Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides: Recent Advances 2008–2021: Part I
Abstract
:1. Introduction
2. Iodination of Alkanes
3. Iodination of Alkenes and Alkynes
4. Iodination of Alkyl Carbonyls Compounds to α-Iodo Alkyl Carbonyl Derivatives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Togo, H.; Iida, S. Synthetic Use of Molecular Iodine for Organic Synthesis. Synlett 2006, 2006, 2159–2175. [Google Scholar] [CrossRef]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpenter, L.J.; Luther, G.W., III; Lu, Z.; Jonsson, M.; et al. Commemorating Two Centuries of Iodine Research: An Interdisciplinary Overview of Current Research. Angew. Chem. Int. Ed. 2011, 50, 11598–11620. [Google Scholar] [CrossRef] [PubMed]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic oxidative iodination of ketones catalysed by sodium nitrite “on water” or in a micelle-based aqueous system. Green Chem. 2009, 11, 1262–1267. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Balar, C.R.; Sharma, V.S.; Prajapati, N.P.; Vekariya, M.K.; Sharma, A.S. Preparation of α-Iodocarbonyl Compounds: An Overall Development. ChemistrySelect 2018, 3, 9189–9203. [Google Scholar] [CrossRef]
- Mphahlele, M.J. Molecular Iodine-Mediated α-Iodination of Carbonyl Compounds. J. Chem. Res. 2010, 34, 121–126. [Google Scholar] [CrossRef]
- Stavber, S.; Jereb, M.; Zupan, M. Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides. Synthesis 2008, 2008, 1487–1513. [Google Scholar] [CrossRef]
- Chouthaiwale, P.V.; Suryavanshi, G.; Sudalai, A. NaIO4–KI–NaN3 as a new reagent system for C–H functionalization in hydrocarbons. Tetrahedron Lett. 2008, 49, 6401–6403. [Google Scholar] [CrossRef]
- Giri, R.; Lan, Y.; Liu, P.; Houk, K.N.; Yu, J.-Q. Understanding Reactivity and Stereoselectivity in Palladium-Catalyzed Diastereoselective sp3 C–H Bond Activation: Intermediate Characterization and Computational Studies. J. Am. Chem. Soc. 2012, 134, 14118–14126. [Google Scholar] [CrossRef]
