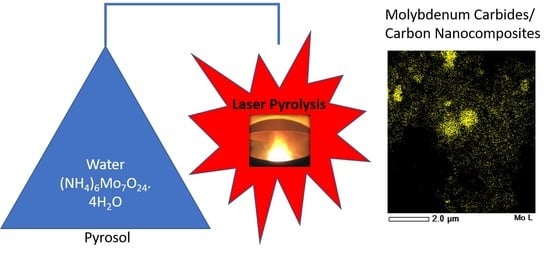
Facile Synthesis and Characterization of Molybdenum Carbides/Carbon Nanocomposites by Laser Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of MoyC/GC Nanocomposites
2.3. Characterization
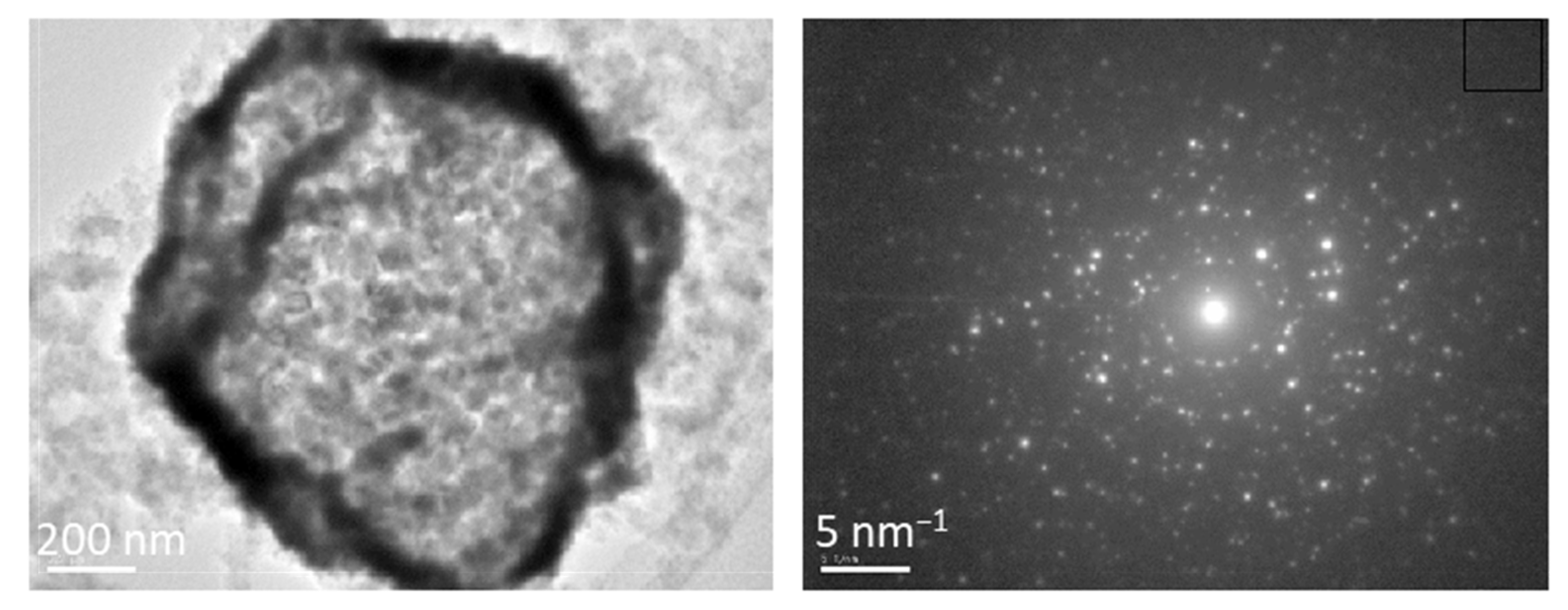
