Al-Mg-Zn(-Cu) Cross-Over Alloys: The New Frontier in High-Strength and Radiation-Resistant Lightweight Materials
Abstract
:1. Introduction
2. AlMgCu Crossover Alloys
3. AlMgZn Crossover Alloys
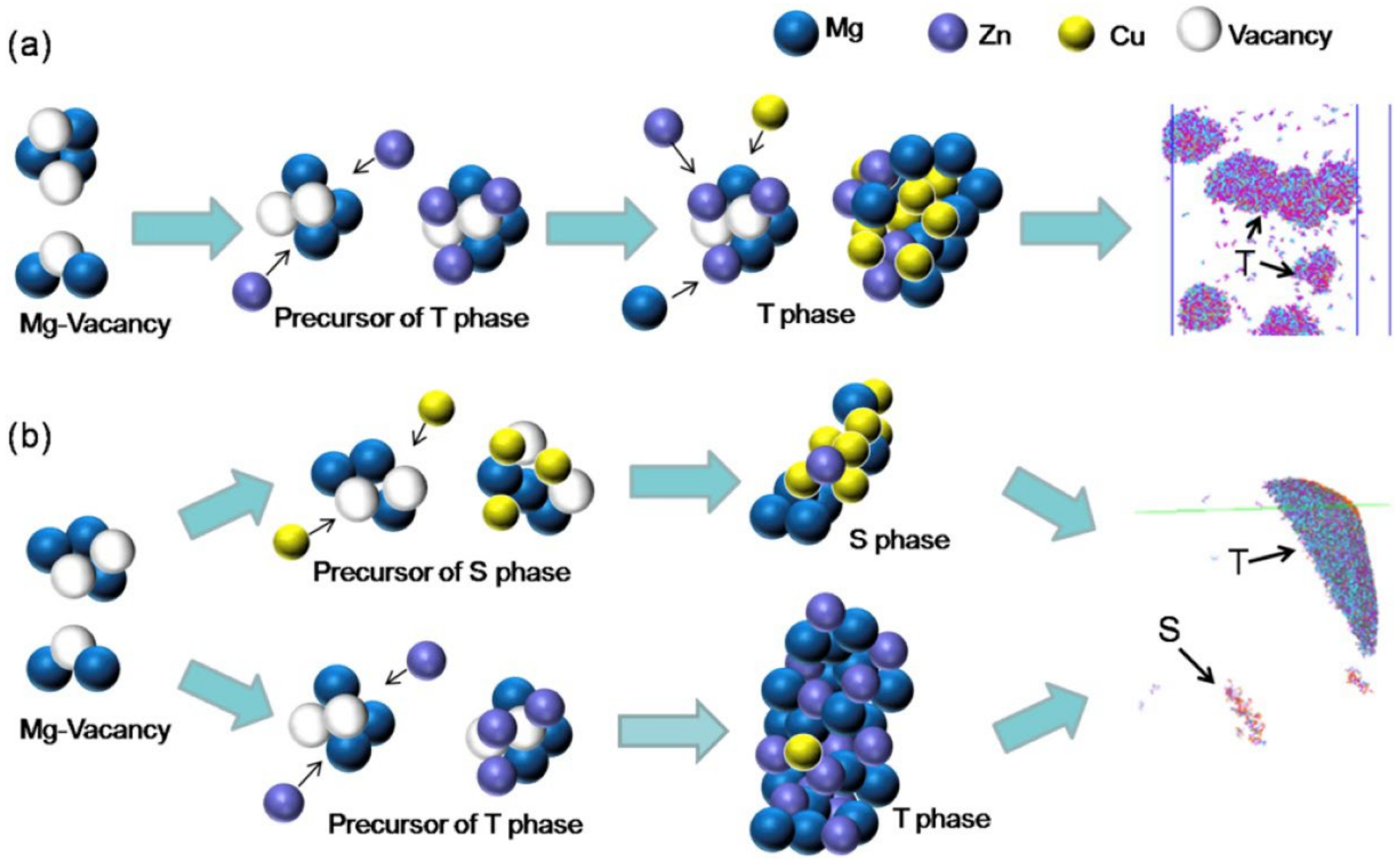
4. AlMgZnCu Crossover Alloys
5. Next-Generation Radiation Resistant Al-Based Crossover Alloys
6. Discussion and Conclusions
- (1)
- Crossover alloys containing Al, Mg, and Cu can reduce or offset the decrease in strength that occurs when cold-deformed AlMg alloys are baked with paint. The extent of compensation depends significantly on the degree of deformation and processing used.