- Zhu, R.-Y.; Saint-Denis, T.G.; Shao, Y.; He, J.; Sieber, J.D.; Senanayake, C.H.; Yu, J.-Q. Ligand-Enabled Pd(II)-Catalyzed Bromination and Iodination of C(sp3)–H Bonds. J. Am. Chem. Soc. 2017, 139, 5724–5727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jereb, M.; Zupan, M.; Stavber, S. Hydrogen peroxide induced iodine transfer into alkenes. Green Chem. 2005, 7, 100–104. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic Oxidative Iodination of Organic Compounds with Iodide Catalyzed by Sodium Nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X. Iodohydroxylation of Alkylidenecyclopropanes. An Efficient Synthesis of Iodocyclopropylmethanol and 3-Iodobut-3-en-1-ol Derivatives. J. Org. Chem. 2008, 73, 4702–4704. [Google Scholar] [CrossRef]
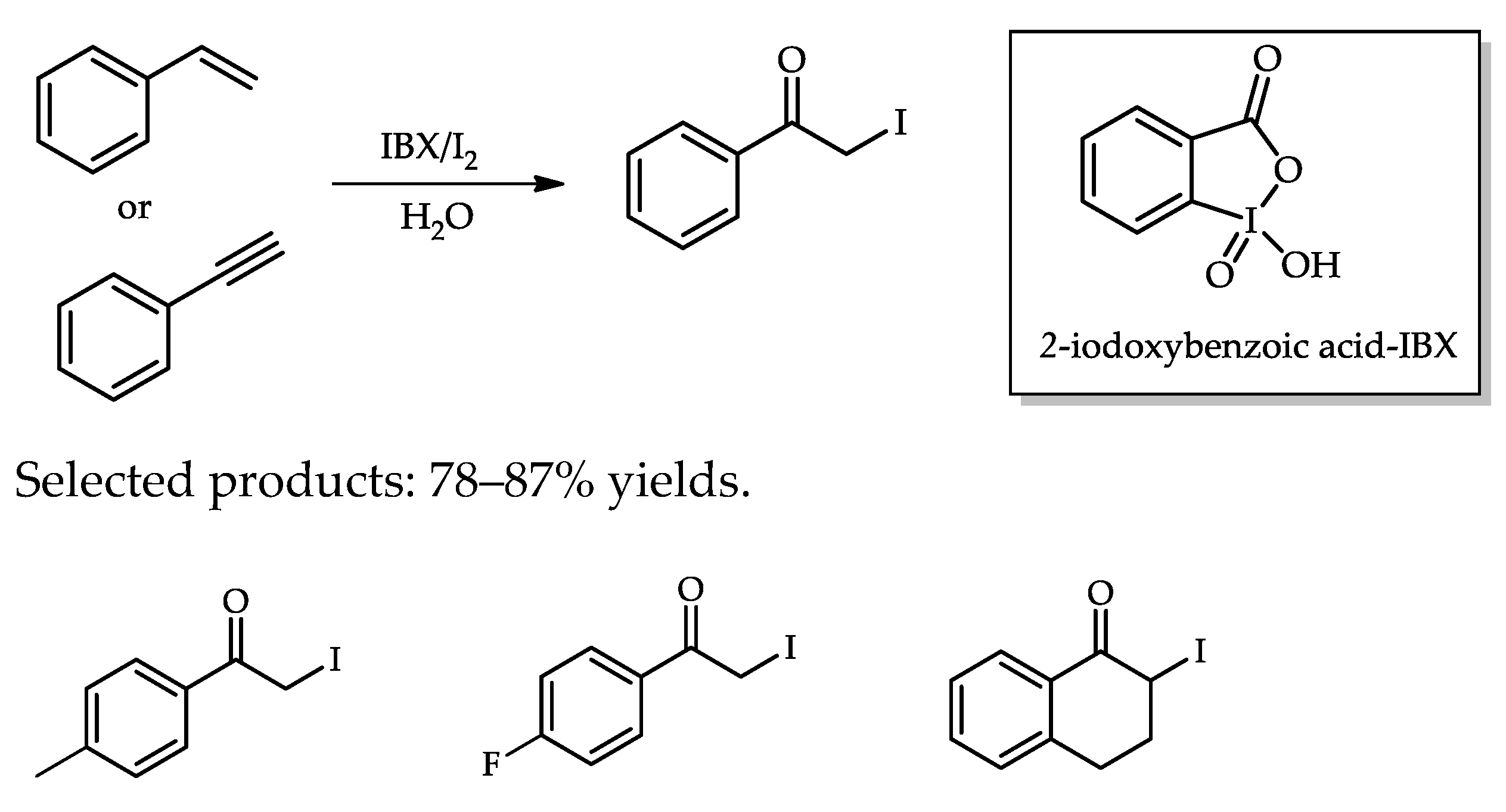
- Yadav, J.S.; Subba Reddy, B.V.; Singh, A.P.; Basak, A.K. IBX/I2-mediated oxidation of alkenes and alkynes in water: A facile synthesis of α-iodoketones. Tetrahedron Lett. 2008, 49, 5880–5882. [Google Scholar] [CrossRef]
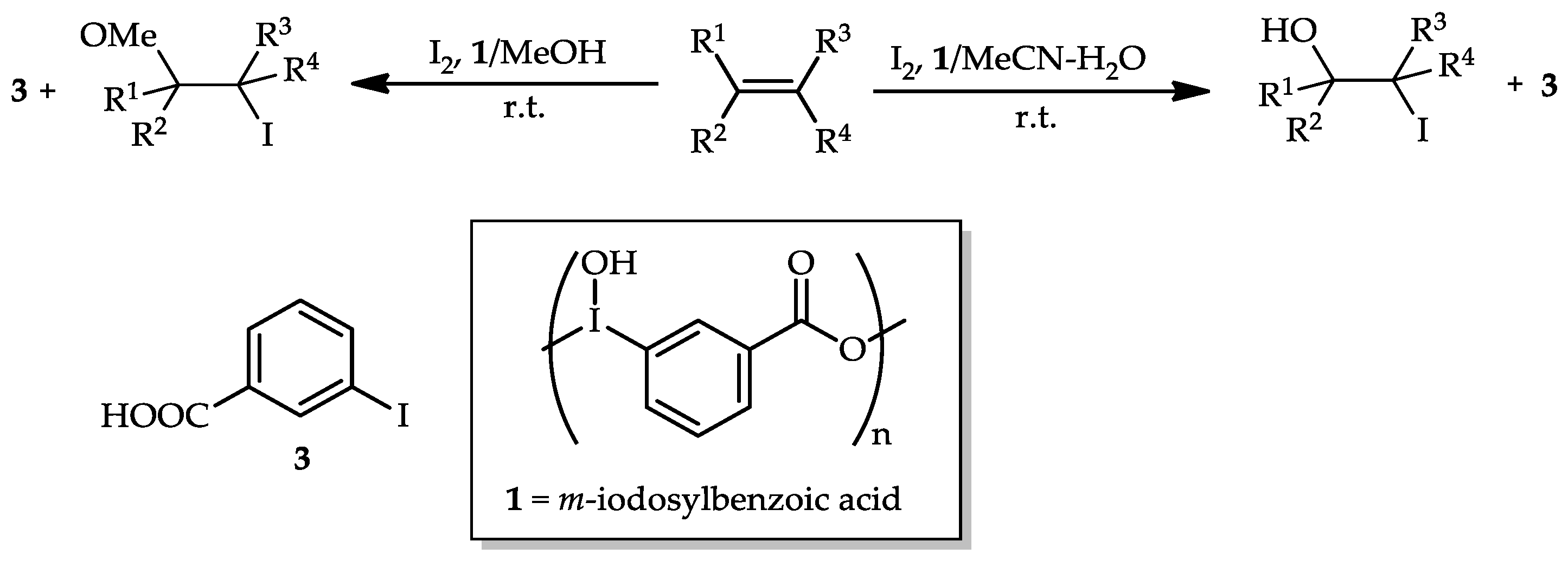
- Yusubov, M.S.; Yusubova, R.Y.; Kirschning, A.; Park, J.Y.; Chi, K.-W. m-Iodosylbenzoic acid, a tagged hypervalent iodine reagent for the iodo-functionalization of alkenes and alkynes. Tetrahedron Lett. 2008, 49, 1506–1509. [Google Scholar] [CrossRef]
- Yang, H.; Xu, B.; Hammond, G.B. Highly Regioselective Fluorination and Iodination of Alkynyl Enolates. Org. Lett. 2008, 10, 5589–5591. [Google Scholar] [CrossRef] [PubMed]
- Sakee, U.; Nasuk, C.; Grigg, R. Synthesis of 1-C-(tetra-O-acetyl-β-d-galactopyranosyl)-2,3-diiodo-1-propene and its reaction with primary amines. Carbohydr. Res. 2009, 344, 2096–2099. [Google Scholar] [CrossRef]
- Agrawal, M.K.; Adimurthy, S.; Ganguly, B.; Ghosh, P.K. Comparative study of the vicinal functionalization of olefins with 2:1 bromide/bromate and iodide/iodate reagents. Tetrahedron 2009, 65, 2791–2797. [Google Scholar] [CrossRef]
