Processing and Characterization of Nickel Matrix Nanocomposites Reinforced with Layered Nickel Aluminide Intermetallics Using Mechanical Alloying and Spark Plasma Sintering
Abstract
:1. Introduction
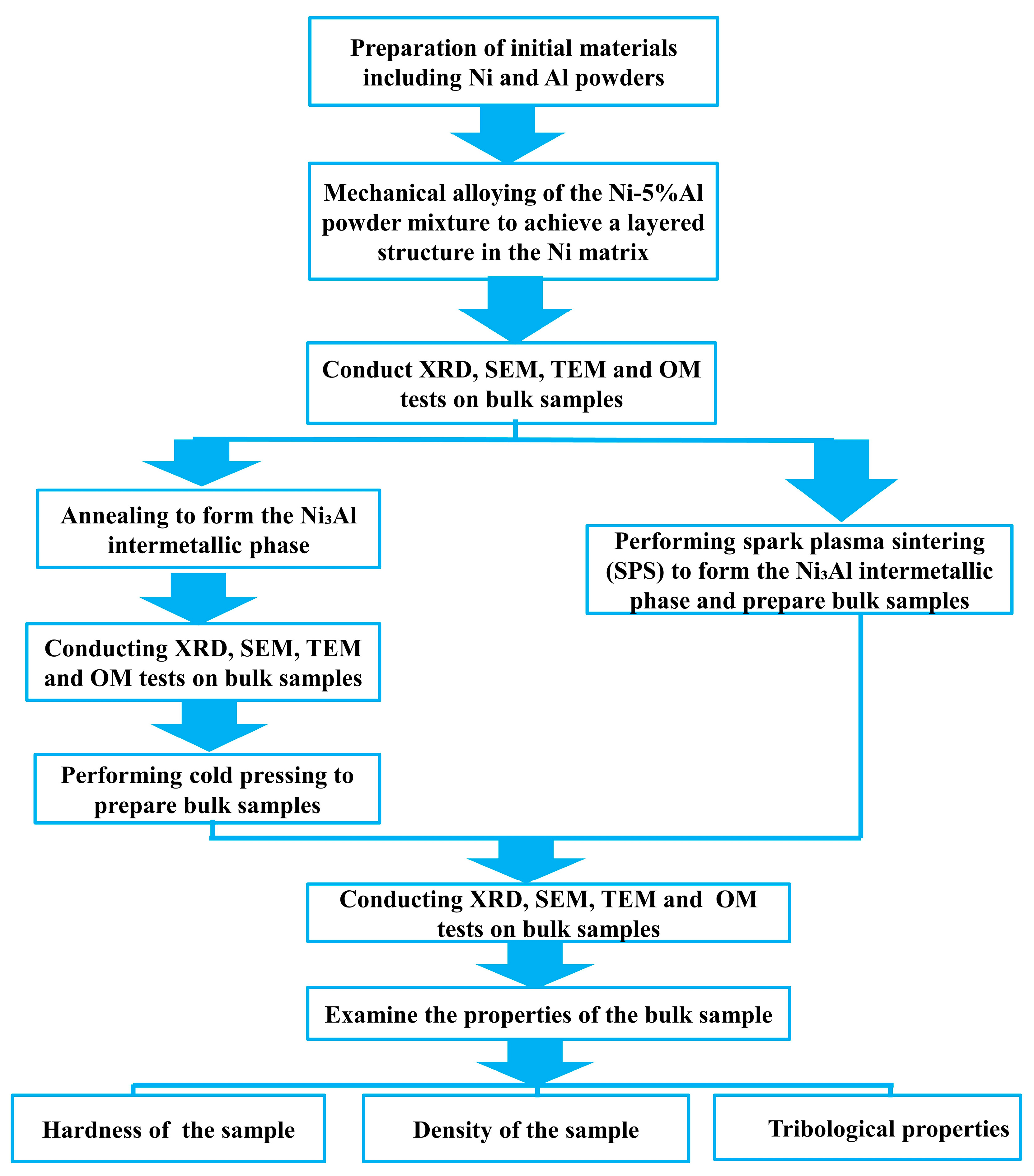
2. Materials and Methods
2.1. Mechanical Alloying
2.2. Spark Plasma Sintering and Annealing Process
2.3. Electron Microscopy and X-Ray Diffraction
2.4. Microhardness and Tribological Testing
3. Results
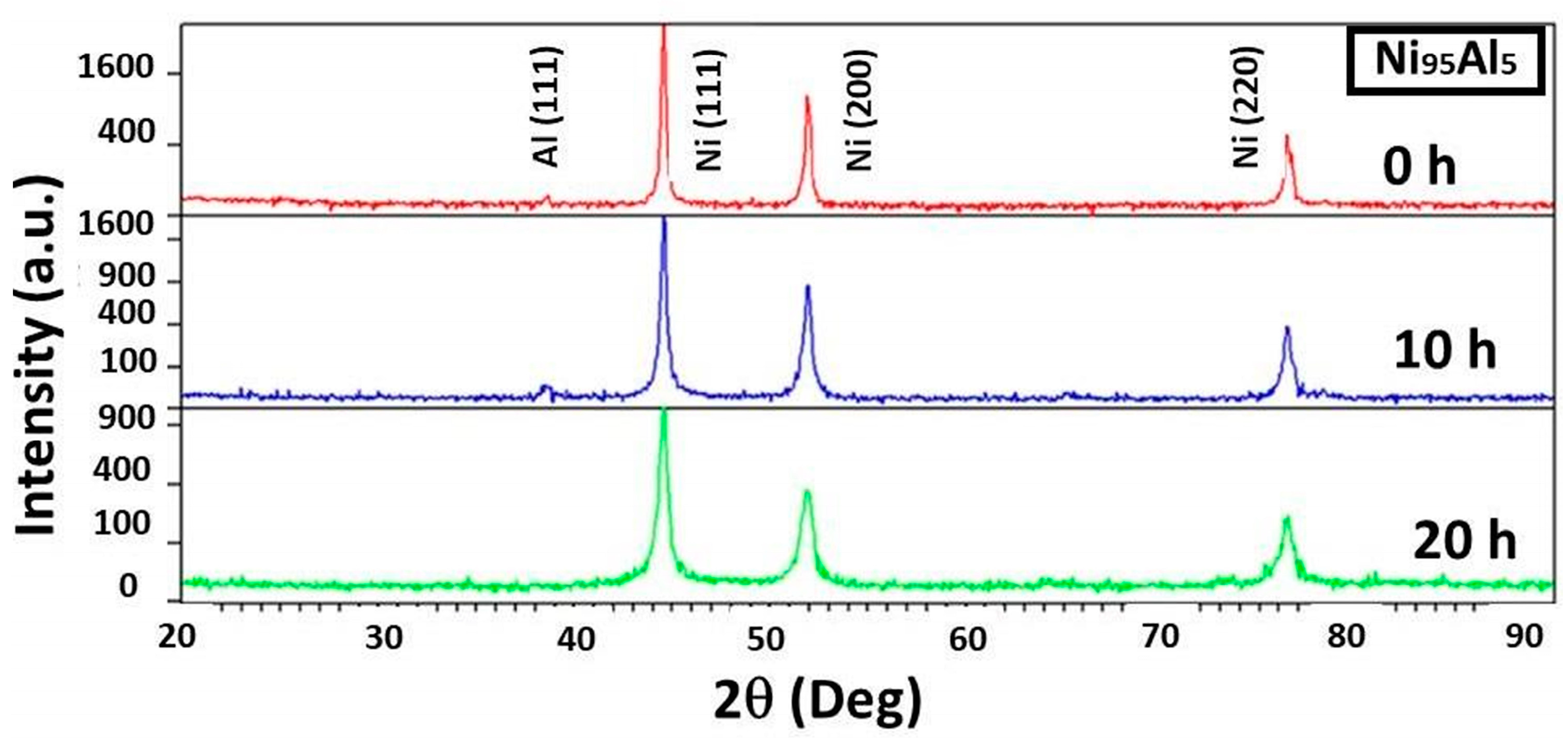
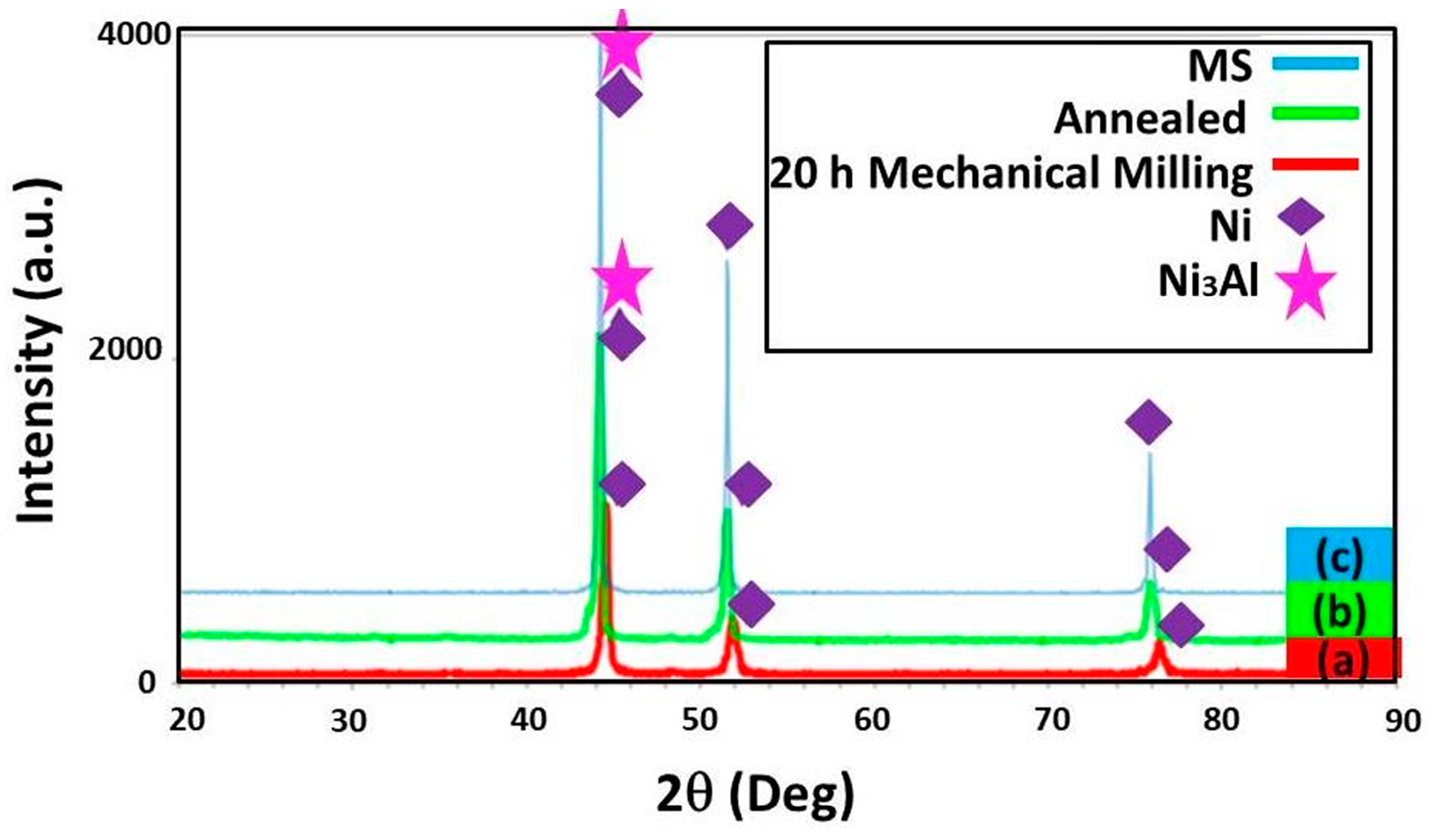
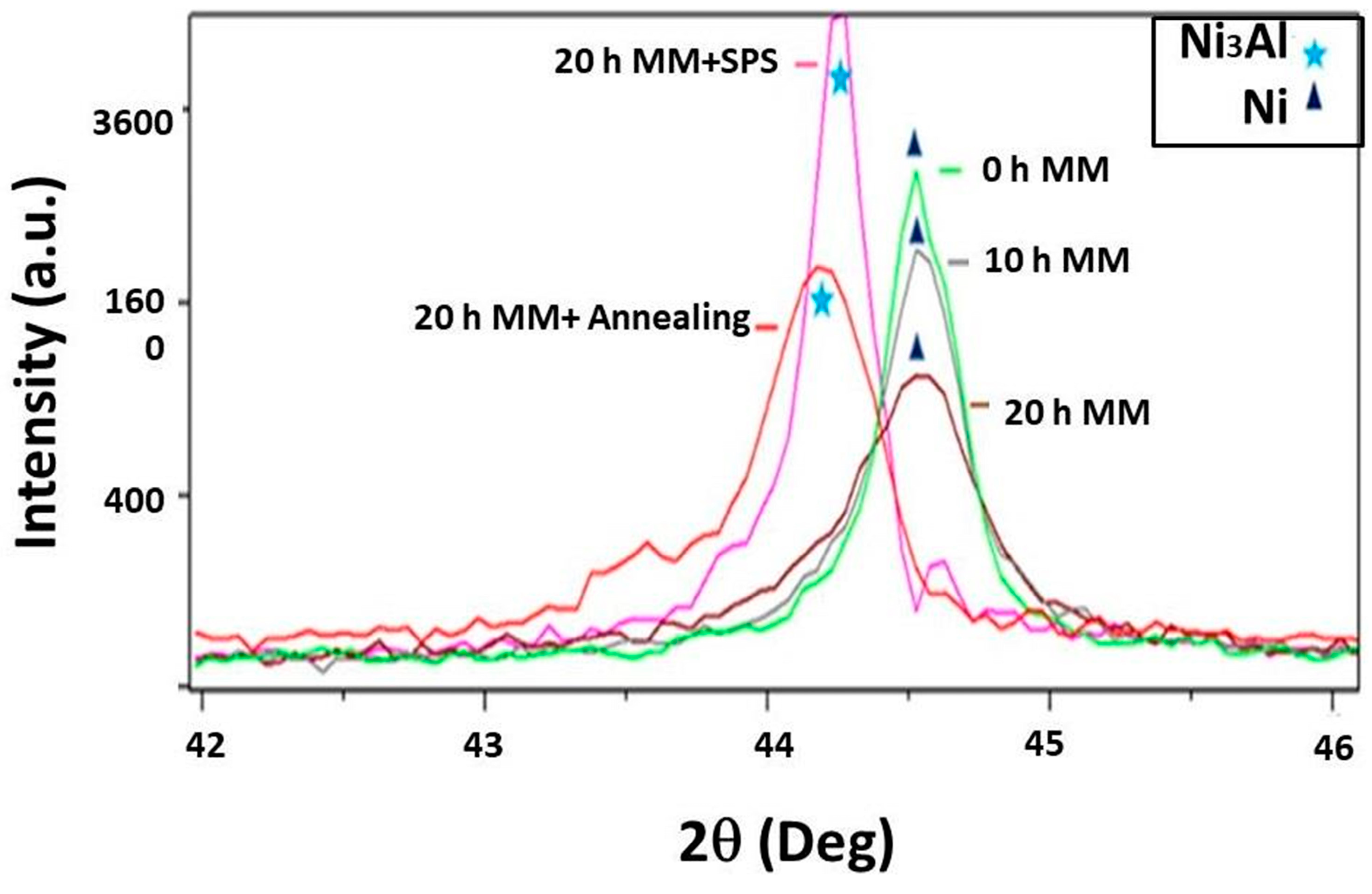
3.1. Structural Changes
3.2. Morphological Investigation
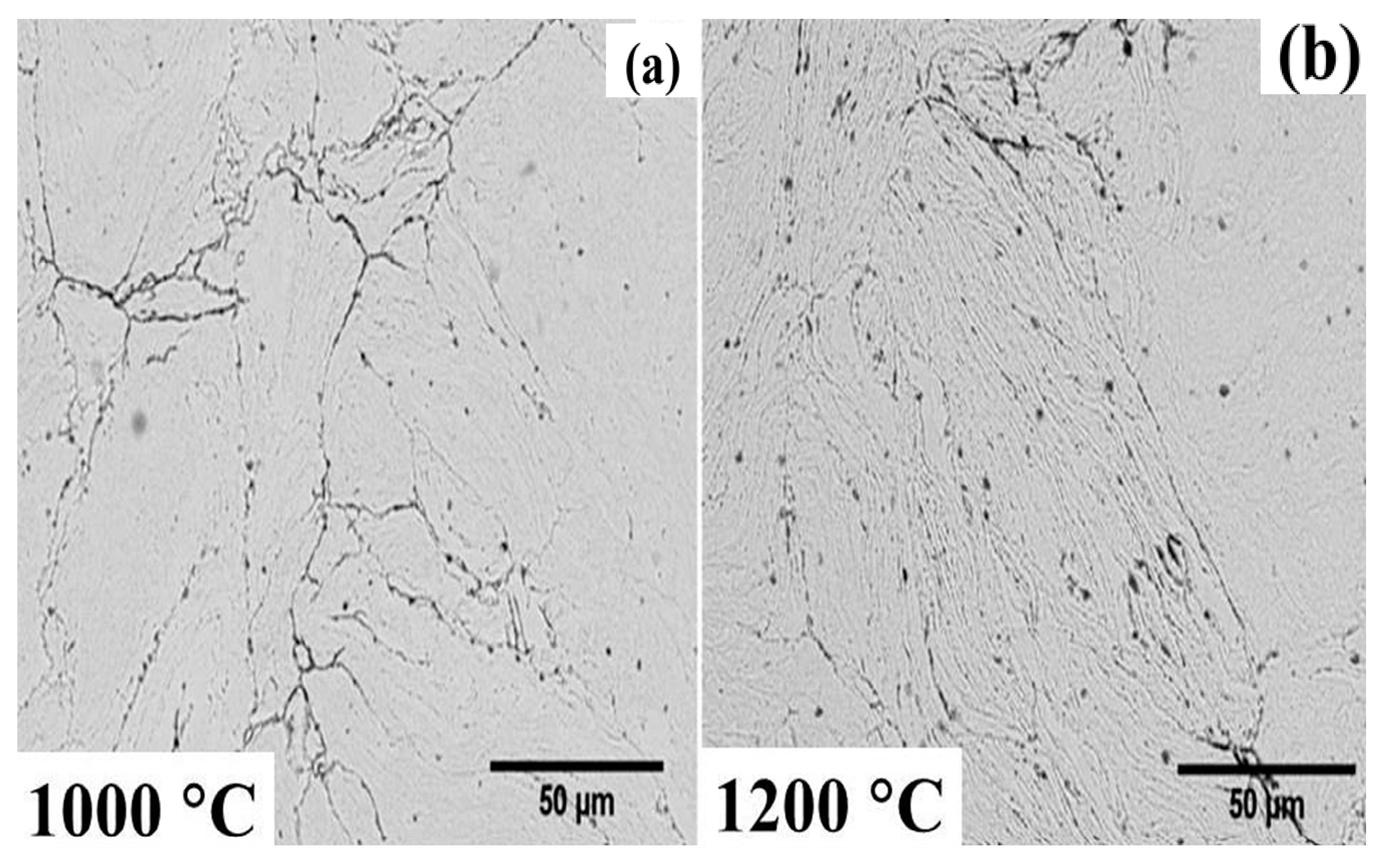
3.3. Microstructural Evolution
3.4. Mechanical Properties
3.4.1. Microhardness
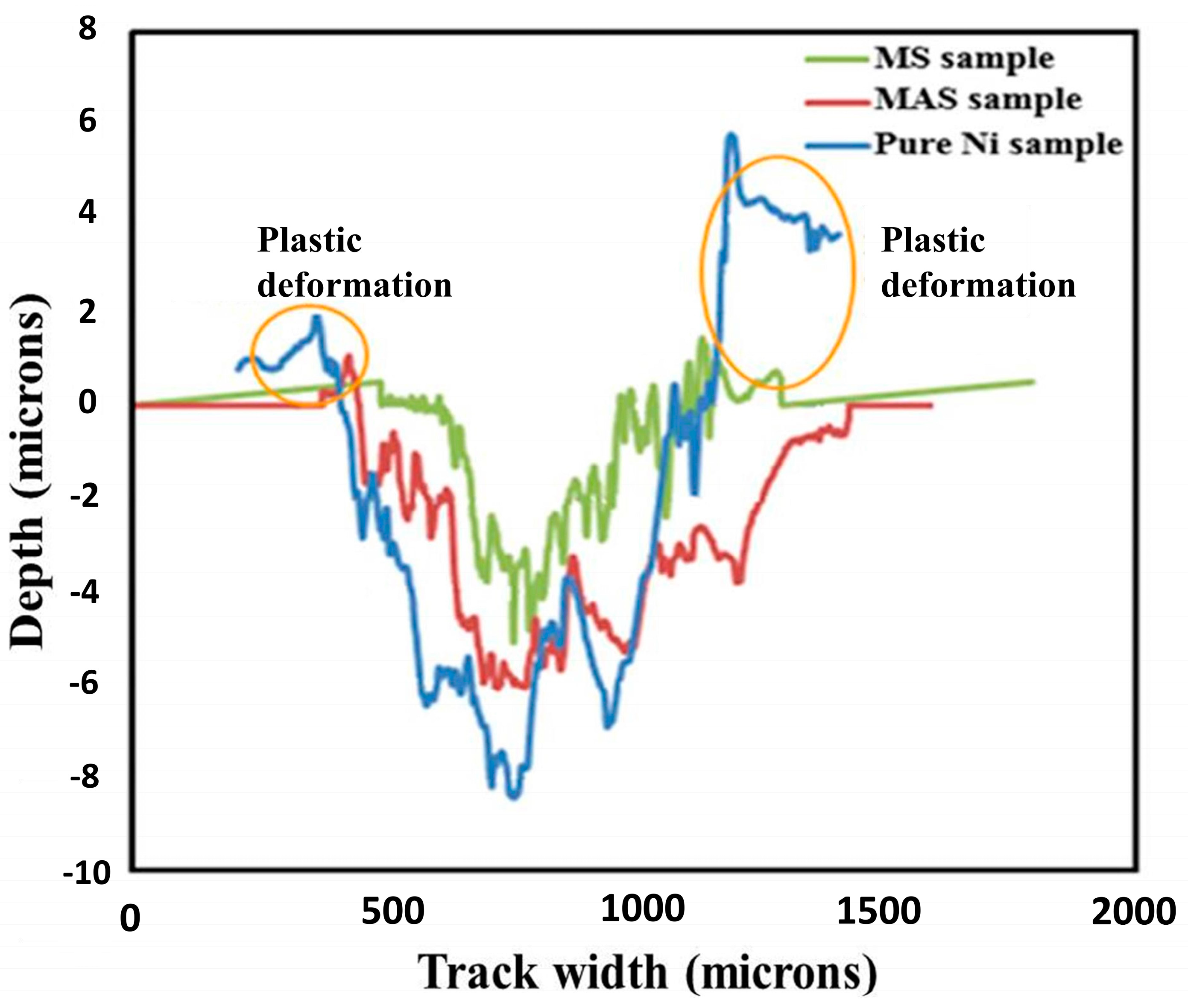
3.4.2. Tribological Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| Original Name | Abbreviation |
| mechanical alloying | MA |
| spark plasma sintering | SPS |
| nickel matrix nanocomposite | NMN |
| metal matrix composites | MMCs |
| process control agent | PCA |
| ball-to-powder ratio | BPR |
| milling + annealing + SPS | MAS |
| milling + SPS | MS |
| scanning electron microscopy | SEM |
| energy-dispersive X-ray spectroscopy | EDS |
| face-centered cubic | FCC |
| body-centered cubic | BCC |
References
- Cavaliere, P.; Sadeghi, B.; Shabani, A. Spark plasma sintering: Process fundamentals. In Spark Plasma Sintering of Materials Advances in Processing and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–20. [Google Scholar]
