum yields [46,47,48,49,50,51]. These products [52,53,54] are further applied in luminescent materials, including lasers and fluorescence sensors (Figure 12b).
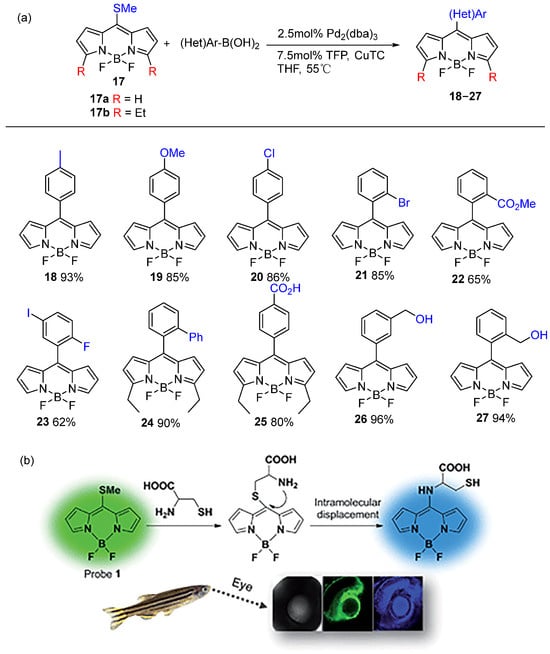
Figure 12.
(a) Synthesis of symmetric BODIPY core reported by Peña-Cabrera et al.; (b) selective detection of cysteine over homocysteine and glutathione by an BODIPY probe for ratiometric imaging in Zebrafish. Reprinted from Ref. [53] with permission; Copyright Royal Society of Chemistry 2015.
In view of the development of this method, it is deemed more suitable for synthesizing symmetric BODIPY precursors, leading to the production of high-quality target compounds. The synthesis commences with the condensation of two pyrrole molecules with one equiv of thiophosgene, yielding a dipyrromethanethione intermediate. This intermediate subsequently undergoes alkylation, followed by standard boron chelation, to afford the corresponding 8-methylthio-substituted BODIPY core. The resulting core can be further functionalized into diverse BODIPY analogues via nucleophilic substitution of the methylthio group or through LSCC reactions. Although this method provides a new synthetic idea for the BODIPY core, it is too cumbersome in terms of steps (Figure 13).
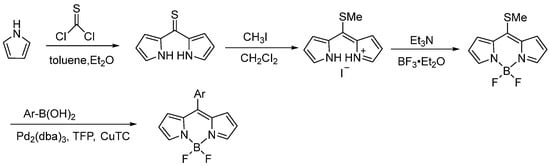
Figure 13.
Synthesis of BODIPY core from thiophosgene and pyrrole.
2.3. Method 4—Synthesis of the BODIPY Core via Transition Metal Catalysis and Organocatalysis
Traditional BODIPY synthesis relies on reactive carbonyl compounds, such as aldehydes or acyl chlorides, as starting materials. Due to the high sensitivity of these substrates to air and moisture, they must be freshly prepared or purified and require careful handling. Additionally, this approach suffers from several drawbacks, including pyrrole polymerization, incompatibility with acid-sensitive functional groups and overall low to moderate reaction yields.
In 2024, Bernhard Breit and colleagues [55] reported a highly efficient five-step reaction sequence for the synthesis of BODIPYs, employing alkenes or aryl bromides as stable and readily available starting materials. These precursors are initially converted into aldehyde intermediates through either rhodium-catalyzed hydroformylation or palladium-catalyzed formylation reactions. The carbonyl functionality of the resulting aldehydes is then selectively activated by a squaramide-based organocatalyst via hydrogen bonding, facilitating the subsequent reaction with pyrrole nucleophiles (Figure 14). This study demonstrated the superiority of this approach over traditional Lewis acid activation, offering improved yields and enhanced functional group tolerance. Notably, this methodology effectively suppresses common side reactions, such as pyrrole polymerization. The use of commercially accessible alkenes and aryl bromides significantly expands the scope of readily obtainable BODIPY derivatives, enabling precise structural tailoring for specific applications. The robustness and versatility of this method were further highlighted by the successful synthesis of 55 diverse derivatives, achieving overall yields of up to 78%. This work represents a significant advancement in BODIPY synthesis.
