MgO–C Refractories with Al2O3 and TiO2 Nano-Additives: Insights from X-Ray Micro-Computed Tomography and Conventional Techniques for Assessing Corrosion and Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Alumina and Titania Nanoparticles
2.1.2. Preparation of Magnesia–Carbon Bricks
2.2. Methods
3. Results and Discussion
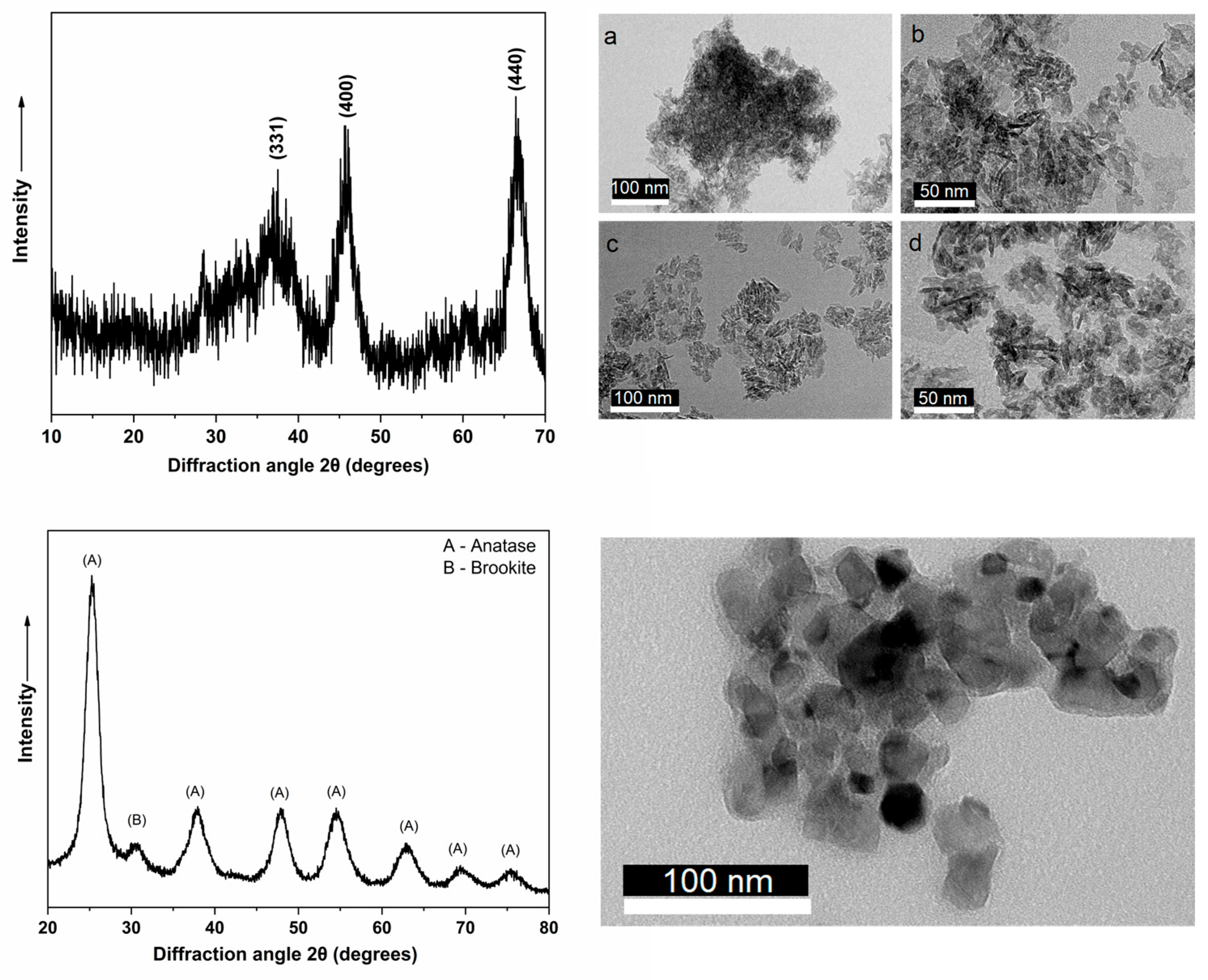
3.1. Characterization of Nanoparticles
3.2. Physical Properties of MgO-C Refractories
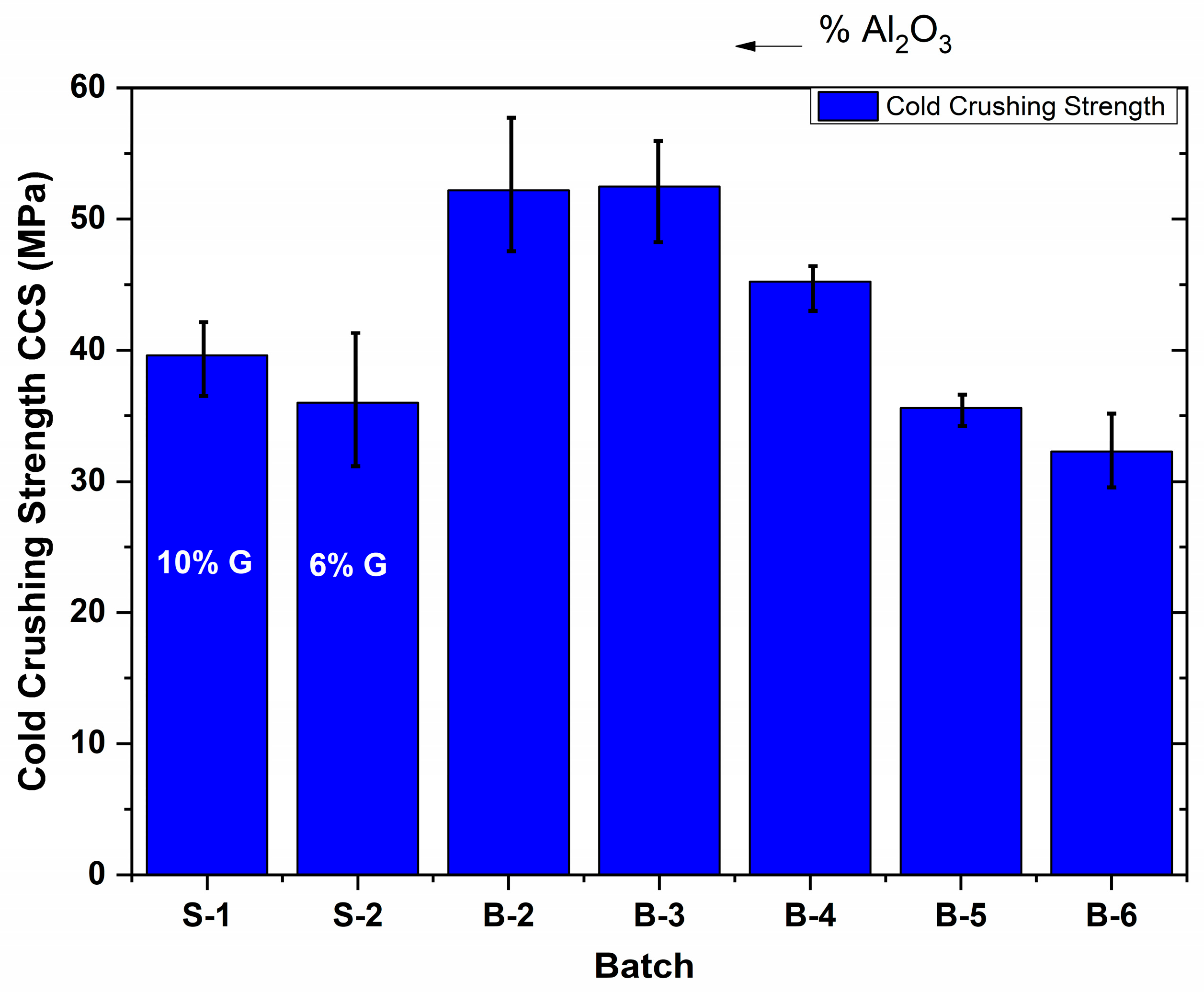
3.3. Mechanical Properties of MgO-C Refractories
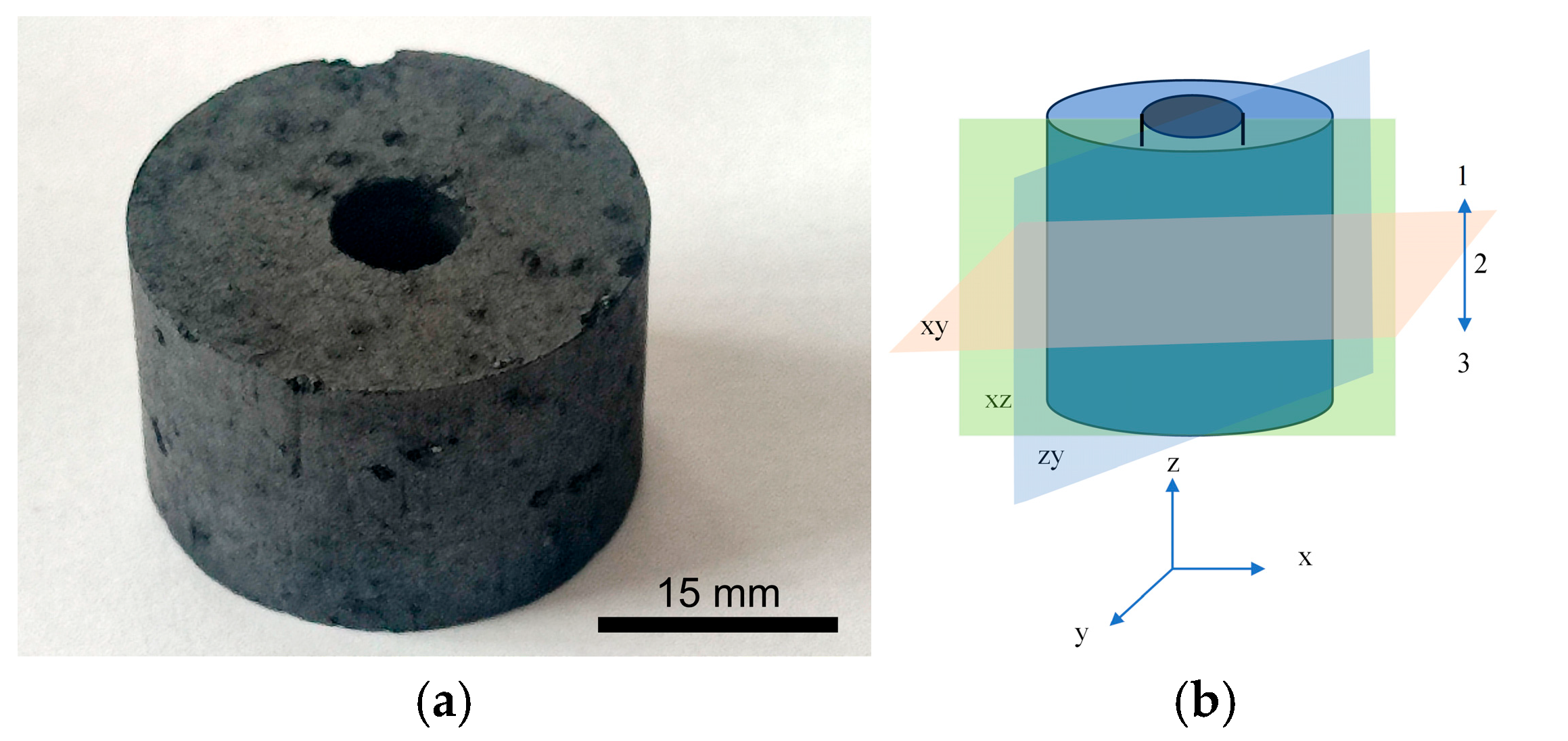
3.4. μCT Study
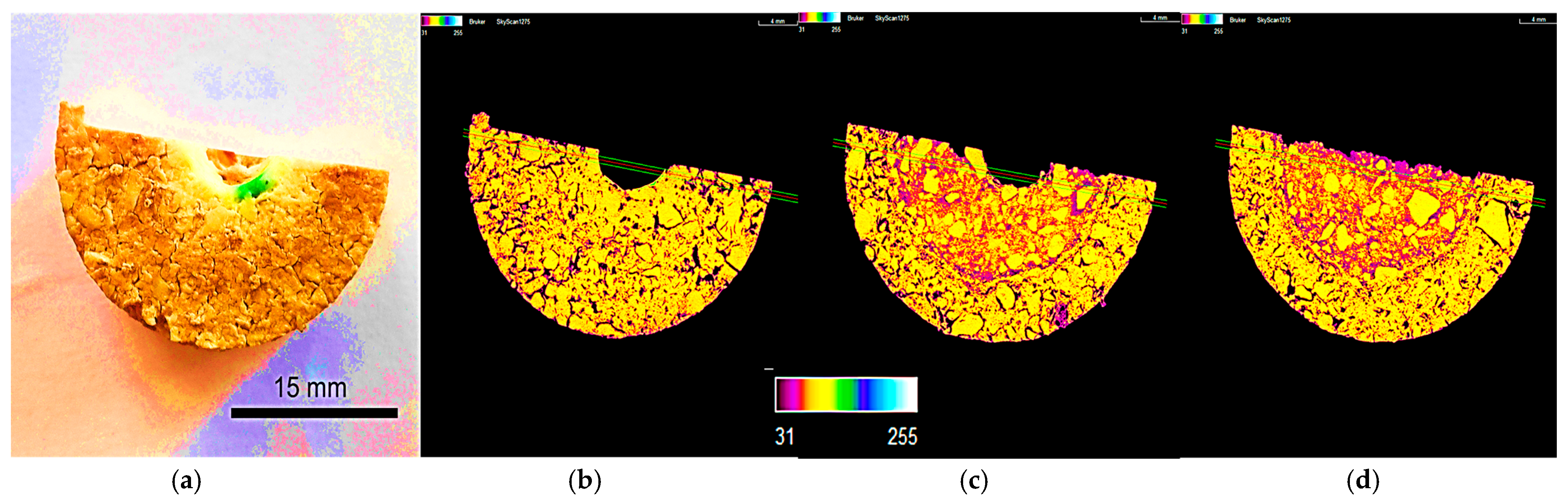
3.4.1. Density Color Mapping
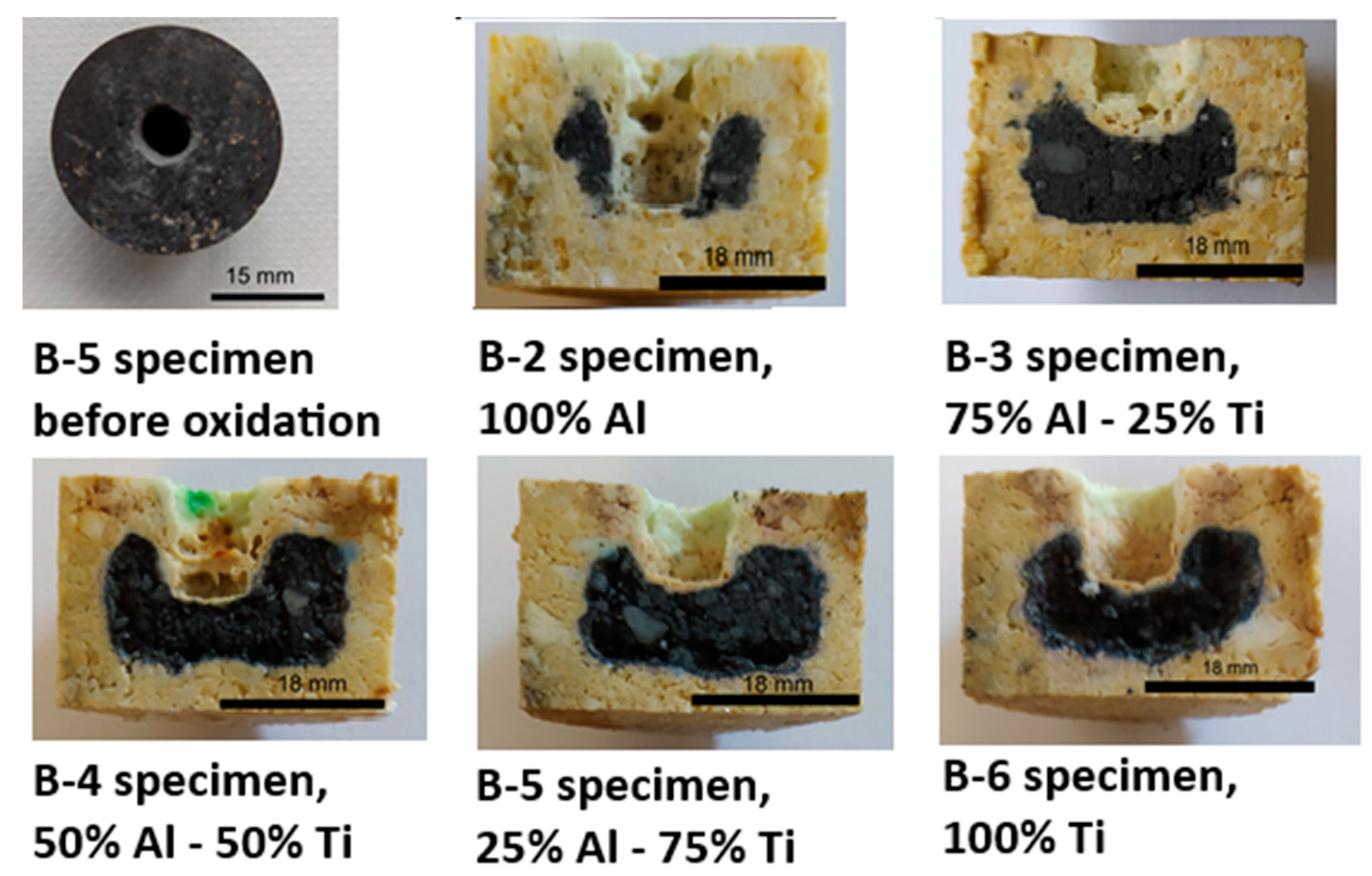
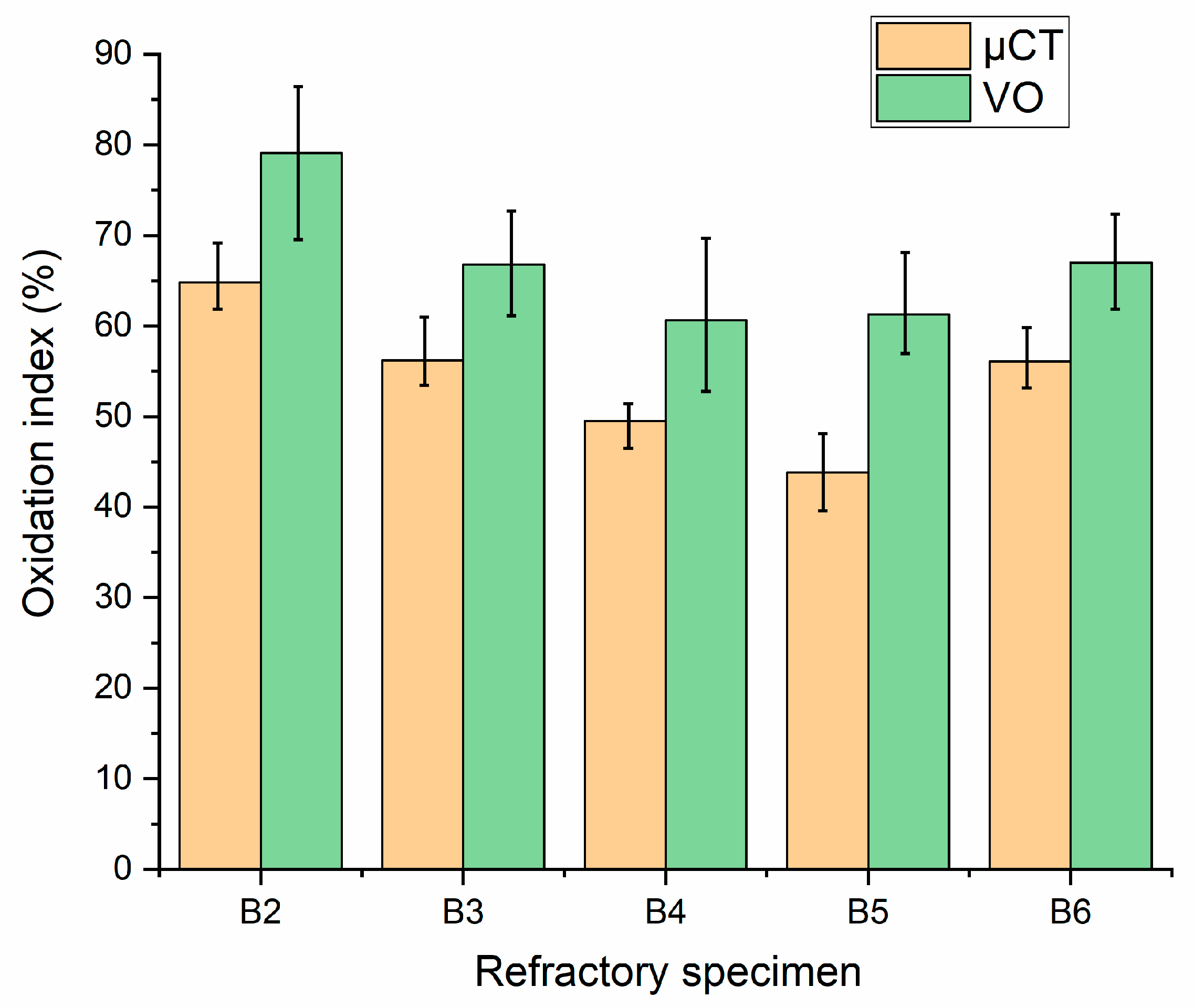
3.4.2. Oxidation Resistance
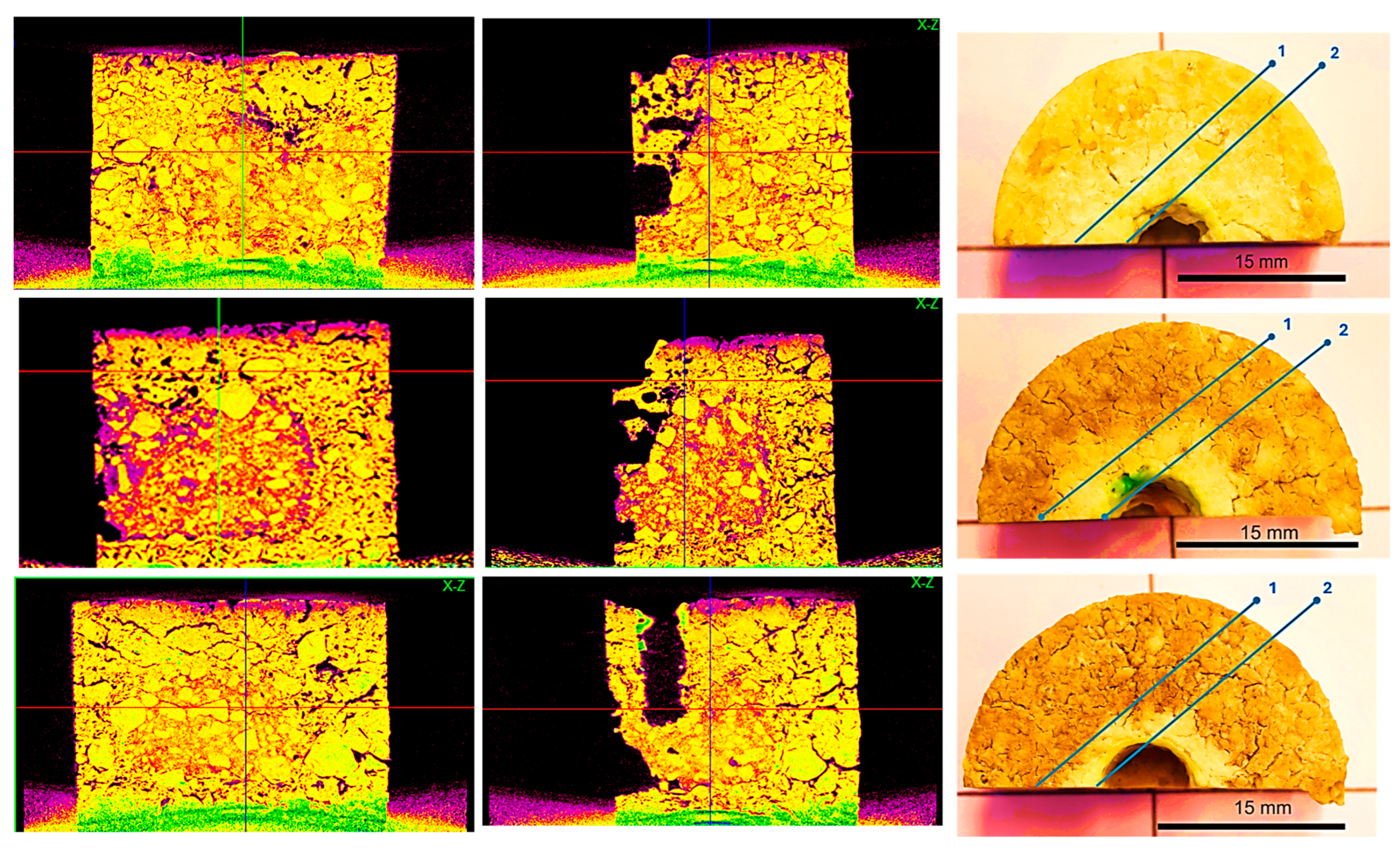
3.4.3. Corrosion Evaluation
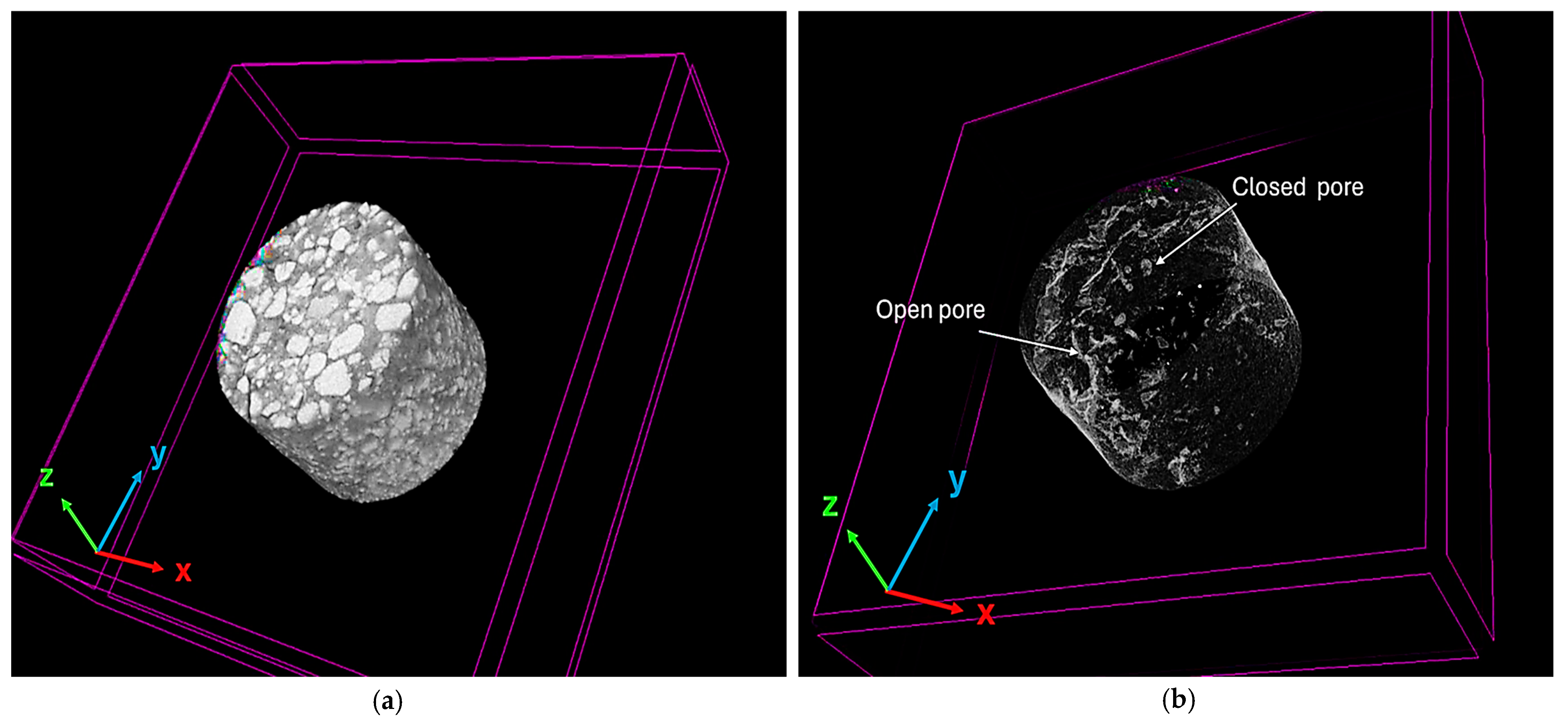
3.4.4. Calculation of Closed Porosity
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vásárhelyi, L.; Kónya, Z.; Kukovecz, Á.; Vajtai, R. Microcomputed tomography–based characterization of advanced materials: A review. Mater. Today Adv. 2020, 8, 100084. [Google Scholar] [CrossRef]
