Abstract
Trypanosoma vivax, a haemoprotozoan of African origin, has spread throughout Latin America, mainly affecting cattle through mechanical transmission by flies. This study reports an outbreak of T. vivax on a dairy farm in the midwestern region of São Paulo State, Brazil. Clinical signs included progressive weight loss, lethargy, hyporexia, reduced milk production, abortions, neurological signs, and deaths. The herd comprised 238 crossbred Holstein-Gir cattle (200 cows, 38 calves). Blood samples were collected from 104 randomly selected cows and all 38 calves to assess hematocrit (HCT) and total plasma protein (TPP), and detect trypomastigote forms using blood and buffy coat smears. PCR was performed on EDTA blood from 30 smear-negative cows. Trypanosoma sp. was identified in 51.9% (54/104) of the smear samples, with morphometric confirmation of T. vivax infection. About 66.6% (20/30) of the smear-negative cows tested positive by PCR, showing the higher sensitivity of molecular tests. Anemia was observed in 56.8% of infected cows, with significantly lower HCT and TPP values than in uninfected animals. The absence of quarantine for new animals, high fly density, and shared needles for oxytocin injections were likely contributing factors. This study underscores the crucial role of molecular diagnostics in accurately investigating outbreaks.
1. Introduction
Trypanosomiasis is a disease caused by protozoan parasites of the genus Trypanosoma, which are unicellular, flagellated haemoprotozoans belonging to the family Trypanosomatidae. While trypanosomiasis occurs globally, Trypanosoma vivax, a species originally described in Africa, was introduced to Latin America around 1830 through the importation of Zebu cattle. Since then, it has spread across the continent, aided by the parasite’s adaptation to mechanical transmission routes [1,2,3,4].
Among the species responsible for trypanosomiasis in domestic ungulates in Latin America, Trypanosoma vivax is particularly significant due to its pathogenicity and the substantial economic impact on the cattle industry [5]. The disease may present in either an acute or a chronic form, with high morbidity and mortality rates [6]. Economic losses include reduced productivity, lower natality rates, and increased medical costs. However, due to the frequent association with secondary infections, quantifying the economic impact directly caused by Trypanosoma infection remains challenging.
Across Africa, hosts can become infected with T. vivax through tsetse flies (Glossina) bites, but mechanical transmission by other biting flies may facilitate the spread of the infection [7]. Previous studies conducted in Brazil indicated that the infection is primarily transmitted by tabanids and stable flies (Stomoxys calcitrans) [4,8,9]. Additionally, iatrogenic transmission by shared needles and syringes has also become an important epidemiological concern [10]. In Brazil, the disease was first documented in cattle in the state of Pará [11], but several cases have been reported in all five regions of the country [5,10,12,13,14,15,16]. Furthermore, T. vivax has also been reported to have infected pigs and horses [17,18,19,20].
The first cases of T. vivax infecting cattle in São Paulo were reported in 2012 and occurred in 2008 in the municipality of Lins, involving high morbidity and mortality of 31 out of an herd of 1080 cattle [21]. Infected cattle presented clinical signs such as anemia, diarrhea, abortion, and neurological alterations, and the diagnosis was confirmed by PCR [21]. Here we report an outbreak of T. vivax infection in dairy cows from the Central-Western region of São Paulo state, Brazil. This report adds valuable evidence of the markedly higher detection rate of T. vivax by PCR compared to conventional smear microscopy, emphasizing the importance of molecular diagnostics for accurate surveillance of this parasite in cattle herds. To our knowledge, this study represents the second documented outbreak in the state.
2. Materials and Methods
2.1. Study Geographical Area and Animal Housing and Management
The outbreak occurred on a rural farm located in the midwestern region of São Paulo state, in the municipality of Anhembi, approximately 206 km from the state capital, São Paulo City. The farm housed a herd consisting of cattle (including 200 dairy cows and 38 calves of crossbred Holstein and Gir), pigs (15), and horses (10). After birth, the calves were allowed to suckle the colostrum for a few hours before they were separated from their dams and housed in a calf barn, where they were fed milk in buckets twice daily. All the cows had been purchased within the six months prior to the outbreak from various rural areas located in the Brazilian states of Goiás, Mato Grosso, Tocantins, and different regions of São Paulo state, without any quarantine protocol.