- Zohari, S.; Sadeghian, Z.; Lotfi, B.; Broeckmann, C. Application of spark plasma sintering (SPS) for the fabrication of in situ Ni–TiC nanocomposite clad layer. J. Alloys Compd. 2015, 633, 479–483. [Google Scholar] [CrossRef]
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced metal matrix nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Walunj, G.; Bearden, A.; Patil, A.; Larimian, T.; Christudasjustus, J.; Gupta, R.K.; Borkar, T. Mechanical and tribological behavior of mechanically alloyed Ni-TiC composites processed via spark plasma sintering. Materials 2020, 13, 5306. [Google Scholar] [CrossRef] [PubMed]
- Chawla, K.K. Composite Materials: Science and Engineering, 4th ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Patil, A.; Walunj, G.; Torgerson, T.B.; Koricherla, M.V.; Khan, M.U.; Scharf, T.W.; Gupta, R.; Borkar, T. Tribological behavior of in situ processed NI-Ti-C nanocomposites. Tribol. Trans. 2021, 64, 53–64. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanical Alloying: Energy Storage, Protective Coatings, and Medical Applications; William Andrew: Oxford, UK; Cambridge, MA, USA, 2020. [Google Scholar]
- Enayati, M.; Sadeghian, Z.; Salehi, M.; Saidi, A. The effect of milling parameters on the synthesis of Ni3Al intermetallic compound by mechanical alloying. Mater. Sci. Eng. A 2004, 375, 809–811. [Google Scholar] [CrossRef]
- Talaş, Ş. Nickel aluminides. In Intermetallic Matrix Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–69. [Google Scholar]
