Powders Synthesized from Calcium Carbonate and Water Solutions of Potassium Hydrosulfate of Various Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses of Powders
2.3. Methods of Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damidot, D.; Glasser, F.P. Thermodynamic investigation of the CaO-Al2O3-CaSO4-K2O-H2O system at 25 °C. Cem. Concr. Res. 1993, 23, 1195–1204. [Google Scholar] [CrossRef]
- Timakov, I.S.; Grebenev, V.V.; Komornikov, V.A.; Zainullin, O.B.; Makarova, I.P.; Selezneva, E.V.; Kuzmin, I.I. Production of Complex Hydrosulphates in the K3H(SO4)2–Rb3H(SO4)2 Series: II. Phase Equilibria in the K2SO4–Rb2SO4–H2SO4–H2O System. Crystallogr. Rep. 2022, 67, 455–463. [Google Scholar] [CrossRef]
- Eriksen, K.M.; Fehrmann, R.; Hatem, G.; Gaune-Escard, M.; Lapina, O.B.; Mastikhin, V.M. Conductivity, NMR, Thermal Measurements, and Phase Diagram of the K2S2O7–KHSO4 System. J. Phys. Chem. 1996, 100, 10771–10778. [Google Scholar] [CrossRef]
- Komornikov, V.A.; Timakov, I.S.; Makarova, I.P.; Selezneva, E.V.; Grebenev, V.V.; Zainullin, O.B. The Study of Phase Equilibria and Superprotonic Crystals in the (NH4)2SO4–K2SO4–H2SO4–H2O system. J. Phys. Conf. Ser. 2020, 1686, 012048. [Google Scholar] [CrossRef]
- Freyer, D.; Voigt, W. Crystallization and phase stability of CaSO4 and CaSO4–based salts. Monatshefte Für Chem./Chem. Mon. 2003, 134, 693–719. [Google Scholar] [CrossRef]
- Safronova, T.V.; Belokozenko, M.A.; Yahyoev, S.O.; Shatalova, T.B.; Kazakova, G.K.; Peranidze, K.K.; Toshev, O.U.; Khasanova, S.S. Ceramics Based on CaSO4·2H2O Powder Synthesized from Ca(NO3)2 and (NH4)2SO4. Inorg. Mater. 2021, 57, 867–873. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, F.; Peng, S.; Xie, H.; Wu, P.; Feng, P.; Gao, C.; Yang, Y.; Guo, W.; Lai, D.; et al. Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite. Appl. Sci. 2016, 6, 411. [Google Scholar] [CrossRef]
- Mananda, A.B.; Harada, H.; Halem, H.I.A.; Mitoma, Y.; Keiko, F. Study of potassium recovery from biomass ash using tartaric acid and syngenite method. J. Mater. Scie. Chem. Eng. 2021, 9, 39–52. [Google Scholar] [CrossRef]
- Otani, T.; Ando, H.; Goshima, T.; Mizuta, K.; Nii, S. Enhanced recovery of potassium from sugarcane molasses for fertilizer. Sugar Tech 2023, 25, 820–826. [Google Scholar] [CrossRef]
- Neto, J.D.S.A.; Angeles, G.; Kirchheim, A.P. Effects of sulfates on the hydration of Portland cement—A review. Constr. Build. Mater. 2021, 279, 122428. [Google Scholar] [CrossRef]
- Arceo, H.B.; Glasser, F.P. Fluxing reactions of sulphate and carbonates in cement clinkering. I. Systems CaSO4-K2SO4 and K2SO4-CaCO3. Cem. Concr. Res. 1990, 20, 862–868. [Google Scholar] [CrossRef]
- Schiefer, C.; Plank, J. Mechanochemical syngenite as hydration accelerator for anhydrite-based self-levelling floor screeds. Constr. Build. Mater. 2021, 308, 124982. [Google Scholar] [CrossRef]
- Pandey, A.; Sonkawade, R.G.; Sahare, P.D. Thermoluminescence and photoluminescence characteristics of nanocrystalline K2Ca2(SO4)3:Eu. J. Phys. D 2002, 35, 2744. [Google Scholar] [CrossRef]
- Safronova, T.V.; Akhmedov, M.M.; Shatalova, T.B.; Tikhonova, S.A.; Kazakova, G.K. Ceramics in the K2O–CaO–SO3–P2O5 System. Russ. J. Inorg. Chem. 2021, 66, 1057–1066. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Safronova, T.V.; Shatalova, T.B.; Filippov, Y.Y.; Vaymugin, L.A.; Vlasenko, V.S.; Likhanov, M.S. Materials in the CaO-K2O-SO3-H2O System Based on Powder Mixtures including Calciolangbeinite K2Ca2(SO4)3 and Calcium Sulfate Anhydrite CaSO4. Ceramics 2023, 6, 1434–1448. [Google Scholar] [CrossRef]
- García-Florentino, C.; Gomez-Nubla, L.; Huidobro, J.; Torre-Fdez, I.; Ruíz-Galende, P.; Aramendia, J.; Hausrath, E.M.; Castro, K.; Arana, G.; Madariaga, J.M. Interrelationships in the gypsum–syngenite–görgeyite system and their possible formation on mars. Astrobiology 2021, 21, 332–344. [Google Scholar] [CrossRef]
- Huidobro, J.; Aramendia, J.; Arana, G.; Hausrath, E.M.; Madariaga, J.M. The effect of low temperature on the Raman spectra of calcium-rich sulfates on Mars. Ann. Glaciol. 2023, 64, 57–62. [Google Scholar] [CrossRef]
- Ju, E.; Shi, E.; Xin, Y.; Cao, H.; Liu, C.; Liu, P.; Chen, J.; Fu, X.; Ling, Z. Laboratory synthesis, spectroscopic characteristics, and conversion relationships of five calcium sulfate double salts relevant to Mars. Icarus 2023, 401, 115610. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Bolanz, R.M.; Ciobotă, V.; Kampe, B.; Rösch, P.; Majzlan, J.; Popp, J. Raman spectroscopic study of calcium mixed salts of atmospheric importance. Vib. Spectrosc. 2012, 61, 206–213. [Google Scholar] [CrossRef]
- Jurišová, J.; Danielik, V.; Fellner, P.; Lencsés, M.; Králik, M. Phase diagram of the system CaSO4-K2SO4-KNO3-Ca(NO3)2-H2O. Acta Chim. Slov. 2014, 7, 20–24. [Google Scholar] [CrossRef]
- Goncharik, I.I.; Shauchuk, V.V.; Kudzina, O.A.; Mozheyko, F.F. Preparation of potassium sulfate in a reaction of potassium chloride and calcium sulfate. Proc. Natl. Acad. Sci. Belarus Chem. Ser. 2017, 98–103. (In Russian). Available online: https://vestichem.belnauka.by/jour/article/view/276 (accessed on 15 August 2024).