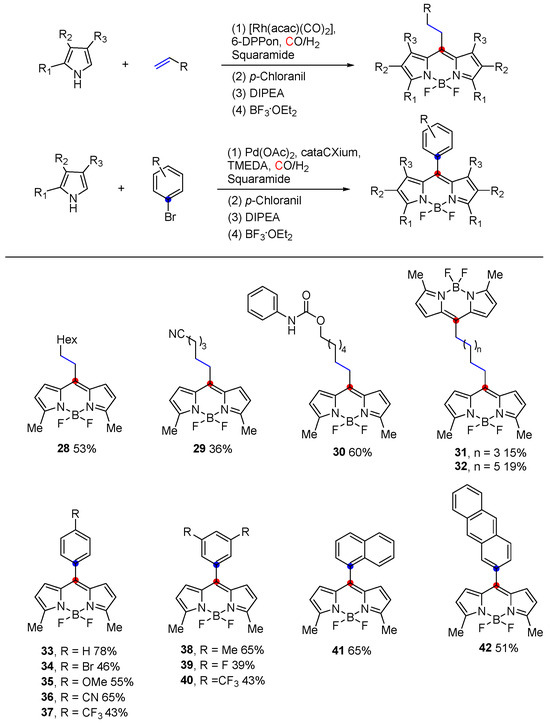
Figure 14.
Synthesis of the BODIPY core via transition metal catalysis and organocatalysis.
2.4. Method 5—Modular Enantioselective Assembly of Multi-Substituted Boron-Stereogenic BODIPYs
BODIPYs are some of the most popular and indispensable tetracoordinate boron compounds. The structural diversity of BODIPY derivatives is inherently extraordinary, because at least five sites of chiral BODIPY can be conveniently modified with different functional substituents. However, the vast potential of boron-stereogenic BODIPYs remains largely untapped due to the absence of general and efficient asymmetric synthetic methodologies. Currently, the synthesis of boron-stereogenic BODIPYs is still in its infancy, facing three major challenges: (1) limited structural diversity in existing boron-stereogenic BODIPYs; (2) a severe lack of efficient synthetic strategies for their construction; and (3) the long-standing difficulty in achieving enantiocontrol at the boron center, owing to the configurational instability of tetracoordinate boron compounds [56,57,58]. To address these challenges, He and colleagues [59] pioneered a modular synthetic approach starting from prochiral BODIPY cores, establishing an efficient route for the enantioselective assembly of boron-stereogenic BODIPYs. The key precursor, dipyrromethane, was readily synthesized from commercially available benzaldehyde and pyrrole via a hydrochloric acid-catalyzed condensation in aqueous media at room temperature, with the product easily isolated by filtration. Next, treatment of the dipyrromethane with N-chlorosuccinimide (NCS) and DDQ, followed by electrophilic borylation using potassium organotrifluoroborate salts, afforded prochiral BODIPY cores bearing two α C-Cl bonds in a scalable manner. With the prochiral BODIPY core in hand, the researchers then investigated the pivotal asymmetric Suzuki cross-coupling reaction, breaking its mirror symmetry via the enantiocontrolled introduction of an aromatic group on the BODIPY core (Figure 15). The strategy is characterized by its generality, modularity, excellent enantioselectivity, and scalability. The success of this method hinges on the palladium/phosphoramidite-catalyzed asymmetric Suzuki coupling, which allows for the selective differentiation of the two α C-Cl bonds in the designed prochiral BODIPY scaffold. This breakthrough not only expands the chemical space of chiral BODIPY dyes but also opens new avenues in chiral boron chemistry, providing a transformative strategy for the synthesis of asymmetric BODIPY cores.
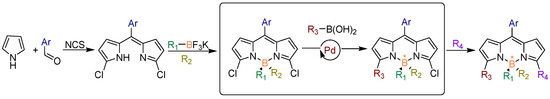
Figure 15.
The design plan for the modular enantioselective assembly of multi-substituted boron-stereogenic BODIPYs. “*” indicates a chiral atomic center.
2.5. Emerging Synthetic Strategies and Other Methods
In addition to the aforementioned synthetic methods for BODIPY core, a variety of novel synthetic strategies have emerged in recent years. These approaches not only feature milder and more environmentally friendly reaction conditions but also expand the structural diversity and functionality of BODIPYs. For instance, green biosynthesis utilizes microbial metabolites as pyrrole sources. As exemplified by Cuiyun Ma et al., prodigiosin derived from Serratia marcescens y2 was successfully used as a precursor to synthesize 3-pyrrolyl BODIPYs, avoiding the use of traditional toxic pyrrole reagents and demonstrating excellent biocompatibility and cell imaging performance [60]. Furthermore, strategies such as electrochemical synthesis and photocatalytic activation are gradually being introduced for the construction of BODIPYs and their dipyrromethane precursors. For example, electropolymerization on electrode surfaces can form BODIPY-functionalized polymers for sensing or optoelectronic materials [61]. Furthermore, Solvent-free or green catalytic systems, such as metal–organic framework (MOF)-supported iodine catalysts, copper nanoparticles (CuNPs), and functionalized ionic liquids, have also shown high efficiency and recyclability in the synthesis of dipyrromethanes, providing green precursors for subsequent BODIPY construction [62,63,64]. These methods not only enhance the atom economy and environmental friendliness of the synthesis but also directly influence the photophysical properties and bioapplication potential of BODIPYs by modulating the reaction pathway. For example, green-synthesized BODIPYs exhibit lower toxicity and deeper tissue penetration capability in live-cell imaging [60]. In the future, a tighter integration of synthetic strategies with downstream functionalities will promote the precise application of BODIPY materials in biomedicine and optoelectronics [65,66,67,68].