- Kong, Y.; Kato, M.; Kurumisawa, K. Recent Advances in X-ray Computed Tomography for Alkali-Activated Materials: A Review. J. Adv. Concr. Technol. 2023, 21, 573–595. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT—A digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, Y.; Gao, Y.; Zhang, X.; Jiang, P. Evaluation of the morphology and pore characteristics of silica refractory using X-ray computed tomography. Ceram. Int. 2021, 47, 18084–18093. [Google Scholar] [CrossRef]
- Razavi, A.; Hopp, V.; Hahn, D.; Sax, A.; Quirmbach, P. Microstructural X-Ray Computed Tomography Investigation of the Defect Evolution in Refractory Castings Based on Andalusite. Ceramics 2024, 7, 1867–1879. [Google Scholar] [CrossRef]
- Stec, J.; Tarasiuk, J.; Wroński, S.; Kubica, P.; Tomala, J.; Filipek, R. Investigation of Molten Metal Infiltration into Micropore Carbon Refractory Materials Using X-ray Computed Tomography. Materials 2021, 14, 3148. [Google Scholar] [CrossRef] [PubMed]
- Pankka, I.; Ahmed, M.S.; Tammela, J.; Taskinen, P.; Lindberg, D. Investigation of End-of-Life Chrome-Magnesia Refractories Using X-Ray Computed Tomography. JOM 2024, 76, 6650–6659. [Google Scholar] [CrossRef]
- Razavi, A.; Stein, A.; Quirmbach, P. Tomographic Imaging of Bauxite Grains Leached Using Hydrochloric Acid. Minerals 2023, 13, 884. [Google Scholar] [CrossRef]
