Abstract
Silver nanowires (AgNWs) have garnered significant attention in nanotechnology due to their unique mechanical and electrical properties and versatile applications. This review explores the synthesis of AgNWs, with a specific focus on the utilization of millifluidic flow reactors (MFRs) as a promising platform for controlled and efficient production. It begins by elucidating the exceptional characteristics and relevance of AgNWs in various technological domains and then delves into the principles and advantages of MFRs by showcasing their pivotal role in enhancing the precision and scalability of nanowire synthesis. Within this review, an overview of the diverse synthetic methods employed for AgNW production using MFRs is provided. Special attention is given to the intricate parameters and factors influencing synthesis and how MFRs offer superior control over these critical variables. Recent advances in this field are highlighted, revealing innovative strategies and promising developments that have emerged. As with any burgeoning field, challenges are expected, so future directions are explored, offering insights into the current limitations and opportunities for further exploration. In conclusion, this review consolidates the state-of-the-art knowledge in AgNW synthesis and emphasizes the critical role of MFRs in shaping the future of nanomaterial production and nanomanufacturing.
1. Introduction
Over the past 20 years, the design and manufacturing of electronics has rapidly evolved and become a fast-growing industry worldwide. Transparent conductive films (TCFs) have furthered this evolution in electronics and are the primary components found in sensors [1], touch screens [2], and energy-harvesting [3] electronic devices. Commonly, indium tin oxide (ITO) is used as the conductive medium in TCFs because of its chemical stability, optical transparency, and electrical conductivity [4,5]. However, there are limitations. ITO is a costly raw material, becomes brittle under deformation, and must undergo a post-annealing process at high temperatures. Due to these reasons, ITO is not an ideal candidate for use as the conductive medium in flexible substrate applications [6]. When considering a conductive material for flexible TCFs, silver nanowires (AgNWs) are an excellent option [7,8,9]. Compared with ITO, AgNWs can produce thinner TCFs, exhibit lower haze, and offer 80% higher optical transmittance [10].
AgNWs are commonly synthesized using templating or polyol methods. Templating involves preparing a template to promote Ag nanocluster formation and growth in one dimension (1D). Template methods are divided into hard and soft categories based on the template material [11]. Nanoporous hard template synthesis produces AgNWs by forming and growing Ag nanoclusters within the membrane pores in 1D. The Ag nanoclusters are formed through either electrochemical or chemical reduction of Ag+. Hard templates offer the advantage of easier morphological control; however, separating the AgNWs from the template for purification is challenging and often results in damage or breakage. Consequently, hard template methods are not ideal for industrial scaling. To overcome the challenges of separation and purification associated with hard templating, solution-based soft templates have gained more popularity. These templates can dissolve in solution, simplifying the process. Moreover, these reactions are based on Ag seed-based growth and carried out in solution, which allows for potential scalability. Soft template syntheses typically involve aqueous reaction solutions that include a reducing agent, a capping agent, and the chosen soft templates. During the reaction, the soft templates chemically absorb onto Ag seeds and kinetically control the growth rate on the template surface through adsorbing and desorbing from the Ag seed surface. While soft templates are advantageous for ease of purification, they have drawbacks, such as low yields (the number of AgNWs per total number of nanostructures), low aspect ratios (the ratio of length and diameter), irregular morphologies, and polycrystallinity [12].
Scalability is a major issue associated with AgNWs being the conductive medium in flexible TCFs. Traditionally, the polyol method has been used to synthesize AgNWs with high aspect ratios in batch reactors, as shown in Figure 1 [13]. These reactions suffer from low AgNW yields, irregular morphologies, and batch-to-batch variability [14]. Batch reactors are dominated by convective heat and mass transfer and require high Reynolds (Re) numbers for proper mixing [15]. Scaling up AgNW polyol batch reactions is difficult because of the nonlinear relationship between reactor volume and reaction conditions, such as reagent addition and mixing, temperature and chemical gradients, and heat and mass transfer [16].
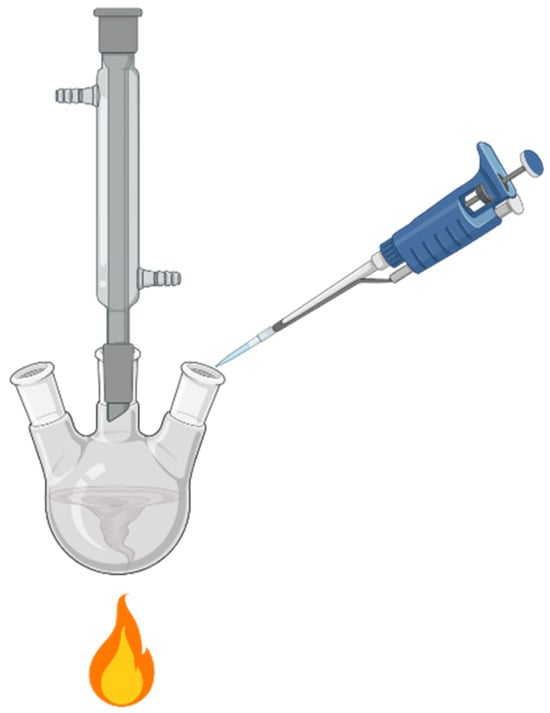
Figure 1.
A typical batch polyol AgNW synthesis setup includes a round flask, heat source, stir bar, condenser, and pipette for reagent addition.