3. Advantages, Disadvantages and Limitations of the Five Methods
In summary, the five synthetic approaches for constructing the BODIPY core exhibit distinct advantages, limitations and applicable scopes (Table 2). Method 1 offers the benefit of shorter synthetic steps, but suffers from substantial byproduct formation and relatively low yields, even with excess pyrrole. In contrast, Method 2 emerges as a targeted “green” evolution, specifically designed to circumvent the excess pyrrole requirement of its predecessor by employing aqueous-phase chemistry. This key advancement allows for high-yielding and pure synthesis of α-unsubstituted BODIPYs, but it introduces a distinct limitation in its incompatibility with α-substituted pyrroles. Notably, growing evidence suggests that slight modifications of Methods 1 and 2 could enable one-step synthesis of BODIPY cores with extended π-conjugation systems. Method 3 provides broad substrate compatibility while maintaining high purity and yield, these advantages come at the expense of prolonged reaction steps and significantly increased time consumption. Method 4, despite its improved yields over Methods 1 and 2 through transition metal-catalyzed optimization, presents challenges including elevated reaction costs, operational complexity, though it does show broader applicability for α-substituted BODIPY derivatives.

Table 2.
Advantages, disadvantages and scope of application of the five methods.
For practical synthetic applications, the optimal method selection depends critically on the target BODIPY structure. When preparing α-unsubstituted BODIPY cores, Methods 2 and 3 should be prioritized as they provide both high yields and excellent product purity. For α-substituted derivatives, researchers may consider: (1) Method 1 for its minimal synthetic steps, (2) Method 3 for its compatibility with 2-ethylpyrrole, or (3) Method 4 for its broad substrate scope. If the product is required to bear functional groups that are incompatible with acidic conditions and are also susceptible to oxidation, Methods 3 and 4 need to be considered first. Method 5 is a novel method concerning the construction of chiral BODIPY matrices, which can be chosen when considering the synthesis of asymmetric BODIPY matrices.
4. Conclusions
BODIPY fluorophores have become indispensable members of the organic fluorescent dye family owing to their outstanding photophysical properties, high stability, and structural versatility. Beyond their established role in fluorescence imaging, sensing, and optoelectronics, their properties can be finely tuned through diverse synthetic transformations such as halogenation, Knoevenagel condensation, nucleophilic substitution, and acylation. Despite the continuous emergence of new BODIPY derivatives and modification strategies, the choice of appropriate precursor synthesis remains a fundamental step for successful molecular design.
In this review, we have summarized five representative synthetic methods for constructing the BODIPY core, outlining their respective advantages, limitations, and application scopes (Figure 16). This comparative analysis is intended to serve as a methodological guide for researchers aiming to design and prepare BODIPY derivatives more efficiently. Our work aims to provide an introductory and systematic guide for researchers or non-expert readers entering the field of BODIPY.
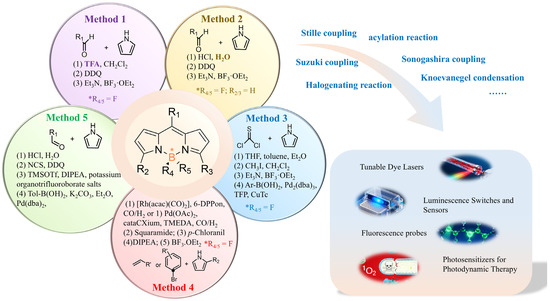
Figure 16.
The summary schematic for diverse synthesis strategies of the BODIPY core and their functional applications. “*” indicates a chiral atomic center.
Looking forward, future progress will likely focus on improving the sustainability and scalability of BODIPY synthesis, expanding compatibility with challenging substrates (e.g., α-substituted pyrroles and electron-rich aromatics), and integrating green chemistry approaches such as aqueous-phase reactions. Furthermore, the development of novel functionalization strategies is expected to open new avenues in advanced materials, bioimaging, photodynamic therapy, and molecular electronics. Although challenges remain—particularly in translating BODIPY chemistry into life sciences—continued innovation in synthetic methodology and molecular engineering will undoubtedly broaden the scope of BODIPY applications, cementing their role as versatile tools in chemistry, biology, and materials science [69,70,71,72,73,74].