- Shevtsova, L.; Mali, V.; Bataev, A.; Anisimov, A.; Dudina, D. Microstructure and mechanical properties of materials obtained by spark plasma sintering of Ni3Al–Ni powder mixtures. Mater. Sci. Eng. A 2020, 773, 138882. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef]
- Enayati, M.; Mohamed, F. Application of mechanical alloying/milling for synthesis of nanocrystalline and amorphous materials. Int. Mater. Rev. 2014, 59, 394–416. [Google Scholar] [CrossRef]
- Awotunde, M.A.; Ayodele, O.O.; Adegbenjo, A.O.; Okoro, A.M.; Shongwe, M.B.; Olubambi, P.A. NiAl intermetallic composites—A review of processing methods, reinforcements and mechanical properties. Int. J. Adv. Manuf. Technol. 2019, 104, 1733–1747. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Reis, M.A.; Vieira, M.F. Aluminum and nickel matrix composites reinforced by CNTs: Dispersion/mixture by ultrasonication. Metals 2017, 7, 279. [Google Scholar] [CrossRef]
- Dutel, G.; Tingaud, D.; Langlois, P.; Dirras, G. Nickel with multimodal grain size distribution achieved by SPS: Microstructure and mechanical properties. J. Mater. Sci. 2012, 47, 7926–7931. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lalianpour, A.; Mehrizi, M.Z.; Beygi, R. Fabrication of NiAl–Al2O3-WC nanocomposite by mechanical alloying and subsequent heat treatment. Ceram. Int. 2019, 45, 19049–19054. [Google Scholar] [CrossRef]