- (2)
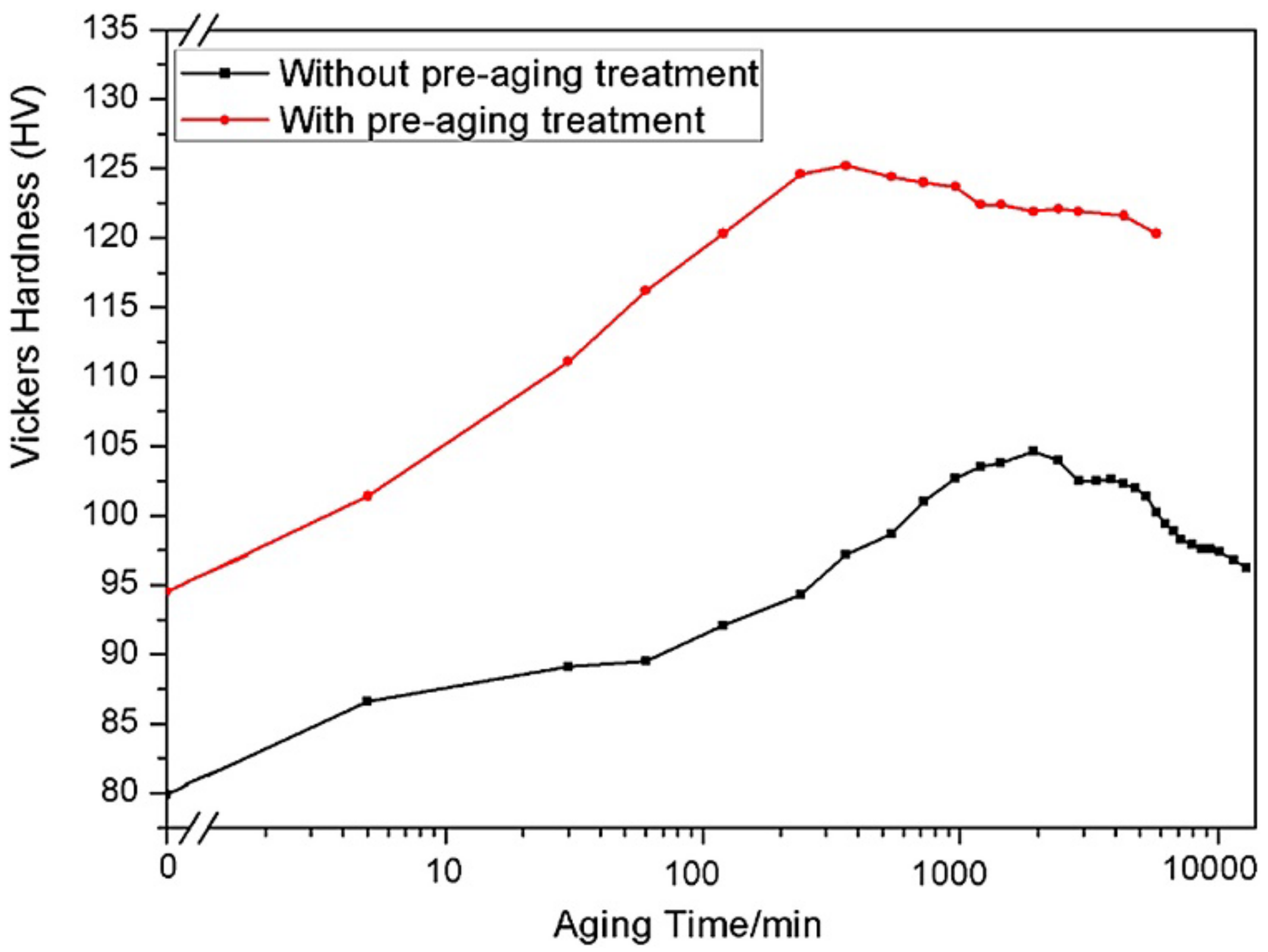
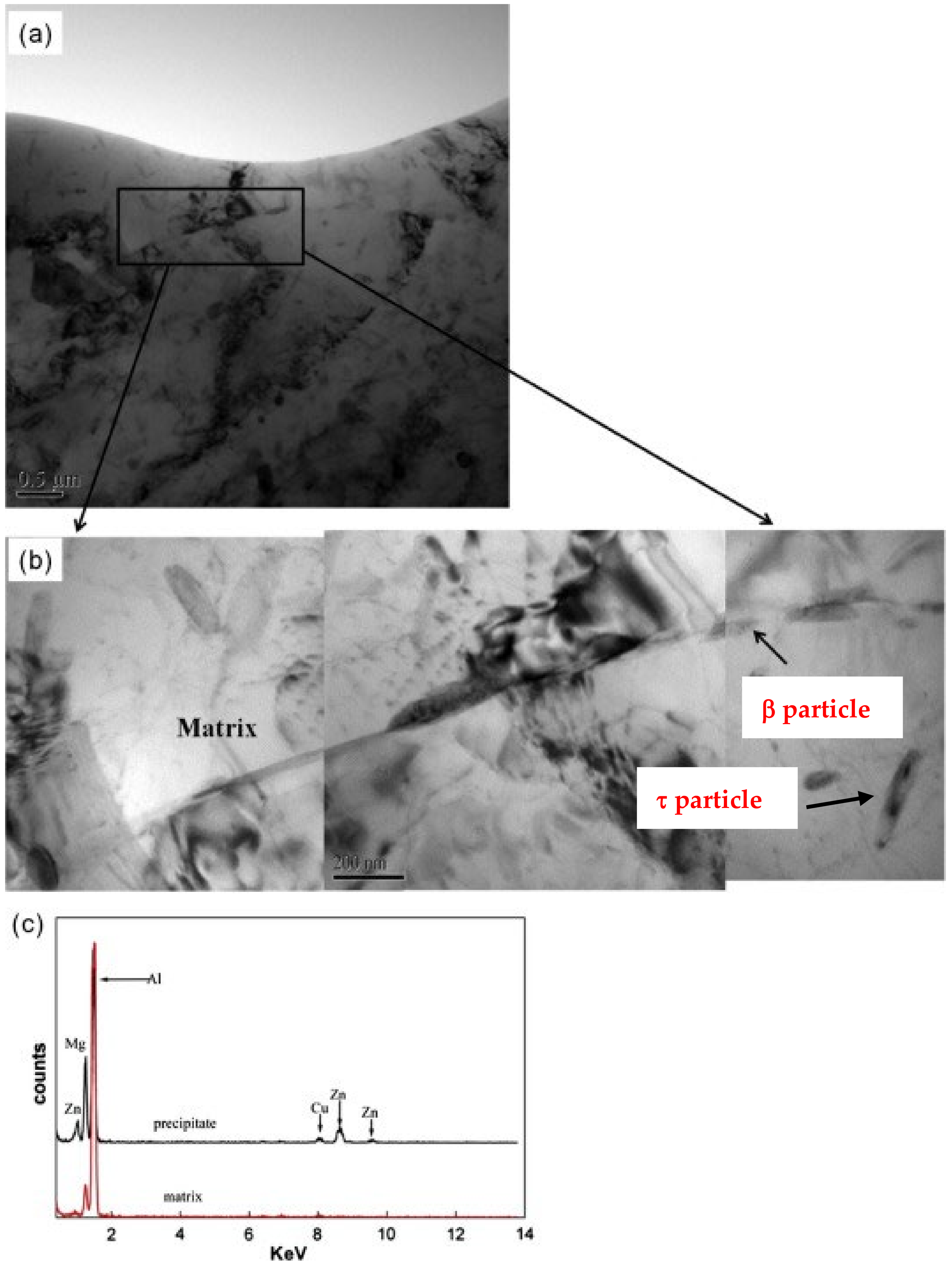
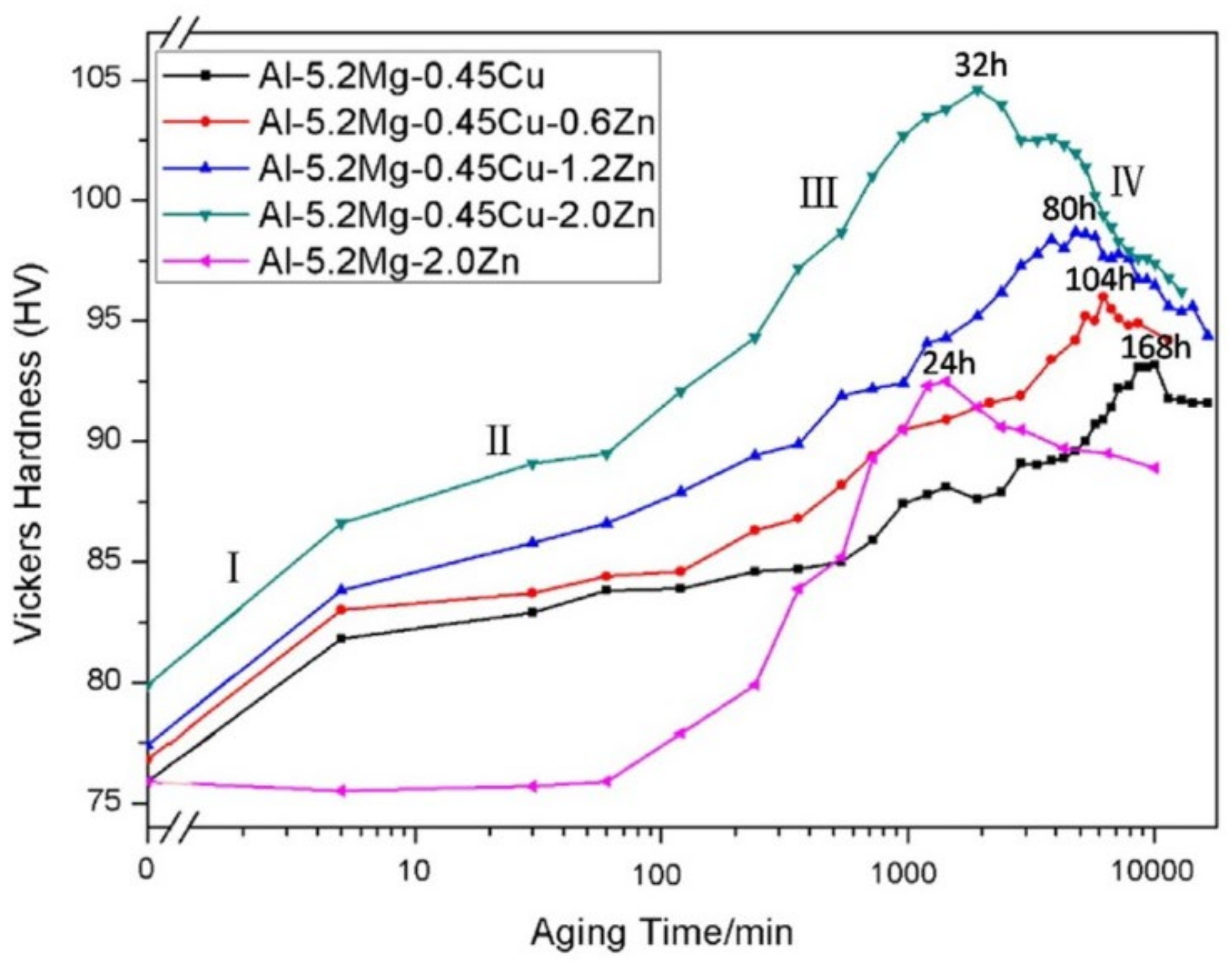
- Crossover alloys of AlMgZn with reduced Zn content demonstrate enhanced resistance to corrosion by inhibiting the formation of the β-phase at grain boundaries and instead dispersing T-phase particles uniformly. When Zn levels are raised and proper processing techniques are employed, the degradation of the surface due to the Lüders band is restricted, and the ability to significantly harden through T-phase precipitation is achieved.