Author Contributions
Conceptualization, R.Y. and H.G.; investigation, methodology and data curation, R.Y., H.G., J.J., T.Z., L.H., Y.Z. and L.T.; writing—original draft preparation, R.Y.; writing—review, editing and supervision, J.W. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was support by the National Natural Science Foundation of China (22065019), the Program for Young and Middle-Aged Academic and Technical Leaders Reserve Talents of Yunnan Province (202105AC160043), the Kunming “Spring City Program” for Youth Top-Notch Talents (C202014001), the Scientific Research Funds of Kunming University (XPZJ2205), and Frontier Research Team of Kunming University 2023.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank the institutions and funding agencies that supported this work, as well as the laboratories and departments that provided infrastructure and access to scientific resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qin, Y.; Liu, X.; Jia, P.P.; Xu, L.; Yang, H.B. BODIPY-based macrocycles. Chem. Soc. Rev. 2020, 49, 5678–5703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Jiao, L.J.; Hao, E. BODIPY-based photocages: Rational design and their biomedical application. Chem. Commun. 2024, 60, 5770–5789. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Q.; Zhang, J.J.; Hong, Z.Y.; Qiu, H.Y.; Li, Y.; Yin, S.C. Architectures and Applications of BODIPY-Based Conjugated Polymers. Polymers 2021, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Liu, J.; Sun, Y.Q.; Liu, M.X.; Guo, W. Carbon-Dipyrromethenes: Bright Cationic Fluorescent Dyes and Potential Application in Revealing Cellular Trafficking of Mitochondrial Glutathione Conjugates. J. Am. Chem. Soc. 2020, 142, 17069–17078. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.D.; Ma, S.Y.; She, M.Y.; Chen, J.; Wang, Z.H.; Liu, P.; Zhang, S.Y.; Li, J.L. Structural modification of BODIPY: Improve its applicability. Chin. Chem. Lett. 2019, 30, 1815–1824. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Treibs, A.; Kreuzer, F.H. Difluorboryl-komplexe von di-und tripyrrylmethenen. Justus Liebigs Ann. Der Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Liu, B.K.; Teng, K.X.; Niu, L.Y.; Yang, Q.Z. Progress in the Synthesis of Boron Dipyrromethene (BODIPY) Fluorescent Dyes. Chin. J. Org. Chem. 2022, 42, 1265–1285. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V.; Ksenofontov, A.A.; Antina, L.A.; Kalyagin, A.A.; Berezin, M.B. Basic structural modifications for improving the practical properties of BODIPY. Coord. Chem. Rev. 2022, 469, 52. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, J.H.; Miao, J.H.; Liu, J.; Wang, L.X. Using bodipy unit to design molecular acceptors with absorption wavelength of > 1000 nm for organic photodetectors. CCS Chem. 2024, 6, 2794–2803. [Google Scholar] [CrossRef]
- Li, P.F.; Wang, Y.; Chai, Z.Y.; Shankar, S.S.; Liang, X.; Xu, H.J.; Sharma, G.D. A new BODIPY dimer containing carbazole group as a small molecule donor for ternary organic solar cells with the PCE up to 14.97%. Dye. Pigment. 2023, 215, 111297. [Google Scholar] [CrossRef]
- Liu, Y.C.; Feng, G.L.; Jie, J.L.; Zhou, W.; Liu, G.J.; Zhang, Y.; Su, H.M.; Xing, G.W. Hepatoma Metastasis-Inhibiting Supramolecular Nanoglycocalyx for Enhanced Type I Photodynamic Therapy. Adv. Healthc. Mater. 2025, 14, 2404253. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Chen, D.D.; Zhu, Y.R.; Zhang, M.; Zhao, H.J. Kinsenoside Suppresses DGAT1-Mediated Lipid Droplet Formation to Trigger Ferroptosis in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 2322. [Google Scholar] [CrossRef]