- Nagura, H.; Kuribayashi, S.; Ishiguro, Y.; Inagi, S.; Fuchigami, T. Electrochemical iodofluorination of electron-deficient olefins. Tetrahedron 2010, 66, 183–186. [Google Scholar] [CrossRef]
- Nobuta, T.; Hirashima, S.-I.; Tada, N.; Miura, T.; Itoh, A. Facile Aerobic Photo-Oxidative Synthesis of Phenacyl Iodides and Bromides from Styrenes Using I2 or Aqueous HBr. Synlett 2010, 2010, 2335–2339. [Google Scholar]
- Chouthaiwale, P.V.; Karabal, P.U.; Suryavanshi, G.; Sudalai, A. Regiospecific Azidoiodination of Alkenes with Sodium Periodate, Potassium Iodide, and Sodium Azide: A High-Yield Synthesis of β-Iodoazides. Synthesis 2010, 2010, 3879–3882. [Google Scholar] [CrossRef]
- Hanessian, S.; Focken, T.; Oza, R. Total Synthesis of Jerangolid A. Org. Lett. 2010, 12, 3172–3175. [Google Scholar] [CrossRef]
- Samakkanad, N.; Katrun, P.; Techajaroonjit, T.; Hlekhlai, S.; Pohmakotr, M.; Reutrakul, V.; Jaipetch, T.; Soorukram, D.; Kuhakarn, C. IBX/I2-Mediated Reaction of Sodium Arenesulfinates with Alkenes: Facile Synthesis of β-Keto Sulfones. ChemInform 2012, 44, 1693–1699. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.-H.; Lee, H.-J.; Kim, J.-N. One-Pot Synthesis of 5-Hydroxypyrrolin-2-one Derivatives from Modified Morita-Baylis-Hillman Adducts via a Consecutive CuI-Mediated Aerobic Oxidation, Allylic Iodination, Hydration of Nitrile, and Lactamization. Bull. Korean Chem. Soc. 2012, 33, 2079–2082. [Google Scholar] [CrossRef] [Green Version]
- Yemets, S.V.; Shubina, T.E.; Krasutsky, P.A. Electrophilic monoiodination of terminal alkenes. Org. Biomol. Chem. 2013, 11, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Moskalik, M.Y.; Shainyan, B.A.; Astakhova, V.V.; Schilde, U. Oxidative addition of trifluoromethanesulfonamide to cycloalkadienes. Tetrahedron 2013, 69, 705–711. [Google Scholar] [CrossRef]
- Gottam, H.; Vinod, T.K. Versatile and Iodine Atom-Economic Co-Iodination of Alkenes. J. Org. Chem. 2011, 76, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, C.; Ma, S. Highly Regio- and Stereoselective Iodohydroxylation of 1,2-Allenylic Sulfoxides in the Presence of Benzyl Thiol. Adv. Synth. Catal. 2011, 353, 1775–1786. [Google Scholar] [CrossRef]
