Abstract
Biodiesel is produced by the transesterification of animal fats and vegetable oils, producing a large amount of glycerol as a by-product. The crude glycerol cannot be used in the food or pharmaceutical industries. It is crucial to transform glycerol into value-added products with applications in different areas to biodiesel be economically viable. One of the possible applications is its use as a precursor for the synthesis of carbon materials. The glycerol-based carbon materials have distinct properties due to the presence of sulfonic acid groups on the material surface, making them efficient catalysts. Additionally, the glycerol-based activated carbon materials show promising results concerning the adsorption of gases and liquid pollutants and recently as capacitors. Despite their potential, currently, little research has been carried out on the synthesis and application of those materials. This review summarized the preparation and application of carbon materials from glycerol, intending to show the potential of these materials.
1. Introduction
Biodiesel is obtained predominantly by transesterification (chemically or enzymatic) of vegetable oils and animal fats and is known for its energy security awareness. It is composed of free fatty acid alkyl esters and has low toxicity and high biodegradability [1]. Biofuels are a clean energy source, whose combustion emits ≈ 35% fewer greenhouse gases compared with diesel fuel [1,2]. However due to technological limitations, biodiesel’s cost is still higher than fossil diesel [1].
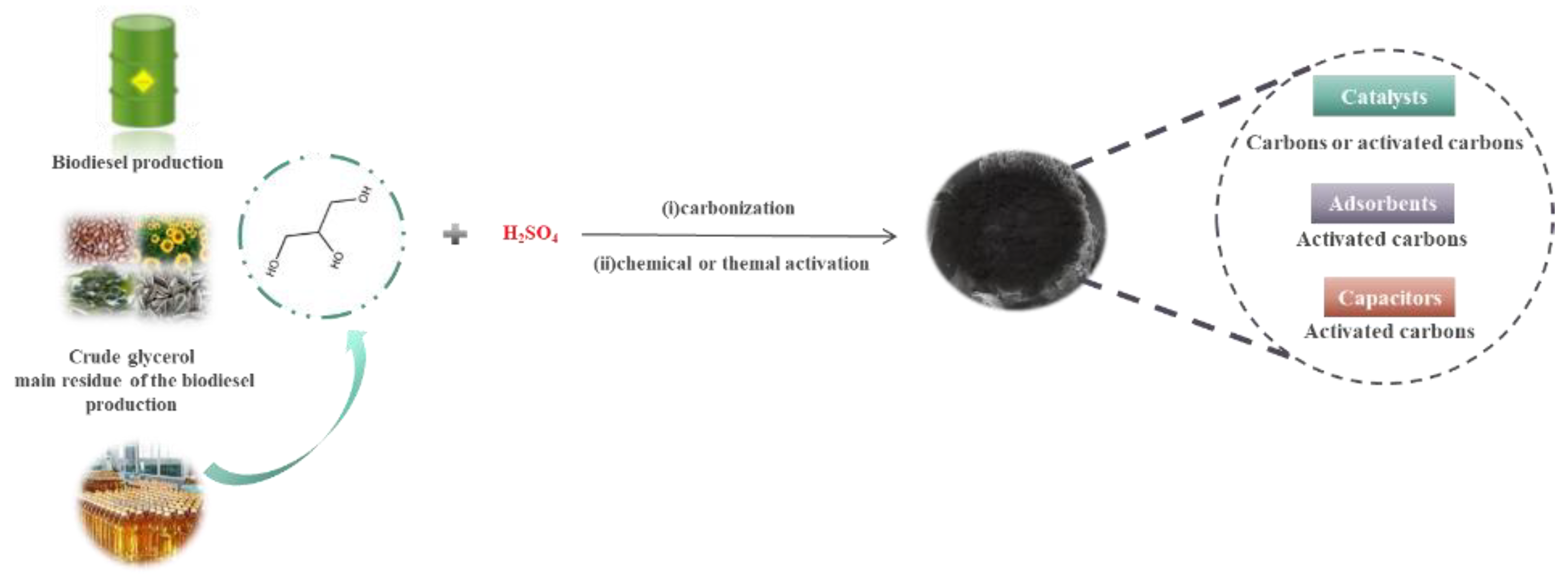
The transesterification of a variety of materials that contain fatty acids that include various vegetable and animal fats, vegetable oils, and edible oil processing residues such as soybean [3], sunflower [4], palm [5], rapeseed [6], canola [7], Jatropha, and cottonseed [8,9,10] generates a large amount of glycerol waste. For instance, for each 10 kg of biodiesel produced, 1 kg of glycerol is generated [11]. The increase of biodiesel production has, as a consequence, an increase of glycerol production. Solutions for this glycerol must be found [12,13,14,15] to ensure the economic feasibility of biodiesel. Yet this crude glycerol cannot be used in most of the traditional applications of glycerol such as food [16,17,18,19] and pharmaceutical industries [20,21,22,23], personal care products [24,25,26], anti-freezers [27,28], e-cigarette liquids [29,30,31], explosives [32] and many other processes as an intermediate compound [33,34,35] due to its poor quality. Additionally, the use of crude glycerol has been gaining ground as a component of heavier fuels and in the processes of obtaining acrylic acid [36]. A much less explored possible use of crude glycerol concerns the development of new solid materials generating high-value products. Coal is traditionally produced from waste, thus adding value to the residue [37,38]. However, the use of glycerol as a precursor in the preparation of carbon materials (carbon and activated carbon, Figure 1) is relatively new and unexplored in comparison with other precursors such as rapeseed [37], potato peel [38,39], sugarcane bagasse [40,41], waste coffee residues [42,43], waste rice husk [44,45], and waste corn [46,47], etc. The glycerol-based carbon materials are obtained in one step by in situ partial carbonization and sulfonation of glycerol with sulfuric acid. In turn, glycerol-based activated carbon materials are prepared in two steps: (i) partial carbonization and sulfonation of glycerol in the presence of sulfuric acid; (ii) chemical or thermal activation of the glycerol-based carbon material. The main advantages of the coal obtained from glycerol are the sulfonic acid groups on the material surface, which give them specific properties for diverse environmental applications such as catalyst, capacitor, and adsorbent materials. This review collected the existing data about the production and use of carbons from glycerol. Despite their potential, most of the research is not contemporary. We intended to show the potential and arouse the interest for this type of material, thus allowing the development of more effective glycerol-based carbons. The synthetic method of the glycerol-based carbons investigated is the same with few variations in the experimental conditions. More in-depth research into the synthesis may lead to carbons with improved properties for the desired applications. The use of these carbons in catalysis is the most explored application. Still, activated carbons have much potential as adsorbents of pollutants and capacitors, yet they are unexplored, and new research is needed, as may be seen in this review. In this work, we intended to call the attention of scientists in the area by showing the potentialities of this by-product in developing advanced carbon materials and promote research in an area with great potential.

Figure 1.
Synthesis of glycerol-based carbons and their main applications.
2. Synthesis of Glycerol-Based Carbon Materials
The synthesis of carbons from glycerol is a relatively new area concerning the use of glycerol. It mainly consists of the partial carbonization and sulfonation of glycerol with sulfuric acid. The main differences observed in the literature concerning the synthesis of carbons are variations in experimental conditions such as glycerol: sulfuric acid mass ratio, reaction temperature, and time. The paper of Devi et al. published in 2009 [48] was the first to synthesize carbon from glycerol. It used the one-pot reaction shown in Figure 1. The partial carbonization and sulfonation of glycerol were carried out with concentrated sulfuric acid (1:4 w/w) using soft experimental conditions. First, the glycerol and sulfuric acid mixture was heated to 180 °C. The mixture was kept at this temperature until foaming ceased. Then, the product was cooled to room temperature and washed with hot water under agitation until reaching neutral pH value. Two years later, the same authors synthesized other carbon using the same procedure but used pitch glycerol as a carbon source and at a different temperature (250 °C) [49]. The reaction yields were 50% and 40%, respectively, and no explanation for why a higher temperature had to be used for this last procedure was presented. The carbons were fully characterized by a great diversity of techniques (elemental analysis, X-ray photoelectron spectroscopy (XPS), X-ray Powder Diffraction (XRD), scanning electron microscopy (SEM), Fourier Transform InfraRed (FTIR), Magic-angle spinning (MAS) NMR 13C, Raman, potentiometric titrations, N2 isotherms, and thermogravimetry/differential thermal analysis (TG/DTA). The obtained carbons had a non-porous nature (<1 m2·g−1), a high density of sulfonic acid groups (–SO3H), and their catalytic capacity in the esterification of palmitic acid, tetrahydropyranylation, and dehydropyranylation was evaluated (See Section 3.1 for more details).