- Schiefer, C.; Plank, J. Solventless Mechanochemical Synthesis of Phase Pure Syngenite. Chem.-Methods 2021, 1, 78–84. [Google Scholar] [CrossRef]
- Safronova, T.V.; Sadilov, I.S.; Chaikun, K.V.; Shatalova, T.B.; Filippov, Y.Y. Synthesis of Monetite from Calcium Hydroxyapatite and Monocalcium Phosphate Monohydrate under Mechanical Activation Conditions. Russ. J. Inorg. Chem. 2019, 64, 1088–1094. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shekhirev, M.A.; Putlyaev, V.I.; Tret’yakov, Y.D. Hydroxyapatite-based ceramic materials prepared using solutions of different concentrations. Inorg. Mater. 2007, 43, 901–909. [Google Scholar] [CrossRef]
- Stepuk, A.A.; Veresov, A.G.; Putlyaev, V.I.; Tret’yakov, Y.D. The influence of NO3−, CH3COO−, and Cl− Ions and the morphology of calcium hydroxyapatite crystals. Dokl. Phys. Chem. 2007, 412, 11–14. [Google Scholar] [CrossRef]
- Smillie, S.; Moulin, E.; Macphee, D.E.; Glasser, F.P. Freshness of cement: Conditions for syngenite CaK2(SO4)2 H2O formation. Adv. Cem. Res. 1993, 19, 93–96. [Google Scholar] [CrossRef]