- (3)
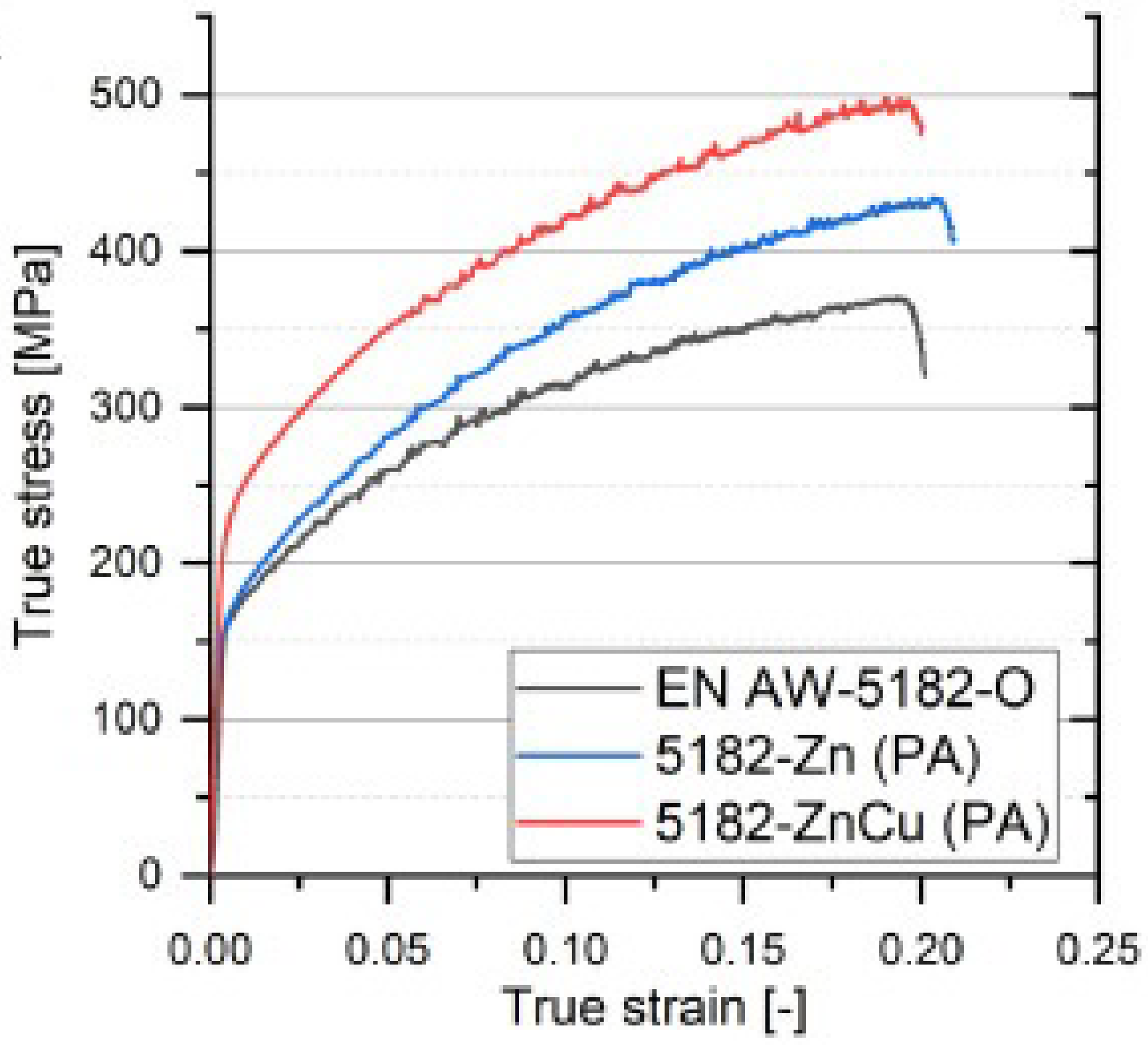
- If the composition and processing of the alloy are carefully optimized, AlMgZn(Cu) crossover alloys have the potential to offer even greater strength, nearly reaching the levels of commercial high-strength AlZnMg(Cu) alloys, and improved corrosion resistance compared to Cu-free AlMgZn crossover alloys. Based on the current technology, the crossover alloys discussed here can offer good formability, which seems to be similar to or even better than commercially available alloys. However, it is important to note that there is still limited experimental data in this area and further research is needed.
