Selective Extraction of Terbium Using Functionalized Metal–Organic Framework-Based Solvent-Impregnated Mixed-Matrix Membranes
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis of Modified MOF
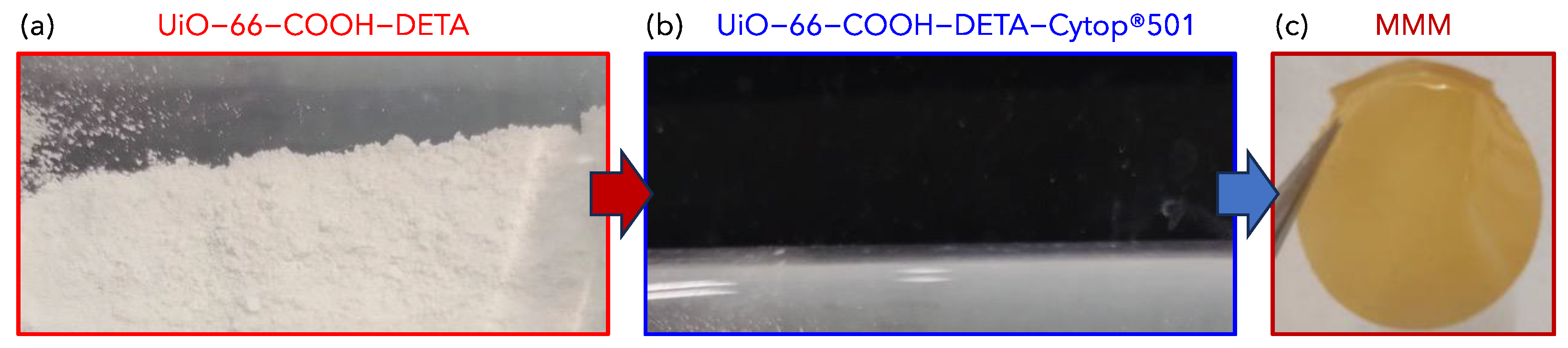
2.3. Preparation of UiO-66-COOH-DETA Impregnated with Cytop®501
2.4. Preparation of MOF-Based Mixed-Matrix Membrane
2.5. Characterization
2.6. Adsorption Experiments
3. Results and Discussion
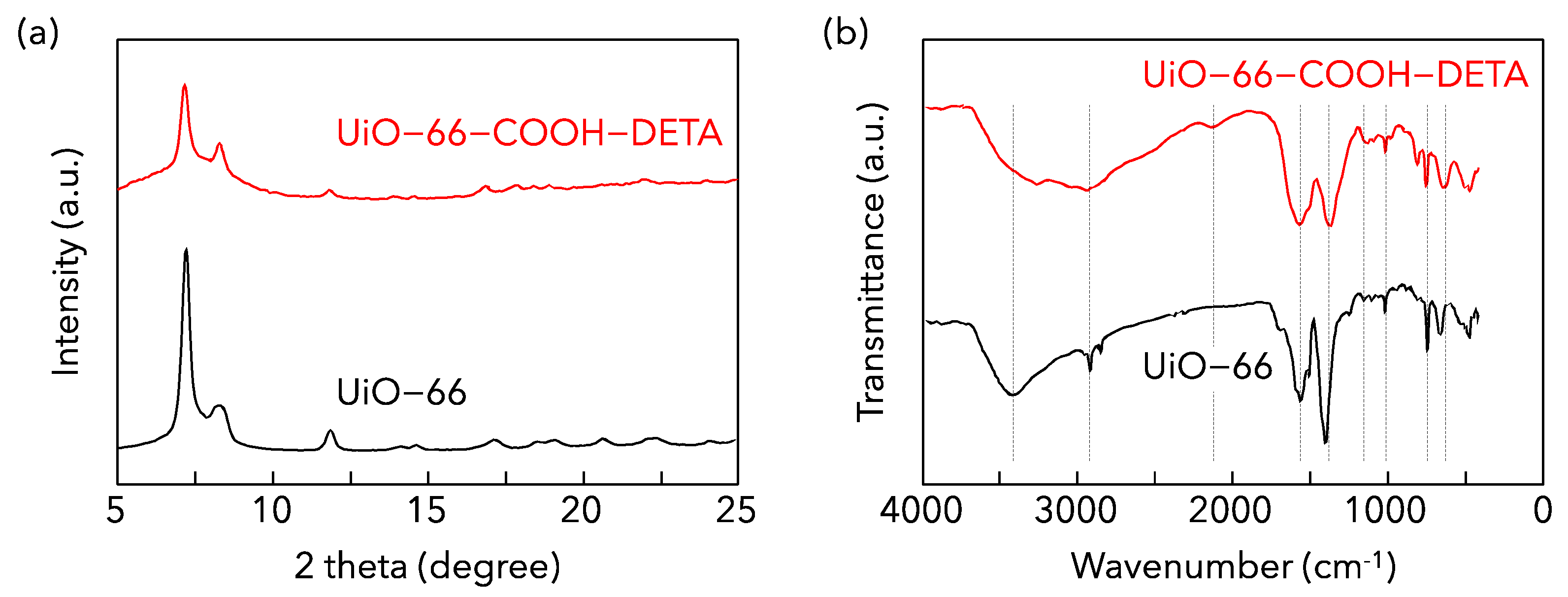
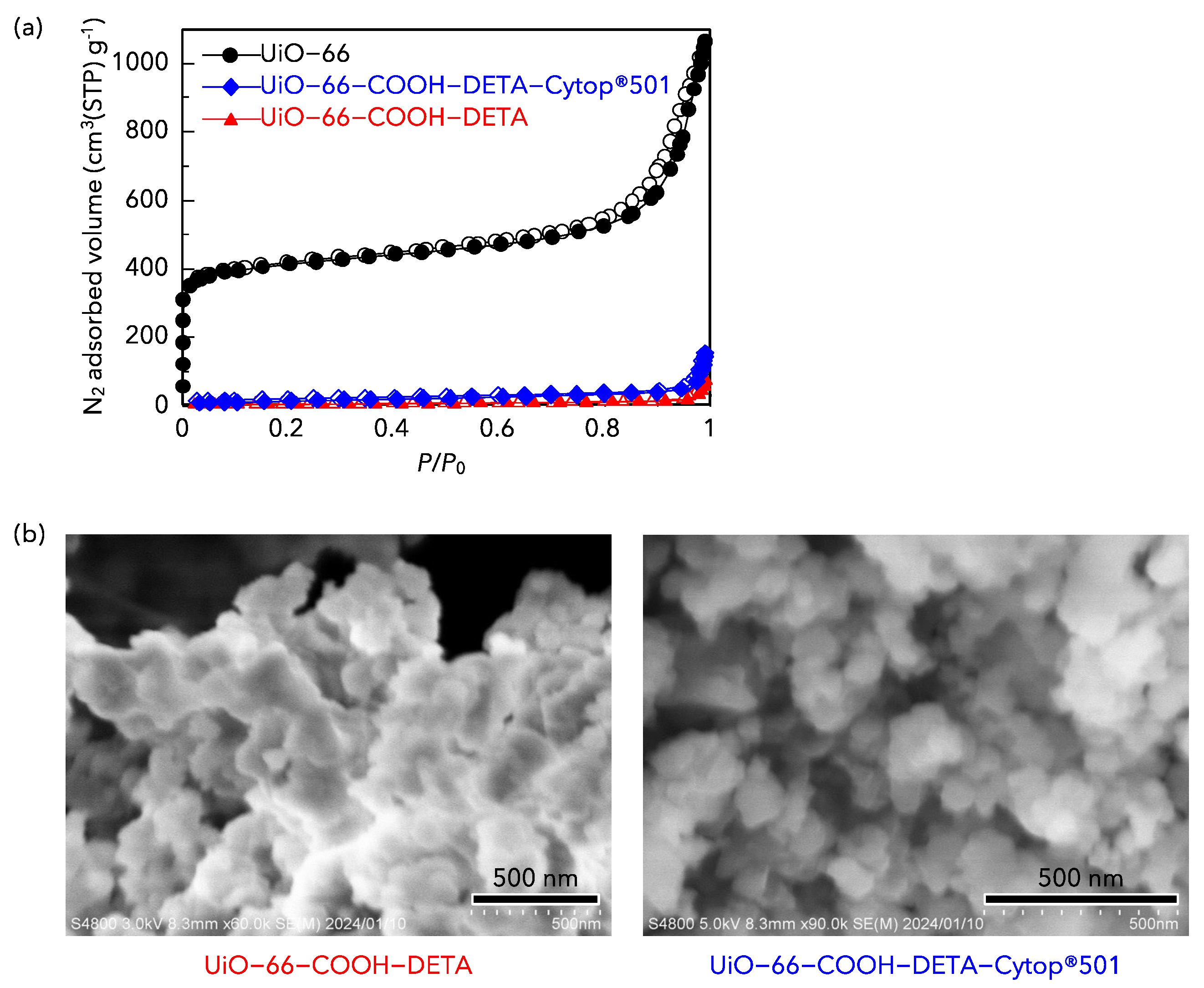
3.1. Characteristics of MOF Adsorbents
3.2. REE Adsorption by the Prepared Membranes
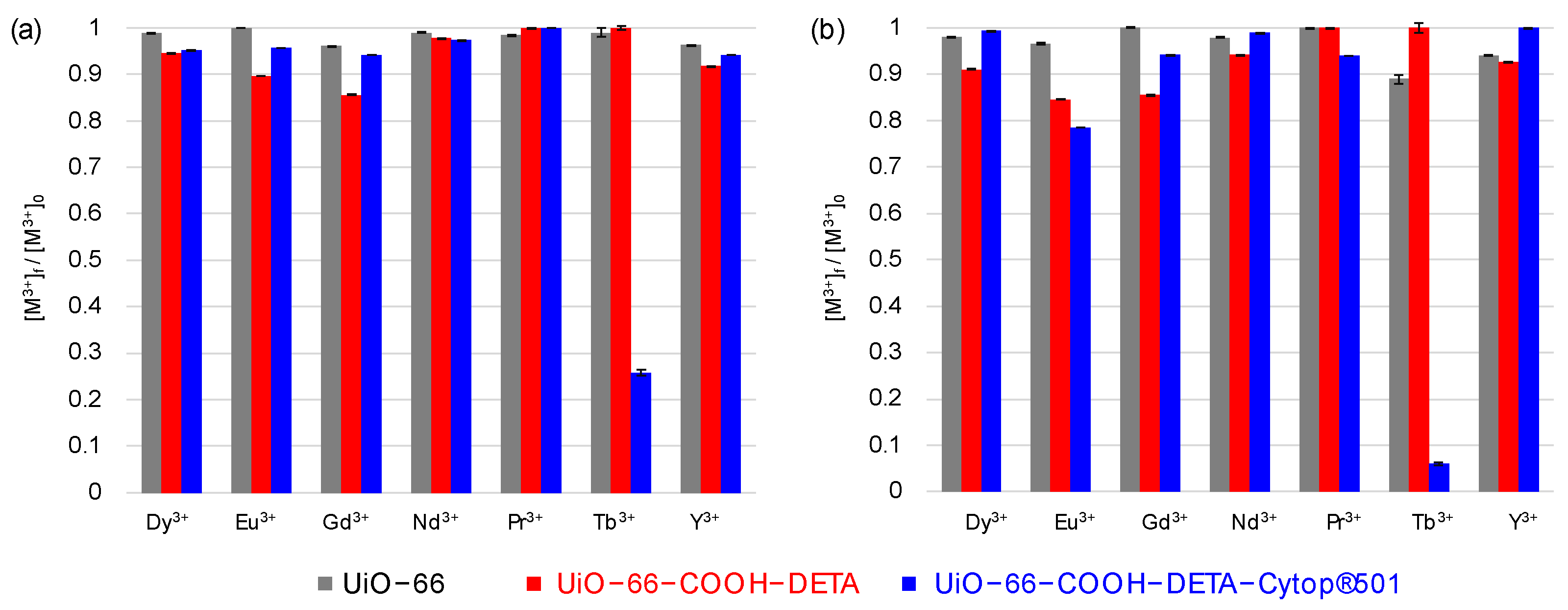
3.3. Selectivity Tests
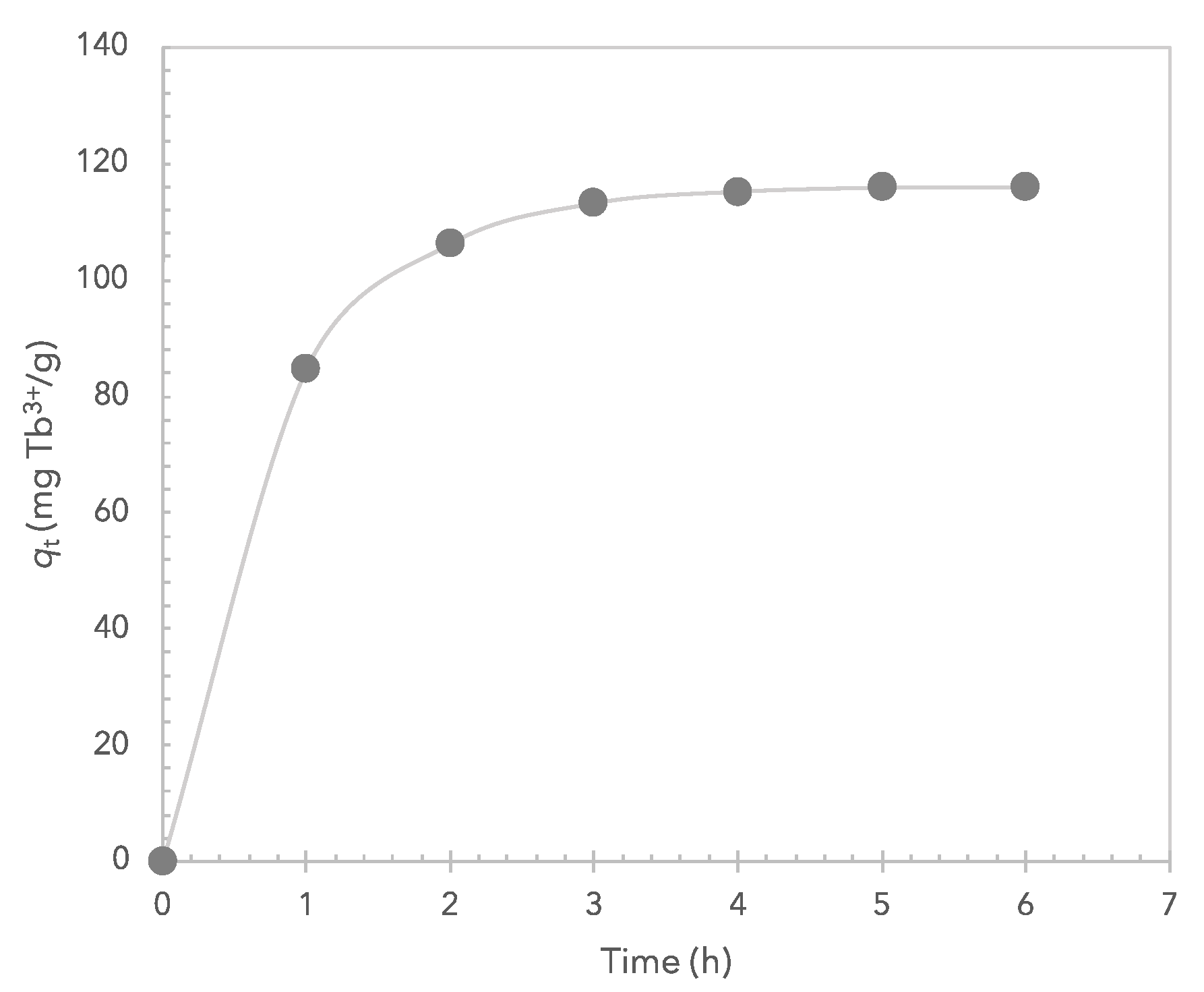
3.4. Adsorption Capacity and Adsorption Kinetics