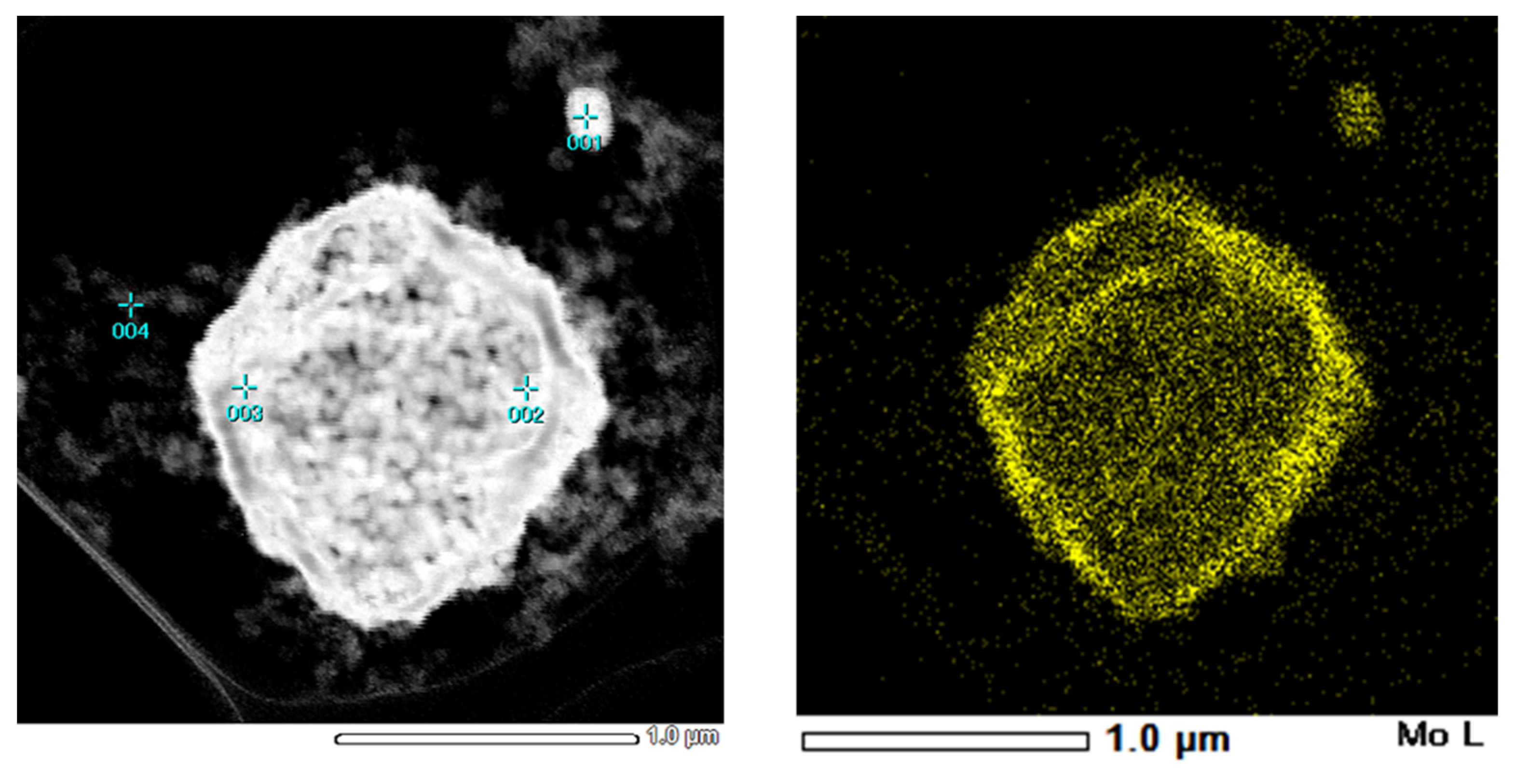
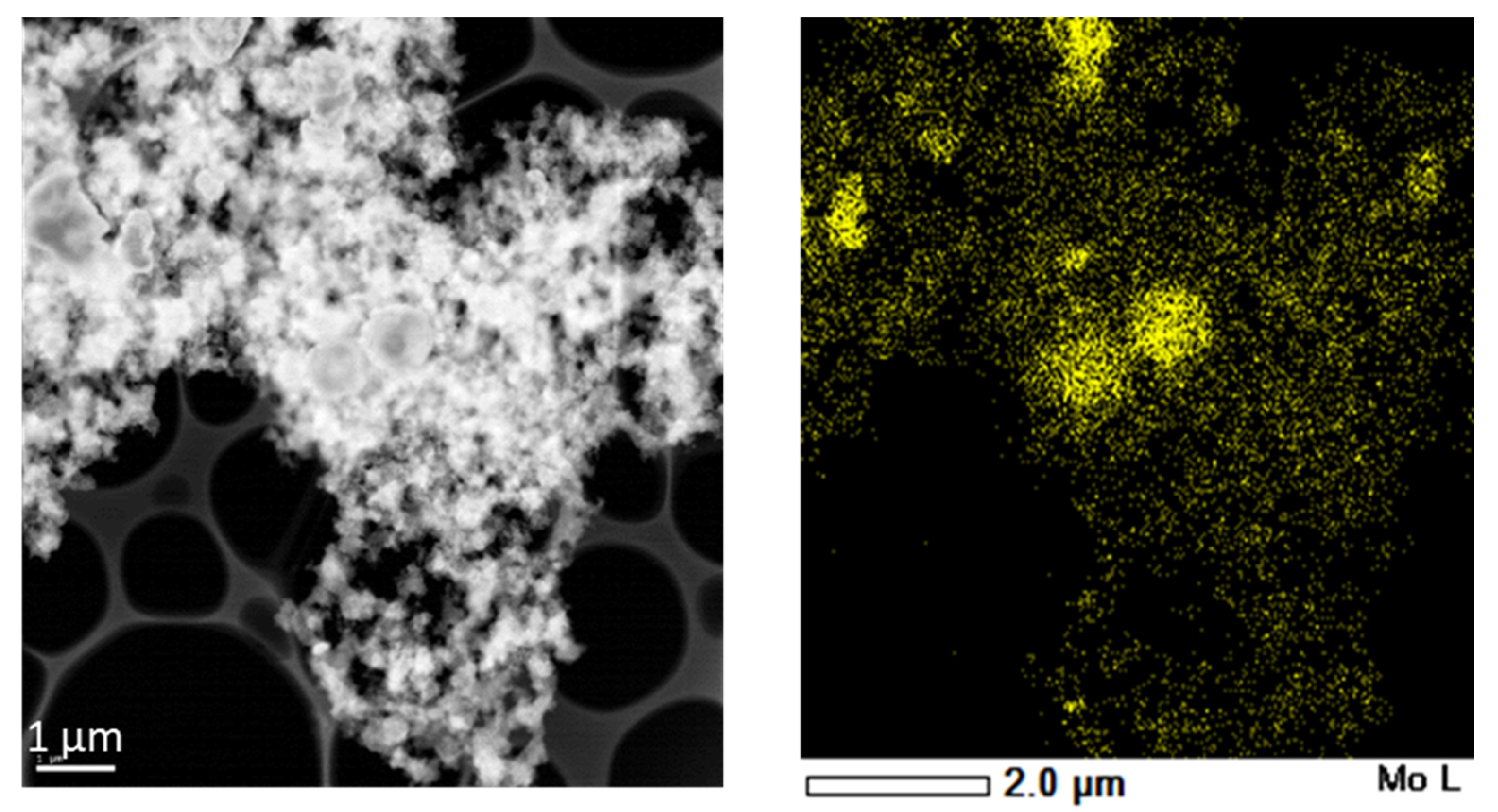
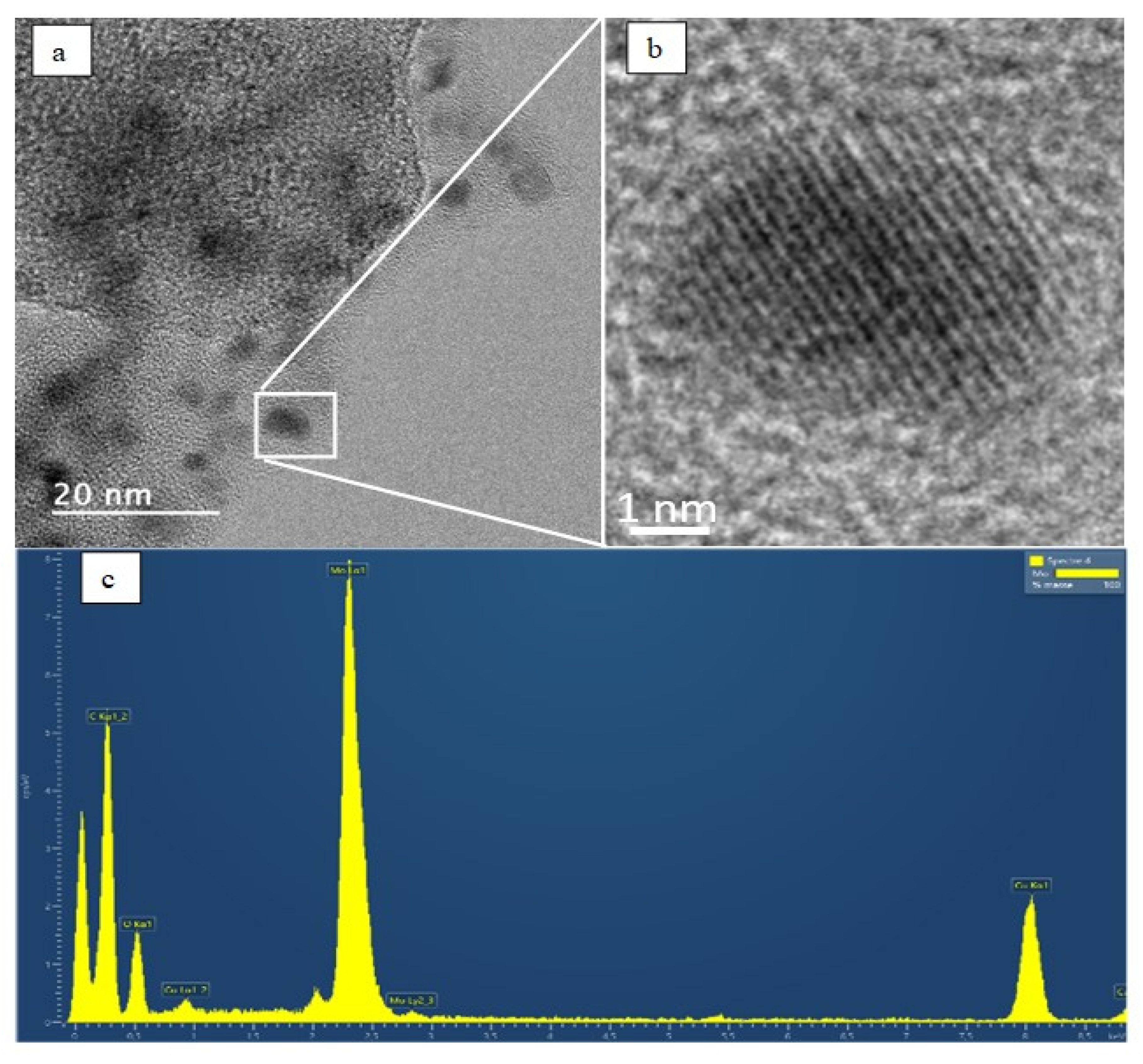
3. Results and Discussion
- Start without refining parameters to generate the hkl information.
- Refine the lattice parameter.
- Refine the Y parameter and GauSize.