- Safronova, T.V. Phase composition of ceramic based on calcium hydroxyapatite powders containing byproducts of the synthesis reaction. Glass. Ceram. 2009, 66, 136–139. [Google Scholar] [CrossRef]
- ICDD. PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley: New York, NY, USA, 1986; pp. 156–159. [Google Scholar]
- Zuo, W.; Zeng, Y.; Yu, X.; Du, L.; Sun, L.; Zhang, F.; Yang, W.; Li, P.; Yang, Q. Solid–Liquid Equilibria of the Quaternary Water–Salt System K+, Mg2+, Ca2+//SO42––H2O at 323.2 K. J. Chem. Eng. Data 2024, 69, 2831–2841. [Google Scholar] [CrossRef]
- Safronova, T.V.; Khantimirov, A.S.; Shatalova, T.B.; Filippov, Y.Y.; Kolesnik, I.V.; Knotko, A.V. Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production. Ceramics 2023, 6, 561–583. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Schuiling, R.D.; Ding, Z.; Hickey, L.; Wharton, D.; Frost, R.L. Vibrational spectroscopic study of syngenite formed during the treatment of liquid manure with sulphuric acid. Vib. Spectrosc. 2002, 28, 209–221. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite–calcite–vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bul. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Thilaga, K.; Selvarajan, P.; Kader, S.A. Investigation on the solubility, growth, vibrational characteristics and linear optical constants of potassium bisulphate single crystals. Phys. Chem. Solid State 2022, 23, 210–215. [Google Scholar] [CrossRef]
- Bukleski, M.; Ivanovski, V.; Petruševski, V.M. IR specular reflectance spectra of KHSO4 single crystal—Dispersion analysis. Vib. Spectrosc. 2011, 57, 15–22. [Google Scholar] [CrossRef]
- Ennaciri, Y.; El Alaoui-Belghiti, H.; Bettach, M. Comparative study of K2SO4 production by wet conversion from phosphogypsum and synthetic gypsum. J. Mater. Res. Technol. 2019, 8, 2586–2596. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ding, Z.; Martens, W.N.; Schuiling, R.D.; Duong, L.V.; Frost, R.L. Thermal decomposition of syngenite, K2Ca(SO4)2·H2O. Thermochim. Acta 2004, 417, 143–155. [Google Scholar] [CrossRef]
- Huang, H.; Wang, C.; Yao, X.; Geng, C. Clinker Slurry for Cementing Across Salt Formations. SPE J. 2024, 29, 1873–1882. [Google Scholar] [CrossRef]
