Chemical Reactivity in Microheterogeneous Media
Conflicts of Interest
References
- Fendler, J.H. Reactivity control in membrane mimetic systems. Pure Appl. Chem. 1982, 54, 1809–1817. [Google Scholar] [CrossRef]
- Dwars, T.; Paetzold, E.; Oehme, G. Reactions in micellar systems. Angew. Chem. Int. Ed. 2005, 44, 7174–7199. [Google Scholar] [CrossRef] [PubMed]
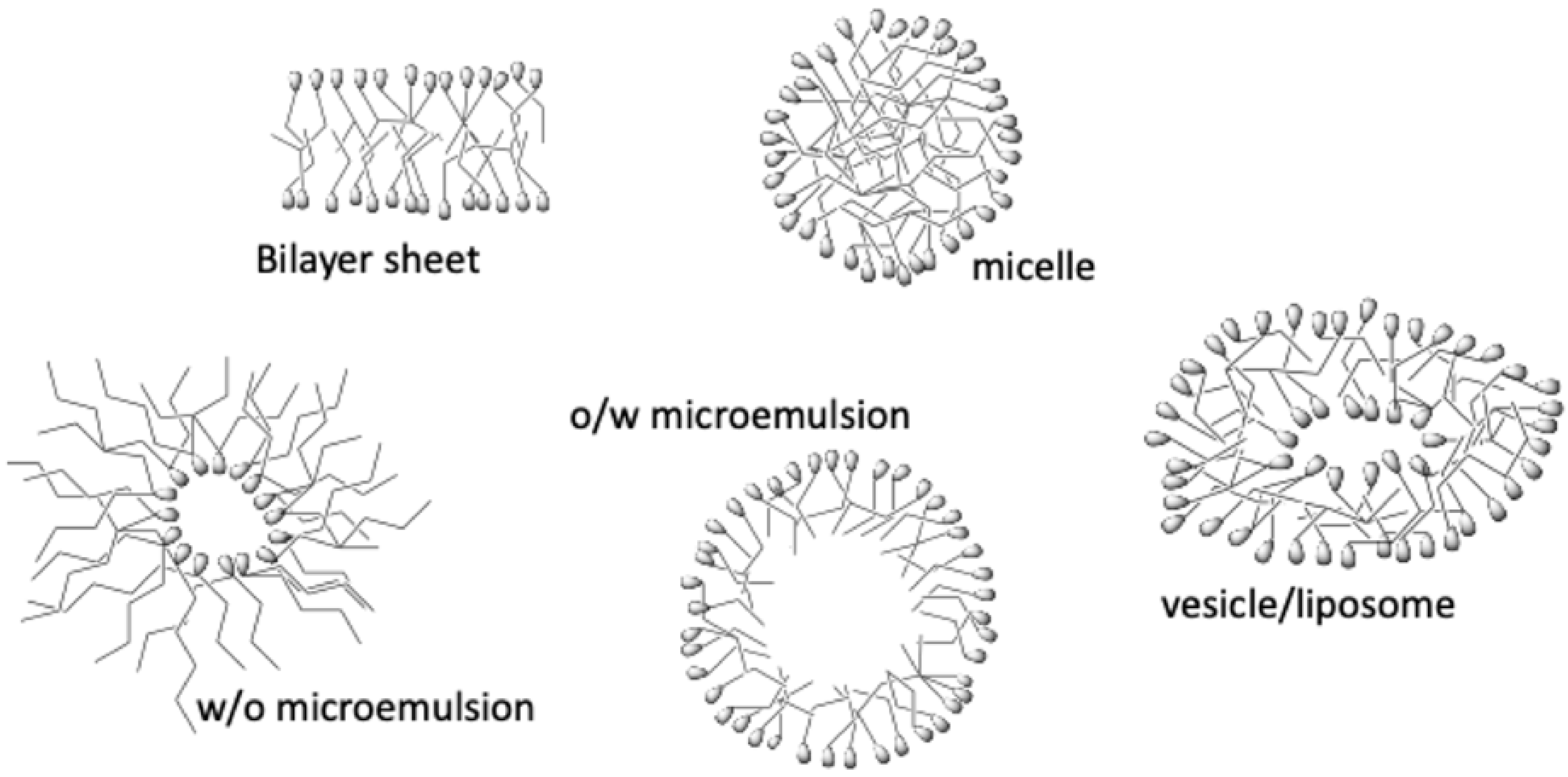
- García-Río, L.; Hervés, P.; Mejuto, J.C.; Pérez-Juste, J.; Rodríguez-Dafonte, P. Comparative study of nitroso group transfer in colloidal aggregates: Micelles, vesicles and microemulsions. New. J. Chem. 2003, 27, 372–380. [Google Scholar] [CrossRef]
- Ghosh, P.; Barnali, K.; Bardhan, S.; Kundu, K.; Saha, S.K.; Paul, B.K.; Das, S. Microemulsion mediated organic synthesis and the possible reaction site. J. Surface Sci. Technol. 2016, 32, 8–16. [Google Scholar] [CrossRef]
- López-Quintela, M.A.; Tojo, C.; Blanco, M.C.; García-Río, L.; Leis, J.R. Microemulsion dynamics and reactions in microemulsions. Curr. Op. Colloid. Interface Sci. 2004, 9, 264–278. [Google Scholar] [CrossRef]
- Costa, M.; Paiva-Martins, F.; Losada-Barreiro, S.; Bravo-Díaz, C. Modeling chemical reactivity at the interfaces of emulsions: Effects of patitioning and temperature. Molecules 2021, 26, 4703. [Google Scholar] [CrossRef]
- Ruasse, M.F.; Blagoeva, I.B.; Ciri, R.; García-Río, L.; Leis, J.R.; Marques, A.; Mejuto, J.C.; Monnier, E. Organic reactions in microorganized media: Why and how? Pure Appl. Chem. 1997, 69, 1923–1932. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Pérez-Lorenzo, M. Microemulsions as microreactors in physical organic chemistry. Pure Appl. Chem. 2007, 79, 1111–1123. [Google Scholar] [CrossRef][Green Version]