- Beltran, R.; Nocquet-Thibault, S.; Blanchard, F.; Dodd, R.H.; Cariou, K. PIFA-mediated ethoxyiodination of enamides with potassium iodide. Org. Biomol. Chem. 2016, 14, 8448–8451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durgaiah, C.; Naresh, M.; Arun Kumar, M.; Swamy, P.; Reddy, M.M.; Srujana, K.; Narender, N. Regio- and stereoselective co-iodination of olefins using NH4I and Oxone. Synth. Commun. 2016, 46, 1133–1144. [Google Scholar] [CrossRef]
- Kaswan, P.; Shelke, G.M.; Rao, V.K.; Kumar, A. Hydroxy-Group-Facilitated Vinylic Iodination of ortho-Vinylnaphthols Using Molecular Iodine. Synlett 2016, 27, 2553–2556. [Google Scholar]
- Xinwei, L.; Song, S.; Ning, J. Oxidative Iodohydroxylation of Olefins with DMSO. Acta Chim. Sinica 2017, 75, 1202–1206. [Google Scholar]
- Wang, H.; Chen, C.; Liu, W.; Zhu, Z. Difunctionalization of alkenes with iodine and tert-butyl hydroperoxide (TBHP) at room temperature for the synthesis of 1-(tert-butylperoxy)-2-iodoethanes. Beilstein J. Org. Chem. 2017, 13, 2023–2027. [Google Scholar] [CrossRef]
- Hokamp, T.; Storm, A.T.; Yusubov, M.; Wirth, T. Iodine Monoacetate for Efficient Oxyiodinations of Alkenes and Alkynes. Synlett 2018, 29, 415–418. [Google Scholar]
- Yi, W.; Wang, P.-F.; Lu, M.; Liu, Q.-Q.; Bai, X.; Chen, K.-D.; Zhang, J.-W.; Liu, G.-Q. Environmentally Friendly Protocol for the Oxidative Iodofunctionalization of Olefins in a Green Solvent. ACS Sustain. Chem. Eng. 2019, 7, 16777–16785. [Google Scholar] [CrossRef]
- Shakhmaev, R.N.; Sunagatullina, A.S.; Ignatishina, M.G.; Yunusova, E.Y.; Zorin, V.V. Synthesis of Ethyl (2E)-5-Phenylpent-2-en-4-ynoate. Russ. J. Org. Chem. 2019, 55, 897–899. [Google Scholar] [CrossRef]
- Meng, L.G.; Cai, P.J.; Guo, Q.X.; Xue, S. Direct Iodination of Monosubstituted Aryl Acetylenes and Acetylenic Ketones. Synth. Commun. 2008, 38, 225–231. [Google Scholar] [CrossRef]