- Matović, V.; Erić, S.; Kremenović, A.; Colomban, P.; Srećković-Batoćanin, D.; Matović, N. The origin of syngenite in black crusts on the limestone monument King’s Gate (Belgrade Fortress, Serbia)—The role of agriculture fertiliser. J. Cult. Herit. 2012, 13, 175–186. [Google Scholar] [CrossRef]
- Clarke, L.; Partridge, E.P. Potassium Sulfate from Syngenite by High–Temperature Extraction with Water. Ind. Eng. Chem. 1934, 26, 897–903. [Google Scholar] [CrossRef]
- Sanytsky, M.; Kropyvnytska, T.; Shyiko, O. Effect of Potassium Sulfate on the Portland Cement Pastes Setting Behavior. Chem. Chem. Technol. 2023, 17, 170–178. [Google Scholar] [CrossRef]
- Wieczorek-Ciurowa, K. Topochemistry of thermal dehydration of syngenite K2Ca(SO4)2·H2O. Thermochim. Acta 1985, 92, 485–487. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Wieczorek-Ciurowa, K. Kinetic study of the thermal dehydration of syngenite K2Ca(SO4)2H2O under isothermal conditions. J. Therm. Anal. Calorim. 1978, 13, 543–552. [Google Scholar] [CrossRef]
