Electronic, Structural, Optical, and Electrical Properties of CsPbX3 Powders (X = Cl, Br, and I) Prepared Using a Surfactant-Free Hydrothermal Approach
Abstract
1. Introduction
2. Materials and Methods
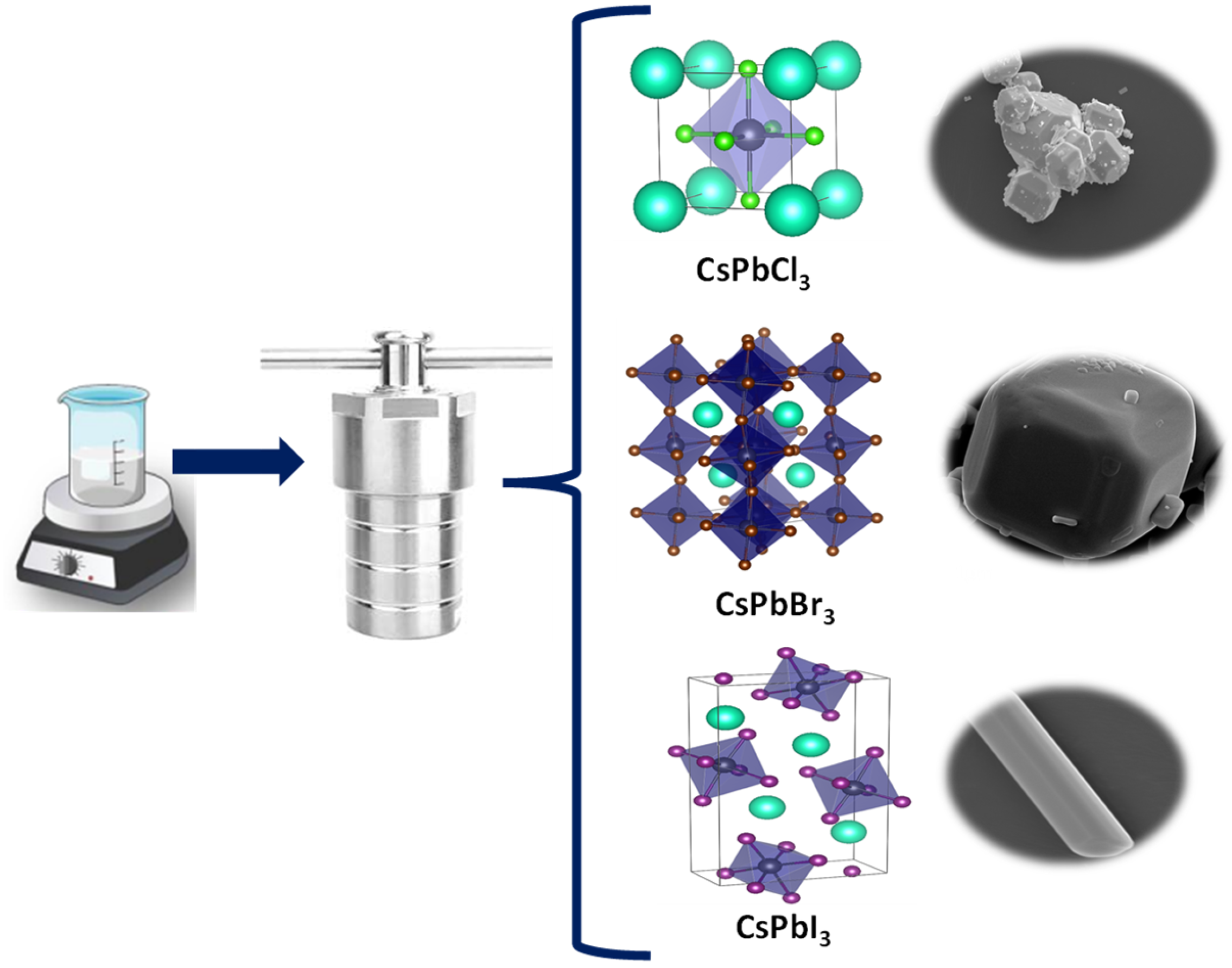
2.1. Materials and Hydrothermal Synthesis of CsPbX3 Powders
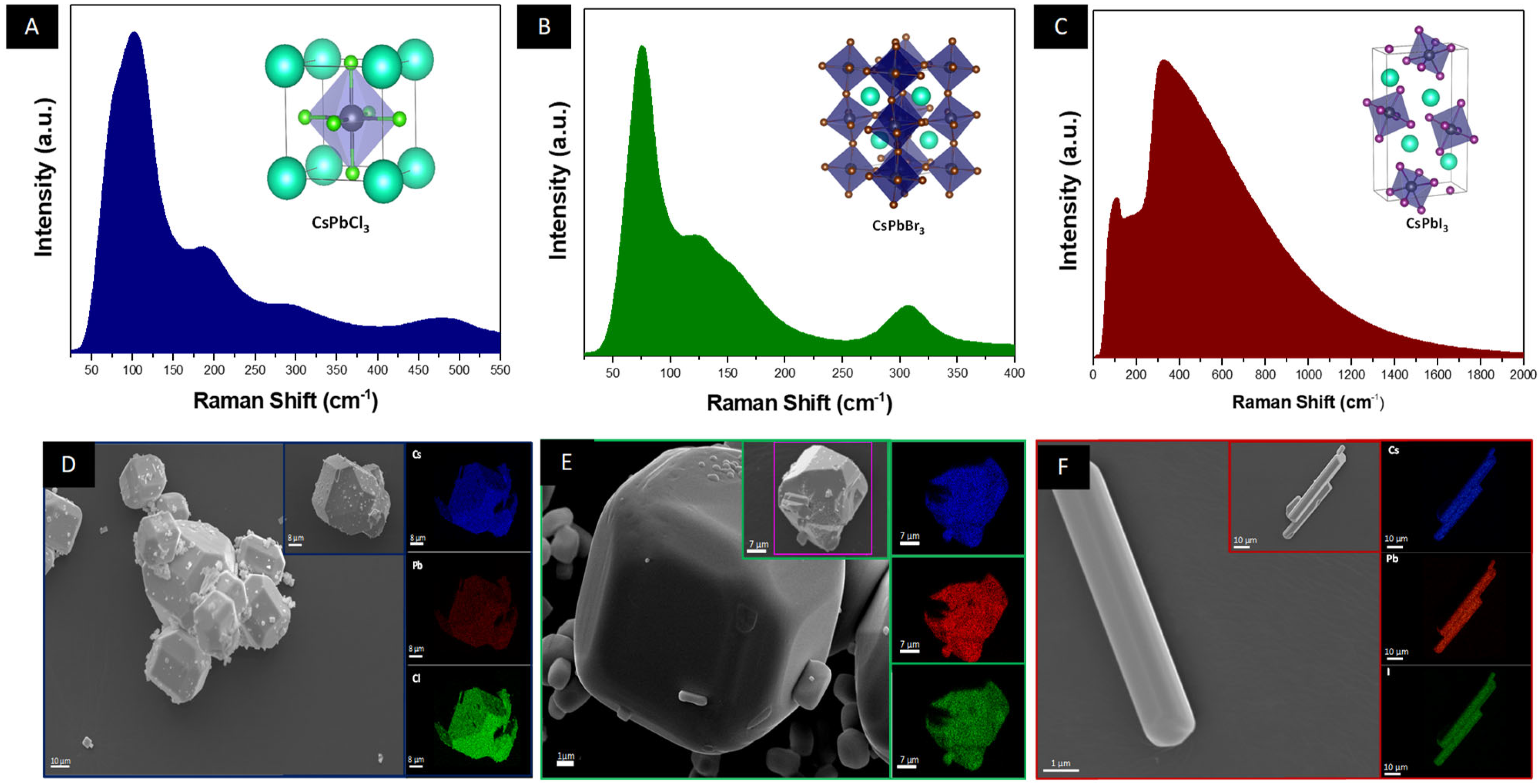
2.2. Characterization
2.3. Computational Method and Models
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, T.M.; Egger, D.A.; Kronik, L.; Hodes, G.; Cahen, D. Hybrid organic—Inorganic perovskites: Low-cost semiconductors with intriguing charge-transport properties. Nat. Rev. Mater. 2016, 1, 15007. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Pinto, F.M.; Dey, S.; Duarte, T.M.; Taft, C.A.; La Porta, F.A. Perovskite-like quantum dots designed for advanced optoelectronic applications. In Functional Properties of Advanced Engineering Materials and Biomolecules; La Porta, F., Taft, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Glazer, A.M. The classification of tilted octahedra in perovskites. Acta Crystallogr. Sect. B 1972, 28, 3384–3392. [Google Scholar] [CrossRef]
- Nakata, M.M.; Mazzo, T.M.; Casali, G.P.; La Porta, F.A.; Longo, E. A large red-shift in the photoluminescence emission of Mg1−xSrxTiO3. Chem. Phys. Lett. 2015, 622, 9–14. [Google Scholar] [CrossRef]
- Sutton, R.J.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Giustino, F.; Snaith, H.J. Cubic or Orthorhombic? Revealing the Crystal Structure of Metastable Black-Phase CsPbI3 by Theory and Experiment. ACS Energy Lett. 2018, 3, 1787–1794. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Abdelhady, A.L.; Manna, L. Zero-Dimensional Cesium Lead Halides: History, Properties, and Challenges. J. Phys. Chem. Lett. 2018, 9, 2326–2337. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 15 January 2022).