Mantovanic et al. [50] and Gonçalves et al. [51] prepared carbons by hydrothermal carbonization using a mixture of glycerol waste and sulfuric acid (different mass ratio) at 150 °C or 180 °C and using several reaction times (0.25–24 h). Interestingly, the authors successfully increased the number of sulfonic groups using sulfuric acid in a post-synthesis treatment [51]. The carbons also had a non-porous nature and a high numbers of acidic surface groups and were tested as catalysts in acetalization and etherification reactions (see Section 3.1 for more details).
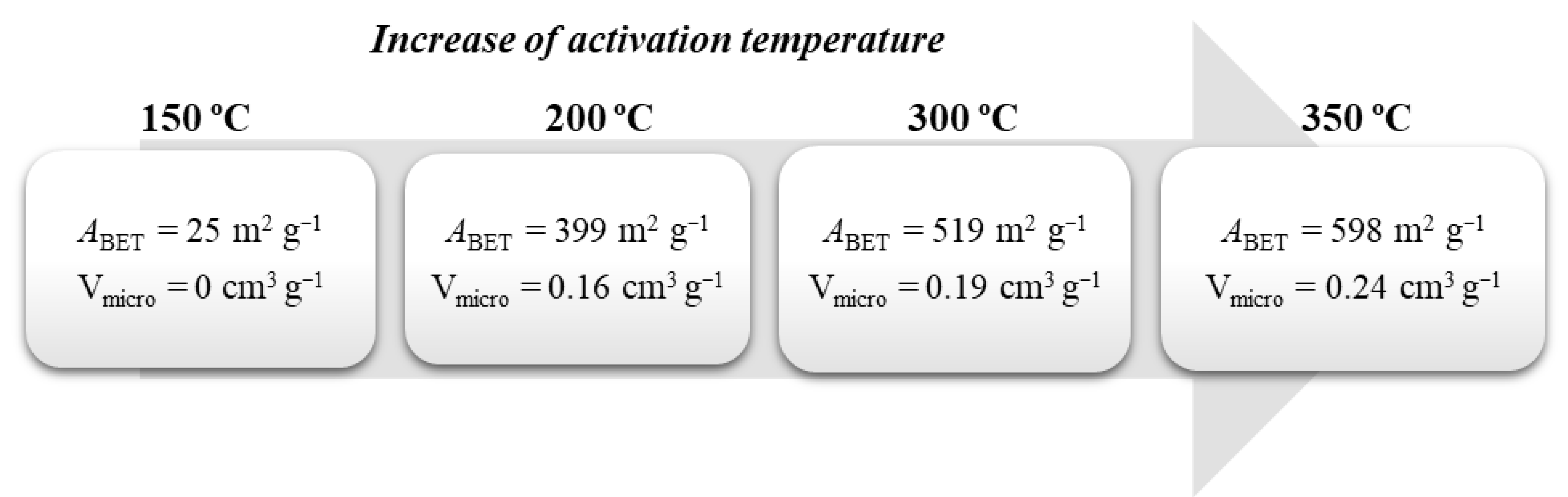
The synthetic procedure described gives rise to non-porous carbons whose main application is in catalysis. For other applications, such as adsorption, the development of a porous structure is crucial. Typically, the synthesis of activated carbons from glycerol requires two steps, carbonization, followed by an activation step [52,53,54]. Some examples of those activated carbons obtained via chemical activation (KOH, ZnCl2, and H3PO4) [52,53] and thermal activation [55,56] may be found in the literature. Different activation agent ratios and temperatures have been tested to vary the porosity of the obtained materials. For instance, the group of Ribeiro et al. [56], after obtaining the carbon using the already described procedure, carried out further calcination (120 °C, 400 °C, 600 °C—60 min in each temperature, plus 800 °C—240 min) under nitrogen flow. The obtained material showed high thermal stability, a basic character due to the decomposition of the sulphonic acid groups, and non-porous nature. This material was then thermally activated under an air atmosphere at different temperatures (150 °C, 200 °C, 300 °C, and 350 °C) for 1 h and generated porosity which increased with the temperature as shown in Figure 2. Additionally, the increase in temperature in the surface oxygen groups (lactones, phenols, and quinones) increases its acid character. Although rare, this work used activated carbons in the catalytic wet peroxide oxidation (CWPO) of 2-nitrophenol (See Section 3.1 for more details).
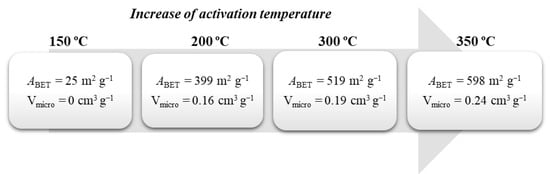
Figure 2.
Influence of the activation temperature in the generation of porosity and development of microporosity.
Another way of obtaining porous carbons may be using a pore-forming agent. The work of Lee et al. [57] used a pure glycerol and crude waste glycerol as a carbon precursor for mesoporous carbon. It also explored glycerol as a pore-forming agent for mesoporous silica. The mesoporous carbon was obtained by the carbonization of glycerol–silica nanoparticles at high temperatures (600 °C) under a nitrogen atmosphere. NaOH solution was used for the removal of the silica–nanoparticle framework. By simply changing the silica particle size in glycerol–silica nanocomposites or changing the silica particle size, it was possible to tailor the pore size and volume, surface area, and pore wall thickness of mesoporous carbon. Due to the presence of other components that may also act as a pore-forming agent in the crude waste glycerol, its use in the synthesis leads to a multimodal pore size distribution of micropores smaller than 2 nm, small mesopores centered at 3.8 nm, and large mesopores above 10 nm.
To the best of our knowledge, the work published in 2016 by Álvarez-Torrellas et al. was the first to study the application of glycerol-based activated carbons as adsorbent materials [55]. The synthesis consisted in the partial carbonization of a glycerol–sulfuric acid mixture, followed by thermal activation. A glycerol and sulfuric acid mixture was heated to 180 °C for 20 min. The resulting material was calcinated in a tube furnace under a nitrogen flow (100 cm3·min−1) at different temperatures (120 °C, 400 °C and 600 °C) during 60 min and 800 °C during 240 min. Then the calcined material (GBCM) was thermally activated at different temperatures (200 °C, 300 °C and 350 °C, for 60 min) in a tube furnace under oxidative atmosphere (flow of 100 cm3·min−1). The textural properties were studied by N2 adsorption-desorption isotherms. The presence of the oxygenated groups was investigated by zeta potential and FTIR data. All obtained acid activated carbons (GBCM200, GBSM300 and GBCM350—where the subscript represents the activation temperature) presented high surface area and microporous structure developed. Their adsorption capacities were evaluated through flumequine and tetracycline (See Section 3.2 for more details).
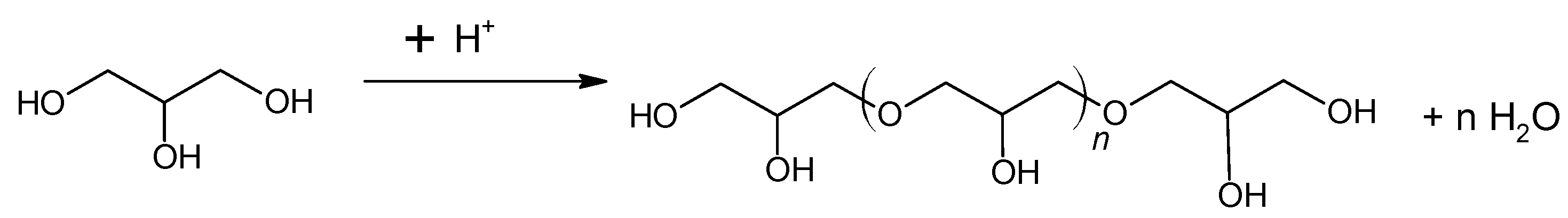
Cui et al. [54] investigated glycerol as a liquid precursor for the preparation of activated carbon. The authors concluded that glycerol pyrolysis in the absence of acid generates no carbon material. This was justified by the evaporation of glycerol (boiling point of 290 °C) before it was carbonized. The description of different acids’ roles and the absence of acid in carbon formation was reported. For this effect, glycerol was mixed with an acid (H2SO4, H3PO4, HCl, or CH3COOH) at volume ratios (10:1, 10:2 and 10:3 v/v). The solutions were added to a quartz boat and heated on the tube furnace in N2 atmosphere to 400 °C, 500 °C, 600 °C, 700 °C, or 800 °C for 1 h. The glycerol pyrolysis in the presence of HCl or CH3COOH did not produce carbon material. However, with H2SO4 or H3PO4 addition, glycerol pyrolysis generated carbon materials. According to the authors, the glycerol is dehydrated and polymerized when exposed to the presence of acids (H2SO4 or H3PO4) at moderate temperatures (<200 °C). Both acids induce dehydration of alcohol groups via protonation of the alcoholic oxygen (Figure 3).
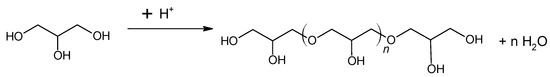
Figure 3.