- Anselmi-Tamburini, U.; Gennari, S.; Garay, J.; Munir, Z. Fundamental investigations on the spark plasma sintering/synthesis process: II. Modeling of current and temperature distributions. Mater. Sci. Eng. A 2005, 394, 139–148. [Google Scholar] [CrossRef]
- Laszkiewicz-Łukasik, J.; Putyra, P.; Klimczyk, P.; Podsiadło, M.; Bednarczyk, K. Spark plasma sintering/field assisted sintering technique as a universal method for the synthesis, densification and bonding processes for metal, ceramic and composite materials. J. Appl. Mater. Eng. 2020, 60, 53–69. [Google Scholar] [CrossRef]
- Azarmi, F.; Tangpong, X.; Chandanayaka, T. Investigation on mechanical properties of cold sprayed Ni–Ni3Al composites. Surf. Eng. 2015, 31, 832–839. [Google Scholar] [CrossRef]
- Udhayabanu, V.; Ravi, K.; Murugan, K.; Sivaprahasam, D.; Murty, B. Development of Ni-Al2O3 in-situ nanocomposite by reactive milling and spark plasma sintering. Metall. Mater. Trans. A 2011, 42, 2085–2093. [Google Scholar] [CrossRef]
- Anvari, S.; Karimzadeh, F.; Enayati, M. Synthesis and characterization of NiAl–Al2O3 nanocomposite powder by mechanical alloying. J. Alloys Compd. 2009, 477, 178–181. [Google Scholar] [CrossRef]
- Blau, P.J.; Budinski, K.G. Development and use of ASTM standards for wear testing. Wear 1999, 225, 1159–1170. [Google Scholar] [CrossRef]
- Yazdani, N.; Toroghinejad, M.R.; Shabani, A.; Cavaliere, P. Effects of process control agent amount, milling time, and annealing heat treatment on the microstructure of alcrcufeni high-entropy alloy synthesized through mechanical alloying. Metals 2021, 11, 1493. [Google Scholar] [CrossRef]