- Chen, S.-N.; Hung, T.-T.; Lin, T.-C.; Tsai, F.-Y. Reusable and Efficient Cul/TBAB-Catalyzed Iodination of Terminal Alkynes in Water under Air. J. Chin. Chem. Soc. 2009, 56, 1078–1081. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kitamura, T. Iodoarylation of Arylalkynes with Molecular Iodine in the Presence of Hypervalent Iodine Reagents. Molecules 2009, 14, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Rajender Reddy, K.; Venkateshwar, M.; Uma Maheswari, C.; Santhosh Kumar, P. Mild and efficient oxy-iodination of alkynes and phenols with potassium iodide and tert-butyl hydroperoxide. Tetrahedron Lett. 2010, 51, 2170–2173. [Google Scholar] [CrossRef]
- Kawaguchi, S.-I.; Ogawa, A. Highly Selective Hydroiodation of Alkynes Using an Iodine−Hydrophosphine Binary System. Org. Lett. 2010, 12, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Gharibyan, H.A.; Makaryan, G.M.; Hovhannisyan, M.R.; Kinoyan, F.S.; Chobanyan, Z.A. Some special features of hydroalumination-iodination of alkyne-1,4-diols. Russ. J. Gen. Chem. 2014, 84, 457–464. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, J.-Y.; Guo, C.-C. (NH4)2S2O8-Mediated Diiodination of Alkynes with Iodide in Water: Stereospecific Synthesis of (E)-Diiodoalkenes. Synthesis 2015, 47, 2081–2087. [Google Scholar] [CrossRef]
- Astakhova, V.V.; Ushakov, I.A.; Shainyan, B.A. Oxidative iodination of N-propargyltriflamide. Russ. J. Org. Chem. 2017, 53, 953–954. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Li, C.; Liu, P. Chloramine Salt Mediated Oxidative Halogenation of Terminal Alkynes with KI or NaBr: Practical Synthesis of 1-Bromoalkynes and 1-Iodoalkynes. Synlett 2018, 29, 2051–2055. [Google Scholar] [CrossRef] [Green Version]
- Banothu, R.; Peraka, S.; Kodumuri, S.; Chevella, D.; Gajula, K.S.; Amrutham, V.; Yennamaneni, D.R.; Nama, N. An aqueous medium-controlled stereospecific oxidative iodination of alkynes: Efficient access to (E)-diiodoalkene derivatives. New J. Chem. 2018, 42, 17879–17883. [Google Scholar] [CrossRef]