Researchers have directed attention to continuous flow reactors (CFRs) to solve the issues associated with the industrial scaling of AgNW synthesis [17,18,19,20]. The two major types of CFRs are micro- and milli-fluidic flow reactors, which have inner diameters at the micro- and milli-scales, respectively. When considering CFRs for AgNW synthesis, the inner tubing diameter must be considered to avoid clogging and agglomeration. Millifluidic flow reactors (MFRs) have proven to reliably produce high-aspect-ratio AgNWs using less waste and shorter reaction times [14,19,21,22,23,24]. MFRs operate at relatively low fluid speeds, where the flow characteristic is considered laminar. Efficient mixing is facilitated by the short diffusional path lengths inside the tubing, which can be further enhanced with curved reaction tubing configurations, as shown in Figure 2 [13]. Flow reactors simplify the scale-up process from lab to production by enabling scaling-out or numbering-up approaches, thereby avoiding the need for reactor resizing and redesigning reaction conditions [23]. Scale-out involves increasing the inner tubing diameter to accommodate larger volumes flowing through the reactors, whereas numbering-up utilizes multiple reactors running in parallel to increase production capacity [25]. Wet chemical AgNW reactions, like the polyol method, are challenging to reproduce and scale up using batch reactors, but they can easily be replicated and scaled using MFRs [14,22].
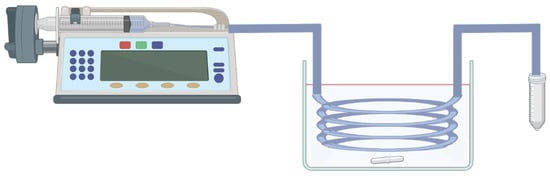
Figure 2.
A typical MFR setup includes syringes and a syringe pump for reagent addition, tubing, the oil bath, and the heat source for the synthesis.
AgNW-based flexible TCFs are commonly manufactured using screen printing [26,27,28], inkjet printing [29,30,31], and, more recently, direct ink writing (DIW) processes [32,33,34]. Screen printing is a versatile technique applicable to various materials, characterized by a mesh screen, a pattern, conductive ink, and a scraper. Factors influencing the screen-printing process include the smoothness of the patterned mesh, screen angle, ink composition, and pressure applied during scraping. Conductive inks used in screen printing processes typically require high viscosity for shear-thinning behavior and low-volatility formulations to prevent the ink from drying out [35]. Although screen printing provides facile printing and high-efficiency TCFs, low resolution, material waste, and difficulty with industrial scaling make it a poor printing candidate for mass TCF manufacturing [36]. Inkjet printing involves ejecting conductive ink dropwise from a nozzle onto a substrate, like 3D printing. Preparing inkjet-printable conductive inks requires adjusting the viscosity and optimizing the wettability to ensure drop-wise printability and adhesion to the substrate. While inkjet printing minimizes waste and supports continuous processes, the deposited ink can sometimes distribute non-uniformly and form a “coffee-ring” pattern upon drying, leading to electrical inconsistencies [35]. DIW, shown in Figure 3 [13], can successfully print AgNW-based conductive ink patterns onto substrates, ensuring reliable TCF manufacturing [33,37]. AgNW suspensions are commonly aqueous, necessitating an increase in viscosity to achieve shear-thinning behavior for use in the DIW process. Ink for DIW should possess excellent wettability and relatively high viscosity to ensure printability and adhesion to the substrate. The advantages of DIW include the ability to print complex patterns, reduction in material waste, and on-demand printing [36]. Additionally, DIW offers advantages for continuous processes suitable for industrial scaling [38].
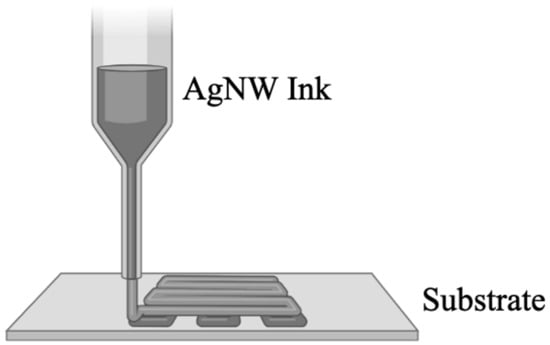
Figure 3.
DIW printers utilize printing software to direct a nozzle in the x–y–z plane that expresses conductive inks into a pattern on a substrate.
In this review, we primarily focus on research published between 2015 and 2025, highlighting key advancements in polyol AgNW synthesis using MFRs for DIW conductive patterns in TCF applications. A search in Google Scholar using the keywords “polyol”, “silver nanowire”, “continuous”, and “millifluidic” revealed that approximately 16 papers have been published on this topic during this period, indicating that the area is steadily gaining momentum in research activity. This selection ensures a comprehensive yet focused discussion on the most recent developments in the field. In Section 2, the operation, design, and flow characteristics of MFRs are covered. In Section 3, the unique properties of AgNWs, the proposed mechanism of the polyol AgNW reaction, and recent advances in polyol AgNW syntheses using MFRs are provided. In Section 4, the use of AgNWs synthesized with the polyol method for DIW conductive pathways in TCFs is discussed. Finally, in Section 5, current issues and future directions in this field are outlined.
2. Millifluidic Flow Reactors
2.1. Classification and Configuration
MFRs are constructed from various tubing materials and feature inner diameters on the millimeter scale. These reactors can be configured in multiple ways for different synthesis needs. The main configurations include straight tubing [23], which provides a simple and direct flow path; coiled tubing [22], which enhances mixing and heat transfer; and segmented tubing [24], where flow is divided into discrete segments to control reaction parameters more precisely while minimizing fouling. Additionally, combinations of these three main configurations can be utilized to optimize the reaction environment for specific synthetic processes, offering control over the synthetic process [14]. This adaptability makes MFRs particularly ideal for scalable production processes, allowing for control over reaction conditions and the size and morphology of AgNWs [23].
2.2. Reaction Environment Characteristics
Small inner dimensions of MFR tubing create a unique reaction environment that affords superior control of AgNW syntheses. Mass AgNW synthesis can be attained while ensuring the production of high-quality, reproducible products. The tubing is relatively long compared with the inner diameter, resulting in a high surface area-to-volume ratio. This high ratio is a critical factor as it enhances heat and mass transfer, leading to more uniform and consistent reaction conditions throughout the entire length of the tubing [39]. The enhanced control over reaction parameters, such as reactant concentration and temperature gradients, allows for precise manipulation of the synthetic process, leading to consistent AgNW dimensions with desirable properties [15].