- Saraf, P.; Shrivastava, P.; Sarkar, M.; Kumar, D. A Facile and Efficient Synthesis of BODIPY-Based Fluorescent Probes for Selective Detection of Hydrazine. Chem.-Asian J. 2025, 20, e202401197. [Google Scholar] [CrossRef] [PubMed]
- Cugnasca, B.S.; Duarte, F.; Santos, H.M.; Capelo-Martínez, J.L.; Bértolo, E.; Dos Santos, A.A.; Lodeiro, C. Ammonia and temperature sensing applications using fluorometric and colorimetric microparticles and polymeric films doped with BODIPY-emitters. Microchim. Acta 2024, 191, 746. [Google Scholar] [CrossRef]
- Liu, M.Y.; Li, W.R.; Peng, Z.X.; Shao, X.X.; Liu, J.; Wang, L.X. Electron Acceptors Based on Resonant N―B←N Unit with Improved Exciton Dissociation for High-Performance Short-Wavelength Infrared Organic Photodetectors. Angew. Chem.-Int. Ed. 2025, 64, e202506116. [Google Scholar] [CrossRef]
- Shen, J.P.; Xu, B.; Zheng, Y.; Zhao, X.Y.; Qi, H.X.; Tang, Y.G.; Lin, W.H.; Li, S.L.; Zhong, Z.Y. Near-Infrared Light-Responsive Immunomodulator Prodrugs Rejuvenating Immune Microenvironment for “Cold” Tumor Photoimmunotherapy. Angew. Chem.-Int. Ed. 2025, 64, e202425309. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Liao, H.T.; Guo, T.Y.; Jiang, W.J.; Liu, R.; Zhu, H.J. High-resolution visualization of level 3 details for latent fingerprints with color-tunable BODIPY dyes. Sens. Actuators B-Chem. 2025, 433, 137518. [Google Scholar] [CrossRef]
- Bruckner, C.; Karunaratne, V.; Rettig, S.J.; Dolphin, D. Synthesis of meso-phenyl-4,6-dipyrrins, preparation of their Cu(II), Ni(II), and Zn(II) chelates, and structural characterization of bis meso-phenyl-4,6-dipyrrinato Ni(II). Can. J. Chem.-Rev. Can. Chim. 1996, 74, 2182–2193. [Google Scholar] [CrossRef]
- Gryko, D.T.; Gryko, D.; Lee, C.H. 5-Substituted dipyrranes: Synthesis and reactivity. Chem. Soc. Rev. 2012, 41, 3780–3789. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.F. The acid-catalyzed polymerization of pyrroles and indoles. Adv. Heterocycl. Chem. 1963, 2, 287–309. [Google Scholar] [CrossRef]
- Lee, C.H.; Lindsey, J.S. One-flask synthesis of meso-substituted dipyrromethanes and their application in the synthesis of trans-substituted porphyrin building blocks. Tetrahedron 1994, 50, 11427–11440. [Google Scholar] [CrossRef]
- Wagner, R.W.; Lindsey, J.S. Boron-dipyrromethene dyes for incorporation in synthetic multi-pigment light-harvesting arrays. Pure Appl. Chem. 1996, 68, 1373–1380. [Google Scholar] [CrossRef]
- Zhong, G.J.; Yang, X.F.; Zhuang, J.Q.; Zhang, Y.F.; Yang, Y.; Wang, Y.; Yao, H.; Wang, C.Y.; Chong, H. A Platinum Complex Containing a BODIPY-Based Photosensitizer, Preparation Method, and Application. CN202411654477.1, 18 February 2025. (In Chinese). [Google Scholar]
- Liu, J.; Liu, J.; Li, H.; Bin, Z.; You, J. Boron-dipyrromethene-based fluorescent emitters enable high-performance narrowband red organic light-emitting diodes. Angew. Chem.-Int. Ed. 2023, 62, e202306471. [Google Scholar] [CrossRef]
- Dutta, D.; Nair, R.R.; Neog, K.; Nair, S.A.; Gogoi, P. Mitochondria-targeted biotin-conjugated BODIPYs for cancer imaging and therapy. RSC Med. Chem. 2023, 14, 2358–2364. [Google Scholar] [CrossRef]
- Khan, T.K.; Pissurlenkar, R.R.S.; Shaikh, M.S.; Ravikanth, M. Synthesis and studies of covalently linked meso-furyl boron-dipyrromethene-ferrocene conjugates. J. Organomet. Chem. 2012, 697, 65–73. [Google Scholar] [CrossRef]