General representation of the polymerization reaction.
In the case of HCl, it has a low boiling point (48 °C), and in the case of CH3COOH, because it is a weak acid, it cannot initiate the glycerol dehydration. In this context, carbon materials with various functional groups and porosities were prepared via sulfuric or phosphoric acid-mediated polymerization and carbonization followed by steam or CO2 activation. The porosity in the activated carbons reached surface areas up to 2470 m2·g−1 and pore volumes up to 1.44 cm3·g−1. The samples prepared with H3PO4 were consistently more mesoporous than samples prepared with H2SO4. The adsorption capacity of those materials was evaluated for the removal of gas phase volatile organic compounds (VOCs) and aqueous phase chromium Cr(VI) (See Section 3.2 for more details).
Naverkar et al. [58] prepared glycerol-based carbon by partial carbonization of glycerol using concentrated sulfuric acid (molar ratio 1:4) followed by thermal treatment. The sulfuric acid was added dropwise to glycerol (10 g) and stirred for 20 min at 180 °C. The carbonized material was further treated at 120 °C and 350 °C to obtain the samples GBC-120 and GBC-350. The carbon materials were characterized by XRD, FTIR, thermal analysis (TG/DTG/DTA), pHPZC measurements, SEM, and N2 adsorption-desorption at low temperature. The samples GBC-120 and GBC-350 presented BET surface areas of 21 m2·g−1 and 464 m2·g−1, respectively. They were studied for the adsorption of methylene blue (See Section 3.2 for more details).
Gonçalves et al. [53] also prepared glycerol-based activated carbon via two steps (polymerization + chemical activation). Firstly, the glycerol polymer was prepared by glycerol polymerization under reflux in the presence of sulfuric acid. The glycerol polymer was chemically activated with ZnCl2 or H3PO4. The authors also investigated several activated carbon synthesis conditions such as the type of activating agent (ZnCl2 or H3PO4), the impregnation ratio (ZnCl2 (XZn = 0.4 and 0.8) and H3PO4 (XP = 0.3 or 0.6) activating agent mass/polymer mass), activation time, and temperature. They were evaluated as supercapacitor electrode and for the adsorption of organic contaminants (See Section 3.2 and Section 3.3 for more details).
Glycerol-based magnetic carbon composites were synthesized by Medeiros et al. [59]. The carbon composites were prepared by mixing glycerol waste and iron(III) salt (heating to 380 °C, 600 °C, or 800 °C) for 3 h in a vertical reflux reactor. The textural properties of GFe3-380 (5 m2·g−1), GFe3-600 (140 m2·g−1), and GFe3-800 (136 m2·g−1) composites were evaluated by N2 adsorption–desorption isotherms. The carbon composites presented the following surface area 5 m2·g−1 (GFe3-380), 140 m2·g−1 (GFe3-600) and 136 m2·g−1 (GFe3-800). According to the pore size distribution for composites, the samples GFe3-600 and GFe3-800 contain both micropores and mesopores (essentially). The composites (GFe3-600 and GFe3-800) were tested as adsorbents of dyes (methylene blue and indigo carmine) (See Section 3.2 for more detail).
More recently, Batista et al. [52] prepared a series of glycerin-activated carbons from crude glycerin (82% glycerol) for application in the gas separation by adsorption processes. Glycerin-activated carbons were prepared via a two-step procedure involving carbonization followed by chemical activation with KOH. A mixture of industrial crude glycerin (82% glycerol) and concentrated sulfuric acid was prepared using a volume ratio of 1:0.5 (glycerol:H2SO4). The acid carbonization process was carried out in a Teflon lined Hydrothermal Autoclave at 180 °C for 6 h in an oven. The carbonized (glycerin-char) was washed with distilled water until the washing was neutral and dried (100 °C); The obtained solid (glycerin-char, crushed to fine powder of dimension < 0.297 mm) was mixed with an activating agent (KOH) in distilled water, been stirring for 2 h (at ambient temperature) and when dried (100 °C). It was used two activation temperatures (700 °C and 800 °C) and weight ratios (1:1, 2:1 and 3:1, KOH:glycerin-char). The mixture (activating agent:glycerin-char) was activated in a horizontal furnace Thermolyne 21100 (under N2 flow 5 cm3·s−1 and 10 °C·min−1·h−1). The glycerin-activated carbons were washed with distilled water until the washing was neutral and dried at 100 °C. The prepared samples (G@700/1, G@700/2, G@700/3 and G@800/1, G@800/2 and G@800/3—the 700/800 corresponds to the activation temperature and the 1, 2 and 3 to the KOH:glycerin-char ratio) presented high surface areas (1166–2150 m2·g−1) and pore volumes between 0.63 and 1.03 cm3·g−1. These glycerin-activated carbons were evaluated as adsorbents for the adsorption separation of ethane and ethylene (See Section 3.2 for more details).
In another work, Batista et al. [60] modified a glycerin-activated carbon and zeolite type A surfaces with chitosan. The purpose of this work was different from the other works presented here. It was to evaluate the potential of those materials as H2S donors for therapeutic application. The activated carbon (Gta@600) was prepared by a combination of acid carbonization with H2SO4 followed by thermal activation (in a nitrogen flow rate = 5 cm3·min−1 at 600 °C for 1 h). The modification of the material surface was obtained by adding chitosan dissolved in acetic acid solution (1 wt%). to a suspension of Gta@600. The chitosan-based carbon (Gta@600Chi) was characterized (FTIR, SEM, XDR, Elemental analysis and N2 adsorption–desorption isotherms). The adsorption capacity of H2S by Gta@600 and Gta@600Chi was performed to evaluate their use as H2S donors (See Section 3.2 for more information).
3. Principal Uses of Carbons from Glycerol
3.1. Catalysis
The carbons synthesized by R.B.N Prasad et al. [48,49,61,62,63], using the procedure previously described, were tested as a solid-acid catalyst for a diversity of one-pot reactions (described in the following paragraphs) showing very good performance with their activity maintained during several cycles. Yet, no investigation was carried out about leaching into the reaction medium and only one catalyst concentration (10 wt%) was tested.
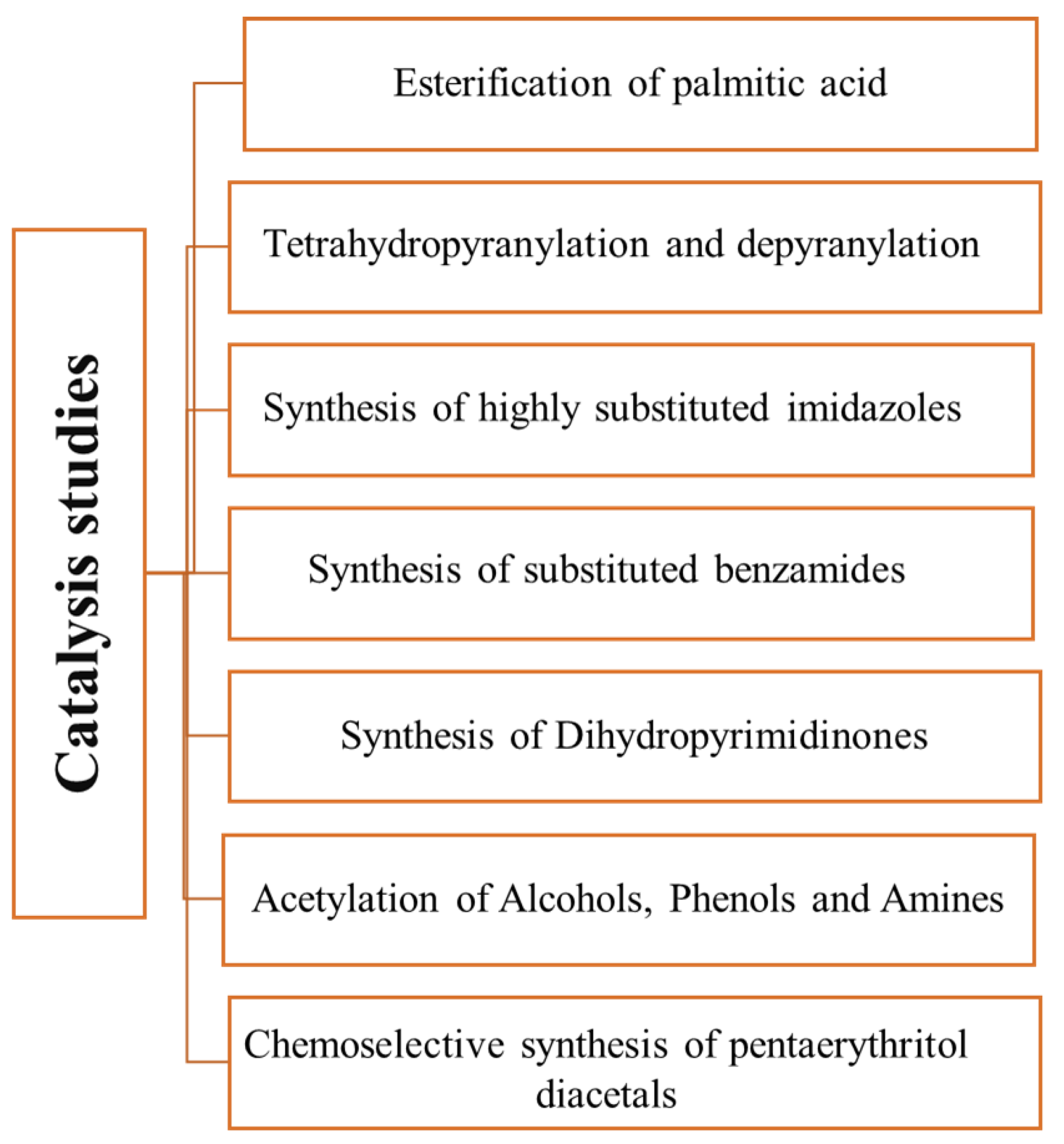