- Saliba, M.; Matsui, T.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; Gratzel, M. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Pinto, F.M.; de Conti, M.C.M.D.; Dey, S.; Velilla, E.; Taft, C.A.; La Porta, F.A. Emerging Metal-Halide Perovskite Materials for Enhanced Solar Cells and Light-Emitting Applications. In Research Topics in Bioactivity, Environment and Energy; Engineering Materials; Taft, C.A., de Lazaro, S.R., Eds.; Springer: Cham, Switzerland, 2022; pp. 45–85. [Google Scholar]
- Jacak, J.E.; Jacak, W.A. Routes for Metallization of Perovskite Solar Cells. Materials 2022, 15, 2254–2279. [Google Scholar] [CrossRef]
- Laska, M.; Krzemińska, Z.; Kluczk-Korch, K.; Schaadt, D.; Popko, E.; Jacak, W.A.; Jacak, J.E. Metallization of solar cells, exciton channel of plasmon photovoltaic effect in perovskite cells. Nano Energy 2020, 75, 104751. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Lu, H.; Chen, C.; Liu, Y.; Bai, X.; Yang, L.; Yu, W.W.; Dai, Q.; Zhang, Y. CsPbBr3 perovskite nanoparticles as additive for environmentally stable perovskite solar cells with 20.46% efficiency. Nano Energy 2019, 59, 517–526. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.; Yakunin, S.; Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Comm. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Nazarenko, O.; Dirin, D.N.; Kovalenko, M.V. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, L.; Li, J.; Liu, X.; Wang, R.; Wang, L.; Tu, G. Growth mechanism of CsPbBr3 perovskite nanocrystals by a co-precipitation method in a CSTR system. Nano Res. 2019, 12, 121–127. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.F.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- Kerner, R.A.; Zhao, L.; Xiao, Z.; Rand, B.P. Ultrasmooth metal halide perovskite thin films via sol–gel processing. J. Mater. Chem. A 2016, 4, 8308–8315. [Google Scholar] [CrossRef]
- Liang, J.; Wang, C.; Zhao, P.; Lu, Z.; Xu, Z.; Wang, Y.; Zhu, H.; Hu, Y.; Zhu, G.; Ma, L.; et al. Solution synthesis and phase control of inorganic perovskites for high-performance optoelectronic devices. Nanoscale 2017, 9, 11841. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, C.C.; Muñoz Flores, B.M.; Jiménez Pérez, V.M. Recent Advances in Synthesis and Properties of Hybrid Halide Perovskites for Photovoltaics. Nano-Micro Lett. 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef]
- Kayalvizhi, T.; Sathaya, A.; Preethi Meher, K.R.S. Hydrothermal Synthesis of Perovskite CsPbBr3: Spotlight on the Role of Stoichiometry in Phase Formation Mechanism. J. Electron. Mater. 2022, 51, 3466–3475. [Google Scholar] [CrossRef]
- Kayalvizhi, T.; Sathaya, A.; Preethi Meher, K.R.S. Facile synthesis of monoclinic CsPbBr3—For promising photovoltaic characteristics. AIP Conf. Proc. 2020, 2265, 030654. [Google Scholar]
- La Porta, F.A.; Masi, S. Solvent-Mediated Structural Evolution Mechanism from Cs4PbBr6 to CsPbBr3 Crystals. Nanomanufacturing 2021, 1, 67–74. [Google Scholar] [CrossRef]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic-inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Bibi, A.; Lee, I.; Nah, Y.; Allam, O.; Kim, H.; Quan, L.N.; Tang, J.; Walsh, A.; Jang, S.S.; Sargent, E.H.; et al. Lead-free halide double perovskites: Toward stable and sustainable optoelectronic devices. Mater. Today 2021, 49, 123–144. [Google Scholar] [CrossRef]