Lactating cows were allowed to graze during the day and received oxytocin before being mechanically milked twice daily. It had been reported that farm workers routinely reused a single disposable syringe and needle per day for the administration of oxytocin intravenously to all the animals prior to mechanical milking. Suboptimal farm management practices observed during farm visits, along with favorable weather conditions, may have led to a significant increase in fly and tabanid populations.
The horses’ grazing area was next to the cows’ pasture, while the pigs’ barns were 200 m away.
2.2. Sample Collection and Blood Analyses
Due to the suspicion of trypanosomiasis and in order to identify the infection status of the herd, blood samples were collected through jugular venipuncture from 104 dairy cows, which were selected randomly among animals that could be safely restrained, and from all 38 calves. The blood samples were collected into tubes containing anticoagulant (EDTA Vacutainer®, BD, Juiz de Fora, MG, Brazil) for hematological analysis (hematocrit (HCT) and total plasma protein (TPP) concentrations) and for the search of trypomastigote forms in whole blood and/or in buffy coat blood smears. Additionally, blood samples were collected from seven pigs and six horses, also arbitrarily selected, via jugular venipuncture into EDTA Vacutainer® (BD, Juiz de Fora, MG, Brazil) tubes to screen for trypomastigote forms in whole blood and buffy coat smears.
The blood samples were centrifuged in capillary tubes (12,000 rpm for six minutes) to prepare buffy coat smears. Whole blood and buffy coat smears were stained with Giemsa and examined under a Nikon E200 optical microscope (Nikon Instruments Inc., Melville, NY, USA) at 1000× magnification.
All the animals that tested positive for trypomastigotes in total blood and/or in the buffy coat blood smears were considered infected.
2.3. Morphometric Analysis
For biometric characterization, five smear slides from Trypanosoma-positive animals (one per animal) were selected to provide a representative baseline of morphometric features. This sample size was sufficient to provide a baseline of morphometric features for confirmation of T. vivax infection in this outbreak. On each smear slide, 20 trypomastigotes were measured, resulting in a total of 100 trypomastigotes analyzed with the OPTHD Microscope Imaging Software (version x64 4.10). The following measurements were conducted: (1) distance from the posterior end to the kinetoplast (PK); (2) from the kinetoplast to the center of the nucleus (KN); (3) posterior end to the center of the nucleus (PN); (4) nucleus to the anterior extremity (NA); (5) flagellum free distance (F); (6) total length including free flagellum (L) [22,23]. Additionally, two ratios were calculated: PN/KN and PN/NA.
2.4. Molecular Diagnosis
DNA was extracted from blood samples collected from 30 cows that tested negative for trypanosomes by both the total blood and buffy coat smears, as well as from 20 calves selected from the 38 examined. The animals were arbitrarily selected, also based on the availability of sufficient remaining blood volume. Extractions were performed using the Illustra Blood GenomicPrep Mini Spin Kit (GE Healthcare, Buckinghamshire, UK) following the manufacturer’s instructions. DNA purity was evaluated at the A260:A280 and A260:A230 ratios using the Nanodrop (Thermo Scientific®, Waltham, MA, USA). PCR was performed using the primers TviCatL1 (GCCATCGCCAAGTACCTCGCCGA) and DTO155 (TTAAAGCTTCCACGAGTTCTTGATGATCCAGTA) previously described [24]. The primers TviCatL1 and DTO155 target a conserved region within the central (catalytic) domain of the cathepsin L-like (CatL-like) gene family of T. vivax. The CatL-like sequences are species-specific and were validated as reliable targets for PCR diagnosis of T. vivax in both South American and African isolates [24]. PCR contained a total of 25 µL, being 12.5 µL of GoTaq® Master Mix (Promega Co., Madison, WI, USA), 300 nM of each primer, 8.8 µL of nuclease-free water, and 2.5 µL of DNA sample. A negative control, no template-DNA, was performed each time a PCR was made. The amplification conditions were 3 min at 94 °C, followed by 30 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min. A final extension of 10 min at 72 °C was also performed. All the samples were analyzed in a 1.5% agarose gel electrophoresis.