The first carbon obtained by partial carbonization and sulfonation of glycerol [48] was tested as a catalyst in the esterification of palmitic acid with methanol at 65 °C. It revealed a high activity (99% conversion in 4 h). The carbon obtained from pitch glycerol using the same experimental procedure was tested as catalyst for tetrahydropyranylation (THP ether synthesis) and dehydropyranylation using a wide variety of alcohols and phenols (17 total) [49]. The tetrahydropyranylation reactions were performed in dichloromethane at room temperature. The THP ethers were obtained in 80–98% yield, the yield being dependent on the alcohol structure, the lowest yields being observed for phenols due to the lower nucleophilic character of the phenolic oxygen. On the other hand, for the dehydropyranylation reactions, the structure of the substrate had no influence, and reactions yields of 95–99% were obtained using methanol as a solvent. Other reactions were also investigated. For instance, highly substituted imidazole derivatives were obtained from 1,2-diketones, aldehydes, NH4OAc, and amines [61]. A study on the reaction temperature and solvents showed the use of acetonitrile at 50–55 °C gives the highest yields (70–84%). The catalyst was also effective for the synthesis of diverse dihydropyrimidinones in refluxing acetonitrile (yields 80–92%) [63]. The introduction of a halogen onto the aromatic ring yields or replaces urea with thiourea originated the lowest yields. Additionally, the same authors explored the use of the catalysts in the obtention of substituted benzamides (71–78% yield) using different aldehydes and amines as substrates [62]. Many parameters were optimized, and the best condition was attained using acetonitrile at 60–65 °C and Cs2CO3 as a base. Finally, using slightly different conditions, two other reactions were investigated using the same type of catalysts [64]. First, the acetylation of alcohols, phenols, and aromatic amines using acetic anhydride at 65 °C (no-solvent and 15% catalyst) was investigated. The acetylation reactions of the alcohols and phenols had a 75–96% yield, while for aromatic amines had a 95–97% yield. The yields and reaction time of the acetylation of alcohol and phenol were related to their structure. The primary and secondary alcohols had higher yields and rapid reaction times, while the phenols showed slow reactions. Interesting, selectivity for the acetylation of the amine group was observed, a fact that may be explained by more nucleophilicity of amines than phenols. Secondly, using different aldehydes and 2,2-bis (hydroxymethyl) propane-1,3-diol different pentaerythritol diacetals were obtained [22]. Among the investigated reaction conditions (catalyst content and reaction temperature), the best results were obtained using 5 wt% catalyst in toluene at 80 °C. Aliphatic aldehydes showed no reactivity. The catalyst showed selectivity towards aromatic aldehydes. Among the aromatic aldehydes, the presence of the electron-donor group showed less reactivity, while the presence of electron-withdrawing groups enhanced the reaction rate. The catalyst was also effective in the deprotection of the diacetals in methanol in reflux within 30 min. Figure 4 summarizes the studies carried out up to this point.
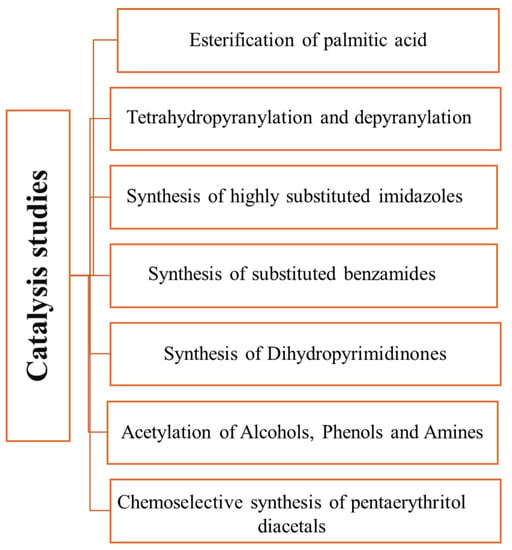
Figure 4.
Reactions studied [22,33,48,49,61,62,63,64] using carbons obtained from glycerol as catalysts.
The group of Gonçalves et al. [50,51] used a glycerin-based carbon obtained by hydrothermal carbonization of a mixture of glycerol waste and sulfuric acid as a catalyst in the glycerol acetalization reaction. The acid character attributed to the –SO3H and –COOH groups at the material surface and the excess of sulfuric acid used during the synthetic procedure affected the final surface chemistry of the material by increasing the surface acidity of these catalysts. The catalyst activity of the carbons through the glycerol acetalization with acetone was dependent on the carbon used as a catalyst. For instance, the 3:1 showed no activity, probably due to the low concentration and absence of –COOH and –SO3H groups, respectively. The importance of the sulfonic groups on the surface was once more confirmed and was highly related to the high catalytic activity of the carbon. For instance, the 3:1 and 2:1 carbon (the ones with higher sulfonic groups) had an 82% conversion of glycerol with almost complete selectivity for solketal. Further studies, using the 2:1 carbon, were conducted to evaluate the effect of different variables in the catalytic activity. They revealed that an increase in the glycerol: acetone molar ratio provided a relevant increase in the glycerol conversion; additionally, an increase in the glycerol conversion was observed. Increasing the amount of catalyst by more than 3% brought no advantage. Regards the temperature reaction, it was observed that at room temperature, the reaction was slightly slower than at 40 °C and 65 °C. However, the process reaches equilibrium at the same level of conversion independent of temperature. The leaching tests showed no appreciable leaching of any active groups present over the surface of the solids.
The same group used carbons obtained by hydrothermal carbonization of a mixture of glycerol waste and sulfuric acid and post-synthesis modified as catalysts for the glycerol etherification reaction [65]. The catalytic activity was evaluated through glycerol etherification with tert-butyl alcohol using a 5 wt% catalyst at 120 °C. The 10:1 carbon showed negligible catalytic activity while the 1:3 carbon had a high catalytic activity (better than Amberlyst resin), a fact that may be attributed to the lower concentration of acidic sites on the 10:1 carbon surface, which is essential for etherification reactions. Interestingly, the post-synthesis modification of the 10:1 carbon led to a substantial improvement in the catalytic (similar to the 1:3 carbon). This improvement was attributed to the introduction of sulfonic acid groups but also to other surface functional groups, such as carboxylic acids. Figure 5 shows the reactions studied by Gonçalves et al. [50,51,65].
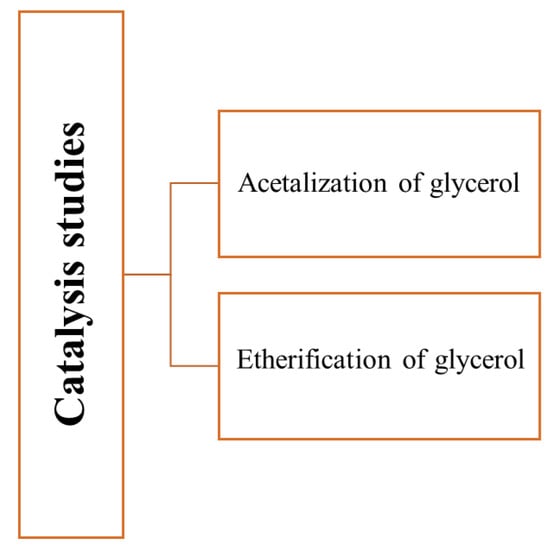
Figure 5.
Reactions studied [50,51,65] using carbons obtained from glycerol as catalysts.
The different reactions in which carbons from glycerol may be used as catalysts are shown in Table 1.

Table 1.
Review of the most relevant data for the indicated reactions using glycerol-based non-porous carbon as catalysts.