- Littler, B.J.; Miller, M.A.; Hung, C.H.; Wagner, R.W.; O’Shea, D.F.; Boyle, P.D.; Lindsey, J.S. Refined synthesis of 5-substituted dipyrromethanes. J. Org. Chem. 1999, 64, 1391–1396. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Xu, H.J.; Xue, Z.L.; Hoffmann, K.; Shen, Z.; Weller, M.G.; You, X.Z.; Rurack, K. Phenanthrene-fused boron-dipyrromethenes as bright long-wavelength fluorophores. Org. Lett. 2008, 10, 1581–1584. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Q.H.; Wang, S.W.; Yu, C.J.; Li, J.; Hao, E.H.; Wei, Y.; Mu, X.L.; Jiao, L.J. Conformation-Restricted Partially and Fully Fused BODIPY Dimers as Highly Stable Near-Infrared Fluorescent Dyes. Org. Lett. 2015, 17, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos-Prieto, J.; Agarrabeitia, A.R.; Garcia-Moreno, I.; Lopez-Arbeloa, I.; Costela, A.; Infantes, L.; Perez-Ojeda, M.E.; Palacios-Cuesta, M.; Ortiz, M.J. Controlling Optical Properties and Function of BODIPY by Using Asymmetric Substitution Effects. Chem.-A Eur. J. 2010, 16, 14094–14105. [Google Scholar] [CrossRef] [PubMed]
- Sobral, A.; Rebanda, N.; da Silva, M.; Lampreia, S.H.; Silva, M.R.; Beja, A.M.; Paixao, J.A.; Gonsalves, A. One-step synthesis of dipyrromethanes in water. Tetrahedron Lett. 2003, 44, 3971–3973. [Google Scholar] [CrossRef]
- Král, V.; Vasek, P.; Dolensky, B. Green chemistry for preparation of oligopyrrole macrocycles precursors: Novel methodology for dipyrromethanes and tripyrromethanes synthesis in water. Collect. Czechoslov. Chem. Commun. 2004, 69, 1126–1136. [Google Scholar] [CrossRef]
- Rohand, T.; Dolušić, E.; Ngo, T.H.; Maes, W.; Dehaen, W. Efficient synthesis of aryldipyrromethanes in water and their application in the synthesis of corroles and dipyrromethenes. Arkivoc 2007, 10, 307–324. [Google Scholar] [CrossRef]
- Zoli, L.; Cozzi, P.G. Electrophilic Activation of Aldehydes “On Water”: A Facile Route to Dipyrromethanes. Chemsuschem 2009, 2, 218–220. [Google Scholar] [CrossRef]
- Li, W.W.; Gong, Q.B.; Guo, X.; Wu, Q.H.; Chang, F.; Wang, H.; Zhang, F.; Hao, E.H.; Jiao, L.J. Synthesis, Reactivity, and Properties of a Class of π-Extended BODIPY Derivatives. J. Org. Chem. 2021, 86, 17110–17118. [Google Scholar] [CrossRef]
- Goud, T.V.; Tutar, A.; Biellmann, J.F. Synthesis of 8-heteroatom-substituted 4,4-difluoro-4-bora-3a, 4a-diaza-s-indacene dyes (BODIPY). Tetrahedron 2006, 62, 5084–5091. [Google Scholar] [CrossRef]
- Liebeskind, L.S.; Srogl, J. Thiol ester-boronic acid coupling. A mechanistically unprecedented and general ketone synthesis. J. Am. Chem. Soc. 2000, 122, 11260–11261. [Google Scholar] [CrossRef]
- Liebeskind, L.S.; Srogl, J. Heteroaromatic thioether-boronic acid cross-coupling under neutral reaction conditions. Org. Lett. 2002, 4, 979–981. [Google Scholar] [CrossRef]
- Peña-Cabrera, E.; Aguilar-Aguilar, A.; González-Domínguez, M.; Lager, E.; Zamudio-Vázquez, R.; Godoy-Vargas, J.; Villanueva-García, F. Simple, general, and efficient synthesis of meso-substituted borondipyrromethenes from a single platform. Org. Lett. 2007, 9, 3985–3988. [Google Scholar] [CrossRef]
- Betancourt-Mendiola, L.; Valois-Escamilla, I.; Arbeloa, T.; Bañuelos, J.; Arbeloa, I.L.; Flores-Rizo, J.O.; Hu, R.; Lager, E.; Gómez-Durán, C.F.A.; Belmonte-Vázquez, J.L.; et al. Scope and Limitations of the Liebeskind-Srogl Cross-Coupling Reactions Involving the Biellmann BODIPY. J. Org. Chem. 2015, 80, 5771–5782. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, I.J.; Hu, R.; Tang, B.Z.; López, F.I.; Peña-Cabrera, E. 8-Alkenylborondipyrromethene dyes. General synthesis, optical properties, and preliminary study of their reactivity. Tetrahedron 2011, 67, 7244–7250. [Google Scholar] [CrossRef]