Among the Trypanosoma-positive cows by buffy coat smear, four of them were arbitrarily selected for DNA extraction and for serving as positive controls in PCR analyses. PCR specificity was confirmed by Sanger sequencing of the positive PCR products from four cows that tested positive by buffy coat smear. The obtained sequences were compared using the Basic Local Alignment Search Tool (National Center for Biotechnology Information, Bethesda, MD, USA).
2.5. Statistical Analysis
Descriptive statistical analyses were used to present means and standard deviations. Data normality was assessed by the Shapiro-Wilk test. The HCT and TPP values for the Trypanosoma-negative animals (based on smear tests and PCR), Trypanosoma-positive animals (based only on smear tests), and Trypanosoma-positive animals (based on both smear tests and PCR) were compared using one-way ANOVA. Mean differences were assessed using the post hoc Tukey test. Values of p < 0.05 were considered statistically significant. All the statistical analyses were performed using GraphPad Prism version 8.2.1.
3. Results
Thirty-seven cows (18.5%, 37/200) presented progressive weight loss, lethargy, anorexia, and reduced milk yield. Additionally, two cases of abortion and three cases of neurological signs (including blindness, paraparesis, and ataxia) were observed. Seventeen cows died within the 30 days preceding blood sample collection.
Among the sampled cows, 51.9% (54/104) were Trypanosoma-positive in either the total blood smear or the buffy coat smear. Both methods demonstrated equivalent efficiency in detecting positive cases. Thirty cows that tested negative for trypanosomes by both whole blood and buffy coat smear microscopy were randomly selected for PCR amplification. Amplification of DNA fragments of expected size (band of 177 bp) was observed in 20 animals, with 66.7% (20/30) testing positive for T. vivax by PCR. These results confirm the high sensitivity of PCR for the detection of T. vivax infections. Partial DNA sequencing (CatL-amplified fragments) from four PCR-positive samples revealed high homology with the T. vivax sequences available in GenBank, supporting the molecular identification. We sequenced only four samples due to budget constraints. However, to ensure representative coverage, we selected samples from different batches to account for potential variation between experimental runs.
Significant differences in the HCT and TPP levels were observed between the Trypanosoma-negative animals and Trypanosoma-positive animals. For HCT, the differences were significant both when positivity was determined solely by smear tests (p = 0.0002) and when determined by the combination of smear tests and PCR (p < 0.0001). A similar pattern was observed for TPP, with statistically significant differences between the Trypanosoma-negative and Trypanosoma-positive animals based on smear tests alone (p = 0.0014) and on combined smear and PCR results (p < 0.0001) (Figure 1).
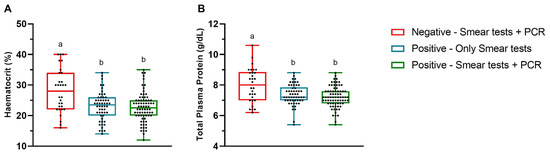
Figure 1.
Mean hematocrit (A) and total plasma protein concentration (B) values in the Trypanosoma-positive and -negative cows based on the total blood smear, buffy coat smear, and polymerase chain reaction (PCR) tests in the state of São Paulo, Brazil. Different lowercase letters above the boxes indicate statistically significant differences. The ends of the box correspond to the upper and lower quartiles, with the median indicated by a horizontal line inside the box. The whiskers extend outward to the maximum and minimum observed values.
Trypanosoma-negative cows (PCR-negative) had HCT and TPP values within the reference range for the species, with means of 28.0% (±7.1) and 7.99 g/dL (±1.12), respectively. The mean HCT values for Trypanosoma-positive cows based only on smear tests and on both smear tests and PCR were 23.0% (±4.6) and 22.7% (±4.8), respectively. The HCT values in the Trypanosoma-positive cows were slightly below the reference range for cattle [25], with anemia detected in 56.8% (42/74) of the positive cows. Additionally, the mean TPP values for the Trypanosoma-positive cows based only on smear tests and those positive on both smear tests and PCR were 7.35 g/dL (±0.68) and 7.21 g/dL (±0.68), respectively, remaining within the species’ reference interval.
All the calves tested negative for trypanosomes by both total blood smear and buffy coat smear, with mean HCT and TPP values of 30.7% (±8.6) and 6.43 g/dL (±0.91), respectively. Additionally, calves, pigs, and horses were PCR-negative when tested with specific primers for T. vivax.