2.3. Flow Behavior and Characteristics
The dimensionless Reynolds number (Re) quantifies the ratio of inertial to viscous forces in a fluid and can be calculated to categorize flow as laminar, transitionary, or turbulent. It depends on the fluid properties and inner tubing diameter, as shown in Equation (1).
where is the fluid density, is the fluid speed, is the inner reactor diameter, and is the dynamic viscosity of the fluid. In MFRs, the flow is typically laminar (Re < 2100) due to small inner tubing diameters and relatively low fluid velocities. Mixing in MFRs occurs through passive micro-mixing, driven by molecular diffusivity under laminar and segmented flow conditions without additional mechanical energy beyond reagent pumping [39].
Secondary forces called Lagrangian turbulences or Dean vortices enhance mixing in CFRs and are quantified by the dimensionless Dean () number. The number depends on the ratio of the inner tubing radius to the radius of the tubing coil and the number, as shown in Equation (2) [40].
where is the tubing radius and is the radius of the tubing coil. The dimensionless helical () number is like the number but considers the effect of changes in the height variation on the fluid flow through a tubing coil, as shown in Equation (3) [19].
where is the critical radius of the tubing coil. Using Equation (4) [41], the can be calculated using the pitch length, , between the tubing coils [19]. The parameters to calculate the De, He, and Rc values are shown in Figure 4 [13].
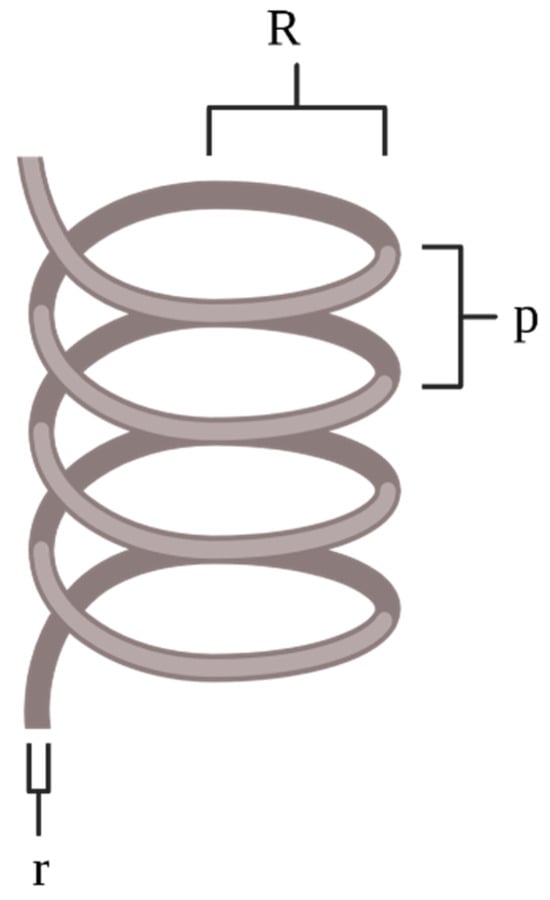
Figure 4.
When calculating the De, He, and Rc for a coiled MFR configuration, the radius of the coil, R, radius of the tubing, r, and height between the coils, p, are considered.
2.4. Transport and Kinetic Effects
The Damkohler number () relates the reaction rate to the mass transfer rate in reactors and can be calculated for second-order, irreversible reactions using Equation (5) [41].
where is the space time, is the growth rate constant, and is the initial concentration. Systems with > 1, commonly batch, indicate insufficient mixing, resulting in kinetically limited reactions and lower AgNW yields with various AgNPs. In contrast, < 1 indicates well-mixed conditions suitable for high-yield AgNW production. In MFRs, the number remains stable when scaling up or extending synthesis times for the mass production of AgNWs [15].
Due to the small inner tubing diameter, diffusive mass and conductive heat transfer dominate in CFRs. The high surface area-to-volume ratio allows for efficient heat transfer to occur in MFRs, which can minimize temperature transients during the reaction and shorten reaction times [15]. The dimensionless Peclet () number can be used to determine transport phenomena and the orientation of the AgNWs in the MFR tubing channel during synthesis and is dependent on the hydrodynamic diameter of AgNWs, velocity, shear rate, and diffusion coefficients, as shown in Equations (6) and (7) [22].
where is the molecular number, is the velocity, is the hydrodynamic diameter of the AgNWs, is the molecular diffusion coefficient, is the rotational Pe number, is the shear rate, and is the rotational diffusion coefficient. numbers characterize the relative importance of convective and diffusive transport within the reactor. In MFRs, a high number suggests that convective transport dominates, which can enhance the mass transport and reaction kinetics. This is crucial for processes like AgNW synthesis, where the efficient mixing and transport of reactants are required [22].
To understand the momentum and heat transport of MFRs, the dimensionless Prandtl () and Nusselt () numbers can be quantified. The number is dependent on the heat capacity and dynamic viscosity, as shown in Equation (8).
where is the heat capacity, and is the dynamic viscosity. For MFRs, a higher number indicates that momentum diffusivity dominates over thermal diffusivity.
Conduction heat transfer dominates in MFRs, and the number can be used to determine how well a fluid transfers heat. The Manlapaz–Churchill correlation can be applied to MFRs because of the relatively low numbers exhibited in reactions and is shown in Equation (9) [23].
Since MFRs are dominated by conductive heat transfer, achieving a higher number indicates more efficient convective heat transfer. This is essential for controlling temperature gradients, which is essential for maintaining product quality and reproducibility in AgNW synthesis [19]. These dimensionless numbers play an important role in MFR design optimization and operation in synthesis of AgNWs for TCFs. By manipulating these dimensionless numbers, the efficiency, reproducibility, and scalability of the synthesis of AgNWs conducted in MFRs can be enhanced. The dimensionless numbers and their relevance to MFRs are shown in Table 1.

Table 1.
Applicable dimensionless numbers, their significance, and nominal values to analyze MFR kinetics and fluid flow parameters.