- (4)
- The potential of crossover alloys to surpass existing commercial aluminum alloys comes from their superior performance in areas such as mechanical strength and corrosion resistance, which are essential engineering criteria. Additionally, research suggests that it is feasible to create a single alloy system that combines high strengthening ability and good formability, making crossover alloys a viable option for reducing the need for a wide variety of materials used for lightweighting. This, in turn, can contribute to a more sustainable life cycle in the traffic and transportation sectors. Based on the evidence presented in this review, this class of alloys also holds significant promise for further scientific exploration and subsequent technological advancements, which could lead to the emergence of an entirely new commercial aluminum alloy class. Furthermore, it is believed that incorporating controlled amounts of Cu, Zn, or Cu + Zn can greatly enhance the recyclability of the crossover alloys. Evaluating this aspect further is crucial, as it is a key requirement in meeting the current standards of a circular economy. While the current findings show promise, further investigation is necessary to fully capitalize on the potential of aluminum crossover alloys. This exploration should not be restricted to just the crossover between 5xxx/2xxx and 5xxx/7xxx, as discussed here, but should potentially encompass the entire range of aluminum alloy compositions.
- (5)
- The adoption of a T-phase precipitation reinforcement mechanism allows this kind of alloy to achieve an incredible resistance to radiation, thanks to the giant unit cell and, at the same time, highly negative enthalpy of formation. This effectively prevents displacement damage from occurring and is the ideal solution for the development of next-generation materials for space applications, lightweight and, at the same time, radiation-resistant.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, G.; Chen, X.; Yan, J.; Fan, L.; Yang, Z.; Zhang, J.; Guan, R. A Review of Progress in the Study of Al-Mg-Zn(-Cu) Wrought Alloys. Metals 2023, 13, 345. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Zhuang, L.; Zhang, J. Improved Age-Hardening Response and Altered Precipitation Behavior of Al-5.2Mg-0.45Cu-2.0Zn (Wt%) Alloy with Pre-Aging Treatment. J. Alloys Compd. 2017, 691, 40–43. [Google Scholar] [CrossRef]
- Alil, A.; Popović, M.; Radetić, T.; Zrilić, M.; Romhanji, E. Influence of Annealing Temperature on the Baking Response and Corrosion Properties of an Al–4.6 Wt% Mg Alloy with 0.54 Wt% Cu. J. Alloys Compd. 2015, 625, 76–84. [Google Scholar] [CrossRef]
- Hou, S.; Liu, P.; Zhang, D.; Zhang, J.; Zhuang, L. Precipitation Hardening Behavior and Microstructure Evolution of Al–5.1 Mg–0.15Cu Alloy with 3.0Zn (Wt%) Addition. J. Mater. Sci. 2018, 53, 3846–3861. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Pan, Y.; Jiang, Y.; Zhuang, L.; Zhang, J.; Zhang, X. Current-Driving Intergranular Corrosion Performance Regeneration below the Precipitates Solvus Temperature in Al–Mg Alloy. J. Mater. Sci. Technol. 2020, 53, 132–139. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Li, S.; Chen, R.; Nan, Y.; Li, L.; Wang, P.; Li, B.; Cui, J.; Nagaumi, H. Evolution of Microstructure, Mechanical Properties and Corrosion Behavior of Al-4Mg-2Zn-0.3Ag (Wt.%) Alloy Processed by T6 or Thermomechanical Treatment. Corros. Sci. 2021, 188, 109551. [Google Scholar] [CrossRef]
- Tunes, M.A.; Stemper, L.; Greaves, G.; Uggowitzer, P.J.; Pogatscher, S. Prototypic Lightweight Alloy Design for Stellar-Radiation Environments. Adv. Sci. 2020, 7, 2002397. [Google Scholar] [CrossRef]
- Schuster, P.A.; Österreicher, J.A.; Kirov, G.; Sommitsch, C.; Kessler, O.; Mukeli, E. Characterisation and Comparison of Process Chains for Producing Automotive Structural Parts from 7xxx Aluminium Sheets. Metals 2019, 9, 305. [Google Scholar] [CrossRef]
- Jain, M.; Allin, J.; Bull, M.J. Deep Drawing Characteristics of Automotive Aluminum Alloys. Mater. Sci. Eng. A 1998, 256, 69–82. [Google Scholar] [CrossRef]
- Engler, O.; Marioara, C.D.; Hentschel, T.; Brinkman, H.-J. Influence of Copper Additions on Materials Properties and Corrosion Behaviour of Al–Mg Alloy Sheet. J. Alloys Compd. 2017, 710, 650–662. [Google Scholar] [CrossRef]