- Palazon, F.; El Ajjouri, Y.; Sebastia-Luna, P.; Lauciello, S.; Manna, L.; Bolink, H.J. Mechanochemical synthesis of inorganic halide perovskites: Evolution of phase-purity, morphology, and photoluminescence. J. Mater. Chem. C 2019, 7, 11406. [Google Scholar] [CrossRef]
- Rosales, B.A.; Wei, L.; Vela, J. Synthesis and mixing of complex halide perovskites by solvent-free solid-state methods. J. Solid State Chem. 2019, 271, 206–215. [Google Scholar] [CrossRef]
- Pal, P.; Saha, S.; Banik, A.; Sarkar, A.; Biswas, K. All-Solid-State Mechanochemical Synthesis and Post-Synthetic Transformation of Inorganic Perovskite-type Halides. Chem. Eur. J. 2018, 24, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Mertzsch, N. Hydrothermal processes in industry. ChemTexts 2020, 6, 21. [Google Scholar] [CrossRef]
- De Conti, M.C.M.D.; Dey, S.; Pottker, W.E.; La Porta, F.A. An overview into advantages and applications of conventional and unconventional hydro(solvo)thermal approaches for novel advanced materials design. In ChemRxiv; Cambridge Open Engage: Cambridge, UK, 2022. [Google Scholar]
- Pinto, F.M.; Suzuki, V.Y.; Silva, R.C.; La Porta, F.A. Oxygen defects and surface chemistry of reducible oxides. Front. Mater. 2019, 6, 260. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Web Standards and Reference Data for First-Principles Simulations. Available online: http://www.quantum-simulation.org (accessed on 5 May 2019).