3. Polyol AgNW Syntheses Using MFRs
3.1. AgNW Properties
AgNWs are considered one-dimensional (1D) by having one dimension, diameter, on the nanoscale. Diameters typically range from 10 to 200 nm, and lengths range from 5 to 100 µm. To be classified as a AgNW, the aspect ratio should be greater than 10; aspect ratios less than 10 are classified as silver nanorods (AgNRs) [12]. AgNWs are excellent candidates for TCF manufacturing due to their strong conductivity, mechanical flexibility, and optical transparency [10]. However, small-aspect-ratio AgNWs and high-AgNW-density depositions can negatively impact the electrical and optical properties of TCFs. Long, thin AgNWs enable low-density, well-percolated conductive networks on thin films, providing sufficient light transmittance [6]. The unique properties of Ag make AgNWs an exceptional conductive material to replace ITO in TCF manufacturing.
3.2. Polyol AgNW Formation Mechanism
The polyol method is a wet chemical reaction with good potential for scale-up. This approach utilizes a silver salt to introduce silver ions (Ag+) into the solution. Glycol serves as both a solvent and a reducing agent. Upon heating, the glycol converts to an aldehyde, effectively reducing Ag+ to Ag0. Reduced silver atoms then nucleate into multiply twinned Ag seeds, initiating 1D growth facilitated by a capping agent [42]. A halide salt mediator has two functions in the polyol reaction, including scavenging oxygen and forming a Ag precipitate to prevent AgNP formation. During nucleation, Ag precipitates are formed with halide salt mediator anions to release Ag+ slowly. During AgNW growth, the surfaces of the multiply twinned Ag seeds are susceptible to oxygen adsorption. The halide-based salt mediator cation scavenges the oxygen and preserves the AgNW morphology. For example, in the case of copper chloride (CuCl2) as the salt mediator, a Cu2+ is reduced to Cu+ by the aldehyde reducing agent. These Cu+ ions then react with the adsorbed oxygen on the surfaces of the silver seeds, converting back into Cu2+ ions. This cycle allows Cu2+ ions to scavenge additional oxygen during the AgNW growth process [42]. Capping agents exhibit a preferential affinity for the {100} facet of AgNWs, promoting the selective attachment of silver atoms to the {111} facets. Multiple twinned seeds are decahedral in shape, with twin boundaries extending outward in a five-fold symmetry to include ten {111} facets. Silver atoms are attracted to the high-energy twin boundary surfaces through the Ostwald ripening process, selectively undergoing crystallization and elongation [12]. A generic polyol process is depicted in Figure 5 and covers the AgNW formation, the role of the capping agent, and the two functions of the halide salt mediator [13].
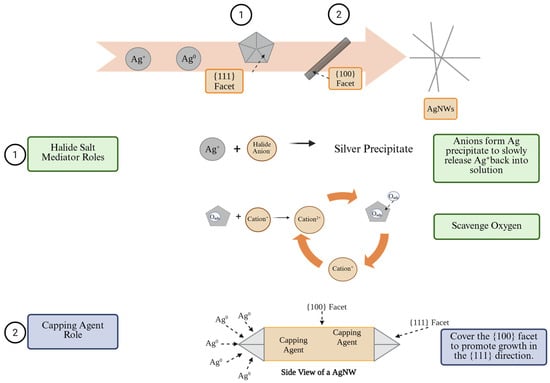
Figure 5.
The AgNW polyol process requires a halide salt mediator and a capping agent to synthesize uniform AgNWs with long lengths and small diameters.
3.3. Advances Using MFRs for Polyol AgNW Synthesis
In recent years, scientists have turned to CFRs to address the scalability challenges associated with batch AgNW processes. Gottesman et al. explored the benefits of CFRs for AgNW synthesis, successfully utilizing the polyol method in an MFR configuration. Their setup involved polytetrafluoroethylene (PTFE) tubing coiling around an aluminum cylinder for enhanced heat transfer, housed in a split furnace at 198 °C. Polyol reagents were pumped into the reactor, and the AgNWs were collected from the outlet tubing, yielding 92% AgNWs with diameters of 71 nm ± 2 after 30 min of residence time [20]. Espinosa et al. compared batch and MFR AgNW syntheses, focusing on the AgNW lifecycle in electrodes [21]. Their MFR reactor setup included coiled stainless-steel tubing inside a furnace at 152 °C, achieving AgNW diameters of 50–200 nm and lengths of 10–20 µm with an optimal residence time of 41–46 min. They concluded that MFRs offered shorter reaction times and ease of operation compared with batch reactors [21]. The early literature emphasized temperature and residence time as critical factors, yet little was known about how MFRs enhance AgNW synthesis efficiency compared with batch methods.
As described by Coskun et al., the synthesis of AgNWs is driven by several factors, including the precursor concentration, reaction time, temperature, and surfactant concentration [43]. These factors play a critical role in dictating the morphology, size, and purity of the nanowires, and understanding their interplay is essential for optimizing synthesis methods [43]. Hemmati et al. compared batch and MFR methods for synthesizing AgNWs using the polyol method, employing PTFE tubing as the reaction environment and a silicone oil bath for heat control [22]. They investigated the growth of AgNWs over time and analyzed the flow dynamics by calculating the numbers, diffusion coefficients (molecular and rotational), and Peclet () numbers. The MFR achieved 100% AgNW synthesis, even at low temperatures down to 130 °C. Flow analysis indicated laminar conditions where AgNWs traveled parallel to flow, maintaining a fixed radial position with a high rotational number. These conditions facilitated efficient mass and heat transfer via diffusion and axial convection within the tubing during synthesis. The study found that residence time governed the AgNW length (7–19 µm) and diameter (98–182 nm) in the MFR setup [22]. Williams et al. optimized polyol reaction conditions to produce a 100% AgNW yield in an MFR using a design of experiments (DoE) [44]. The authors utilized input parameters including reagent concentrations and reaction temperatures and the output parameter as the AgNW yield derived from the DoE. They employed the decision tree (DT) and random forest (RF) machine learning (ML) algorithms to predict the yield of AgNWs for a given reaction, achieving accuracies of 96.9% and 97.5%, respectively. The optimal polyol reaction conditions resulted in a 100% AgNW yield, with average concentrations of 16 mg/mL, lengths of 32 μm (σ ± 3.5 μm), diameters of 68 nm (σ ± 12 nm), and aspect ratios of 475 [44].