The previously described work concerns the use of carbons without activation for the use as catalysts. As already referred, the use of activated carbon is not so usual, and only one work exists that effectively used activated carbons as a catalyst [56]. The catalytic activity of carbons was investigated in the catalytic wet peroxide oxidation (CWPO) of 2-nitrophenol. Adsorption studies revealed that some adsorption on the material surface occurred, yet catalytic activity of the carbons was observed, especially to carbon activated at 300 °C. The characteristics of these materials (developed porosity allied to high basicity and lower oxygen content) seemed to explain their catalytic activity. On the other hand, the higher removal of 2-nitrophenol by the material activated at 350 °C may be explained by a high contribution of the adsorption process. Further studies with the carbon activated at 300 °C revealed its catalytic efficiency was increased when the CWPO process was conducted under intensified conditions (T = 50 °C, pH = 3, stoichiometric amount of H2O2 and a pollutant/catalyst mass ratio = 2). However, the recyclability of the catalyst was studied. The catalyst lost activity after the first cycle due to the adsorption process and the deactivation of the carbon active sites responsible for hydrogen peroxide decomposition, yet its activity may be restored by a simple oxidative thermal regeneration.
3.2. Adsorption
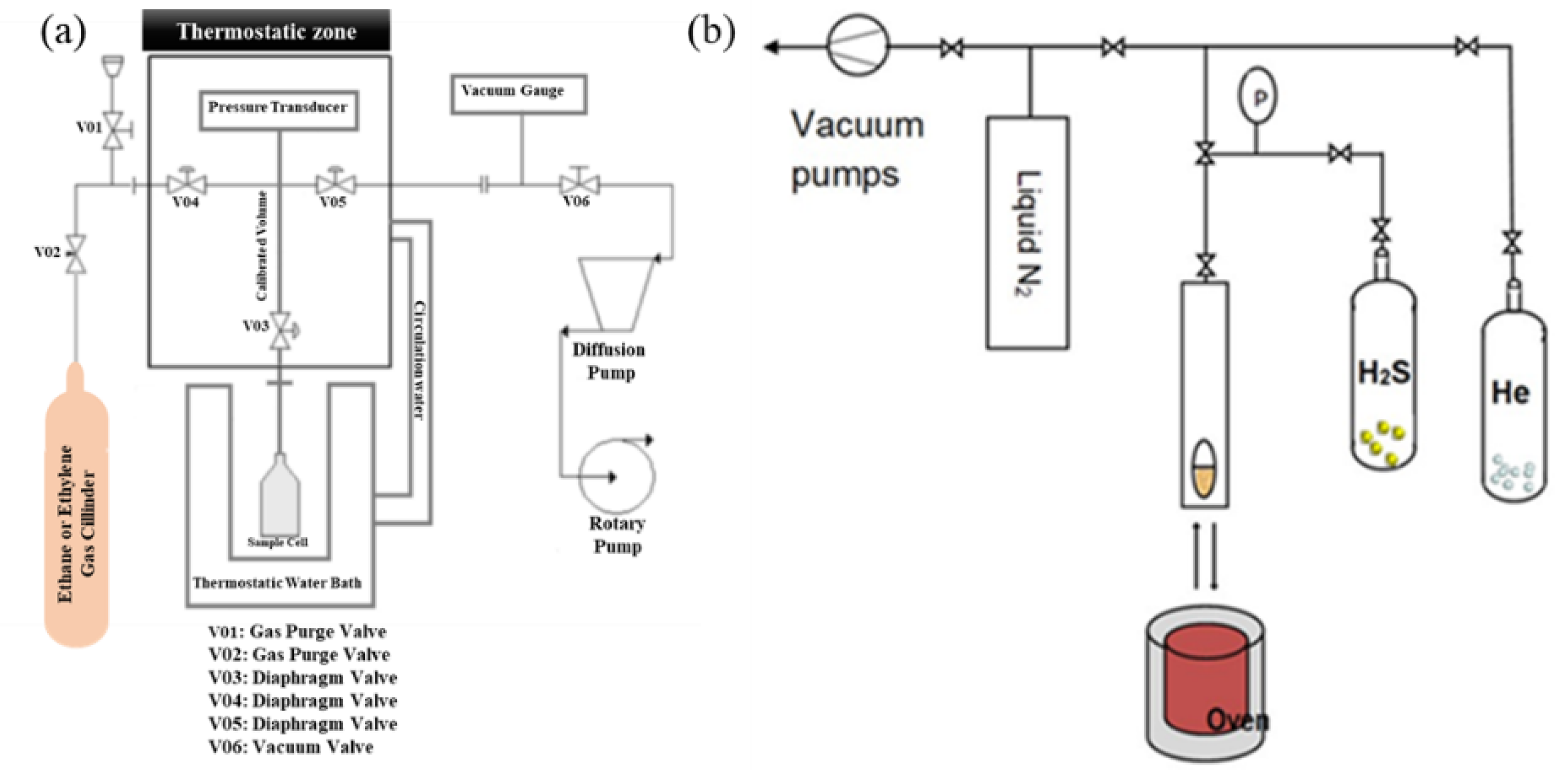
As mentioned, glycerol can be converted into a material that has promising properties for application as adsorbent materials. Their adsorbent capacity was examined to different adsorbates such as medicines (flumequine, tetracycline and paracetamol) [54,65], aqueous phase chromium Cr(VI), dyes (methylene blue and indigo carmine), VOCs (toluene and hexane), and ethene, ethylene. The adsorption studies (Figure 6) in aqueous solutions are different from the gas adsorption, which normally requires special equipment based on gravimetric or volumetric methods. The schematic representation of a volumetric apparatus is shown in Figure 7a, for the ethene/ethylene separation, and in Figure 7b, for H2S adsorption.
Figure 6.
Representation of the adsorption studies.
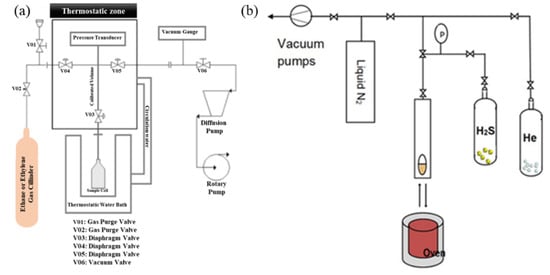
Figure 7.
Volumetric system used for ethane/ethylene separation (a) and H2S adsorption (b) studies. Reproduced with permission from [66], Wyley, 2022.
As already mentioned, to the best of our knowledge, the work published in 2016 by Álvarez-Torrellas et al. was the first to study the application of glycerol-based activated carbons as adsorbent materials [55]. The authors focused on the preparation of 3 activated carbon (GBCM200, GBSM300 and GBCM350) and their application as adsorbent materials for the removal of the antibiotic compounds (flumequine and tetracycline) from aqueous solution. The adsorption of flumequine was found to be dependent on the textural properties of the glycerol-based activated carbon materials. The maximum adsorption capacity (41.5 mg·g−1) was verified onto sample GBCM350. The sequence of flumequine adsorption capacity in the glycerol-activated carbon series was the following: GBCM350 > GBCM300 > GBCM200 with adsorption capacities of 41.5 mg·g−1, 33.7 mg·g−1 and 0.9 mg·g−1, respectively. For tetracycline this sequence was GBCM350 > GBCM200 > GBCM300 (58.1 mg·g−1, 53.9 mg·g−1 and 51.3 mg·g−1, respectively). The activated carbons showed a higher adsorption capacity for tetracycline and its adsorption was almost the same for all three activated carbons, showing that the adsorption of this antibiotic was not dependent on the structural differences obtained at the different activation temperatures used. Additionally, no relation between the antibiotic structure and activated carbon properties was found.
Cui et al. [54] investigated the obtained activated carbon from liquid glycerol as adsorbent for the removal of gas phase volatile organic compounds (VOCs) and aqueous phase chromium Cr(VI). The adsorption capacities reported for toluene, hexane, and Cr(VI) were 1.5 g·g−1, 1.1 g·g−1, and 56 mg·g−1, respectively. The adsorption of the compound in aqueous solutions was much lower than the VOCs, probably due to the competing adsorption of water, making the comparison among them difficult.
Naverkar et al. [58] examined the adsorption of methylene blue by glycerol-based carbons (GBC-120 and GBC-350). The samples GBC-120 and GBC-350 presented BET surface areas of 21 m2·g−1 and 464 m2·g−1, respectively. The sample (GBC-120) exhibited maximum methylene blue adsorption of 1050 mg·g−1. According to the authors, the higher equilibrium adsorption of 1050 mg−1 on GBC-120 was attributed to the presence of a large amount of –SO3H groups compared with GBC-350, where several surface functionalities were lost upon thermal treatment.