- Rowe, J.J.; Morey, G.W.; Hansen, I.D. The binary system K2SO4-CaSO4. J. Inorg. Nucl. Chem. 1965, 27, 53–58. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zelenski, M.E.; Zubkova, N.V.; Yapaskurt, V.O.; Chukanov, N.V.; Belakovskiy, D.I.; Pushcharovsky, D.Y. Calciolangbeinite, K2Ca2(SO4)3, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Mineral. Mag. 2012, 76, 673–682. [Google Scholar] [CrossRef]
- Rowe, J.J.; Morey, G.W.; Silber, C.C. The ternary system K2SO4-MgSO4-CaSO4. J. Inorg. Nucl. Chem. 1967, 29, 925–942. [Google Scholar] [CrossRef]
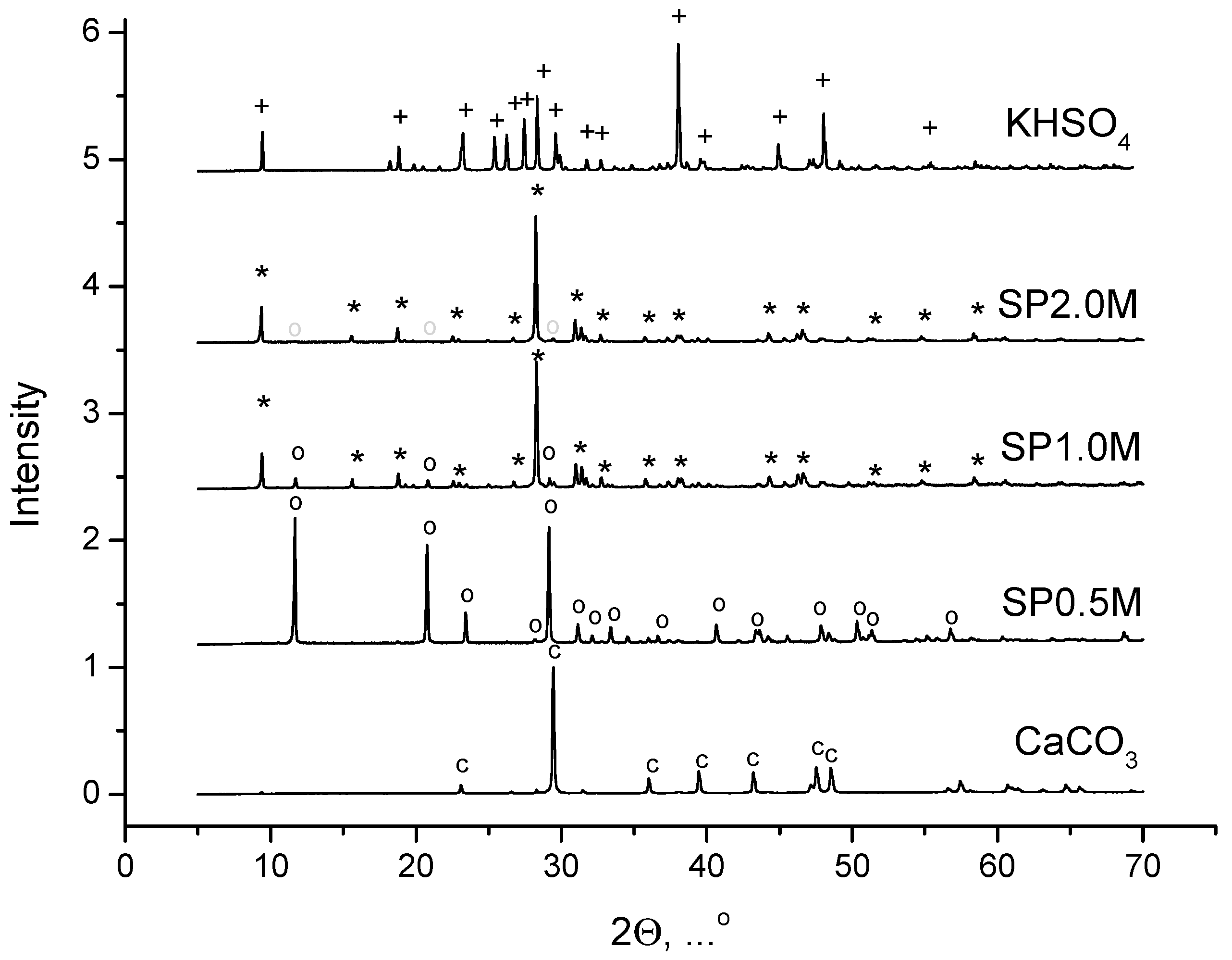
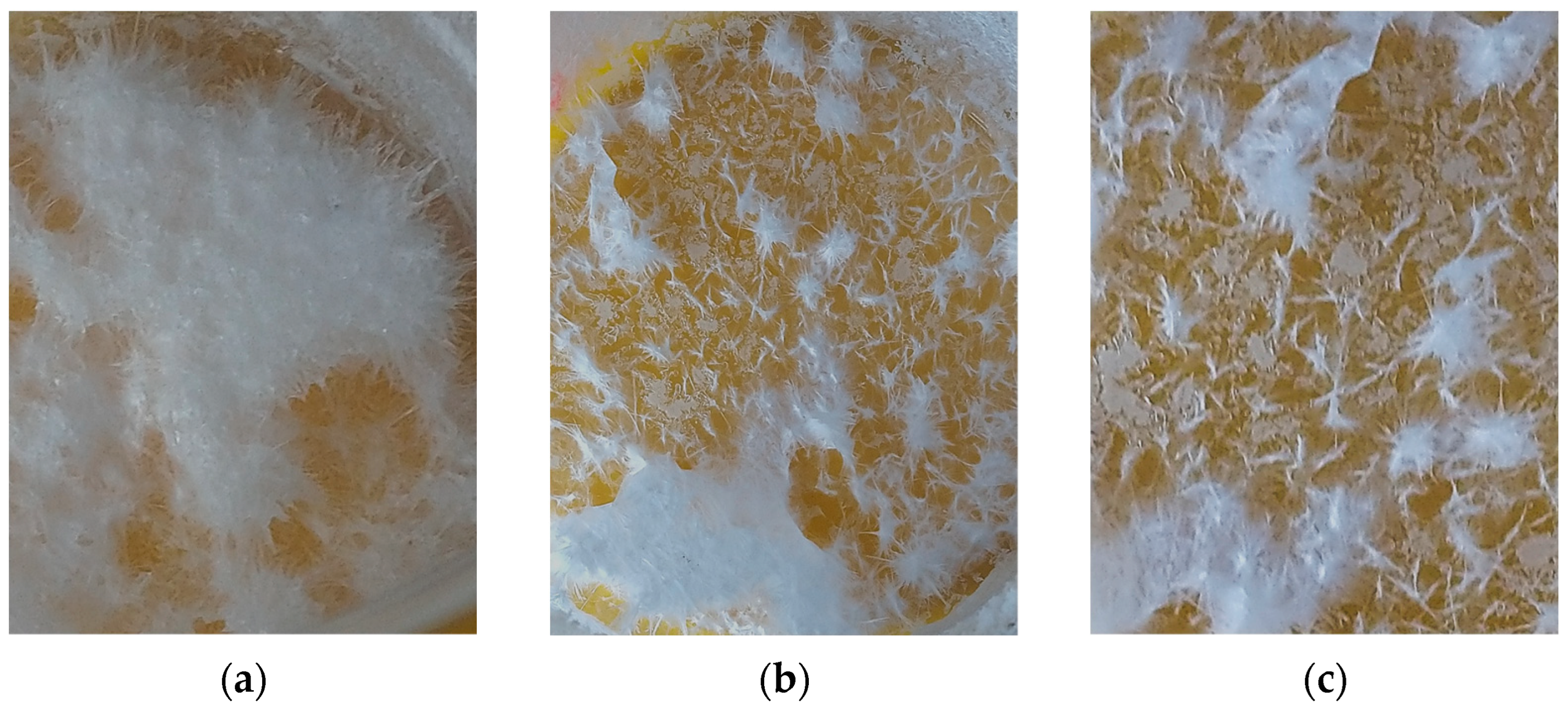
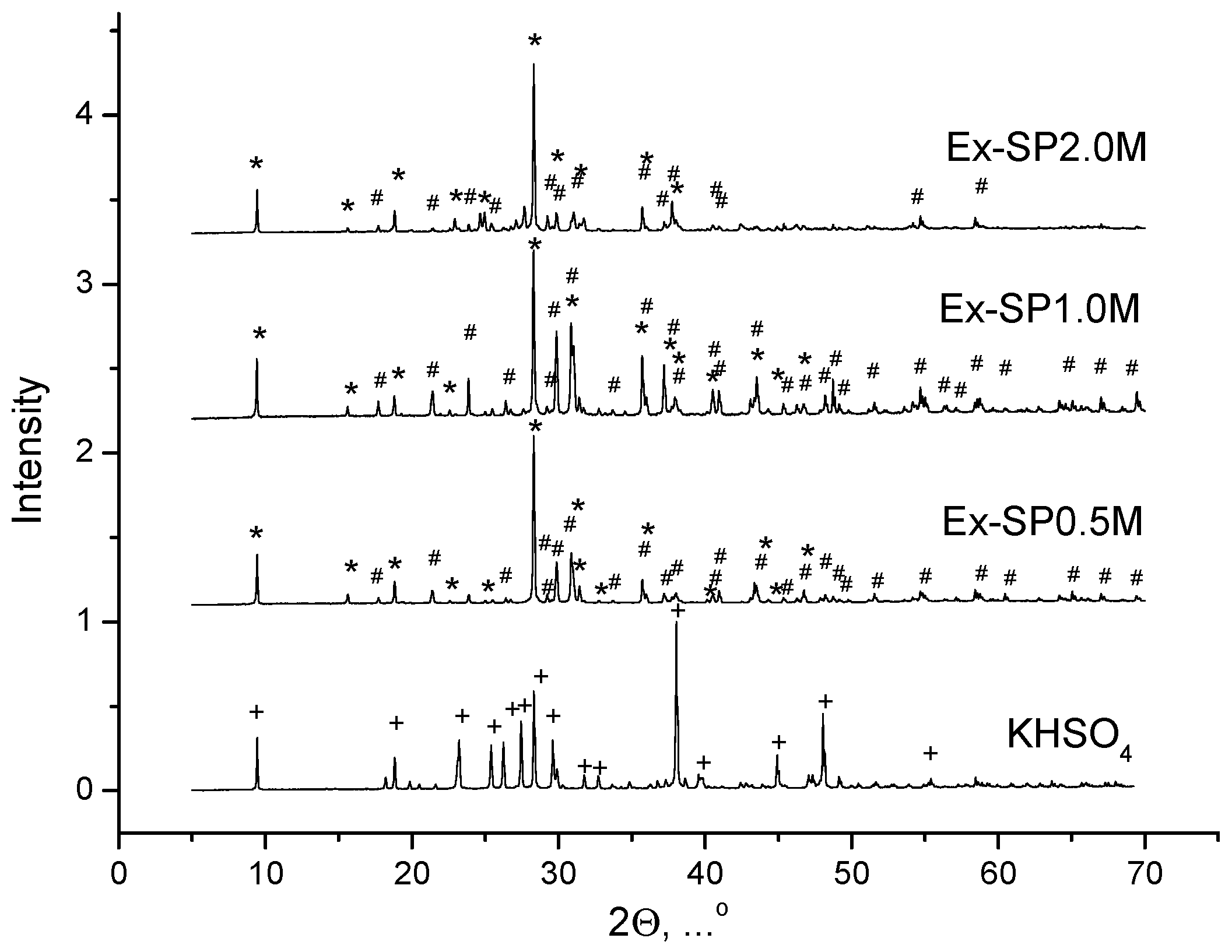

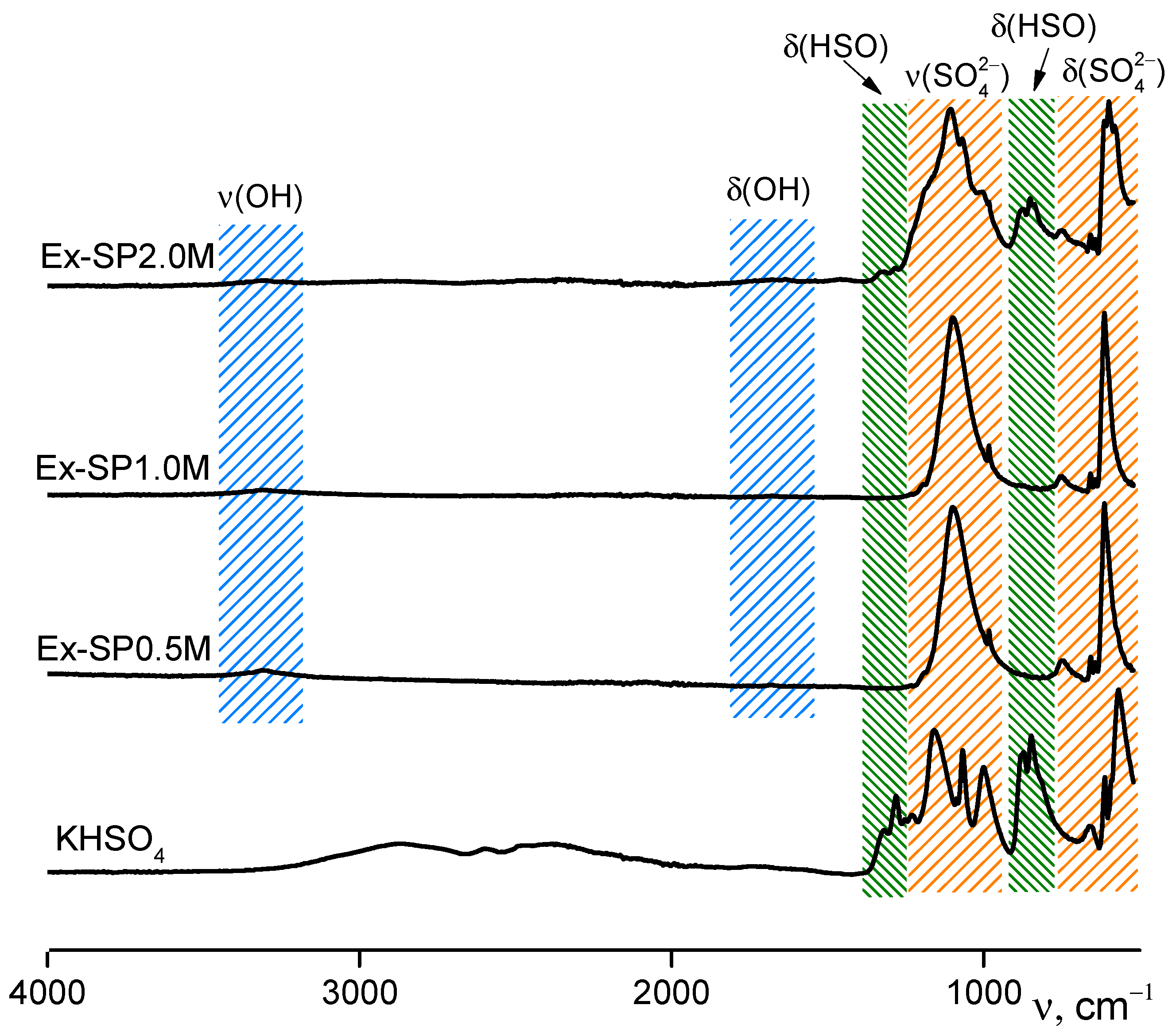

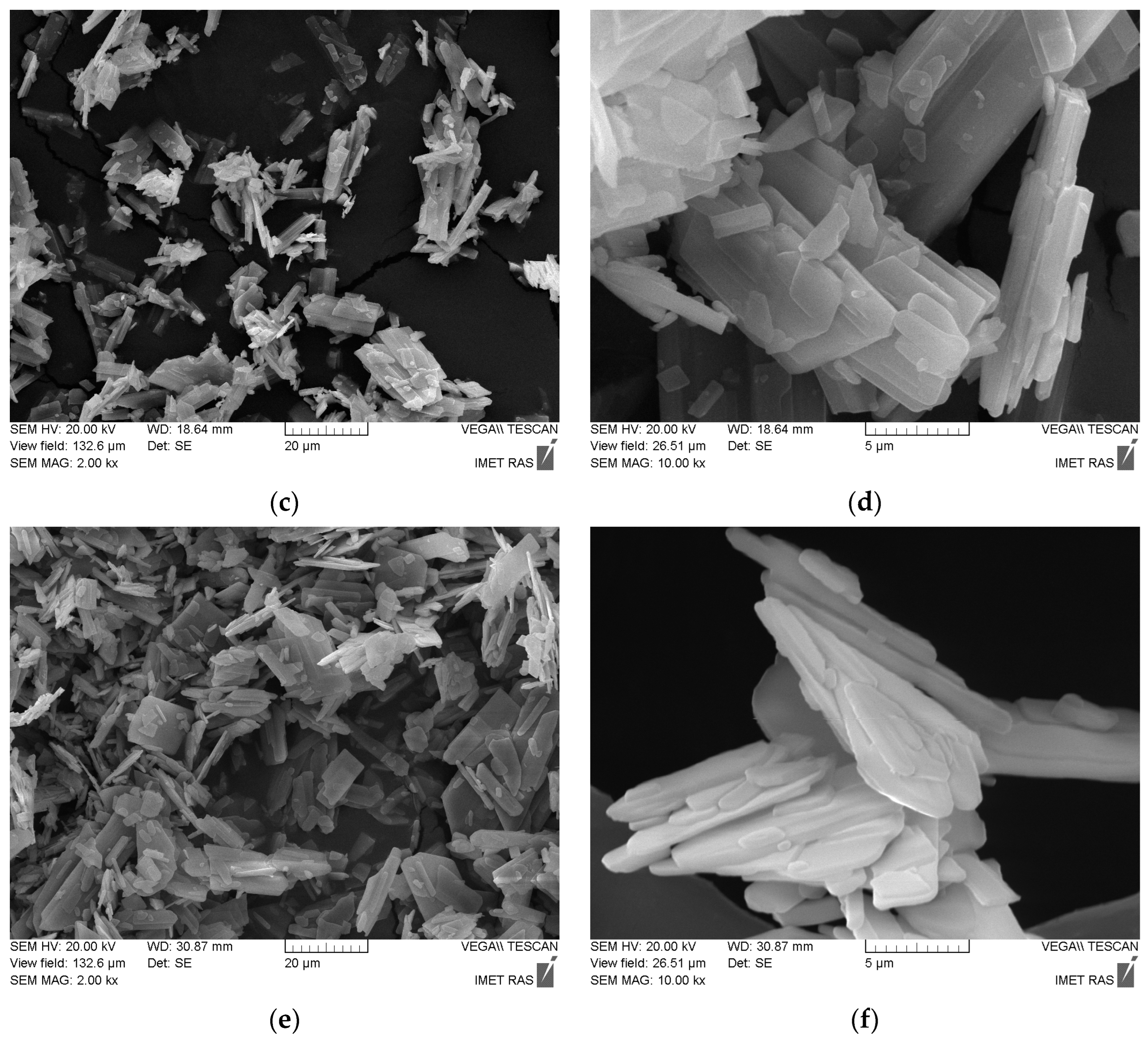
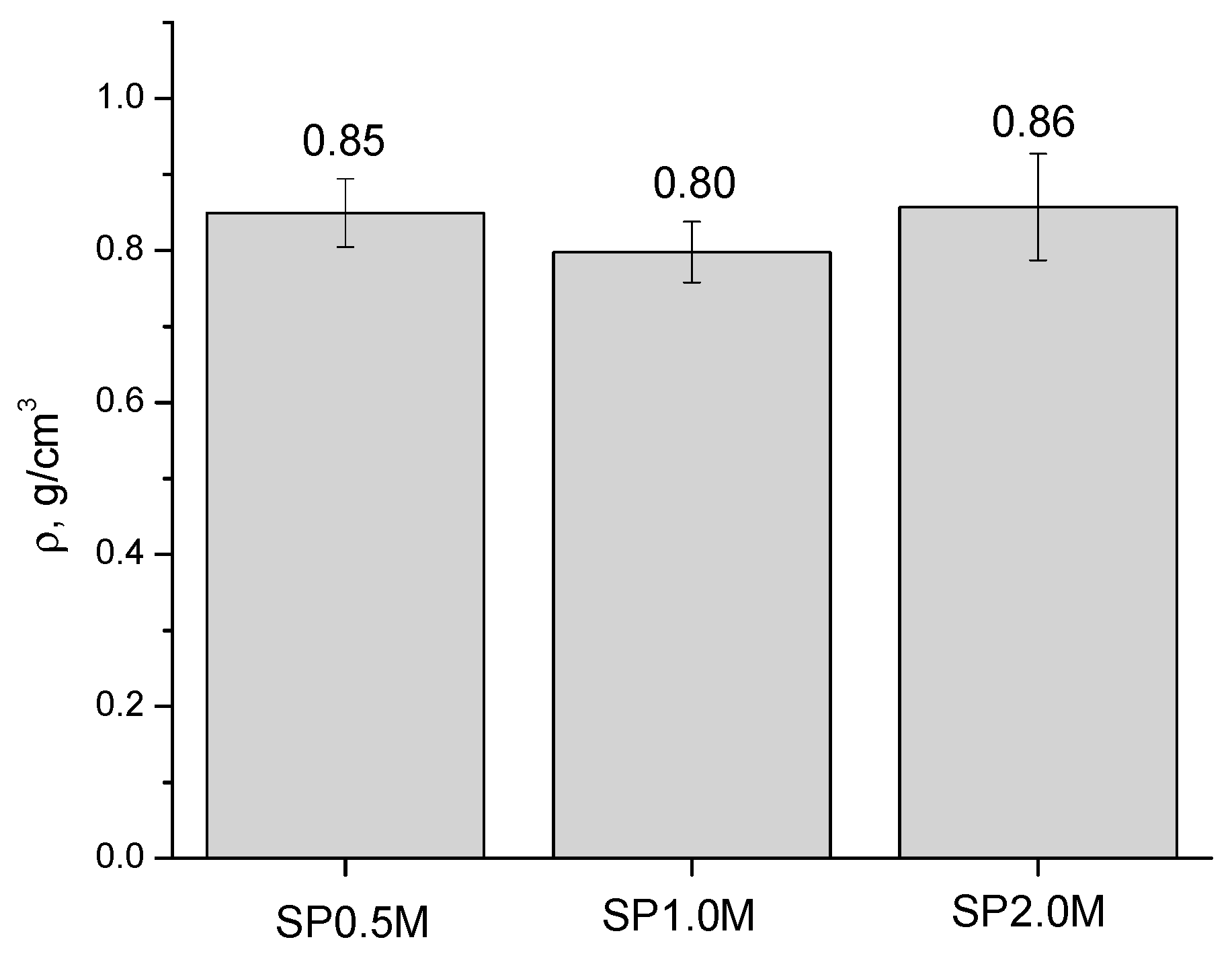


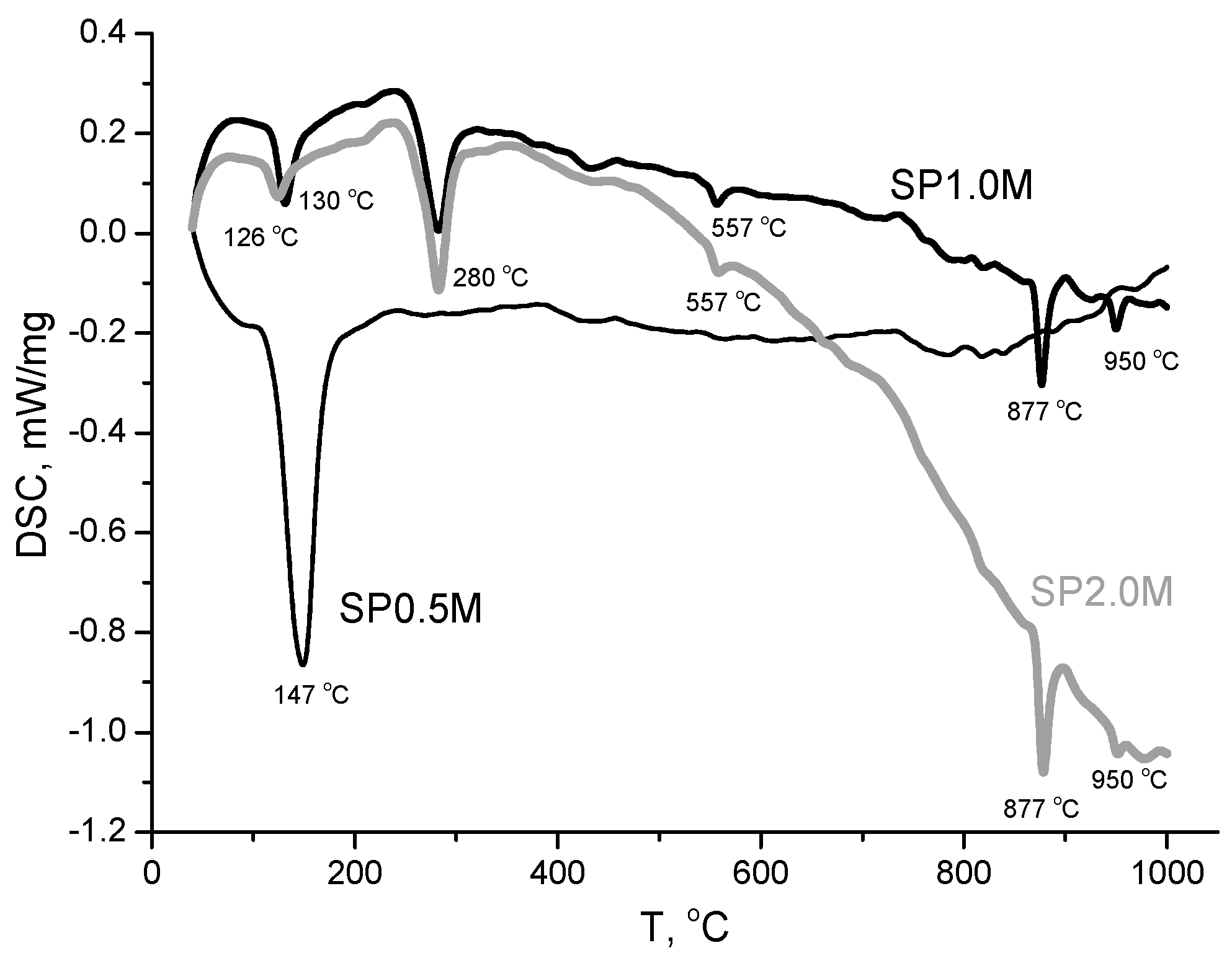
| Labeling 1 | Concentration of KHSO4 Solution, mol/L | Volume of Solution, mL | Amount of Substance by Reaction, mol | Mass of Reagents, g | Expected Mass of K2Ca(SO4)2·H2O, g | ||
|---|---|---|---|---|---|---|---|
| KHSO4 | CaCO3 | KHSO4 | CaCO3 | ||||
| SP0.5M | 0.5 | 400 | 0.2 | 0.1 | 27.2 | 10.0 | 32.8 |
| SP1.0M | 1 | 400 | 0.4 | 0.2 | 54.4 | 20.0 | 65.6 |
| SP2.0M | 2 | 400 | 0.8 | 0.4 | 108.8 | 40.0 | 131.2 |