The reactor configuration significantly influences the morphology of AgNWs, particularly within the controlled environments of CFRs. Kinhal et al. compared batch reactors with straight and coiled MFR configurations for synthesizing silver nanoparticles (AgNPs) [23]. They investigated the effects of varying capping agent, metal precursor, and reducing agent concentrations. High capping agent concentrations resulted in AgNPs across all reactors, while low capping agent concentrations combined with high metal precursor concentrations produced silver nanorods (AgNRs) and AgNWs in the straight and coiled MFRs, respectively. Exclusively, the coiled MFR synthesized AgNWs with lengths averaging 134 nm ± 38 [23]. Another method to modify an MFR configuration is by implementing flow segmentation, where immiscible fluids are used to segment the reaction fluids. Lau et al. applied flow segmentation in a CFR by utilizing FC-70 fluid to synthesize AgNWs using the polyol method in a coiled MFR [45]. The study focused on investigating how the polymer chain length of the capping agent affects AgNW synthesis. Perfluoroalkoxy (PFA) reaction tubing was placed inside a tubing furnace maintained at 130 °C for the synthetic process, and AgNWs along with FC-70 were collected at the reactor outlet. The density difference between the fluids facilitated the straightforward separation of the AgNW product. The reported dimensions included lengths of 36.4 μm and diameters of 95 nm, achieved using a capping agent with a polymer chain length of approximately 1.3 million in a segmented flow reactor [45].
Considering scalability for AgNW syntheses, MFRs offer the advantage of easy scalability through numbering up or scaling out without requiring extensive redesign of the reaction setup. Yu et al. explored the scale-out strategies for MFR synthesis of AgNWs [14]. They employed a segmented-flow MFR submerged in a silicone oil bath heated to 160 °C to optimize the reaction conditions. The tubing diameter in the benchtop reactor was increased from 1 mm to 6 mm, with the temperature and residence time maintained constant. Initially, AgNPs formed in solution, which prompted the authors to adjust the residence time by increasing the reaction flow rate. A residence time of 60 min yielded AgNWs measuring 47 ± 3 μm in length and 117 ± 10 nm in diameter within the 6 mm inside diameter tubing MFR. The authors successfully scaled AgNW production from milligram-scale benchtop reactors to gram-per-hour large-scale reactors and anticipate achieving kilogram-scale production by numbering up channels in the reactor, as shown in Figure 6 [14].
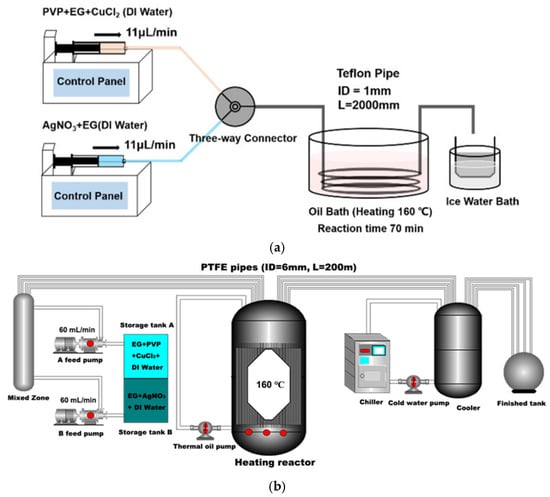
Figure 6.
The milligram-scale reactor setup used to synthesize AgNWs using a millifluidic reactor (a) and the reactor setup for scaling out to a gram-per-hour production rate (b). Reproduced from [14] in Nanomaterials (2022, 12, 1018).
A crucial part of a polyol AgNW reaction design is to understand the kinetics of the process by assessing the rate of nucleation (k1) and growth (k2). A study by Williams et al. utilized a non-linear Finke–Watzky model to quantify the nucleation and growth rate constants for both the MFR and batch reactors [46]. The authors discovered that the k1 and k2 for the MFRs were approximately four and two times larger, respectively, than the batch reactor rate constants. Additionally, the AgNW synthesis in the MFRs was about three times faster than in the batch reactor, and the coiled configuration of the MFRs promoted AgNW growth, minimized temperature transients, and enhanced reagent mixing caused by Dean vortices [46].
4. Polyol-Synthesized AgNW-Based TCF Applications
Given the scalability potential of polyol-based AgNW synthesis with MFRs, DIW emerges as a promising method for the continuous production of reliable AgNW-based TCFs for electronic applications. However, there is currently a literature gap in studies addressing this approach. Recently, some research has focused on synthesizing AgNWs using the polyol method and subsequently using DIW to create 3D-printed TCFs. Yang et al. synthesized AgNWs in a batch reactor using the polyol method and utilized them to manufacture AgNW-based stretchable, wearable sensors via DIW. They deposited an aqueous droplet of AgNW suspension onto a polyethylene terephthalate (PET) substrate and used compressed air under convective flow to induce a capillary force that lifted and captured the AgNWs in the droplet at the surface. As the water evaporated, the droplet area decreased, densely packing the AgNWs at the interface. The authors employed DIW to encapsulate the AgNWs on the PET substrate using silicone to form an elastomer matrix. The fabricated sensors could withstand over 500% strain, exhibited an electrical response range from 10% to 120%, and remained electrically reliable after more than 500 cycles of stretching and releasing. Additionally, the AgNW-based TCFs demonstrated antibacterial properties, enhancing the functionality of the sensors [33]. Kong et al. synthesized AgNWs using the polyol method in a batch reactor to develop soft electro-adhesion grippers using a sodium alginate (SA) conductive ink formulation via DIW. The authors reported AgNWs with average lengths and diameters of 17 µm and 23 nm, respectively. The conductive inks were formulated using SA, deionized (DI) water, and Triton X-100 as needed to improve wettability on the PET substrate. The TCFs for the soft electro-adhesion grippers exhibited excellent conductivity of up to 126,000 S cm−1 on a silicon wafer, and demonstrated shear adhesion forces of as high as 15.5 kPa under a driving voltage of 3 kV. The high resolution and conductivity achieved through DIW using AgNWs enables the continuous, customized manufacturing of soft and wearable electronics [37].
