Since the second half of the last century, the science of colloids has undergone a true revolution, from being little more than a collection of qualitative observations of the macroscopic behavior of some complex systems to becoming a discipline with substantial theoretical foundations. Almost all experimental techniques and theoretical procedures, both from a physical and chemical point of view, have been applied to the study of colloidal aggregates. These facts allow us to form a general idea of the complexity that these systems contain.
One of the most critical colloidal systems is those substances that, under certain circumstances, can associate to give rise, in solution, to aggregates made up of many units and that, in many cases, self-assemble (surfactants in solution). These systems are characterized by the fact that the contact area between the aggregates that form and the medium that contains them is relatively large. Another interesting aspect is that these aggregates can take many forms, depending on the conditions in which they were formed or the structure of the units that form them. Thus, we have micelles, vesicles, bilayers, lamellae, microemulsions, and nanoemulsions (see Figure 1). Of course, all of them have a great interest in practically all scientific and technological development fields.
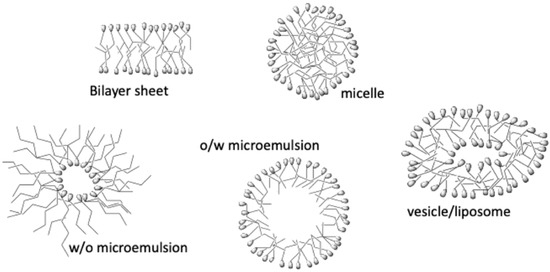
Figure 1.
Structure of different self-assembled microheterogeneous aggregates.
Specifically, the characteristics presented by these structures in which there is a separation into two domains (the hydrocarbon region with a marked hydrophobic character and a highly polar aqueous solution) are used to reproduce "in vitro" certain biological aspects, such as chemical reactions that take place at the cellular level, the movement of substances through membranes, and the properties of water trapped in restricted media. Specifically, the study of micellar systems, microemulsions, or synthetic vesicles has experienced a robust boom in the last fifty years, in such a way that this area of research has acquired its name and is known as biomimetic chemistry [1].
Additionally, the great interest in these compounds should be highlighted due to their ability to solubilize many compounds with very different hydrophobic/hydrophilic properties. For this reason, colloidal aggregates constitute an alternative reaction medium to phase transfer catalysis systems, facilitating the reaction between substances insoluble in water with others highly soluble in this medium [2,3]. Thus, these systems can act as chemical reactors. Their great capacity to concentrate reagents in a small volume (including ionic reagents associated with the surfactants’ heads) gives them a remarkable catalytic capacity [4,5,6]. On the other hand, different hydrophobic/hydrophilic domains can cause the physical separation of the reagents, causing significant inhibition of chemical reactions.
In addition, the structure of these self-aggregates is variable, so we could "design" the reaction medium, turning it into a scalable chemical nanoreactor [7,8,9]: we can change the micellar volume, the radius of the microemulsion droplet, or the size of the vesicle without the need of a synthetic manipulation, solely varying the surfactant concentration or any of the components, adding reagents that act as cosurfactants, or modifying the packaging/packing parameters of these systems. In this way, one could speak of nanoreactors that provide a reaction medium with specific and modulable characteristics, which can resemble biological systems [10,11].
Thus, microemulsions and nanoemulsions are likely to play a much more critical role in our future industries and daily lives, driven by the increasing importance of energy, environmental concerns, demand for food additives and natural preservatives, and stringent provisions, regulations, and standards for the approval of any artificial food ingredient. Incorporating microemulsions and nanoemulsions loaded with bioactive compounds into more realistic environments, such as food matrices, and evaluating their behavior under different process conditions may give some more practical hints to the food and beverage industry. Future research is also likely to address other features such as the simultaneous encapsulation of bioactive compounds in foods, simulation of digestion/absorption, and organoleptic characteristics of developed formulations to assess overall product acceptance by consumers. In addition, in vivo studies are of great importance in advancing the understanding of the functionality of bioactive compounds under realistic gastrointestinal conditions [12].
Research must also consider the reagents incorporated into their formulation to extend their duration with time and the influence of various factors (daylight, temperature, UV radiation, and oxygen) on the oxidation extent of nanodroplets in emulsions [13].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fendler, J.H. Reactivity control in membrane mimetic systems. Pure Appl. Chem. 1982, 54, 1809–1817. [Google Scholar] [CrossRef]
- Dwars, T.; Paetzold, E.; Oehme, G. Reactions in micellar systems. Angew. Chem. Int. Ed. 2005, 44, 7174–7199. [Google Scholar] [CrossRef] [PubMed]
- García-Río, L.; Hervés, P.; Mejuto, J.C.; Pérez-Juste, J.; Rodríguez-Dafonte, P. Comparative study of nitroso group transfer in colloidal aggregates: Micelles, vesicles and microemulsions. New. J. Chem. 2003, 27, 372–380. [Google Scholar] [CrossRef]
- Ghosh, P.; Barnali, K.; Bardhan, S.; Kundu, K.; Saha, S.K.; Paul, B.K.; Das, S. Microemulsion mediated organic synthesis and the possible reaction site. J. Surface Sci. Technol. 2016, 32, 8–16. [Google Scholar] [CrossRef]
- López-Quintela, M.A.; Tojo, C.; Blanco, M.C.; García-Río, L.; Leis, J.R. Microemulsion dynamics and reactions in microemulsions. Curr. Op. Colloid. Interface Sci. 2004, 9, 264–278. [Google Scholar] [CrossRef]
- Costa, M.; Paiva-Martins, F.; Losada-Barreiro, S.; Bravo-Díaz, C. Modeling chemical reactivity at the interfaces of emulsions: Effects of patitioning and temperature. Molecules 2021, 26, 4703. [Google Scholar] [CrossRef]
- Ruasse, M.F.; Blagoeva, I.B.; Ciri, R.; García-Río, L.; Leis, J.R.; Marques, A.; Mejuto, J.C.; Monnier, E. Organic reactions in microorganized media: Why and how? Pure Appl. Chem. 1997, 69, 1923–1932. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Pérez-Lorenzo, M. Microemulsions as microreactors in physical organic chemistry. Pure Appl. Chem. 2007, 79, 1111–1123. [Google Scholar] [CrossRef][Green Version]
- Forconi, M. Medium effects in biologically related catalysis. Adv. Phys. Org. Chem. 2015, 49, 57–101. [Google Scholar] [CrossRef]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as nanoreactors for synthesis of biopolymer nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Arsene, M.L.; Răut, I.; Călin, M.; Jecu, M.L.; Doni, M.; Gurban, A.M. Versatility of reverse micelles: From biomimetic models to nano (bio)sensor design. Processes 2021, 9, 345. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Abbasi, S.; Emmambux, M.N. Background, Limitations, and Future Perspectives in Food Grade Microemulsions and Nanoemulsions. Food Rev. Int. 2022, 1–39. [Google Scholar] [CrossRef]
- Szumała, P.; Wysocka, I. Effect of gelation and storage conditions on the oxidative stability of microemulsion and nanoemulsion delivery systems. Eur. J. Pharm. Sci. 2018, 124, 17–25. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).