- Medrano, S.; Zhao, H.; Gault, B.; De Geuser, F.; Sinclair, C.W. A Model to Unravel the Beneficial Contributions of Trace Cu in Wrought Al–Mg Alloys. Acta Mater. 2021, 208, 116734. [Google Scholar] [CrossRef]
- Ringer, S.P.; Hono, K.; Sakurai, T.; Polmear, I.J. Cluster Hardening in an Aged Al-Cu-Mg Alloy. Scr. Mater. 1997, 36, 517–521. [Google Scholar] [CrossRef]
- Hino, M.; Koga, S.; Oie, S.; Yanagawa, M. Properties of Al-Mg Based Alloys for Automonile Body Panel. Konelco Technol. Rev. 1991, 11, 1–5. [Google Scholar]
- Ratchev, P.; Verlinden, B.; De Smet, P.; Van Houtte, P. Precipitation Hardening of anAl–4.2wt% Mg–0.6wt% Cu Alloy. Acta Mater. 1998, 46, 3523–3533. [Google Scholar] [CrossRef]
- Marceau, R.K.W.; Sha, G.; Ferragut, R.; Dupasquier, A.; Ringer, S.P. Solute Clustering in Al–Cu–Mg Alloys during the Early Stages of Elevated Temperature Ageing. Acta Mater. 2010, 58, 4923–4939. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Leitner, H.; Ebner, T.; Uggowitzer, P.J. Mechanisms Controlling the Artificial Aging of Al–Mg–Si Alloys. Acta Mater. 2011, 59, 3352–3363. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Ebner, T.; Prillhofer, R.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Design Strategy for Controlled Natural Aging in Al–Mg–Si Alloys. Acta Mater. 2016, 118, 296–305. [Google Scholar] [CrossRef]
- Schmid, F.; Uggowitzer, P.J.; Schäublin, R.; Werinos, M.; Ebner, T.; Pogatscher, S. Effect of Thermal Treatments on Sn-Alloyed Al-Mg-Si Alloys. Materials 2019, 12, 1801. [Google Scholar] [CrossRef]
- Prillhofer, R.; Rank, G.; Berneder, J.; Antrekowitsch, H.; Uggowitzer, P.; Pogatscher, S. Property Criteria for Automotive Al-Mg-Si Sheet Alloys. Materials 2014, 7, 5047–5068. [Google Scholar] [CrossRef]
- Green, W.P.; Kulas, M.-A.; Niazi, A.; Taleff, E.M.; Oishi, K.; Krajewski, P.E.; McNelley, T.R. Deformation and Failure of a Superplastic AA5083 Aluminum Material with a Cu Addition. Met. Mater. Trans. A 2006, 37, 2727–2738. [Google Scholar] [CrossRef]
- Ebenberger, P.; Uggowitzer, P.J.; Gerold, B.; Pogatscher, S. Effect of Compositional and Processing Variations in New 5182-Type AlMgMn Alloys on Mechanical Properties and Deformation Surface Quality. Materials 2019, 12, 1645. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Houtte, P.V.; Verlinden, B. Influence of Previous Ageing Treatments on the Strength of an Al-4.3 Wt.% Mg-1.2 Wt.% Cu Alloy Processed by ECAE. Int. J. Mater. Res. 2008, 99, 273–280. [Google Scholar] [CrossRef]
- Vidal, V.; Zhang, Z.R.; Verlinden, B. Precipitation Hardening and Grain Refinement in an Al–4.2wt%Mg–1.2wt%Cu Processed by ECAP. J. Mater. Sci. 2008, 43, 7418–7425. [Google Scholar] [CrossRef]
- Löffler, H.; Kovács, I.; Lendvai, J. Decomposition Processes in Al-Zn-Mg Alloys. J. Mater. Sci. 1983, 18, 2215–2240. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Structure of the Aluminium: Magnesium: Zinc Alloys. Metall. Rev. 1971, 16, 95–124. [Google Scholar] [CrossRef]
- Inoue, H.; Sato, T.; Kojima, Y.; Takahashi, T. The Temperature Limit for GP Zone Formation in an Al-Zn-Mg Alloy. Met. Trans. A 1981, 12, 1429–1434. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Guo, M.; Cao, L. Investigation on Microstructure and Mechanical Properties of Al-Zn-Mg-Cu Alloys with Various Zn/Mg Ratios. J. Mater. Sci. Technol. 2021, 85, 106–117. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, X.; Tang, S.; Zhu, Q.; Song, H.; Cao, L. Co-Precipitation of T′ and H′ Phase in Al-Zn-Mg-Cu Alloys. Mater. Charact. 2020, 169, 110610. [Google Scholar] [CrossRef]
- Bergman, G.; Waugh, J.L.T.; Pauling, L. The Crystal Structure of the Metallic Phase Mg32(Al, Zn)49. Acta Cryst. 1957, 10, 254–259. [Google Scholar] [CrossRef]
- Bigot, A.; Auger, P.; Chambreland, S.; Blavette, D.; Reeves, A. Atomic Scale Imaging and Analysis of T’ Precipitates in Al-Mg-Zn Alloys. Microsc. Microanal. Microstruct. 1997, 8, 103–113. [Google Scholar] [CrossRef]