- Chaira, D. Powder metallurgy routes for composite materials production. Encycl. Mater. Compos. 2021, 2, 588–604. [Google Scholar]
- Enayati, M.H. Formation of nanoscale layered structures and subsequent transformations during mechanical alloying of Ni60Nb40 powder mixture in a low energy ball mill. Kona Powder Part. J. 2015, 32, 196–206. [Google Scholar] [CrossRef]
- Azhagarsamy, P.; Sekar, K.; Murali, K. Nickel Aluminide intermetallic composites fabricated by various processing routes—A review. Mater. Sci. Technol. 2022, 38, 556–571. [Google Scholar] [CrossRef]
- Paul, A.R.; Mukherjee, M.; Singh, D. A critical review on the properties of intermetallic compounds and their application in the modern manufacturing. Cryst. Res. Technol. 2022, 57, 2100159. [Google Scholar] [CrossRef]
- Munroe, P.; George, M.; Baker, I.; Kennedy, F. Microstructure, mechanical properties and wear of Ni–Al–Fe alloys. Mater. Sci. Eng. A 2002, 325, 1–8. [Google Scholar] [CrossRef]
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Mardiha, P.; Bahrami, A.; Mohammadnejad, A. Towards a high strength ductile Ni/Ni3Al/Ni multilayer composite using spark plasma sintering. Sci. Sinter. 2019, 51, 401–408. [Google Scholar] [CrossRef]
- Saba, F.; Zhang, F.; Liu, S.; Liu, T. Reinforcement size dependence of mechanical properties and strengthening mechanisms in diamond reinforced titanium metal matrix composites. Compos. Part B Eng. 2019, 167, 7–19. [Google Scholar] [CrossRef]