- -
- Average apparent size for (±1 nm);
- -
- Average apparent size for (± 1 nm).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Zheng, Y.; Kozinski, J.A.; Dalai, A.K. Insights on pathways for hydrogen generation from ethanol. Sustain. Energy Fuels 2017, 1, 1232. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919. [Google Scholar] [CrossRef] [PubMed]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442. [Google Scholar] [CrossRef]
- Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H.M.; Tews, P.; Panster, P.; Diehl, M.; Lang, J.; Kreuzer, T.; et al. Platinum group metals and compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production—A review. Renew. Sustain. Energy Rev. 2017, 75, 1101. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Sun, H.H.; Xu, F.; Wang, J.G. A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met. 2020, 39, 335. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354. [Google Scholar] [CrossRef]
- Chen, H.; Zou, X. Intermetallic borides: Structures, synthesis and applications in electrocatalysis. Inorg. Chem. Front. 2020, 7, 2248. [Google Scholar] [CrossRef]
- Gujral, H.S.; Singh, G.; Baskar, A.V.; Guan, X.; Geng, X.; Kotkondawar, A.V.; Rayalu, S.; Kumar, P.; Karakoti, A.; Vinu, A. Metal nitride-based nanostructures for electrochemical and photocatalytic hydrogen production. Sci. Technol. Adv. Mater. 2022, 23, 76. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. 2012, 124, 12875. [Google Scholar] [CrossRef] [Green Version]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube–graphene hybrid support. ACS Nano 2014, 8, 5164. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cheng, N.; Liu, Q.; Ge, C.; Asiri, A.M.; Sun, X. Mo2C nanoparticles decorated graphitic carbon sheets: Biopolymer-derived solid-state synthesis and application as an efficient electrocatalyst for hydrogen generation. ACS Catal. 2014, 4, 2658. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C nanoparticles dispersed on hierarchical carbon microflowers for efficient electrocatalytic hydrogen evolution. ACS Nano 2016, 10, 11337. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Huang, Z.; Yang, Q.; Wei, G.; Chen, Z.; Humphrey, M.G.; Zhang, C. Ultrafast synthesis of molybdenum carbide nanoparticles for efficient hydrogen generation. J. Mater. Chem. A 2017, 5, 22805. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, R.; Gautam, S.; De Sarkar, A.; Tiwari, N.; Nath Jha, S.; Bhattacharya, D. Nano-structured hybrid molybdenum carbides/nitrides generated in situ for HER applications. J. Mater. Chem. A 2017, 5, 7764. [Google Scholar] [CrossRef]
- Jothi, P.R.; Zhang, Y.; Scheifers, J.P.; Park, H.; Fokwa, B.P.T. Molybdenum diboride nanoparticles as a highly efficient electrocatalyst for the hydrogen evolution reaction. Sustain. Energy Fuels 2017, 1, 1928. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12, 12761. [Google Scholar] [CrossRef]
- Guy, K.; Tessier, F.; Kaper, H.; Grasset, F.; Dumait, N.; Demange, V.; Nishio, M.; Matsushita, Y.; Matsui, Y.; Takei, T.; et al. Original synthesis of molybdenum nitrides using metal cluster compounds as precursors: Applications in heterogeneous catalysis. Chem. Mater. 2020, 32, 6026. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, S.; Liu, Y.; Liu, D.; Razal, J.M.; Lei, W. Interfacial engineering of 3D hollow Mo-based carbide/nitride nanostructures. ACS Appl. Mater. Interfaces 2021, 13, 50524. [Google Scholar] [CrossRef]
- Available online: https://www.iris-france.org/wp-content/uploads/2019/01/Policy-PAPER-GENERATE-janvier-2019.pdf (accessed on 31 January 2019).