- Weber, E.; Fernandez, M.; Wapner, P.; Hoffman, W. Comparison of X-ray micro-tomography measurements of densities and porosity principally to values measured by mercury porosimetry for carbon–carbon composites. Carbon 2009, 48, 2151–2158. [Google Scholar] [CrossRef]
- Galvez-Hernandez, P.; Smith, R.; Gaska, K.; Mavrogordato, M.; Sinclair, I.; Kratz, J. The effect of X-ray computed tomography scan parameters on porosity assessment of carbon fibre reinfored plastics laminates. J. Compos. Mater. 2023, 57, 4535–4548. [Google Scholar] [CrossRef]
- Rashidi, A.; Olfatbakhsh, T.; Crawford, B.; Milani, A.S. A Review of Current Challenges and Case Study toward Optimizing Micro-Computed X-Ray Tomography of Carbon Fabric Composites. Materials 2020, 13, 3606. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Sarkar, R. Nano carbon containing low carbon magnesia carbon refractory: An overview. Prot. Met. Phys. Chem. Surf. 2016, 52, 467–474. [Google Scholar] [CrossRef]
- Kundu, R.; Sarkar, R. MgO-C Refractories: A Detailed Review of These Irreplaceable Refractories in Steelmaking. Interceram Int. Ceram. Rev. 2021, 70, 46–55. [Google Scholar] [CrossRef]