5. Conclusions and Future Directions
This review focused on recent research and advancements for polyol AgNW synthesis using MFRs for TCF manufacturing via DIW. While AgNWs exhibit mechanical flexibility, high conductivity, and optical transparency, the high cost of batch AgNW synthesis, lack of scalability for the process, and chemical instability of AgNWs have hindered scale-up [6]. MFRs offer a promising solution to overcome the limitations of batch reactors for industrial-scale and reliable AgNW synthesis through numbering up or scaling out. In addition, while the economic and environmental impacts of polyol batch reactors and polyol millifluidic flow reactors (MFRs) have not been extensively compared in the literature, we suggest that future research should focus on such analyses. This would help assess the potential advantages of MFRs in terms of scalability, cost-efficiency, and sustainability for silver nanowire synthesis, similar to the analysis we conducted for the green and polyol batch processes [47]. AgNW-based TCFs are great candidates to replace ITO-based TCFs in flexible and stretchable electronic applications. However, these films also come with disadvantages. Thermal instability, susceptibility to corrosion, and vulnerability to mechanical stress are among the primary drawbacks. DIW shows the most promise as a printing method to support continuous TCF manufacturing, from AgNW synthesis to conductive ink printing on an industrial scale. However, DIW faces challenges, such as moderate printing uniformity, low resolution, slow printing speed, complex conductive ink formulations, and multi-step TCF manufacturing processes, which are barriers to scaling up [36]. The high surface area of TCFs can lead to moisture and air absorption through the porous materials, leading to corrosion and eventual electrical failure [10]. The long-term electrical and mechanical performances of AgNW-based TCFs are adversely affected by factors such as temperature, light, air, and humidity. Oxidation and sulfidation are common forms of corrosion for AgNW-based TCFs under ambient conditions, leading to increased resistance, reduced conductivity, and degradation of electrical and mechanical properties [10]. As DIW is a relatively new printing method for TCFs, optimizing the ink formulation, printers, and printing processes is crucial to meet the demands of industrial-scale TCF manufacturing. Researchers are exploring methods to chemically and electrically stabilize AgNWs in TCFs, including chemical sintering with spin coatings [48], embedding the AgNWs in a matrix [49], and surface alloying to reduce the surface oxidation potential [50]. To provide continuous, scalable printability, minimize TCF corrosion, and reduce the steps in the TCF manufacturing process, real-time mixing with direct connections from the AgNW synthesis, as proposed in Figure 7, could be required [13].
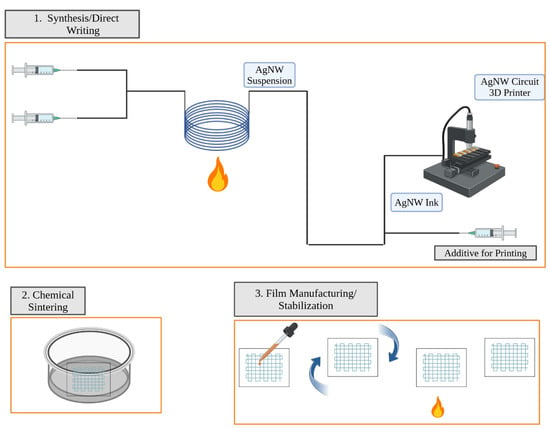
Figure 7.
A proposed method to manufacture electrically and chemically stable AgNW-based TCFs using MFRs, DIW, chemical sintering, and spin coating.
Author Contributions
D.F.W.: conceptualization, methodology, investigation, writing—original draft, and editing. S.H.: conceptualization, methodology, investigation, writing—review, editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation (NSF) under grant numbers 1939018 and 2422696.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, S.; Zhao, W.; Zeng, J.; He, Z.; Wang, X.; Zhu, Z.; Hu, R.; Liu, C.; Wang, Q. Wearable non-invasive glucose sensors based on metallic nanomaterials. Mater. Today Bio 2023, 20, 100638. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, J.P.; Kim, W.H.; Song, Y.H.; Kim, S.; Jeong, H.-J. Large-scale transfer of Ag nanowires from PET to PC film using a roll-to-roll UV lamination process for a capacitive touch sensor. RSC Adv. 2023, 13, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, Y.; Wei, W.; Zhou, Y. Large-Area Flexible Organic Solar Cells with a Robust Silver Nanowire-Polymer Composite as Transparent Top Electrode. Adv. Funct. Mater. 2023, 33, 2210675. [Google Scholar] [CrossRef]
- Wahab, O.J.; Kang, M.; Meloni, G.N.; Daviddi, E.; Unwin, P.R. Nanoscale Visualization of Electrochemical Activity at Indium Tin Oxide Electrodes. Anal. Chem. 2022, 94, 4729–4736. [Google Scholar] [CrossRef]
- Zhao, B.; Nisula, M.; Dhara, A.; Henderick, L.; Mattelaer, F.; Dendooven, J.; Detavernier, C. Atomic Layer Deposition of Indium-Tin-Oxide as Multifunctional Coatings on V2O5 Thin-Film Model Electrode for Lithium-Ion Batteries. Adv. Mater. Interfaces 2020, 7, 2001022. [Google Scholar] [CrossRef]
- Choi, C.; Schlenker, E.; Ha, H.; Cheong, J.Y.; Hwang, B. Versatile Applications of Silver Nanowire-Based Electrodes and Their Impacts. Micromachines 2023, 14, 562. [Google Scholar] [CrossRef]
- Sharma, N.; Koshy, A.M.; Kandregula, G.R.; Ramanujam, K.; Ray, D.; Swaminathan, P. Printed Silver Nanowire-PEDOT:PSS Composite Electrodes for Flexible Transparent Microsupercapacitor Applications. ACS Appl. Energy Mater. 2024, 7, 363–372. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Bi, Y.-G.; Shan, D.-M.; Chen, Z.-Y.; Wang, Y.-F.; Sun, H.-B.; Feng, J. Highly flexible organo-metal halide perovskite solar cells based on silver nanowire–polymer hybrid electrodes. Nanoscale 2023, 15, 5429–5436. [Google Scholar] [CrossRef]
- Lee, M.; Piper, R.T.; Bhandari, B.; Hsu, J.W.P. Multiobjective Optimization of Silver-Nanowire Deposition for Flexible Transparent Conducting Electrodes. ACS Appl. Nano Mater. 2023, 6, 17364–17368. [Google Scholar] [CrossRef]
- You, W.; Liao, B.; Wan, S.; Guo, X. Research progress on the stability of transparent conductive films for silver nanowires. Microelectron. Reliab. 2024, 156, 115394. [Google Scholar] [CrossRef]
- Fu, D.; Yang, R.; Wang, Y.; Wang, R.; Hua, F. Silver Nanowire Synthesis and Applications in Composites: Progress and Prospects. Adv. Mater. Technol. 2022, 7, 2200027. [Google Scholar] [CrossRef]
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y.; Huang, Z.; Wei, Y. Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. B 2017, 223, 1–23. [Google Scholar] [CrossRef]
- BioRender. Available online: https://www.biorender.com/ (accessed on 1 September 2024).