- Gómez-Durán, C.F.A.; García-Moreno, I.; Costela, A.; Martin, V.; Sastre, R.; Bañuelos, J.; Arbeloa, F.L.; Arbeloa, I.L.; Peñ-Cabrera, E. 8-PropargylaminoBODIPY: Unprecedented blue-emitting pyrromethene dye. Synthesis, photophysics and laser properties. Chem. Commun. 2010, 46, 5103–5105. [Google Scholar] [CrossRef] [PubMed]
- Esnal, I.; Urís-Benavides, A.; Gómez-Durán, C.F.A.; Osorio-Martínez, C.A.; García-Moreno, I.; Costela, A.; Bañuelos, J.; Epelde, N.; Arbeloa, I.L.; Hu, R.; et al. Reaction of Amines with 8-MethylthioBODIPY: Dramatic Optical and Laser Response to Amine Substitution. Chem.-Asian J. 2013, 8, 2691–2700. [Google Scholar] [CrossRef]
- Flores-Rizo, J.O.; Esnal, I.; Osorio-Martínez, C.A.; Gómez-Durán, C.F.A.; Bañuelos, J.; Arbeloa, I.L.; Pannell, K.H.; Metta-Magaña, A.J.; Peña-Cabrera, E. 8-Alkoxy- and 8-Aryloxy-BODIPYs: Straightforward Fluorescent Tagging of Alcohols and Phenols. J. Org. Chem. 2013, 78, 5867–5877. [Google Scholar] [CrossRef]
- Gutérrez-Ramos, B.D.; Bañuelos, J.; Arbeloa, T.; Arbeloa, I.L.; González-Navarro, P.E.; Wrobel, K.; Cerdán, L.; García-Moreno, I.; Costela, A.; Peñ-Cabrera, E. Straightforward Synthetic Protocol for the Introduction of Stabilized C Nucleophiles in the BODIPY Core for Advanced Sensing and Photonic Applications. Chem.-A Eur. J. 2015, 21, 1755–1764. [Google Scholar] [CrossRef]
- Gómez-Infante, A.d.J.; Bañuelos, J.; Valois-Escamilla, I.; Cruz-Cruz, D.; Prieto-Montero, R.; López-Arbeloa, I.; Arbeloa, T.; Peña-Cabrera, E. Synthesis, Properties, and Functionalization of Nonsymmetric 8-MethylthioBODIPYs. Eur. J. Org. Chem. 2016, 29, 5009–5023. [Google Scholar] [CrossRef]
- Gómez-Durán, C.F.A.; Esnal, I.; Valois-Escamilla, I.; Urías-Benavides, A.; Bañuelos, J.; Arbeloa, I.L.; García-Moreno, I.; Peñ-Cabrera, E. Near-IR BODIPY Dyes à la Carte—Programmed Orthogonal Functionalization of Rationally Designed Building Blocks. Chem.-A Eur. J. 2016, 22, 1048–1061. [Google Scholar] [CrossRef]
- Kim, D.; Yamamoto, K.; Ahn, K.H. A BODIPY-based reactive probe for ratiometric fluorescence sensing of mercury ions. Tetrahedron 2012, 68, 5279–5282. [Google Scholar] [CrossRef]
- Ma, D.H.; Kim, D.; Seo, E.; Lee, S.-J.; Ahn, K.H. Ratiometric fluorescence detection of cysteine and homocysteine with a BODIPY dye by mimicking the native chemical ligation. Analyst 2015, 140, 422–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Treich, N.R.; Wimpenny, J.D.; Kieffer, I.A.; Heiden, Z.M. Synthesis and characterization of chiral and achiral diamines containing one or two BODIPY molecules. New J. Chem. 2017, 41, 14370–14378. [Google Scholar] [CrossRef]
- Miller, L.; Impelmann, A.; Bauer, F.; Breit, B. Carbonylation as a Key Step in New Tandem Reactions—A Route to BODIPYs. Chem.-A Eur. J. 2024, 30, e202303752. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Nyokong, T.; Kobayashi, N.; Shen, Z. Optically active BODIPYs. Coord. Chem. Rev. 2016, 318, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Song, Q.L. Recent advances in the construction of tetracoordinate boron compounds. Chem. Commun. 2023, 59, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Abdou-Mohamed, A.; Aupic, C.; Fournet, C.; Parrain, J.L.; Chouraqui, G.; Chuzel, O. Stereoselective formation of boron-stereogenic organoboron derivatives. Chem. Soc. Rev. 2023, 52, 4381–4391. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.Q.; Zhan, B.Q.; Zhao, J.Y.; Guo, Y.H.; Zu, B.; Li, Y.Z.; He, C. Modular enantioselective assembly of multi-substituted boron-stereogenic BODIPYs. Nat. Chem. 2025, 17, 83–91. [Google Scholar] [CrossRef]