- Pan, S.; Jin, K.; Wang, T.; Zhang, Z.; Zheng, L.; Umehara, N. Metal matrix nanocomposites in tribology: Manufacturing, performance, and mechanisms. Friction 2022, 10, 1596–1634. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Abadeh, H.K.; Omidvar, H.; Hosseinpour, S. Assessment of Tribological behavior of nickel-nano Si3N4 composite coatings fabricated by pulsed electroplating process. Surf. Topogr. Metrol. Prop. 2020, 8, 025009. [Google Scholar] [CrossRef]
- Wang, L.; Dong, B.; Qiu, F.; Geng, R.; Zou, Q.; Yang, H.; Li, Q.; Xu, Z.; Zhao, Q.; Jiang, Q. Dry sliding friction and wear characterization of in situ TiC/Al-Cu3.7-Mg1.3 nanocomposites with nacre-like structures. J. Mater. Res. Technol. 2020, 9, 641–653. [Google Scholar] [CrossRef]
- Rivero, L.E.H.S.; Pizzatto, A.; Teixeira, M.F.; Rabelo, A.; Falcade, T.; Scheid, A. Effect of Laser Power and Substrate on the Hastelloy C276 TM Coatings Features Deposited by Laser Cladding. Mater. Res. 2020, 23, e20200067. [Google Scholar] [CrossRef]
- Choudhury, Y.; Gupta, P. Wear behavior of composites and nanocomposites: A new approach. Recent Trends Nanomater. Synth. Prop. 2017, 29–48. [Google Scholar]
- Zhu, H.-W.; Yu, B.-Y.; Zhang, H.; Yu, B.-N.; Lv, S.-N.; Zheng, L.; Li, R.-X. Effect of annealing treatment on microstructure and mechanical properties of Al/Ni multilayer composites during accumulative roll bonding (ARB) process. J. Iron Steel Res. Int. 2020, 27, 96–104. [Google Scholar] [CrossRef]