- Forconi, M. Medium effects in biologically related catalysis. Adv. Phys. Org. Chem. 2015, 49, 57–101. [Google Scholar] [CrossRef]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Arsene, M.L.; Răut, I.; Călin, M.; Jecu, M.L.; Doni, M.; Gurban, A.M. Versatility of reverse micelles: From biomimetic models to nano (bio)sensor design. Processes 2021, 9, 345. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Abbasi, S.; Emmambux, M.N. Background, Limitations, and Future Perspectives in Food Grade Microemulsions and Nanoemulsions. Food Rev. Int. 2022, 1–39. [Google Scholar] [CrossRef]
- Szumała, P.; Wysocka, I. Effect of gelation and storage conditions on the oxidative stability of microemulsion and nanoemulsion delivery systems. Eur. J. Pharm. Sci. 2018, 124, 17–25. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejuto, J.C.; Cid-Samamed, A. Chemical Reactivity in Microheterogeneous Media. Compounds 2022, 2, 193-195. https://doi.org/10.3390/compounds2030015
Mejuto JC, Cid-Samamed A. Chemical Reactivity in Microheterogeneous Media. Compounds. 2022; 2(3):193-195. https://doi.org/10.3390/compounds2030015
Chicago/Turabian StyleMejuto, Juan C., and Antonio Cid-Samamed. 2022. "Chemical Reactivity in Microheterogeneous Media" Compounds 2, no. 3: 193-195. https://doi.org/10.3390/compounds2030015
APA StyleMejuto, J. C., & Cid-Samamed, A. (2022). Chemical Reactivity in Microheterogeneous Media. Compounds, 2(3), 193-195. https://doi.org/10.3390/compounds2030015