- Ma, C.; Luo, H.; Zhang, F.; Guo, D.; Chen, S.; Wang, F. Green biosynthesis, photophysical properties and application of 3-pyrrolyl BODIPY. Chin. J. Org. Chem. 2024, 44, 216–223. [Google Scholar] [CrossRef]
- Walesa-Chorab, M.; Banasz, R.; Kubicki, M.; Patroniak, V. Dipyrromethane functionalized monomers as precursors of electrochromic polymers. Electrochim. Acta 2017, 258, 571–581. [Google Scholar] [CrossRef]
- Rangaraj, P.; Parshamoni, S.; Konar, S. MOF as a syringe pump for the controlled release of iodine catalyst in the synthesis of meso-thienyl dipyrromethanes. Chem. Commun. 2015, 51, 15526–15529. [Google Scholar] [CrossRef] [PubMed]
- Megarajan, S.; Ahmed, K.B.A.; Rajmohan, R.; Vairaprakash, P.; Anbazhagan, V. An easily accessible and recyclable copper nanoparticle catalyst for the solvent-free synthesis of dipyrromethanes and aromatic amines. Rsc Adv. 2016, 6, 103065–103071. [Google Scholar] [CrossRef]
- Senapak, W.; Saeeng, R.; Jaratjaroonphong, J.; Kasemsuk, T.; Sirion, U. Green synthesis of dipyrromethanes in aqueous media catalyzed by SO3H-functionalized ionic liquid. Org. Biomol. Chem. 2016, 14, 1302–1310. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cheng, C.; Kang, Z.X.; Miao, W.; Liu, Q.Y.; Wang, H.; Hao, E.H. Organotrifluoroborate salts as complexation reagents for synthesizing BODIPY dyes containing both fluoride and an organo substituent at the boron center. J. Org. Chem. 2019, 84, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.F.O.; Lopes, S.M.M.; Pineiro, M.; Melo, T. Current advances in the synthesis of valuable dipyrromethane scaffolds: Classic and new methods. Molecules 2019, 24, 4348. [Google Scholar] [CrossRef] [PubMed]
- Gopala, L.; Yan, Y.J.; Chen, Z.L. Detail synthetic study of infrared fluorescent dyes: Design, synthesis and chemical properties of their photodynamic therapy probes. Chem. Rev. Lett. 2022, 5, 12–67. [Google Scholar] [CrossRef]
- Krasnopyorov, A.I.; Larkina, E.A. Features of F2-BODIPY synthesis (a review). Russ. J. Org. Chem. 2024, 60, 789–805. [Google Scholar] [CrossRef]
- Clarke, R.G.; Hall, M.J. Recent developments in the synthesis of the BODIPY dyes. Adv. Heterocycl. Chem. 2019, 128, 181–261. [Google Scholar] [CrossRef]
- Wu, S.M.; Gai, L.Z.; Zhou, Z.K.; Lu, H. Recent advances in zig-zag-fused BODIPYs. Org. Chem. Front. 2022, 9, 5989–6000. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Guo, X.; Kang, Z.X.; Wu, Q.H.; Li, H.; Cheng, C.; Yu, C.J.; Jiao, L.J.; Hao, E.R. Aryl-Boron-Substituted BODIPYs: Direct Access via Aluminum-Chloride-Mediated Arylation from Arylstannanes and Tuning the Optoelectronic Properties. Org. Lett. 2023, 25, 744–749. [Google Scholar] [CrossRef]
- He, L.M.; Li, L.; Zhao, M.Y.; Zhao, Y.X.; Li, Y.Q.; Li, X.G.; Yang, Y.H.; Gao, S.L.; Lei, P.; Wang, Z.H.; et al. Dibenzothieno and dibenzothieno [2,3-d] thieno [a]-fused BODIPYs: Synthesis, unique structure and photophysical properties. Mater. Chem. Front. 2024, 8, 3266–3271. [Google Scholar] [CrossRef]
- Li, J.; Xue, N.; Gao, S.L.; Yang, Y.H.; Weng, Z.H.; Ju, H.D.; Wang, Z.H.; Li, X.G.; Jiang, W. Dithio-Fused Boron Dipyrromethenes: Synthesis and Impact of S-Heteroaromatic Annulation Mode. Org. Lett. 2024, 26, 5472–5477. [Google Scholar] [CrossRef]
- Meng, Y.D.; Fang, W.; Pei, Z.H.; Chen, W.H.; Ding, S.Y.; Shen, M.L.; Bu, Y.C.; Yao, C.Z.; Li, Q.K.; Yu, J.; et al. Organocatalyzed Enantioselective C-N Bond-Forming SNAr Reactions for Synthesizing Stereogenic-at-Boron BODIPYs. Jacs Au 2025, 5, 1965–1973. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).