- Mishra, S.; Chandra, K.; Prakash, S. Dry sliding wear behaviour of nickel-, iron-and cobalt-based superalloys. Tribol.-Mater. Surf. Interfaces 2013, 7, 122–128. [Google Scholar] [CrossRef]
- Yakovtseva, O.; Mochugovskii, A.; Emelina, N.; Zanaeva, E.; Prosviryakov, A.; Mikhaylovskaya, A. Strenghthening features of mechanically alloyed Al-Mn-Cu alloy. Metallurgist 2024, 68, 672–682. [Google Scholar] [CrossRef]
- Pinate, S.; Ghassemali, E.; Zanella, C. Strengthening mechanisms and wear behavior of electrodeposited Ni–SiC nanocomposite coatings. J. Mater. Sci. 2022, 57, 16632–16648. [Google Scholar] [CrossRef]











| Weight (g) | Diameter (mm) | Number of Balls |
|---|---|---|
| 4 | 5 | 10 |
| 12 | 5 | 15 |
| 24 | 5 | 20 |
| No. | Specimen | MM | Annealing | SPS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Time (h) | Tem (°C) | Time (min) | Tem (°C) | Pressure (MPa) | Porosity (%) | Theoretical Density (%) | ||
| 1 | Pure Ni | 20 | 5 | 1200 | 40 | 1.7 | 98.2 | ||
| 2 | MAS | 20 | 1 | 700 | 5 | 1200 | 40 | 4 | 96 |
| 3 | MS | 20 | 5 | 1200 | 40 | 5 | 95.2 | ||
| 4 | MS | 20 | 5 | 1000 | 40 | 6 | 94 | ||
| 5 | MAS | 20 | 1 | 700 | 5 | 1000 | 40 | 7.6 | 92.5 |
| No.Specimen | MA | Annealing | SPS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Time (h) | Temp (°C) | Time (min) | Temp (°C) | Pressure (MPa) | Grain Size (nm) | Hardness (HV) | ||
| 1 | Pure Ni | 20 | 5 | 1200 | 40 | 90 | 200 ± 5 | ||
| 2 | MAS | 20 | 1 | 700 | 5 | 1200 | 40 | 85 | 310 ± 15 |
| 3 | MS | 20 | 5 | 1200 | 40 | 65 | 350 ± 15 | ||