- Hamann, D.R. Optimized norm-conserving Vanderbilt pseudopotentials. Phys. Rev. B 2017, 88, 085117. [Google Scholar] [CrossRef]
- Thesika, K.; Murugan, A.V. Microwave-Enhanced Chemistry at Solid–Liquid Interfaces: Synthesis of All-Inorganic CsPbX3 Nanocrystals and Unveiling the Anion-Induced Evolution of Structural and Optical Properties. Inorg. Chem. 2020, 59, 6161–6175. [Google Scholar] [CrossRef]
- Cha, J.-H.; Han, J.H.; Yin, W.; Park, C.; Park, Y.; Ahn, T.K.; Cho, J.H.; Jung, D.-Y. Photoresponse of CsPbBr3 and Cs4PbBr6 Perovskite Single Crystals. J. Phys. Chem. Lett. 2017, 8, 565–570. [Google Scholar] [CrossRef]
- Moller, C.K. Crystal structure and photoconductivity of cesium plumbohalides. Nature 1958, 182, 143. [Google Scholar] [CrossRef]
- Trots, D.M.; Myagkota, S.V. High-Temperature Structural Evolution of Caesium and Rubidium Triiodoplumbates. J. Phys. Chem. Solids 2008, 69, 2520–2526. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, T.; Xie, Q.; Gao, L.; Lu, L.; Tang, Y. First-principles study on the electronic and optical properties of the orthorhombic CsPbBr3 and CsPbI3 with Cmcm space group. New J. Chem. 2021, 45, 15857–15862. [Google Scholar] [CrossRef]
- Liao, M.; Shan, B.; Li, M. In Situ Raman Spectroscopic Studies of Thermal Stability of All-Inorganic Cesium Lead Halide (CsPbX3, X = Cl, Br, I) Perovskite Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Kong, Q.; Bischak, C.G.; Yu, Y.; Dou, L.; Eaton, S.W.; Ginsberg, N.S.; Yang, P. Structural, optical, and electrical properties of phase-controlled cesium lead iodide nanowires. Nano Res. 2017, 10, 1107–1114. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Dong, J.; Yu, H.; Zhou, C.; Zhang, B.; Xu, Y.; Jie, W. Centimeter-sized inorganic lead halide perovskite CsPbBr3 crystals grown by an improved solution method. Cryst. Growth Des. 2017, 17, 6426–6431. [Google Scholar] [CrossRef]
- Linaburg, M.R.; McClure, E.T.; Majher, J.D.; Woodward, P.M. Cs1–xRbxPbCl3 and Cs1–xRbxPbBr3 Solid Solutions: Understanding Octahedral Tilting in Lead Halide Perovskites. Chem. Mater. 2017, 29, 3507–3514. [Google Scholar] [CrossRef]
- Xu, S.; Libanori, A.; Luo, G.; Chen, J. Engineering bandgap of CsPbI3 over 1.7 eV with enhanced stability and transport properties. IScience 2021, 24, 102235. [Google Scholar] [CrossRef]