- Berg, L.K.; Gjønnes, J.; Hansen, V.; Li, X.Z.; Knutson-Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L.R. GP-Zones in Al–Zn–Mg Alloys and Their Role in Artificial Aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant Hardening Response in AlMgZn(Cu) Alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Carroll, M.C.; Gouma, P.I.; Mills, M.J.; Daehn, G.S.; Dunbar, B.R. Effects of Zn Additions on the Grain Boundary Precipitation and Corrosion of Al-5083. Scr. Mater. 2000, 42, 335–340. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Zhuang, L.; Zhang, J. Correlations between Stress Corrosion Cracking, Grain Boundary Precipitates and Zn Content of Al–Mg–Zn Alloys. J. Alloys Compd. 2016, 655, 178–187. [Google Scholar] [CrossRef]
- Xu, X.; Jin, X.; Liu, Z.; Zhang, B.; Zhang, R.; Zhuang, Y.; Zhang, P.; Wei, H. Influence of Large Amount Zn on Mechanical Properties and Corrosion Resistance of 5083 Hot Rolled Aluminum Alloy. Appl. Phys. A 2020, 126, 713. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Cui, H.; Zhuang, L.; Zhang, J. Mechanical Properties, Intergranular Corrosion Behavior and Microstructure of Zn Modified Al–Mg Alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; He, Z.; Zhuang, L.; Zhang, J. Enhanced and Accelerated Age Hardening Response of Al-5.2Mg-0.45Cu (Wt%) Alloy with Zn Addition. Mater. Sci. Eng. A 2016, 666, 34–42. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Oberhauser, P.; Uggowitzer, P.J.; Pogatscher, S. Age-Hardening Response of AlMgZn Alloys with Cu and Ag Additions. Acta Mater. 2020, 195, 541–554. [Google Scholar] [CrossRef]
- Yun, J.; Kang, S.; Lee, S.; Bae, D. Development of Heat-Treatable Al-5Mg Alloy Sheets with the Addition of Zn. Mater. Sci. Eng. A 2019, 744, 21–27. [Google Scholar] [CrossRef]
- Stemper, L.; Mitas, B.; Kremmer, T.; Otterbach, S.; Uggowitzer, P.J.; Pogatscher, S. Age-Hardening of High Pressure Die Casting AlMg Alloys with Zn and Combined Zn and Cu Additions. Mater. Des. 2019, 181, 107927. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, F.; Wang, S.; Ji, S. The Development of Low-Temperature Heat-Treatable High-Pressure Die-Cast Al–Mg–Fe–Mn Alloys with Zn. J. Mater. Sci. 2021, 56, 11083–11097. [Google Scholar] [CrossRef]
- Vlach, M.; Cizek, J.; Kodetova, V.; Leibner, M.; Cieslar, M.; Harcuba, P.; Bajtosova, L.; Kudrnova, H.; Vlasak, T.; Neubert, V.; et al. Phase Transformations in Novel Hot-Deformed Al–Zn–Mg–Cu–Si–Mn–Fe(–Sc–Zr) Alloys. Mater. Des. 2020, 193, 108821. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Gault, B.; Makineni, S.K.; Ponge, D.; Raabe, D. (Al, Zn)3Zr Dispersoids Assisted H′ Precipitation in anAl-Zn-Mg-Cu-Zr Alloy. Materialia 2020, 10, 100641. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, D.; Wang, X.; Ma, Q.; Zhuang, L.; Zhang, J. Effects of Cu Addition on the Precipitation Hardening Response and Intergranular Corrosion of Al-5.2Mg-2.0Zn (Wt.%) Alloy. Mater. Charact. 2016, 122, 177–182. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, D.; Zhang, J.; Zhuang, L. On the Suppression of Lüders Elongation in High-Strength Cu/Zn Modified 5xxx Series Aluminum Alloy. J. Alloys Compd. 2020, 834, 155138. [Google Scholar] [CrossRef]
- Österreicher, J.A.; Kirov, G.; Gerstl, S.S.A.; Mukeli, E.; Grabner, F.; Kumar, M. Stabilization of 7xxx Aluminium Alloys. J. Alloys Compd. 2018, 740, 167–173. [Google Scholar] [CrossRef]
- Österreicher, J.A.; Tunes, M.A.; Grabner, F.; Arnoldt, A.; Kremmer, T.; Pogatscher, S.; Schlögl, C.M. Warm-Forming of Pre-Aged Al-Zn-Mg-Cu Alloy Sheet. Mater. Des. 2020, 193, 108837. [Google Scholar] [CrossRef]
- Omer, K.; Abolhasani, A.; Kim, S.; Nikdejad, T.; Butcher, C.; Wells, M.; Esmaeili, S.; Worswick, M. Process Parameters for Hot Stamping of AA7075 and D-7xxx to Achieve High Performance Aged Products. J. Mater. Process. Technol. 2018, 257, 170–179. [Google Scholar] [CrossRef]
- Longgang, H.; Jiajia, Y.; Di, Z.; Linzhong, Z.; Li, Z.; Jishan, Z. Corrosion Behavior of Friction Stir Welded Al-Mg-(Zn) Alloys. Rare Met. Mater. Eng. 2017, 46, 2437–2444. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, D.; Zhuang, L.; Zhang, J. Intergranular Corrosion Resistance of Zn Modified 5××× Series Al Alloy during Retrogression and Re-Aging Treatment. Mater. Charact. 2018, 144, 264–273. [Google Scholar] [CrossRef]
- Carroll, M.C.; Gouma, P.I.; Daehn, G.S.; Mills, M.J. Effects of Minor Cu Additions on a Zn-Modified Al-5083 Alloy. Mater. Sci. Eng. A 2001, 319, 425–428. [Google Scholar] [CrossRef]
- Carroll, M.C.; Buchheit, R.G.; Daehn, G.S.; Mills, M.J. Optimum Trace Copper Levels for SCC Resistance in a Zn-Modified Al-5083 Alloy. Mater. Sci. Forum 2002, 396, 1443–1448. [Google Scholar] [CrossRef]