| Labeling | Expected Mass of K2Ca(SO4)2·H2O, g | Mass of Synthesized Powder, g | Phase Composition of Synthesized Powder 1, Mass % | |
|---|---|---|---|---|
| K2Ca(SO4)2·H2O (#96-900-8129) 1 | CaSO4·2H2O (#96-901-7314) 1 | |||
| SP0.5M | 32.8 | 15.5 | 0 | 100 |
| SP1.0M | 65.6 | 58.2 | 92.9 | 7.1 |
| SP2.0M | 131.2 | 114.3 | 98.7 | 1.3 |
| Labeling | Mass of Extracted Product, g | Phase Composition of Extracted Products 1, Mass % | Mass of K2SO4 in Extracted Product, g | |
|---|---|---|---|---|
| K2Ca(SO4)2·H2O (#96-900-8129) 1 | K2SO4 (#96-900-7570) 1 | |||
| Ex-SP0.5M | 17.2 | 62.2 | 37.8 | 6.51 |
| Ex-SP1.0M | 5.8 | 49.1 | 50.9 | 2.95 |
| Ex-SP2.0M | 5.6 | 83.3 | 16.7 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronova, T.V.; Laptin, P.D.; Zybina, A.I.; Liao, X.; Shatalova, T.B.; Boytsova, O.V.; Khayrutdinova, D.R.; Akhmedov, M.M.; Xu, Z.; Kolesnik, I.V.; et al. Powders Synthesized from Calcium Carbonate and Water Solutions of Potassium Hydrosulfate of Various Concentrations. Compounds 2024, 4, 650-663. https://doi.org/10.3390/compounds4040039