| 4 | MS | 20 | 5 | 1200 | 40 | 34 | 250 ± 20 | ||
| 5 | MAS | 20 | 1 | 700 | 5 | 1200 | 40 | 48 | 280 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adabavazeh, Z.; Shiranibidabadi, A.H.; Enayati, M.H.; Karimzadeh, F. Processing and Characterization of Nickel Matrix Nanocomposites Reinforced with Layered Nickel Aluminide Intermetallics Using Mechanical Alloying and Spark Plasma Sintering. Nanomanufacturing 2025, 5, 1. https://doi.org/10.3390/nanomanufacturing5010001
Adabavazeh Z, Shiranibidabadi AH, Enayati MH, Karimzadeh F. Processing and Characterization of Nickel Matrix Nanocomposites Reinforced with Layered Nickel Aluminide Intermetallics Using Mechanical Alloying and Spark Plasma Sintering. Nanomanufacturing. 2025; 5(1):1. https://doi.org/10.3390/nanomanufacturing5010001
Chicago/Turabian StyleAdabavazeh, Zary, Amir Hossein Shiranibidabadi, Mohammad Hossein Enayati, and Fathallah Karimzadeh. 2025. "Processing and Characterization of Nickel Matrix Nanocomposites Reinforced with Layered Nickel Aluminide Intermetallics Using Mechanical Alloying and Spark Plasma Sintering" Nanomanufacturing 5, no. 1: 1. https://doi.org/10.3390/nanomanufacturing5010001
APA StyleAdabavazeh, Z., Shiranibidabadi, A. H., Enayati, M. H., & Karimzadeh, F. (2025). Processing and Characterization of Nickel Matrix Nanocomposites Reinforced with Layered Nickel Aluminide Intermetallics Using Mechanical Alloying and Spark Plasma Sintering. Nanomanufacturing, 5(1), 1. https://doi.org/10.3390/nanomanufacturing5010001