- Yu, J.; Yang, L.; Jiang, J.; Dong, X.; Cui, Z.; Wang, C.; Lu, Z. Scalable Production of High-Quality Silver Nanowires via Continuous-Flow Droplet Synthesis. Nanomaterials 2022, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.J.; Karadaghi, L.R.; Wang, L.; Malmstadt, N.; Brutchey, R.L. Continuous Flow Methods of Fabricating Catalytically Active Metal Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 27479–27502. [Google Scholar] [CrossRef] [PubMed]
- Pussepitiyalage, V.B.; Hemmati, S. Sustainable, Green, and Continuous Synthesis of Fivefold Palladium Nanorods Using l-Ascorbic Acid in a Segmented Millifluidic Flow Reactor. Langmuir 2022, 38, 4200–4212. [Google Scholar] [CrossRef]
- Neyt, N.C.; Riley, D.L. Application of reactor engineering concepts in continuous flow chemistry: A review. React. Chem. Eng. 2021, 6, 1295–1326. [Google Scholar] [CrossRef]
- Hu, C. Reactor design and selection for effective continuous manufacturing of pharmaceuticals. J. Flow Chem. 2021, 11, 243–263. [Google Scholar] [CrossRef]
- Lin, X.Z.; Terepka, A.D.; Yang, H. Synthesis of Silver Nanoparticles in a Continuous Flow Tubular Microreactor. Nano Lett. 2004, 4, 2227–2232. [Google Scholar] [CrossRef]
- Gottesman, R.; Tangy, A.; Oussadon, I.; Zitoun, D. Silver nanowires and nanoparticles from a millifluidic reactor: Application to metal assisted silicon etching. New J. Chem. 2012, 36, 2456–2459. [Google Scholar] [CrossRef]
- Espinosa, N.; Søndergaard, R.R.; Jørgensen, M.; Krebs, F.C. Flow Synthesis of Silver Nanowires for Semitransparent Solar Cell Electrodes: A Life Cycle Perspective. ChemSusChem 2016, 9, 893–899. [Google Scholar] [CrossRef]
- Hemmati, S.; Barkey, D.P.; Eggleston, L.; Zukas, B.; Gupta, N.; Harris, M. Silver Nanowire Synthesis in a Continuous Millifluidic Reactor. ECS J. Solid State Sci. Technol. 2017, 6, P144–P149. [Google Scholar] [CrossRef]
- Kinhal, K.V.; Bhatt, N.; Subramaniam, P. Transport and Kinetic Effects on the Morphology of Silver Nanoparticles in a Millifluidic System. Ind. Eng. Chem. Res. 2019, 58, 5820–5829. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. Continuous, green, and room-temperature synthesis of silver nanowires in a helically-coiled millifluidic reactor. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130806. [Google Scholar] [CrossRef]
- Monbaliu, J.-C.M.; Legros, J. Will the next generation of chemical plants be in miniaturized flow reactors? Lab A Chip 2023, 23, 1349–1357. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Shan, J.; Liu, C.; Guo, X.; Zhao, X.; Yang, H. Facile fabrication of large-scale silver nanowire transparent conductive films by screen printing. Mater. Res. Express 2022, 9, 66401. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Li, Y.; Yi, Z.; Zhou, G. Fabrication of Screen Printing-Based AgNWs Flexible Transparent Conductive Film with High Stability. Micromachines 2020, 11, 1027. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Chen, X.; Lai, W.; Huang, W. A Simple Strategy towards Highly Conductive Silver-Nanowire Inks for Screen-Printed Flexible Transparent Conductive Films and Wearable Energy-Storage Devices. Adv. Mater. Technol. 2019, 4, 1900196. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Huang, Q.; Li, Z.; Ng, T.N.; Zhao, H. Direct Embedment and Alignment of Silver Nanowires by Inkjet Printing for Stretchable Conductors. ACS Appl. Electron. Mater. 2020, 2, 3289–3298. [Google Scholar] [CrossRef]
- Patil, P.; Patil, S.; Kate, P.; Kulkarni, A.A. Inkjet printing of silver nanowires on flexible surfaces and methodologies to improve the conductivity and stability of the printed patterns. Nanoscale Adv. 2021, 3, 240–248. [Google Scholar] [CrossRef]
- Park, J.; Kim, G.; Lee, B.; Lee, S.; Won, P.; Yoon, H.; Cho, H.; Ko, S.H.; Hong, Y. Highly Customizable Transparent Silver Nanowire Patterning via Inkjet-Printed Conductive Polymer Templates Formed on Various Surfaces. Adv. Mater. Technol. 2020, 5, 2000042. [Google Scholar] [CrossRef]
- Kong, X.; Chen, H.; Li, H.; Yue, L.; Gong, M.; Lin, X.; Zhang, M.; Zhang, L.; Wang, D. Hierarchically Ordered Grid-Type Silver Nanowire Microelectrodes via Direct Ink Writing. ACS Appl. Nano Mater. 2024, 7, 14898–14905. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Narayanan, S.S.; Wang, X.; Zhao, H. Advanced Printing Transfer of Assembled Silver Nanowire Network into Elastomer for Constructing Stretchable Conductors. Adv. Eng. Mater. 2023, 25, 2300675. [Google Scholar] [CrossRef]