3.5. Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Sun, B.; Wang, C.; Lim, M.K.; Wang, P.; Geng, X.; Chen, W.Q. Impacts of China’s exports decline in rare earth primary materials from a trade network-based perspective. Resour. Policy 2023, 81, 103321. [Google Scholar] [CrossRef]
- Liu, S.L.; Fan, H.R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Li, X.C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Kujawa, J.; Al Gharabli, S.; Szymczyk, A.; Terzyk, A.P.; Boncel, S.; Knozowska, K.; Kujawski, W. On membrane-based approaches for rare earths separation and extraction–Recent developments. Coord. Chem. Rev. 2023, 493, 215340. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Separation of critical metals by membrane technology under a circular economy framework: A review of the state-of-the-art. Processes 2023, 11, 1256. [Google Scholar] [CrossRef]
- Da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of rare-earth metals from aqueous solutions by bio/adsorption using non-conventional materials: A review with recent studies and promising approaches in column applications. J. Rare Earths 2020, 38, 339–355. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best practices for the synthesis, activation, and characterization of metal–organic frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, K.; Cui, J.; Yu, H.; Wang, Y.; Shan, W.; Xiong, Y. 3D-agaric like core-shell architecture UiO-66-NH2@ZIF-8 with robust stability for highly efficient REEs recovery. Chem. Eng. J. 2020, 386, 124023. [Google Scholar] [CrossRef]
- Paz, R.; Gupta, N.K.; Viltres, H.; Leyva, C.; Romero-Galarza, A.; Srinivasan, S.; Rajabzadeh, A.R. Lanthanides adsorption on metal-organic framework: Experimental insight and spectroscopic evidence. Sep. Purif. Technol. 2022, 298, 121606. [Google Scholar] [CrossRef]