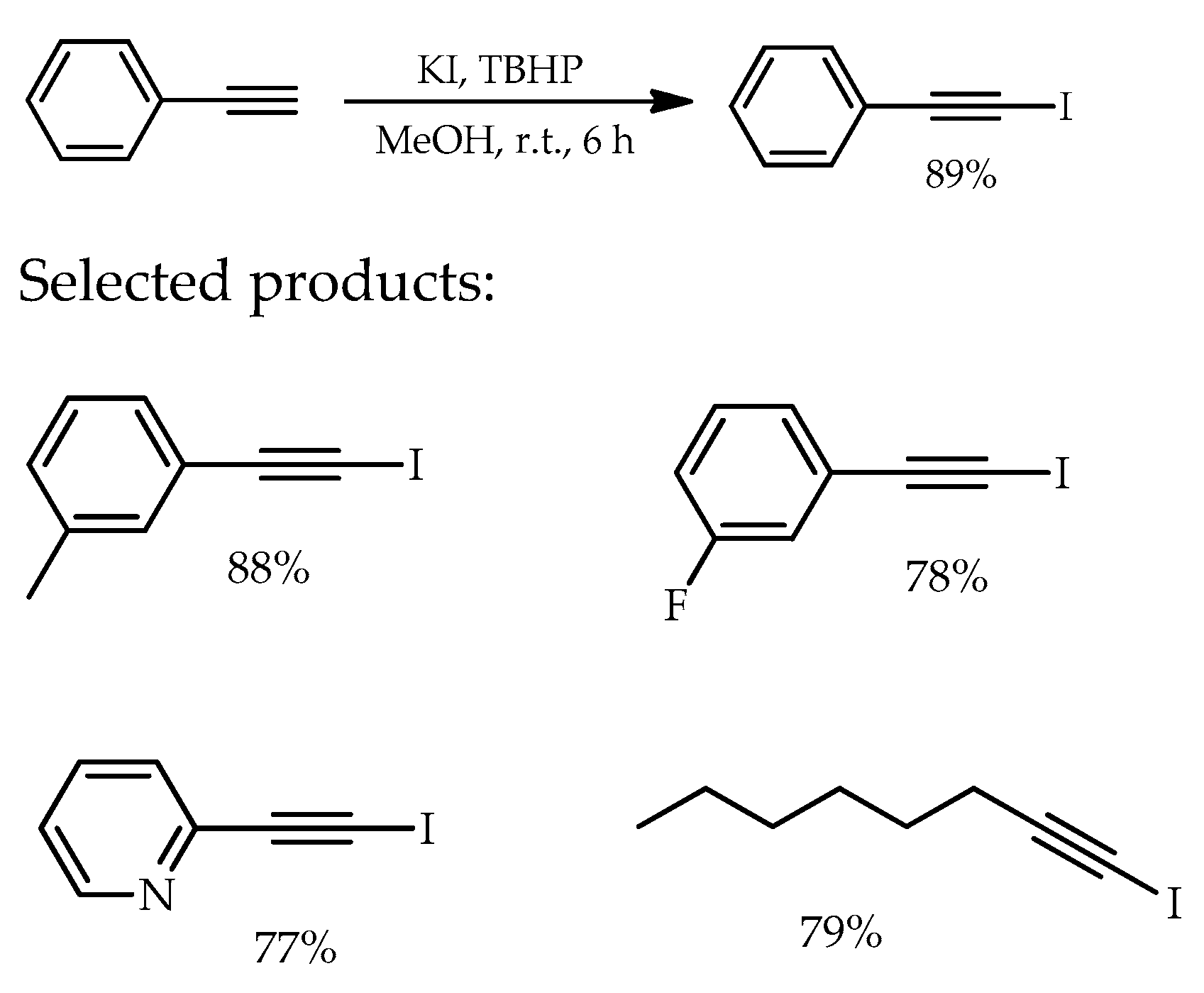
- Ferris, T.; Carroll, L.; Mease, R.C.; Spivey, A.C.; Aboagye, E.O. Iodination of terminal alkynes using KI/CuSO4–A facile method with potential for radio-iodination. Tetrahedron Lett. 2019, 60, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Han, L.-B. Ready access to organoiodides: Practical hydroiodination and double-iodination of carbon-carbon unsaturated bonds with I2. Tetrahedron 2019, 75, 3510–3515. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, R.; Chattopadhyay, S.K. Oxidant- and additive-free simple synthesis of 1,1,2-triiodostyrenes by one-pot decaroboxylative iodination of propiolic acids. Tetrahedron Lett. 2020, 61, 152378. [Google Scholar] [CrossRef]
- Lingling, L.; Yiming, L.; Xuefeng, J. Visible-Light-Promoted Diiodination of Alkynes Using Sodium Iodide. Chinese J. Org. Chem. 2020, 40, 3354–3361. [Google Scholar]
- Zhou, P.; Feng, S.; Qiu, H.; Zhang, J. Sodiump-Toluenesulfinate/KI-Mediated Aerobic Oxidative Iodination of Terminal Alkynes for Synthesis of 1-Iodoalkynes and 1,3-Diynes. Chinese J. Org. Chem. 2021, 41, 394–399. [Google Scholar] [CrossRef]
- Pavlinac, J.; Laali, K.K.; Zupan, M.; Stavber, S. Iodination of Organic Compounds with Elemental Iodine in the Presence of Hydrogen Peroxide in Ionic Liquid Media. Aust. J. Chem. 2008, 61, 946–955. [Google Scholar] [CrossRef]
- Pavlinac, J.; Zupan, M.; Stavber, S. Iodination of Organic Compounds Using the Reagent System Ι2–30% aq. H2O2 under Organic Solvent-free Reaction Conditions. Acta Chim. Slov. 2008, 55, 841–849. [Google Scholar]
- Iskra, J.; Stavber, S.; Zupan, M. Aerobic oxidative iodination of organic molecules activated by sodium nitrite. Tetrahedron Lett. 2008, 49, 893–895. [Google Scholar] [CrossRef]