- Rauta, P.R.; Sahoo, N. Properties enhancement of refractory bricks by incorporation of nano materials. In Proceedings of the 2015 International Conference on Nascent Technologies in the Engineering Field (ICNTE), Navi Mumbai, India, 9–10 January 2015; pp. 1–6. [Google Scholar]
- Salomão, R.; Souza, A.; Fernandes, L.; Arruda, C. Advances in nanotechnology for refractories: When very small meets hot, heavy, and large. Am. Ceram. Soc. Bull. 2013, 92, 22–27b. [Google Scholar]
- Sobolev, K. How Nanotechnology Can Change the Concrete World Part Two of a Two-Part Series. Am. Ceram. Soc. Bull. 2005, 84, 5. [Google Scholar]
- Antonovič, V.; Pundiene, I.; Stonys, R.; Česniene, J.; Keriene, J. A review of the possible applications of nanotechnology in refractory concrete. J. Civ. Eng. Manag. 2010, 16, 595–602. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E. Impact of Titania nanoparticles addition on the microstructure and properties of MgO-C refractories. Ceram. Int. 2017, 43, 15472–15477. [Google Scholar] [CrossRef]
- Kusiorowski, R. Effect of titanium oxide addition on magnesia refractories. J. Aust. Ceram. Soc. 2020, 56, 1583–1593. [Google Scholar] [CrossRef]
- Aneziris, C.G.; Hubálková, J.; Barabás, R. Microstructure evaluation of MgO–C refractories with TiO2- and Al-additions. J. Eur. Ceram. Soc. 2007, 27, 73–78. [Google Scholar] [CrossRef]