Gonçalves et al. [53] also investigated the adsorption of organic contaminants from water: a dye (methylene blue) and a drug (paracetamol) on glycerol-based carbons. The activated carbons from glycerol were also tested as capacitor materials (described in Section 3.3). More recently, glycerol-based magnetic carbon composites were synthesized by Medeiros et al. [59]. The composites (GFe3-600 and GFe3-800) were tested as adsorbents of dyes (methylene blue and indigo carmine). The sample GFe3-800 showed a higher adsorption capacity than GFe3-600 for methylene blue, adsorbed up to 82% and 62% in 60 min, respectively.
In 2021, Batista et al. [52] prepared a series of glycerin-activated carbons from crude glycerin (82% glycerol) for gas separation by adsorption processes. The glycerin-activated carbons were evaluated as adsorbents for the adsorption of ethane and ethylene. All the adsorbents were shown to be ethane selective. The materials exhibited a higher adsorption capacity of ethane (8.92–14.81 mmol·g−1) than ethylene (8.27–12.63 mmol·g−1). The glycerin-activated carbons (except for the sample G@700/3) after two regeneration cycles presented ~100% of the adsorption capacity. In addition, in another work, M. Batista et al. [59] used the glycerin-based activated carbon (Gta@600) and its chitosan-based carbon (Gta@600Chi) as H2S adsorbents. The chitosan-based carbon (Gta@600Chi) presented a H2S insignificant release due to its chemical adsorption. However, the Gta@600 adsorbed a significant amount of H2S and it could be investigated for other applications such as natural gas purification.
The results already available in the literature clearly show activated carbons obtained from glycerol can adsorb compounds with different structures and properties (Table 2), and therefore indicate the importance to extend the research to the adsorption of other class of chemicals. Most of the studies may have applications in environmental problems. However, as was shown they may also be used for separation processes. Nevertheless, and despite their potential, more studies should be conducted, namely regeneration studies. At this moment no commercial products exist, and their development is dependent on more research to be possible obtain high effective products at low production cost. In Table 2 is presented the adsorption data on glycerol-based carbons.

Table 2.
Review of the textural properties and adsorption capacity of glycerol-activated carbon materials.
3.3. Capacitors
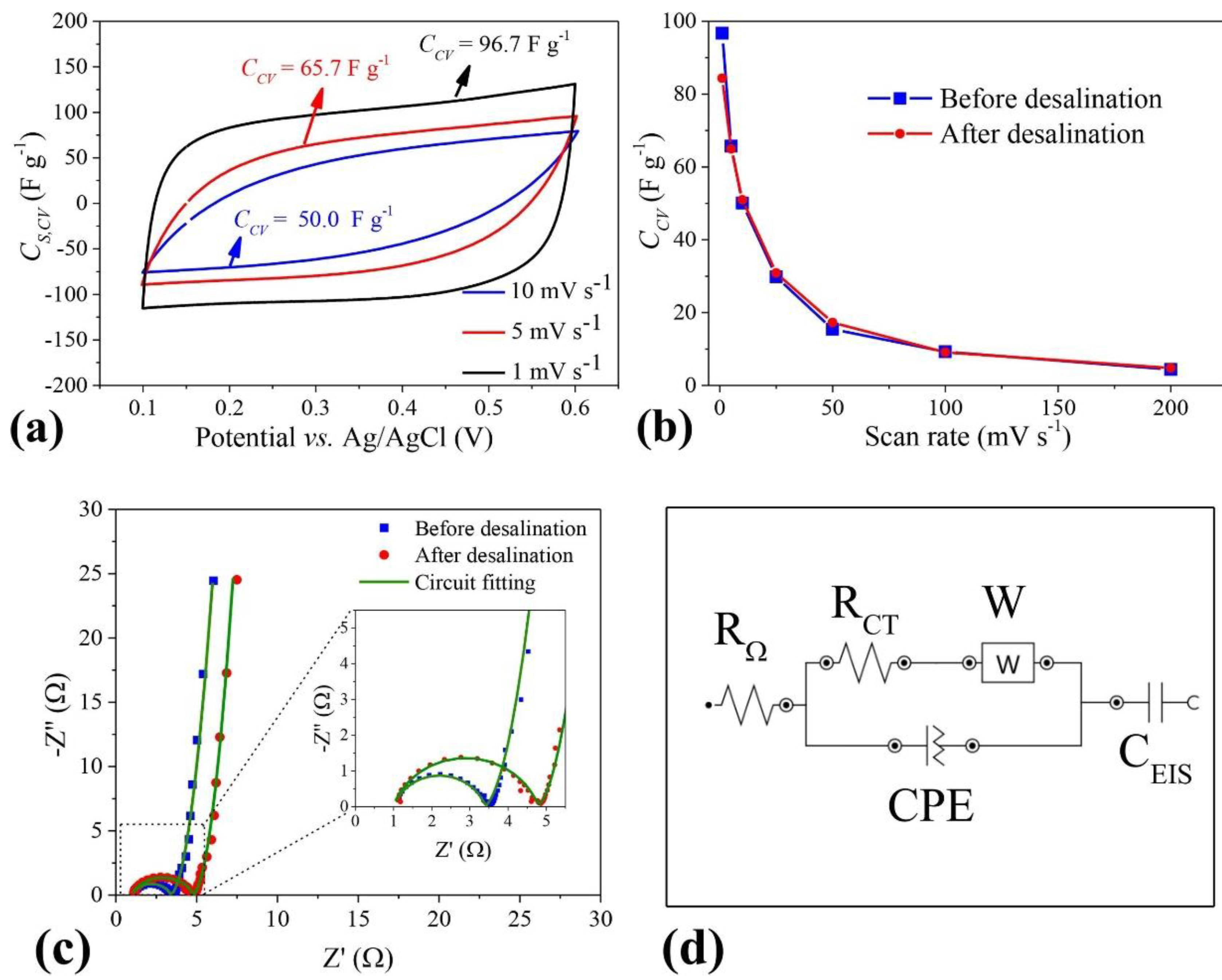
The ability that activated carbons obtained from glycerol may have as capacitors has been investigated very recently and a brief overview of the work done so far is presented. The first reference to this possibility was in 2019 by Gonçalves et al. and is a small part of the work concerning the adsorption capacity of activated carbon described before [53]. The authors selected three activated carbons: two with larger surface areas activated differently and one with the higher micropores/mesopores ratio. Electrodes were prepared by pressing a mixture of activated, multiwalled carbon nanotubes. The more suitable activated carbon was obtained from using ZnCl2 as the activating agent. It presented the higher micropores/mesopores ratio and not the largest surface. This characteristic was attributed to the more suitable pore distribution in this carbon and the higher micropores/mesopores ratio. More recently, Narvekar et al. [67] synthesized carbon from glycerol which was chemically activated with KOH at 800 °C under N2 atmosphere for 2 h. Cyclic voltammetry studies showed the activated carbon had a much higher capacitance than the commercial carbons (Vulcan XC-72 or CNT), a fact that was attributed to the carbonyl and sulphonyl surface functionalities and large surface area with favorable pore size distribution wherein the pores are accessible to form an extended electrical double layer. More recently, Juchen et al. [68] synthesized KOH activated carbon from crude glycerol. The electrodes were prepared by mixing 90 wt% of chemically activated carbon and 10 wt% of Polyvinylidene fluoride (PVDF) in n-methyl-pyrrolidone (NMP) solvent and used for the desalination of brackish water. Figure 8 shows the results of cyclic voltammetry experiences showing the electrode capacitance, resistivity, and mass transfer effects in the desalination process. The electrodes remained stable over 50 desalination/regeneration cycles applying potentials lower than 1.2 V.
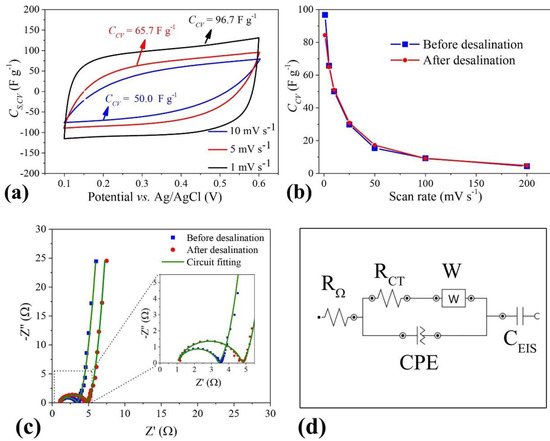
Figure 8.
(a) Specific capacitance from cyclic voltammograms recorded at different scan rates, before desalination; (b) total specific capacitance, as a function of scan rate, before and after desalination applying 1.2 V; (c) Nyquist plots before and after desalination applying 1.2 V; (d) modified Randle equivalent circuit. Working and counter electrodes: PGAC. Electrolyte: 1 mol·L−1 NaCl. Reproduced with permission from [68], Elsevier, 2022.