- Yadav, J.S.; Kondaji, G.; Shiva Ram Reddy, M.; Srihari, P. Facile synthesis of α-iodo carbonyl compounds and α-iodo dimethyl ketals using molecular iodine and trimethylorthoformate. Tetrahedron Lett. 2008, 49, 3810–3813. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, G.; Qin, J.; Gao, M.; Cao, L.; Wu, A. An Efficient Method for the Selective Iodination of α,β-Unsaturated Ketones. Synthesis 2008, 2008, 3675–3681. [Google Scholar]
- Terent’ev, A.O.; Borisov, A.M.; Platonov, M.M.; Starikova, Z.A.; Chernyshev, V.V.; Nikishin, G.I. Reaction of Enol Ethers with the I2-H2O2 System: Synthesis of 2-Iodo-1-methoxy Hydroperoxides and Their Deperoxidation and Demethoxylation to 2-Iodo Ketones. Synthesis 2009, 2009, 4159–4166. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, J.-M.; Park, H.-J.; Kwag, B.-M.; Lee, S.-B. Direct Metal-free α-Iodination of Arylketones Induced by Iodine or Iodomethane with HTIB in Ionic Liquid. Bull. Korean Chem. Soc. 2010, 31, 1385–1386. [Google Scholar] [CrossRef] [Green Version]
- Yusubov, M.S.; Yusubova, R.Y.; Funk, T.V.; Chi, K.-W.; Kirschning, A.; Zhdankin, V.V. m-Iodosylbenzoic Acid as a Convenient Recyclable Hypervalent Iodine Oxidant for the Synthesis of α-Iodo Ketones by Oxidative Iodination of Ketones. Synthesis 2010, 2010, 3681–3685. [Google Scholar] [CrossRef]
- Khan, A.T.; Ali, S. A Useful and Convenient Synthetic Protocol for Iodination of Organic Substrates Using a Combination of Vanadyl Acetylacetonate, Hydrogen Peroxide, and Sodium Iodide. Bull. Chem. Soc. Jpn. 2012, 85, 1239–1243. [Google Scholar] [CrossRef]
- Moriya, T.; Yoneda, S.; Kawana, K.; Ikeda, R.; Konakahara, T.; Sakai, N. Indium(III)-Catalyzed Reductive Bromination and Iodination of Carboxylic Acids to Alkyl Bromides and Iodides: Scope, Mechanism, and One-Pot Transformation to Alkyl Halides and Amine Derivatives. J. Org. Chem. 2013, 78, 10642–10650. [Google Scholar] [CrossRef]
- Prebil, R.; Stavber, S. Aerobic oxidative α-iodination of carbonyl compounds using molecular iodine activated by a nitrate-based catalytic system. Tetrahedron Lett. 2014, 55, 5643–5647. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Zdvizhkov, A.T.; Kulakova, A.N.; Novikov, R.A.; Arzumanyan, A.V.; Nikishin, G.I. Reactions of mono- and bicyclic enol ethers with the I2–hydroperoxide system. RSC Adv. 2014, 4, 7579–7587. [Google Scholar] [CrossRef]