- Gómez Rodríguez, C.; Das Roy, T.K.; Shaji, S.; Castillo Rodríguez, G.A.; García Quiñonez, L.; Rodríguez, E.; González, J.O.; Aguilar-Martínez, J.A. Effect of addition of Al2O3 and Fe2O3 nanoparticles on the microstructural and physico-chemical evolution of dense magnesia composite. Ceram. Int. 2015, 41, 7751–7758. [Google Scholar] [CrossRef]
- ASTM C20-00; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM International: West Conshohocken, PA, USA, 2022; p. 19428–2959. [CrossRef]
- ASTM-C133-97-2015; Standard Test Methods for Cold Crushing Strength and Modulus of Rupture of Refractories. ASTM International: West Conshohocken, PA, USA, 2024.
- Jansson, S.; Brabie, V.; Jönsson, P. Corrosion mechanism and kinetic behaviour of MgO–C refractory material in contact with CaO–Al2O3–SiO2–MgO slag. Scand. J. Metall. 2005, 34, 283–292. [Google Scholar] [CrossRef]
- Siahpoosh, S.M.; Salahi, E.; Hessari, F.A.; Mobasherpour, I. Facile Synthesis of γ-Alumina Nanoparticles via the Sol-Gel Method in Presence of Various Solvents. Sigma J. Eng. Nat. Sci. 2017, 35, 441–456. [Google Scholar]
- Siahpoosh, S.M.; Salahi, E.; Hessari, F.A.; Mobasherpour, I. Synthesis of γ-Alumina Nanoparticles with High-Surface-Area via Sol-Gel Method and their Performance for the Removal of Nickel from Aqueous Solution. Bull. Société R. Sci. Liège 2016, 85, 812–934. [Google Scholar] [CrossRef]