- Tang, H.-P.; Wang, Q.-D.; Luo, C.; Lei, C.; Liu, T.-W.; Li, Z.-Y.; Jiang, H.-Y.; Ding, W.-J.; Fang, J.; Zhang, J.-W. Effects of Aging Treatment on the Precipitation Behaviors and Mechanical Properties of Al-5.0Mg-3.0Zn-1.0Cu Cast Alloys. J. Alloys Compd. 2020, 842, 155707. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Ding, Q.; Zhang, J.; Zhuang, L. Solute Clustering and Precipitation of Al-5.1Mg-0.15Cu-xZn Alloy. Mater. Sci. Eng. A 2019, 759, 465–478. [Google Scholar] [CrossRef]
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.-Y. Diffusion Coefficients of Some Solutes in Fcc and Liquid Al: Critical Evaluation and Correlation. Mater. Sci. Eng. A 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Liu, H.; Zhuang, L.; Zhang, J. Precipitation Hardening and Intergranular Corrosion Behavior of Novel Al–Mg–Zn(-Cu) Alloys. J. Alloys Compd. 2021, 853, 157199. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, D.; Pan, Y.; Hou, S.; Zhuang, L.; Zhang, J. Strengthening Mechanism of Age-Hardenable Al–xMg–3Zn Alloys. Mater. Sci. Technol. 2019, 35, 1071–1080. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, D.; Zuo, J.; Hou, S.; Zhuang, L.; Zhang, J. The Effect of Grain Boundary Character Evolution on the Intergranular Corrosion Behavior of Advanced Al-Mg-3wt%Zn Alloy with Mg Variation. Mater. Charact. 2018, 146, 47–54. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Pan, Y.; Ding, Q.; Long, W.; Zhang, J.; Zhuang, L. Dependence of Microstructure, Mechanical Properties, and Inter-Granular Corrosion Behavior of Al-5.1Mg-3.0Zn-0.15Cu Alloys with High Temperature Pre-Treatment. Mater. Charact. 2020, 168, 110512. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, D.; Liu, H.; Zhang, J. Stress Corrosion Cracking Behavior of Al-xMg-3.1Zn Aluminum Alloys and Its Mechanism. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2020, 49, 1601–1606. [Google Scholar]
- Ding, Q.; Zhang, D.; Yan, Y.; Zhuang, L.; Zhang, J. Role of Subgrain Stripe on the Exfoliation Corrosion of Al-4.6Mg-3.1Zn (Wt.%) Alloy. Corros. Sci. 2020, 169, 108622. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, D.; Zhang, J.; Zhuang, L. Zn/Cu Regulated Critical Strain and Serrated Flow Behavior in Al–Mg Alloys. Mater. Sci. Eng. A 2020, 795, 139991. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Pan, Y.; Li, H.; Zhou, L.; Zhang, J.; Zhuang, L. The Suppression of Solidification Cracking of Al Welds by Regulating Zn/Mg Ratio. Weld. World 2021, 65, 691–698. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Liu, H.; Zhang, Z.; Li, H.; Zhuang, L.; Zhang, J. Reducing Welding Hot Cracking of High-Strength Novel Al–Mg–Zn–Cu Alloys Based on the Prediction of the T-Shaped Device. Sci. Technol. Weld. Join. 2020, 25, 483–489. [Google Scholar] [CrossRef]
- Klein, T.; Schnall, M.; Gomes, B.; Warczok, P.; Fleischhacker, D.; Morais, P.J. Wire-Arc Additive Manufacturing of a Novel High-Performance Al-Zn-Mg-Cu Alloy: Processing, Characterization and Feasibility Demonstration. Addit. Manuf. 2021, 37, 101663. [Google Scholar] [CrossRef]
- Willenshofer, P.D.; Tunes, M.A.; Vo, H.T.; Stemper, L.; Renk, O.; Greaves, G.; Uggowitzer, P.J.; Pogatscher, S. Radiation-Resistant Aluminium Alloy for Space Missions in the Extreme Environment of the Solar System. arXiv 2022, arXiv:2210.03397. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceci, A.; Costanza, G.; Tata, M.E. Al-Mg-Zn(-Cu) Cross-Over Alloys: The New Frontier in High-Strength and Radiation-Resistant Lightweight Materials. Compounds 2024, 4, 664-678. https://doi.org/10.3390/compounds4040040
Ceci A, Costanza G, Tata ME. Al-Mg-Zn(-Cu) Cross-Over Alloys: The New Frontier in High-Strength and Radiation-Resistant Lightweight Materials. Compounds. 2024; 4(4):664-678. https://doi.org/10.3390/compounds4040040
Chicago/Turabian StyleCeci, Alessandra, Girolamo Costanza, and Maria Elisa Tata. 2024. "Al-Mg-Zn(-Cu) Cross-Over Alloys: The New Frontier in High-Strength and Radiation-Resistant Lightweight Materials" Compounds 4, no. 4: 664-678. https://doi.org/10.3390/compounds4040040
APA StyleCeci, A., Costanza, G., & Tata, M. E. (2024). Al-Mg-Zn(-Cu) Cross-Over Alloys: The New Frontier in High-Strength and Radiation-Resistant Lightweight Materials. Compounds, 4(4), 664-678. https://doi.org/10.3390/compounds4040040