- Marri, M.R.; Macharla, A.K.; Peraka, S.; Nama, N. Oxidative iodination of carbonyl compounds using ammonium iodide and oxone®. Tetrahedron Lett. 2011, 52, 6554–6559. [Google Scholar] [CrossRef]
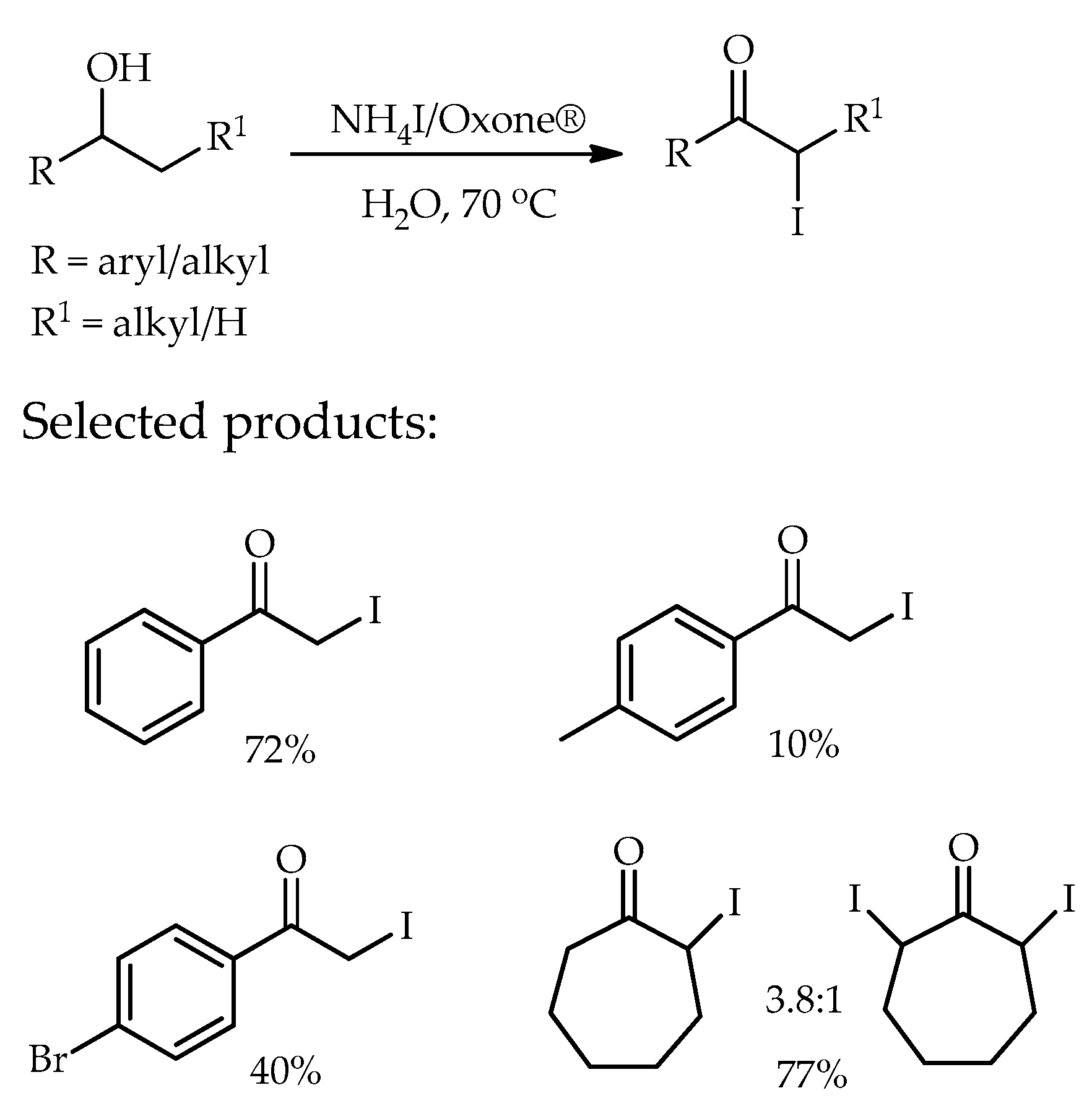
- Reddy, M.M.; Swamy, P.; Naresh, M.; Srujana, K.; Durgaiah, C.; Rao, T.V.; Narender, N. One-pot synthesis of α-iodoketones from alcohols using ammonium iodide and Oxone® in water. RSC Adv. 2015, 5, 12186–12190. [Google Scholar] [CrossRef]
- Zhu, R.-Y.; Liu, L.-Y.; Yu, J.-Q. Highly Versatile β-C(sp3)–H Iodination of Ketones Using a Practical Auxiliary. J. Am. Chem. Soc. 2017, 139, 12394–12397. [Google Scholar] [CrossRef]
- Sanz-Marco, A.; Možina, Š.; Martinez-Erro, S.; Iskra, J.; Martín-Matute, B. Synthesis of α-Iodoketones from Allylic Alcohols through Aerobic Oxidative Iodination. Adv. Synth. Catal. 2018, 360, 3884–3888. [Google Scholar] [CrossRef]




















































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajvazi, N.; Stavber, S. Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides: Recent Advances 2008–2021: Part I. Compounds 2022, 2, 3-24. https://doi.org/10.3390/compounds2010002
Ajvazi N, Stavber S. Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides: Recent Advances 2008–2021: Part I. Compounds. 2022; 2(1):3-24. https://doi.org/10.3390/compounds2010002
Chicago/Turabian StyleAjvazi, Njomza, and Stojan Stavber. 2022. "Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides: Recent Advances 2008–2021: Part I" Compounds 2, no. 1: 3-24. https://doi.org/10.3390/compounds2010002
APA StyleAjvazi, N., & Stavber, S. (2022). Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides: Recent Advances 2008–2021: Part I. Compounds, 2(1), 3-24. https://doi.org/10.3390/compounds2010002