- Khalil, M.; Shehata, M.M.; Ghazy, O.; Waly, S.A.; Ali, Z.I. Synthesis, characterization and γ-rays irradiation of cobalt-based metal-organic framework for adsorption of Ce (III) and Eu (III) from aqueous solution. Radiat. Phys. Chem. 2022, 190, 109811. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Zhao, Y.; Li, Z.; Wang, H.; Du, R. Carboxyl functional poly (ionic liquid) s confined in metal–organic frameworks with enhanced adsorption of metal ions from water. Sep. Purif. Technol. 2022, 299, 121790. [Google Scholar] [CrossRef]
- Kavun, V.; van der Veen, M.A.; Repo, E. Selective recovery and separation of rare earth elements by organophosphorus modified MIL-101(Cr). Microporous Mesoporous Mater. 2021, 312, 110747. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, T.; Wang, M.; Zhu, Y.; Wang, X. Hierarchically structural PAN/UiO-66-(COOH)2 nanofibrous membranes for effective recovery of Terbium (III) and Europium (III) ions and their photoluminescence performances. Chem. Eng. J. 2019, 370, 729–741. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, S.; Wang, R.; Zhang, L.; Wang, Y.; Yin, W. Phosphoric acid functionalized superhydrophilic and underwater superoleophobic UiO-66/polyester fabric composite membrane for efficient oil/water separation and Gd (III) recovery. Desalination 2022, 544, 116141. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Li, Z.; Han, Z.; Fan, J. Review of synthesis and separation application of metal-organic framework-based mixed-matrix membranes. Polymers 2023, 15, 1950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, H.; Yang, Y.; Qian, G.; Cui, Y. Cationic metal–organic framework-based mixed-matrix membranes for fast sensing and removal of Cr2O72− within water. Front. Chem. 2022, 10, 852402. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lee, Y.R.; Yu, K.; Bhattacharjee, S.; Ahn, W.S. Gd3+ adsorption over carboxylic-and amino-group dual-functionalized UiO-66. Ind. Eng. Chem. Res. 2019, 58, 2324–2332. [Google Scholar] [CrossRef]
- Lee, Y.R.; Yu, K.; Ravi, S.; Ahn, W.S. Selective adsorption of rare earth elements over functionalized Cr-MIL-101. ACS Appl. Mater. Interfaces 2018, 10, 23918–23927. [Google Scholar] [CrossRef] [PubMed]
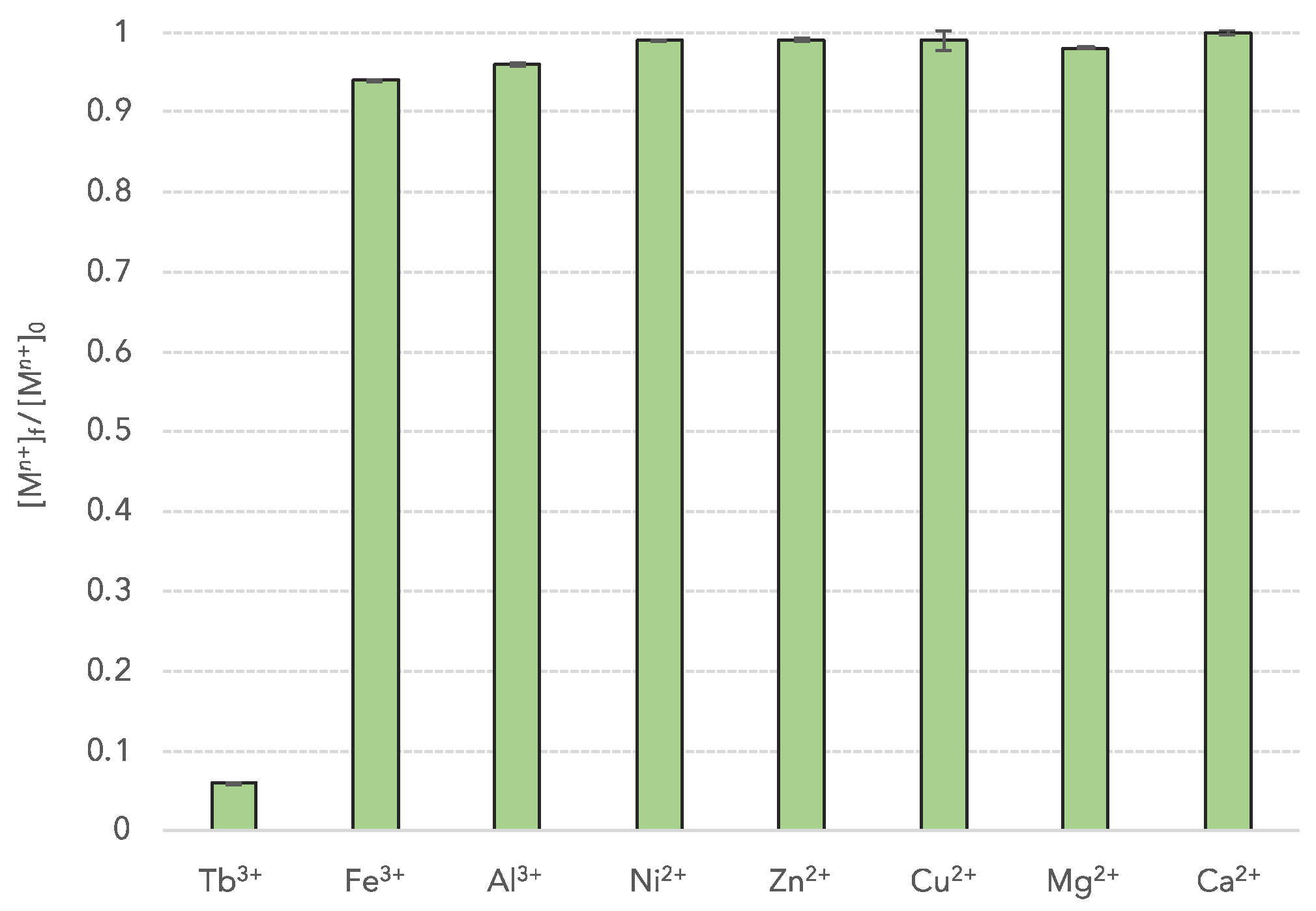
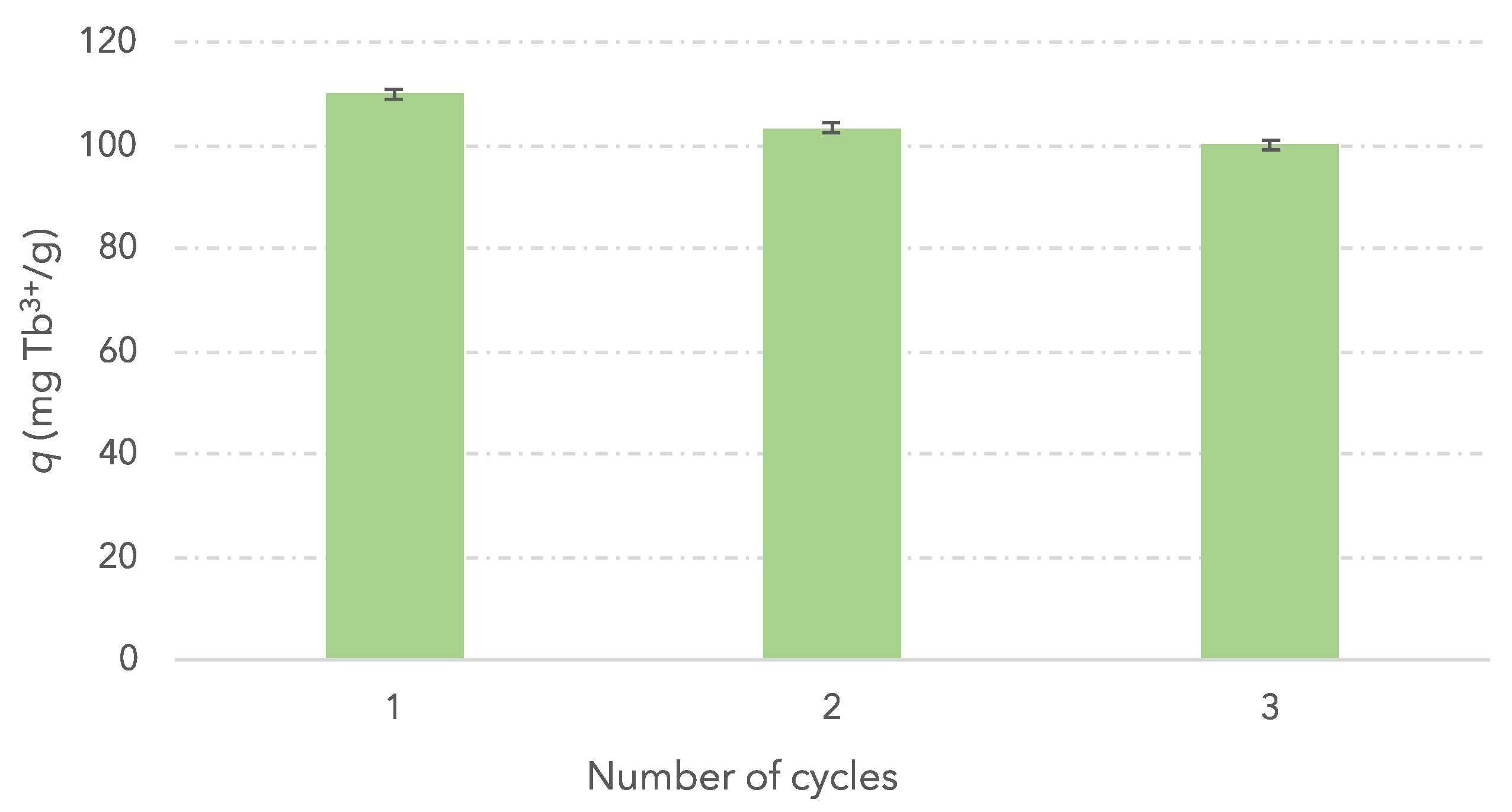
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharaf, M.; Atrees, M.S.; Saleh, G.M.; Mira, H.I.; Tanaka, S. Selective Extraction of Terbium Using Functionalized Metal–Organic Framework-Based Solvent-Impregnated Mixed-Matrix Membranes. Compounds 2024, 4, 679-687. https://doi.org/10.3390/compounds4040041
Sharaf M, Atrees MS, Saleh GM, Mira HI, Tanaka S. Selective Extraction of Terbium Using Functionalized Metal–Organic Framework-Based Solvent-Impregnated Mixed-Matrix Membranes. Compounds. 2024; 4(4):679-687. https://doi.org/10.3390/compounds4040041
Chicago/Turabian StyleSharaf, Maha, Mohamed S. Atrees, Gehad M. Saleh, Hamed I. Mira, and Shunsuke Tanaka. 2024. "Selective Extraction of Terbium Using Functionalized Metal–Organic Framework-Based Solvent-Impregnated Mixed-Matrix Membranes" Compounds 4, no. 4: 679-687. https://doi.org/10.3390/compounds4040041
APA StyleSharaf, M., Atrees, M. S., Saleh, G. M., Mira, H. I., & Tanaka, S. (2024). Selective Extraction of Terbium Using Functionalized Metal–Organic Framework-Based Solvent-Impregnated Mixed-Matrix Membranes. Compounds, 4(4), 679-687. https://doi.org/10.3390/compounds4040041