- Jiang, P.-Z.; Deng, Z.; Min, P.; Ye, L.; Qi, C.-Z.; Zhao, H.-Y.; Liu, J.; Zhang, H.-B.; Yu, Z.-Z. Direct ink writing of multifunctional gratings with gel-like MXene/norepinephrine ink for dynamic electromagnetic interference shielding and patterned Joule heating. Nano Res. 2024, 17, 1585–1594. [Google Scholar] [CrossRef]
- Dimngaihvungi, E.; Singh, M.; Pani, B.; Singh, A.K. Silver and copper nanowire-based nanocomposite for transparent electrodes: Deposition methods and applications in solar cells. Compos. Interfaces 2023, 30, 1449–1481. [Google Scholar] [CrossRef]
- Xu, X.; Xue, P.; Gao, M.; Li, Y.; Xu, Z.; Wei, Y.; Zhang, Z.; Liu, Y.; Wang, L.; Liu, H.; et al. Assembled one-dimensional nanowires for flexible electronic devices via printing and coating: Techniques, applications, and perspectives. Adv. Colloid Interface Sci. 2023, 321, 102987. [Google Scholar] [CrossRef]
- Kong, X.; Li, H.; Wang, J.; Wang, Y.; Zhang, L.; Gong, M.; Lin, X.; Wang, D. Direct Writing of Silver Nanowire Patterns with Line Width down to 50 μm and Ultrahigh Conductivity. ACS Appl. Mater. Interfaces 2023, 15, 9906–9915. [Google Scholar] [CrossRef]
- Lu, H.-C.; Liao, Y.-C. Direct Printed Silver Nanowire Strain Sensor for Early Extravasation Detection. Nanomaterials 2021, 11, 2583. [Google Scholar] [CrossRef]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding Whether to Go with the Flow: Evaluating the Merits of Flow Reactors for Synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 7502–7519. [Google Scholar] [CrossRef]
- Wu, K.-J.; Torrente-Murciano, L. Continuous synthesis of tuneable sized silver nanoparticles via a tandem seed-mediated method in coiled flow inverter reactors. React. Chem. Eng. 2018, 3, 267–276. [Google Scholar] [CrossRef]
- Fogler, H.S. Essential of Chemical Reaction Engineering; Prentice Hall: Saddle River, NJ, USA, 2013. [Google Scholar]
- Ha, H.; Amicucci, C.; Matteini, P.; Hwang, B. Mini review of synthesis strategies of silver nanowires and their applications. Colloid Interface Sci. Commun. 2022, 50, 100663. [Google Scholar] [CrossRef]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol Synthesis of Silver Nanowires: An Extensive Parametric Study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar] [CrossRef]
- Williams, D.F.; Rahimi, N.; Smay, J.E.; Hemmati, S. Optimizing silver nanowire synthesis: Machine learning improves and predicts yield for a polyol, millifluidic flow reactor. Appl. Nanosci. 2023, 13, 6539–6552. [Google Scholar] [CrossRef]
- Lau, K.S.; Chin, S.X.; Tan, S.T.; Lim, F.S.; Chang, W.S.; Yap, C.C.; Jumali, M.H.H.; Zakaria, S.; Chook, S.W.; Chia, C.H. Silver nanowires as flexible transparent electrode: Role of PVP chain length. J. Alloy. Compd. 2019, 803, 165–171. [Google Scholar] [CrossRef]
- Williams, D.F.; Smay, J.E.; Hemmati, S. Kinetic analysis of silver nanowire synthesis: Polyol batch and continuous millifluidic methods. Nanoscale 2025, 17, 7114–7127. [Google Scholar] [CrossRef] [PubMed]
- Kaabipour, S.; Neal, F.; Hemmati, S. High-Yield, Environmentally-Friendly, and Sustainable Synthesis of Silver Nanowires Using Tannic Acid and Their Application in Conductive Ink Preparation: Economic Analysis and Rheological Investigation. Mater. Interfaces 2025, 2, 32–45. [Google Scholar] [CrossRef]
- Bin, P.S.; Geng, W.H.; Wang, T.; Zhu, Q.; Li, M.; Liu, X.L.; Qian, P.F.; Bao, Z.L.; Yang, Z.X.; Geng, H.Z. Aluminum-Doped ZnO Weld Silver Nanowires-Based High Transmittance, Low Sheet Resistance, and Tough Composite Transparent Conductive Films. Adv. Eng. Mater. 2023, 25, 2201444. [Google Scholar] [CrossRef]
- Yao, W.; Yuxin, T.; Meng, L.; Hanming, D.; Demei, K.; Dezeng, L. Sandwich structure silver nanowires transparent conductive films with improved photoelectronic performance. J. Mater. Sci. 2024, 59, 435–446. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.; Seol, J.; Park, C.; Myoung, J.; Jeong, U. Enhanced Chemical Stability of Ag Nanowires by Slight Surface Modification with Pd. Adv. Mater. Interfaces 2018, 5, 1800250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).