4. Summary and Outlook
The amount of glycerol produced as a by-product in the biodiesel industry has been increasing. In addition, the use of waste fats (waste and residues), for sustainability reasons, by the biodiesel industry originated glycerol, which may contain unwanted compounds (contaminants). This causes this glycerol not to be used in certain applications such as food or cosmetics, because they do not have the kosher certification as demanded by the food, pharmaceutical, and cosmetic industries. This fact reinforces the need to quickly discover other applications for this glycerol and its use for the synthesis of carbons may be a solution, as may be seen by the work developed so far and their wide applications. The carbons from glycerol have been successfully used in a wide range of applications such as catalyst for a wide range of reactions such as acetylation, etherification, synthesis of substituted imidazoles, and benzamides, among others. The activated carbons have been used as adsorbent of gases (H2S, VOCs, ethene and ethylene) and liquid (dyes and pharmaceuticals) pollutants, and capacitor materials. Nevertheless, this research is still in its initial stages in comparison with other carbons, and optimization of the synthetic procedures by changing the activated agent, temperatures, and pressure may give rise to more effective materials for a given application. Other possibilities could be surface functional group variation on the activated carbon surface, which may be achieved using different treatment parameters, or by post-synthesis modification, a possibility that has not been investigated so far. A systematic study of surface modification may help in obtaining better materials for the intended application.
Concerning practical applications, the adsorption process is the most promising for the glycerol-based active carbons. Adsorption technology is known for its simplicity, reliability, and low energy and maintenance costs, and it is already being used in many situations. The viability of this process is very dependent on the adsorbent. The use of glycerol-based activated carbons as adsorbent will depend on the possibility of producing this material using an energetic and environmentally sustainable processes.
Author Contributions
Conceptualization, M.B., S.C., J.P., M.L.P. and R.C.; methodology, M.B. and S.C.; validation, M.B. and S.C.; formal analysis, M.B. and S.C.; investigation, M.B. and S.C.; writing—original draft preparation, M.B. and S.C.; writing—review and editing, M.B., S.C., J.P., M.L.P. and R.C.; funding acquisition, J.P. and M.L.P.; M.B. and S.C. contributes equally for the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by Fundação para a Ciência e a Tecnologia (FCT) developed in the scope of the projects UIDB/00100/2020 (CQE), UIDB/04028/2020 & UIDP/04028/2020 (CERENA).
Data Availability Statement
No applicable.
Acknowledgments
This work has received funding from Fundação para a Ciência e a Tecnologia (FCT) in the scope of the projects UIDB/00100/2020 (CQE), UIDB/04028/2020 (CQE), IMS—LA/P/0056/2020 & UIDP/04028/2020 (CERENA). MB and SC acknowledge for FCT-Investigator contract-DL57 and PTDC/MEDQUI/28271/2017, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef] [PubMed]
- Niekurzak, M. Determining the Unit Values of the Allocation of Greenhouse Gas Emissions for the Production of Biofuels in the Life Cycle. Energies 2021, 14, 8394. [Google Scholar] [CrossRef]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Porte, A.F.; Schneider, R.D.C.D.S.; Kaercher, J.A.; Klamt, R.A.; Schmatz, W.L.; da Silva, W.L.T.; Filho, W.A.S. Sunflower biodiesel production and application in family farms in Brazil. Fuel 2010, 89, 3718–3724. [Google Scholar] [CrossRef]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Jang, M.G.; Kim, D.K.; Park, S.C.; Lee, J.S.; Kim, S.W. Biodiesel production from crude canola oil by two-step enzymatic processes. Renew. Energy 2012, 42, 99–104. [Google Scholar] [CrossRef]
- Folaranmi, J. Production of Biodiesel (B100) from Jatropha Oil Using Sodium Hydroxide as Catalyst. J. Pet. Eng. 2013, 2013, 430–438. [Google Scholar] [CrossRef]
- Sharma, L.; Grover, N.K.; Bhardwaj, M.; Kaushal, I. Comparison of Engine Performance of Mixed Jatropha and Cottonseed Derived Biodiesel Blends with Conventional Diesel. Int. J. Emerg. Technol. 2012, 3, 29–32. [Google Scholar]
- Köse, Ö.; Tüter, M.; Aksoy, H.A. Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium. Bioresour. Technol. 2002, 83, 125–129. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Dosuna-Rodríguez, I.; Gaigneaux, E. Glycerol acetylation catalysed by ion exchange resins. Catal. Today 2012, 195, 14–21. [Google Scholar] [CrossRef]
- Choi, W.J. Glycerol-Based Biorefinery for Fuels and Chemicals. Recent Pat. Biotechnol. 2008, 2, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.; Vicente, G.; Morales, G.; Paniagua, M.; Moreno, J.; Roldán, R.; Ezquerro, A.; Pérez, C. Acid-catalyzed etherification of bio-glycerol and isobutylene over sulfonic mesostructured silicas. Appl. Catal. A Gen. 2008, 346, 44–51. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Xia, L.-Z.; Yang, M.; He, M.; Jiang, M.-Z.; Qin, C.; Wei, Z.-J.; Gao, H.-T. Food emulsifier glycerin monostearate aggravates phthalates’ testicular toxicity by disrupting tight junctions’ barrier function in rats. Food Qual. Saf. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.W.; Saini, M.; Lin, L.-J.; Chiang, C.-J.; Chao, Y.-P. Systematic Engineering of Escherichia coli for d-Lactate Production from Crude Glycerol. J. Agric. Food Chem. 2015, 63, 9583–9589. [Google Scholar] [CrossRef]
- Feng, X.; Ding, Y.; Xian, M.; Xu, X.; Zhang, R.; Zhao, G. Production of optically pure d -lactate from glycerol by engineered Klebsiella pneumoniae strain. Bioresour. Technol. 2014, 172, 269–275. [Google Scholar] [CrossRef]
- Khan, A.; Bhide, A.; Gadre, R. Mannitol production from glycerol by resting cells of Candida magnoliae. Bioresour. Technol. 2009, 100, 4911–4913. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. Glycerol: Properties and production. In The Future of Glycerol, 2nd ed.; Green Chemistry Series; RSC: Cambridge, UK, 2010; pp. 1–187. [Google Scholar]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Ummadisetti, C.; Rachapudi, B.N.P.; Bethala, L.A.P.D. Glycerol-based SO3H-Carbon Catalyst: A green recyclable catalyst for the chemoselective synthesis of pentaerythritol diacetals. Eur. J. Chem. 2014, 5, 536–540. [Google Scholar] [CrossRef]
- Hejna, A.; Kosmela, P.; Formela, K.; Piszczyk, Ł; Haponiuk, J.T. Potential applications of crude glycerol in polymer technology–Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 66, 449–475. [Google Scholar] [CrossRef]
- Hara, M.; Verkman, A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7360–7365. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Sparavigna, A. The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 6S–22S. [Google Scholar] [CrossRef]
- Silva, P.H.; Gonçalves, V.L.; Mota, C.J. Glycerol acetals as anti-freezing additives for biodiesel. Bioresour. Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef]
- Trifoi, A.R.; Agachi, P.; Pap, T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew. Sustain. Energy Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Li, L.; Lee, E.S.; Nguyen, C.; Zhu, Y. Effects of propylene glycol, vegetable glycerin, and nicotine on emissions and dynamics of electronic cigarette aerosols. Aerosol Sci. Technol. 2020, 54, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.G.; Dutta, D.; Kazipeta, K.; Chong, N.S. Influence of the E-Cigarette Emission Profile by the Ratio of Glycerol to Propylene Glycol in E-Liquid Composition. ACS Omega 2019, 4, 13338–13348. [Google Scholar] [CrossRef]
- Woodall, M.; Jacob, J.; Kalsi, K.K.; Schroeder, V.; Davis, E.; Kenyon, B.; Khan, I.; Garnett, J.P.; Tarran, R.; Baines, D.L. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L957–L967. [Google Scholar] [CrossRef]
- Rarata, G.; Smętek, J. Explosives Based on Hydrogen Peroxide—A Historical Review and Novel Applications. High-Energ. Mater. 2016, 8, 56–62. [Google Scholar]