- Singh, I.B.; Gupta, A.; Dubey, S.; Shafeeq, M.; Banerjee, P.; Sinha, A.S.K. Sol–gel synthesis of nanoparticles of gamma alumina and their application in defluoridation of water. J. Sol-Gel Sci. Technol. 2016, 77, 416–422. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Bian, X.; Wu, Y. Synthesis and characterization of amorphous Al2O3 and γ-Al2O3 by spray pyrolysis. Green Process. Synth. 2016, 5, 305–310. [Google Scholar] [CrossRef]
- Falk, G.S.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Rodrigues Neto, J.B.; Moreno, R. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanoparticle Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Ghadiry, M.; Gholami, M.; Lai, C.; Ahmad, H.; Chong, W. Ultra-Sensitive Humidity Sensor Based on Optical Properties of Graphene Oxide and Nano-Anatase TiO2. PLoS ONE 2016, 11, e0153949. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Study on low carbon containing MgO-C refractory: Use of nano carbon. Ceram. Int. 2012, 38, 2339–2346. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Nano carbon containing MgO-C refractory: Effect of graphite content. Ceram. Int. 2012, 38, 4909–4914. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Y.; Sang, S.; Jin, S. The influence of Al and Si additives on the microstructure and mechanical properties of low-carbon MgO-C refractories. J. Ceram. Sci. Technol. 2016, 7, 127–134. [Google Scholar]
- Ghasemi-Kahrizsangi, S.; Gheisari Dehsheikh, H.; Boroujerdnia, M. Effect of micro and nano-Al2O3 addition on the microstructure and properties of MgO-C refractory ceramic composite. Mater. Chem. Phys. 2017, 189, 230–236. [Google Scholar] [CrossRef]
- Su, K.; Zhang, Q.; Tian, X.; Ouyang, D.; Liu, X.; Cui, J. Role of nano-Al2O3 particles in improving the properties of MgO–C slide plate materials. Ceram. Int. 2023, 49, 23696–23703. Available online: https://www.sciencedirect.com/science/article/pii/S0272884223011720 (accessed on 25 April 2023). [CrossRef]
- Lee, W.E.; Rainforth, W.M. Ceramic Microstructures: Property Control by Processing; Chapman and Hall: London, UK, 1994; pp. 299–311. [Google Scholar]
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.C.; Mishra, S.K.; Agarwal, A.; Study of structural transformation in TiO2 nanoparticles and its optical properties. 2013, 549, 114–120. Available online: https://www.researchgate.net/publication/232815014_Study_of_structural_transformation_in_TiO2_nanoparticles_and_its_optical_properties (accessed on 2 May 2025).