- Cauchetier, M.; Croix, O.; Luce, M.; Michon, M.; Paris, J.; Tistchenko, S. Laser synthesis of ultrafine powders. Ceram. Int. 1987, 13, 13. [Google Scholar] [CrossRef]
- Cauchetier, M.; Croix, O.; Herlin, N.; Luce, M. Nanometric Si/C/N powder production by laser-aerosol interaction. J. Am. Ceram. Soc. 1994, 77, 993. [Google Scholar] [CrossRef]
- Belchi, R.; Habert, A.; Foy, E.; Gheno, A.; Vedraine, S.; Antony, R.; Ratier, B.; Bouclé, J.; Herlin-Boime, N. One-step synthesis of TiO2/graphene nanocomposites by laser pyrolysis with well-controlled properties and application in perovskite solar cells. ACS Omega 2019, 4, 11906. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Xu, S.; Liu, Z.; Huang, G.; Zhang, C.; Ai, J.; Li, X.; Li, N. Ultra-small molybdenum carbide nanoparticles in situ entrapped in mesoporous carbon spheres as efficient catalysts for hydrogen evolution. ChemCatChem 2019, 11, 2643. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Li, G.D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem. Int. Ed. 2015, 54, 10752. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhang, Y.; Jiang, W.J.; Zhang, X.; Dai, Z.; Wan, L.J.; Hu, J.S. Pomegranate-like N,P-doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution. ACS Nano 2016, 10, 8851. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Hu, B.C.; Wu, P.; Liang, H.W.; Yu, Z.L.; Lin, Y.; Zheng, Y.R.; Li, Z.; Yu, S.H. Mo2C nanoparticles embedded within bacterial cellulose-derived 3D N-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, H.; Jiang, H.; Zhang, H.; Guo, S.; Hu, Y.; Li, C. Mo-based ultrasmall nanoparticles on hierarchical carbon nanosheets for superior lithium ion storage and hydrogen generation catalysis. Adv. Energy Mater. 2017, 7, 1602782. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey reduced graphene oxide coupled with an Mo2N–Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 2018, 30, 1704156. [Google Scholar] [CrossRef]
- Wei, H.F.; Xi, Q.Y.; Chen, X.A.; Guo, D.Y.; Ding, F.; Yang, Z.; Wang, S.; Li, J.; Huang, S.M. Molybdenum carbide nanoparticles coated into the graphene wrapping N-doped porous carbon microspheres for highly efficient electrocatalytic hydrogen evolution both in acidic and alkaline media. Adv. Sci. 2018, 5, 1700733. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Liu, D.; Li, H.; Li, J. Molybdenum carbide-decorated metallic cobalt@nitrogen-doped carbon polyhedrons for enhanced electrocatalytic hydrogen evolution. Small 2018, 14, 1704227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, S.; Tsubaki, N.; Wu, M. Highly dispersed Mo2C anchored on N,P-codoped graphene as efficient electrocatalyst for hydrogen evolution reaction. ChemCatChem 2018, 10, 2300. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.Q.; Wu, C.Y.; Xiao, K.; Liu, Z.Q. Heterostructures composed of N-doped carbon nanotubes encapsulating cobalt and β-Mo2C nanoparticles as bifunctional electrodes for water splitting. Angew.Chem. Int. Ed. 2019, 58, 4923. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K.P. Direct synthesis of large-area 2D Mo2C on in situ grown graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.X.; Das Chowdhury, K.; Ochoa, R.; Lee, W.T.; Bandow, S.; Dresselhaus, M.S.; Eklund, P.C. Structural characterization of nanocrystalline Mo and W carbide and nitride catalysts produced by CO2 laser pyrolysis. MRS Online Proc. Libr. 1994, 368, 69. [Google Scholar] [CrossRef]