- Chhillar, P.; Dhamaniya, B.P.; Pathak, S.K.; Karak, S. Stabilization of Photoactive γ-CsPbI3 Perovskite Phase by Incorporation of Mg. ACS Appl. Electron. Mater. 2022, 4, 5368–5378. [Google Scholar] [CrossRef]
- Filip, M.R.; Eperon, G.E.; Snaith, H.J.; Giustino, F. Steric Engineering of Metal-Halide Perovskites with Tunable Optical Band Gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar] [CrossRef]
- Masi, S.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Stabilization of Black Perovskite Phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020, 5, 1974–1985. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Gong, J.; Guo, P.; Benjamin, S.E.; Gregory Van Patten, P.; Schaller, R.D.; Xu, T. Cation engineering on lead iodide perovskites for stable and high-performance photovoltaic applications. J. Energy Chem. 2018, 27, 1017–1039. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1−xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Luttinger, J.M.; Kohn, W. Motion of electrons and holes in perturbed periodic fields. Phys. Rev. 1955, 97, 869. [Google Scholar] [CrossRef]
- Kohanoff, J. Electronic Structure Calculations for Solids and Molecules: Theory and Computational Methods; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Tanaka, K.; Takahashi, T.; Ban, T.; Kondo, T.; Uchida, K.; Miura, N. Comparative study on the excitons in lead-halide-based perovskite-type crystals CH 3NH 3PbBr 3 CH3NH3PbI3. Solid State Commun. 2003, 127, 619–623. [Google Scholar] [CrossRef]
- Ghaithan, H.M.; Alahmed, Z.A.; Qaid, S.M.H.; Aldwayyan, A.S. Density Functional Theory Analysis of Structural, Electronic, and Optical Properties of Mixed-Halide Orthorhombic Inorganic Perovskites. ACS Omega 2021, 6, 30752–30761. [Google Scholar] [CrossRef]
- García-Fernández, A.; Moradi, Z.; Bermúdez-García, J.M.; Sánchez-Andújar, M.; Gimeno, V.A.; Castro-García, S.; Señarís-Rodríguez, M.A.; Mas-Marzá, E.; Garcia-Belmonte, G.; Fabregat-Santiago, F. Effect of Environmental Humidity on the Electrical Properties of Lead Halide Perovskites. J. Phys. Chem. C 2019, 123, 2011–2018. [Google Scholar] [CrossRef]