- Koparde, V.N.; Cummings, P.T. Phase transformations during sintering of titania nanoparticles. ACS Nano 2008, 2, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Bavand-Vandchali, M.; Golestani-Fard, F.; Sarpoolaky, H.; Rezaie, H.R.; Aneziris, C.G. The influence of in situ spinel formation on microstructure and phase evolution of MgO–C refractories. J. Eur. Ceram. Soc. 2008, 28, 563–569. [Google Scholar] [CrossRef]
- Mahato, S.; Behera, S.K. Oxidation resistance and microstructural evolution in MgO–C refractories with expanded graphite. Ceram. Int. 2016, 42, 7611–7619. [Google Scholar] [CrossRef]
- Nanda, S.; Choudhury, A.; Chandra, K.S.; Sarkar, D. Raw materials, microstructure, and properties of MgO–C refractories: Directions for refractory recipe development. J. Eur. Ceram. Soc. 2023, 43, 14–36. [Google Scholar] [CrossRef]


| Raw Materials/Batch | B-1 | B-2 | B-3 | B-4 | B-5 | B-6 |
|---|---|---|---|---|---|---|
| MgO (3–5 mm) | 3.68 | 3.68 | 3.68 | 3.68 | 3.68 | 3.68 |
| MgO (1–3 mm) | 31.58 | 31.58 | 31.58 | 31.58 | 31.58 | 31.58 |
| MgO (0.5–1 mm) | 14.74 | 14.74 | 14.74 | 14.74 | 14.74 | 14.74 |
| MgO (0–0.5 mm) | 21.05 | 21.05 | 21.05 | 21.05 | 21.05 | 21.05 |
| MgO (200 mesh) | 23.7 | 23.7 | 23.7 | 23.7 | 23.7 | 23.7 |
| Fine-grained graphite | 3 | 3 | 3 | 3 | 3 | 3 |
| Coarse graphite | 3 | 3 | 3 | 3 | 3 | 3 |
| Novolac resin | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Resol resin | 5 | 5 | 5 | 5 | 5 | 5 |
| γ-alumina nanoparticles | - | 7 | 5.25 | 3.5 | 1.75 | - |
| Anatase nanoparticles | - | - | 1.75 | 3.5 | 5.25 | 7 |
| Al metal powder | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Boron carbide powder | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Steps | Mixing Sequence | Mixing Time (min) |
|---|---|---|
| 1 | Coarse and medium magnesia | 1.0 |
| 2 | Addition of graphite, aluminum metal powder, boron carbide powder, novolak resin, and a part of resol | 5.0 |
| 3 | Addition of magnesia 0–0.5 mm, fine magnesia powder, and the remaining resol resin | 10.0 |
| Batch | R (Al2O3/TiO2) | B (g/cm3) | P (%) | W (%) | T |
|---|---|---|---|---|---|
| B-1 | - | 2.88 | 10.65 | 3.70 | 3.22 |
| B-2 | 100 | 2.67 | 19.71 | 7.39 | 3.32 |
| B-3 | 75/25 | 2.78 | 16.76 | 6.03 | 3.34 |
| B-4 | 50/50 | 2.66 | 20.01 | 7.54 | 3.32 |
| B-5 | 25/75 | 2.64 | 19.87 | 7.54 | 2.39 |
| B-6 | 0 | 2.66 | 19.79 | 7.46 | 3.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkiouzel, S.; Ioannou, V.; Gioti, C.; Vasilopoulos, K.C.; Ntaflos, A.; Paipetis, A.S.; Salmas, C.E.; Karakassides, M.A. MgO–C Refractories with Al2O3 and TiO2 Nano-Additives: Insights from X-Ray Micro-Computed Tomography and Conventional Techniques for Assessing Corrosion and Oxidation. Nanomanufacturing 2025, 5, 10. https://doi.org/10.3390/nanomanufacturing5030010
Gkiouzel S, Ioannou V, Gioti C, Vasilopoulos KC, Ntaflos A, Paipetis AS, Salmas CE, Karakassides MA. MgO–C Refractories with Al2O3 and TiO2 Nano-Additives: Insights from X-Ray Micro-Computed Tomography and Conventional Techniques for Assessing Corrosion and Oxidation. Nanomanufacturing. 2025; 5(3):10. https://doi.org/10.3390/nanomanufacturing5030010
Chicago/Turabian StyleGkiouzel, Sevastia, Vasileios Ioannou, Christina Gioti, Konstantinos C. Vasilopoulos, Angelos Ntaflos, Alkiviadis S. Paipetis, Constantinos E. Salmas, and Michael A. Karakassides. 2025. "MgO–C Refractories with Al2O3 and TiO2 Nano-Additives: Insights from X-Ray Micro-Computed Tomography and Conventional Techniques for Assessing Corrosion and Oxidation" Nanomanufacturing 5, no. 3: 10. https://doi.org/10.3390/nanomanufacturing5030010
APA StyleGkiouzel, S., Ioannou, V., Gioti, C., Vasilopoulos, K. C., Ntaflos, A., Paipetis, A. S., Salmas, C. E., & Karakassides, M. A. (2025). MgO–C Refractories with Al2O3 and TiO2 Nano-Additives: Insights from X-Ray Micro-Computed Tomography and Conventional Techniques for Assessing Corrosion and Oxidation. Nanomanufacturing, 5(3), 10. https://doi.org/10.3390/nanomanufacturing5030010