- Ochoa, R.; Hager, G.T.; Lee, W.T.; Bandow, S.; Givens, E.; Eklund, P.C. Relative activity and selectivity of nanoscale Mo2N, Mo2C and MoS2 catalysts synthesized by laser pyrolysis. MRS Online Proc. Libr. 1994, 368, 27. [Google Scholar] [CrossRef]
- Ochoa, R.; Bi, X.X.; Rao, A.M.; Eklund, P.C. The Chemistry of Transition Metal Carbides and Nitrides; Oyama, S.T., Ed.; Springer: Dordrecht, The Netherlands, 1996; p. 489. [Google Scholar]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis Materials Science Forum. In Materials Science Forum; Transtec Publications: Freienbach, Switzerland, 1999; pp. 118–123. [Google Scholar]
- Rodríguez-Carvajal, J. Recent Developments of the Program FULLPROF. In Commission on Powder Diffraction (IUCr); Newsletter: London, UK, 2001; Volume 26, p. 12. [Google Scholar]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80. [Google Scholar] [CrossRef]
- Baek, D.S.; Jung, G.Y.; Seo, B.; Kim, J.C.; Lee, H.W.; Shin, T.J.; Jeong, H.Y.; Kwak, S.K.; Joo, S.H. Ordered mesoporous metastable α-MoC1-x with enhanced water dissociation capability for boosting alkaline hydrogen evolution activity. Adv. Funct. Mater. 2019, 29, 1901217. [Google Scholar] [CrossRef]
- Hu, M.; Chen, H.; Liu, B.; Xu, X.; Cao, B.; Jing, P.; Zhang, J.; Gao, R.; Zhang, J. Coupling ceria with dual-phased molybdenum carbides for efficient and stable hydrogen evolution electrocatalysis at large-current-density in freshwater and seawater. Appl. Catal. B 2022, 317, 121774. [Google Scholar] [CrossRef]


| Synthesis Number | Solution | CO2 Laser Power (W) | C2H4 (sccm) | NH3 (sccm) | Total Production (mg/h) (±1) | % Carbon Elemental Analysis * (±1) |
|---|---|---|---|---|---|---|
| MOC4 | A | 1475 | 200 | 0 | 694 | 86 |
| MOC5 | A | 1475 | 100 | 0 | 180 | 68 |
| MOC6 | B | 1477 | 200 | 0 | 827 | 85 |
| MOC7 | B | 1477 | 100 | 0 | 225 | 71 |
| MOC8 | B | 1477 | 130 | 0 | 253 | 77 |
| MOC9 | B | 1568 | 100 | 0 | 237 | 71 |
| MOC10 | B | 1568 | 100 | 100 | 200 | 74 |
| MOC11 | B | 1527 | 50 | 100 | 67 | n.m. |
| MOC12 | B | 1527 | 0 | 200 | 8 | n.m. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caroff, T.; Badaki, P.; Herbert, N.; Tessier, F.; Berthebaud, D.; Ohashi, N.; Uchikoshi, T.; Lonchambon, P.; Herlin-Boime, N.; Grasset, F. Facile Synthesis and Characterization of Molybdenum Carbides/Carbon Nanocomposites by Laser Pyrolysis. Nanomanufacturing 2022, 2, 112-123. https://doi.org/10.3390/nanomanufacturing2030009
Caroff T, Badaki P, Herbert N, Tessier F, Berthebaud D, Ohashi N, Uchikoshi T, Lonchambon P, Herlin-Boime N, Grasset F. Facile Synthesis and Characterization of Molybdenum Carbides/Carbon Nanocomposites by Laser Pyrolysis. Nanomanufacturing. 2022; 2(3):112-123. https://doi.org/10.3390/nanomanufacturing2030009
Chicago/Turabian StyleCaroff, Théo, Pitalinani Badaki, Nathalie Herbert, Franck Tessier, David Berthebaud, Naoki Ohashi, Tetsuo Uchikoshi, Pierre Lonchambon, Nathalie Herlin-Boime, and Fabien Grasset. 2022. "Facile Synthesis and Characterization of Molybdenum Carbides/Carbon Nanocomposites by Laser Pyrolysis" Nanomanufacturing 2, no. 3: 112-123. https://doi.org/10.3390/nanomanufacturing2030009
APA StyleCaroff, T., Badaki, P., Herbert, N., Tessier, F., Berthebaud, D., Ohashi, N., Uchikoshi, T., Lonchambon, P., Herlin-Boime, N., & Grasset, F. (2022). Facile Synthesis and Characterization of Molybdenum Carbides/Carbon Nanocomposites by Laser Pyrolysis. Nanomanufacturing, 2(3), 112-123. https://doi.org/10.3390/nanomanufacturing2030009