Safronova TV, Laptin PD, Zybina AI, Liao X, Shatalova TB, Boytsova OV, Khayrutdinova DR, Akhmedov MM, Xu Z, Kolesnik IV, et al. Powders Synthesized from Calcium Carbonate and Water Solutions of Potassium Hydrosulfate of Various Concentrations. Compounds. 2024; 4(4):650-663. https://doi.org/10.3390/compounds4040039
Chicago/Turabian StyleSafronova, Tatiana V., Peter D. Laptin, Alexandra I. Zybina, Xiaoling Liao, Tatiana B. Shatalova, Olga V. Boytsova, Dinara R. Khayrutdinova, Marat M. Akhmedov, Zichen Xu, Irina V. Kolesnik, and et al. 2024. "Powders Synthesized from Calcium Carbonate and Water Solutions of Potassium Hydrosulfate of Various Concentrations" Compounds 4, no. 4: 650-663. https://doi.org/10.3390/compounds4040039
APA StyleSafronova, T. V., Laptin, P. D., Zybina, A. I., Liao, X., Shatalova, T. B., Boytsova, O. V., Khayrutdinova, D. R., Akhmedov, M. M., Xu, Z., Kolesnik, I. V., Kaimonov, M. R., Gavlina, O. T., & Akhmedov, M. R. (2024). Powders Synthesized from Calcium Carbonate and Water Solutions of Potassium Hydrosulfate of Various Concentrations. Compounds, 4(4), 650-663. https://doi.org/10.3390/compounds4040039