- Hong, X.; McGiveron, O.; Kolah, A.K.; Orjuela, A.; Peereboom, L.; Lira, C.T.; Miller, D.J. Reaction kinetics of glycerol acetal formation via transacetalization with 1,1-diethoxyethane. Chem. Eng. J. 2013, 222, 374–381. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening. Appl. Energy 2014, 123, 75–81. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Basir, N.I.; Abdullah, A.Z. A review on one-pot synthesis of acrylic acid from glycerol on bi-functional catalysts. J. Ind. Eng. Chem. 2021, 93, 216–227. [Google Scholar] [CrossRef]
- Batista, M.K.S.; Mestre, A.S.; Matos, I.; Fonseca, I.M.; Carvalho, A.P. Biodiesel production waste as promising biomass precursor of reusable activated carbons for caffeine removal. RSC Adv. 2016, 6, 45419–45427. [Google Scholar] [CrossRef]
- Bernardo, M.; Rodrigues, S.; Lapa, N.; Matos, I.; Lemos, F.; Batista, M.K.S.; Carvalho, A.P.; Fonseca, I. High efficacy on diclofenac removal by activated carbon produced from potato peel waste. Int. J. Environ. Sci. Technol. 2016, 13, 1989–2000. [Google Scholar] [CrossRef]
- Osman, A.I.; Blewitt, J.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Production and characterisation of activated carbon and carbon nanotubes from potato peel waste and their application in heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 37228–37241. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Ruiz, M.; Rolz, C. Activated Carbons from Sugar Cane Bagasse. Ind. Eng. Chem. Prod. Res. Dev. 1971, 10, 429–432. [Google Scholar] [CrossRef]
- Kemp, K.; Baek, S.-B.; Lee, W.-G.; Meyyappan, M.; Kim, K.S. Activated carbon derived from waste coffee grounds for stable methane storage. Nanotechnology 2015, 26, 385602. [Google Scholar] [CrossRef] [PubMed]
- Pagalan, E., Jr.; Sebron, M.; Gomez, S.; Salva, S.J.; Ampusta, R.; Macarayo, A.J.; Joyno, C.; Ido, A.; Arazo, R. Activated carbon from spent coffee grounds as an adsorbent for treatment of water contaminated by aniline yellow dye. Ind. Crops Prod. 2020, 145, 111953. [Google Scholar] [CrossRef]
- Riyanto; Astuti, R.; Mukti, B.I. Simple preparation of rice husk activated carbon (RHAC) and applications for laundry and methylene blue wastewater treatment. AIP Conf. Proc. 2017, 1911, 20033. [Google Scholar] [CrossRef]
- Sharath, D.; Ezana, J.; Shamil, Z. Production of activated carbon from solid waste rice peel (husk) using chemical activation. J. Ind. Pollut. Control 2017, 33, 1132–1139. [Google Scholar]
- Tsai, W.; Chang, C.; Lee, S. A low cost adsorbent from agricultural waste corn cob by zinc chloride activation. Bioresour. Technol. 1998, 64, 211–217. [Google Scholar] [CrossRef]
- Medhat, A.; El-Maghrabi, H.H.; Abdelghany, A.; Abdel Menem, N.M.; Raynaud, P.; Moustafa, Y.M.; Elsayed, M.A.; Nada, A.A. Efficiently activated carbons from corn cob for methylene blue adsorption. Appl. Surf. Sci. Adv. 2021, 3, 100037. [Google Scholar] [CrossRef]
- Devi, B.L.A.P.; Gangadhar, K.N.; Prasad, P.S.S.; Jagannadh, B.; Prasad, R.B.N. A Glycerol-based Carbon Catalyst for the Preparation of Biodiesel. ChemSusChem 2009, 2, 617–620. [Google Scholar] [CrossRef]
- Prabhavathi Devi, B.L.A.; Gangadhar, K.N.; Siva Kumar, K.L.N.; Shiva Shanker, K.; Prasad, R.B.N.; Sai Prasad, P.S. Synthesis of sulfonic acid functionalized carbon catalyst from glycerol pitch and its application for tetrahydropyranyl protection/deprotection of alcohols and phenols. J. Mol. Catal. A Chem. 2011, 345, 96–100. [Google Scholar] [CrossRef]
- Mantovani, M.; Aguiar, E.M.; Carvalho, W.A.; Mandelli, D.; Gonçalves, M. Utilization of biodiesel waste for acid carbon preparation with high catalyst activity in the glycerol etherification reaction. Quim. Nova 2015, 38, 526–532. [Google Scholar] [CrossRef]
- Gonçalves, M.; Rodrigues, R.; Galhardo, T.S.; Carvalho, W.A. Highly selective acetalization of glycerol with acetone to solketal over acidic carbon-based catalysts from biodiesel waste. Fuel 2016, 181, 46–54. [Google Scholar] [CrossRef]
- Batista, M.; Pinto, M.L.; Carvalho, R.; Pires, J. Glycerin-based adsorbents for the separation of ethane and ethylene. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127975. [Google Scholar] [CrossRef]
- Gonçalves, M.; Castro, C.S.; Boas, I.K.V.; Soler, F.C.; Pinto, E.D.C.; Lavall, R.L.; Carvalho, W.A. Glycerin waste as sustainable precursor for activated carbon production: Adsorption properties and application in supercapacitors. J. Environ. Chem. Eng. 2019, 7, 103059. [Google Scholar] [CrossRef]
- Cui, Y.; Atkinson, J.D. Tailored activated carbon from glycerol: Role of acid dehydrator on physiochemical characteristics and adsorption performance. J. Mater. Chem. A 2017, 5, 16812–16821. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Ribeiro, R.; Gomes, H.; Ovejero, G.; García, J. Removal of antibiotic compounds by adsorption using glycerol-based carbon materials. Chem. Eng. J. 2016, 296, 277–288. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pinho, M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Development of glycerol-based metal-free carbon materials for environmental catalytic applications. Catal. Today 2015, 240, 61–66. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jin, M.-H.; Park, J.C.; Lee, C.-B.; Oh, D.-K.; Lee, S.-W.; Park, J.-W.; Park, J.-S. Waste-Glycerol-Directed Synthesis of Mesoporous Silica and Carbon with Superior Performance in Room-Temperature Hydrogen Production from Formic Acid. Sci. Rep. 2015, 5, 15931. [Google Scholar] [CrossRef]
- Narvekar, A.A.; Fernandes, J.; Tilve, S. Adsorption behavior of methylene blue on glycerol based carbon materials. J. Environ. Chem. Eng. 2018, 6, 1714–1725. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Ardisson, J.D.; Lago, R.M. Preparation of magnetic mesoporous composites from glycerol and iron(III) salt. J. Chem. Technol. Biotechnol. 2020, 95, 1038–1045. [Google Scholar] [CrossRef]
- Batista, M.; Pinto, M.L.; Antunes, F.; Pires, J.; Carvalho, S. Chitosan Biocomposites for the Adsorption and Release of H2S. Materials 2021, 14, 6701. [Google Scholar] [CrossRef]
- Ramesh, K.; Murthy, S.N.; Karnakar, K.; Nageswar, Y.V.D.; Vijayalakhshmi, K.; Prabhavathi Devi, B.L.A.; Prasad, R.B.N. A novel bioglycerol-based recyclable carbon catalyst for an efficient one-pot synthesis of highly substituted imidazoles. Tetrahedron Lett. 2012, 53, 1126–1129. [Google Scholar] [CrossRef]
- Ramesh, K.; Murthy, S.N.; Karnakar, K.; Reddy, K.H.V.; Nageswar, Y.V.D.; Vijay, M.; Devi, B.P.; Prasad, R.B.N. A mild and expeditious synthesis of amides from aldehydes using bio glycerol-based carbon as a recyclable catalyst. Tetrahedron Lett. 2012, 53, 2636–2638. [Google Scholar] [CrossRef]
- Konkala, K.; Sabbavarapu, N.M.; Katla, R.; Durga, N.Y.V.; Kumar Reddy, T.V.; Prabhavathi, P.D.; Rachapudi, B.N.P. Revisit to the Biginelli reaction: A novel and recyclable bioglycerol-based sulfonic acid functionalized carbon catalyst for one-pot synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones. Tetrahedron Lett. 2012, 53, 1968–1973. [Google Scholar] [CrossRef]
- Gangadhar, K.N.; Vijay, M.; Prasad, R.B.N.; Devi, B.L.A.P. Glycerol-Based Carbon-SO3H Catalyzed Benign Synthetic Protocol for the Acetylation of Alcohols, Phenols and Amines under Solvent-Free Conditions. Green Sustain. Chem. 2013, 03, 122–128. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, M.; Mantovani, M.; Carvalho, W.A.; Rodrigues, R.; Mandelli, D.; Albero, J.S. Biodiesel wastes: An abundant and promising source for the preparation of acidic catalysts for utilization in etherification reaction. Chem. Eng. J. 2014, 256, 468–474. [Google Scholar] [CrossRef]
- Pinto, R.V.; Carvalho, S.; Antunes, F.; Pires, J.; Pinto, M.L. Emerging Nitric Oxide and Hydrogen Sulfide Releasing Carriers for Skin Wound Healing Therapy. ChemMedChem 2022, 17, e202100429. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, A.A.; Fernandes, J.; Naik, S.; Tilve, S. Development of glycerol based carbon having enhanced surface area and capacitance obtained by KOH induced thermochemical activation. Mater. Chem. Phys. 2021, 261, 124238. [Google Scholar] [CrossRef]
- Juchen, P.T.; Barcelos, K.M.; Oliveira, K.S.; Ruotolo, L.A. Using crude residual glycerol as precursor of sustainable activated carbon electrodes for capacitive deionization desalination. Chem. Eng. J. 2022, 429, 132209. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).