- Bisquert, J.; Bertoluzzi, L.; Mora-Sero, I.; Garcia-Belmonte, G. Theory of Impedance and Capacitance Spectroscopy of Solar Cells with Dielectric Relaxation, Drift-Diffusion Transport, and Recombination. J. Phys. Chem. C 2014, 118, 18983–18991. [Google Scholar] [CrossRef]
- Walsh, A.; Stranks, S.D. Taking Control of Ion Transport in Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 1983–1990. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.F.; O’Regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Comm. 2015, 6, 7497. [Google Scholar] [CrossRef]
- Mizusaki, J.; Arai, K.; Fueki, K. Ionic conduction of the perovskite-type halides. Solid State Ion. 1983, 11, 203–211. [Google Scholar] [CrossRef]

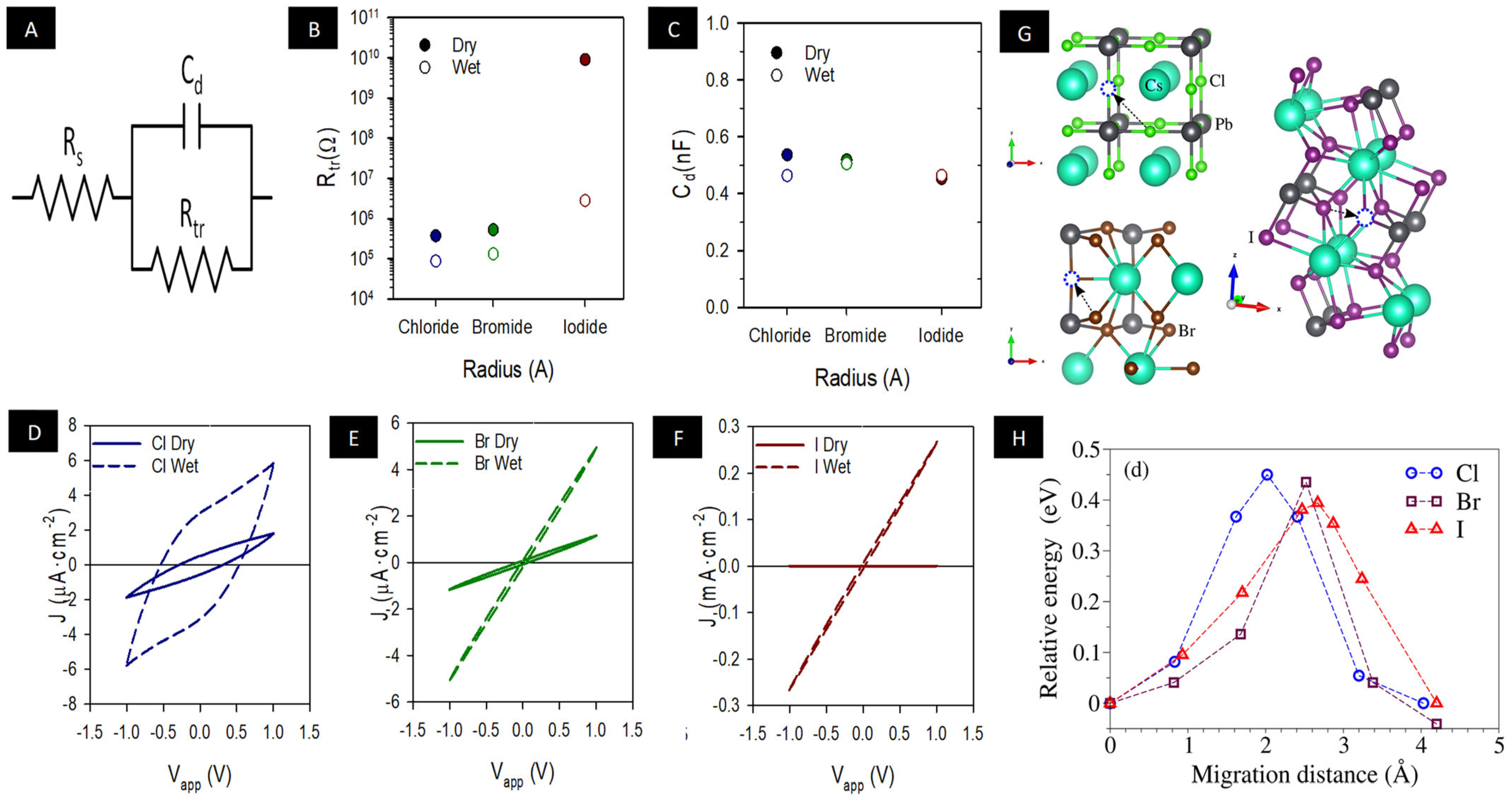
| Model | me | mh | µ |
|---|---|---|---|
| CsPbCl3 | 0.28 | 0.27 | 0.14 |
| CsPbBr3 | 0.24 | 0.25 | 0.12 |
| CsPbI3 | 1.32 | 1.48 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría-Arrondo, C.; Alvarez, A.O.; Masi, S.; Fabregat-Santiago, F.; Porta, F.A.L. Electronic, Structural, Optical, and Electrical Properties of CsPbX3 Powders (X = Cl, Br, and I) Prepared Using a Surfactant-Free Hydrothermal Approach. Nanomanufacturing 2023, 3, 217-227. https://doi.org/10.3390/nanomanufacturing3020013
Echeverría-Arrondo C, Alvarez AO, Masi S, Fabregat-Santiago F, Porta FAL. Electronic, Structural, Optical, and Electrical Properties of CsPbX3 Powders (X = Cl, Br, and I) Prepared Using a Surfactant-Free Hydrothermal Approach. Nanomanufacturing. 2023; 3(2):217-227. https://doi.org/10.3390/nanomanufacturing3020013
Chicago/Turabian StyleEcheverría-Arrondo, Carlos, Agustin O. Alvarez, Sofia Masi, Francisco Fabregat-Santiago, and Felipe A. La Porta. 2023. "Electronic, Structural, Optical, and Electrical Properties of CsPbX3 Powders (X = Cl, Br, and I) Prepared Using a Surfactant-Free Hydrothermal Approach" Nanomanufacturing 3, no. 2: 217-227. https://doi.org/10.3390/nanomanufacturing3020013
APA StyleEcheverría-Arrondo, C., Alvarez, A. O., Masi, S., Fabregat-Santiago, F., & Porta, F. A. L. (2023). Electronic, Structural, Optical, and Electrical Properties of CsPbX3 Powders (X = Cl, Br, and I) Prepared Using a Surfactant-Free Hydrothermal Approach. Nanomanufacturing, 3(2), 217-227. https://doi.org/10.3390/nanomanufacturing3020013