Twenty trypomastigotes observed in the blood smears from five positive animals (totaling 100 trypomastigotes) were morphometrically characterized and identified as T. vivax based on body size, shape, membrane wave development, nucleus, and kinetoplast (Table 1).

Table 1.
Measurements in micrometers (μm) (means ± SD, minimum and maximum) of randomly selected trypomastigotes found (n = 100) in total blood smear of Trypanosoma-positive cows, in the state of São Paulo, Brazil.
All the dairy cows in the herd, regardless of their Trypanosoma infection status, were subjected to a pharmacological treatment with intramuscular administration of diminazene aceturate (3.5 mg/kg BW) and isometamidium chloride (0.5 mg/kg BW). Diminazene aceturate remains the most used drug for managing trypanosomiasis in cattle [26]. Two weeks after the Trypanosoma-positive diagnosis and treatment, 15 cows died, while cows that were not in the severe stages of the disease recovered successfully.
4. Discussion
In this study, 37 cows showed clinical signs suggestive of trypanosomiasis, which motivated further investigation of the cases.
Trypanosoma vivax infects a wide range of wild and domestic animals and is considered one of the most important Trypanosoma species affecting cattle, leading to significant economic losses in herds from Africa, Central America, and South America [19]. The individual susceptibility depends on age, concurrent bacterial infections, pregnancy, lactation, nutritional status, hygiene conditions, and the presence of blood sucking flies or other mechanical vectors such as tabanids [27].
The main predisposing factors for T. vivax infection in this report were the lack of a quarantine protocol, particularly considering that the cows had been purchased from various rural areas and regions, combined with the use of shared needles to administer oxytocin prior to milking. While there is a possibility that the animals were infected before arriving on the farm, they had been there for at least six months before this outbreak. The incubation period of T. vivax ranges from 4 to 40 days and may vary depending on the virulence of the strain. Therefore, the use of shared needles is likely the primary source of transmission of the infection within the herd. The massive presence of flies, also observed during the farm visit, was associated with inadequate hygiene practices and may have contributed to disease transmission. The poor nutritional conditions of some animals may have resulted from the progression of the infection or from underlying nutritional deficiencies. Similarly, it is difficult to determine whether the cases of pneumonia observed in the Trypanosoma-positive animals of this study occurred prior to or as a consequence of the immunosuppression caused by trypanosomiasis [28].
Direct parasitological methods, such as total blood smears and buffy coat smears, are practical for use under field conditions and demonstrate high agreement with reference diagnostic standards [29]. However, they lack sensitivity, as results often depend on the stage of the disease and parasite load [30]. Although this study found a high agreement of total blood smear and buffy coat smear tests in diagnosing Trypanosoma-positive cows, PCR analysis revealed that 20 out of 30 randomly selected smear-negative cows were false negatives and tested positive for T. vivax. These findings highlight the limitations of different diagnostic methods based on microscopical, serological, or molecular bases [19]. PCR exhibited higher sensitivity [24].
Additionally, T. vivax PCR-positive cows had significantly lower HCT and TPP concentrations than PCR-negative cows. These results were expected, as infected cows with T. vivax tend to develop immune-mediated hemolytic anemia and hypoproteinemia [4,31]. Anemia occurs in response to trypomastigote reproduction by binary fission in the bloodstream. The host’s immune system produces antibodies that can target erythrocytes, leading to their lysis [28]. Parasite-induced hypoalbuminemia is commonly observed due to increased vascular permeability and protein loss through damaged tissues [32]. The parasite may also release toxic substances when it is destroyed within the circulatory system, causing damage to blood vessels [28]. Together, these mechanisms can explain the significant reduction in HCT and TPP observed in positive animals.
The morphometric results of blood trypanosomes obtained in this study were consistent with previous reports on T. vivax trypomastigotes [12,23,33,34,35]. In addition, the PCR assay used in this study demonstrated high specificity for the detection of T. vivax in cattle, as confirmed by partial sequencing of CatL-amplified fragments, which showed high homology with the T. vivax sequences available in GenBank. Therefore, these findings confirmed T. vivax as the causative agent of the outbreak among dairy cows. In light of the outbreak, farm owners and workers were advised on key sanitary measures, including avoiding needle sharing, quarantining new animals, and improving hygiene. Regular monitoring and early diagnostic testing were also recommended. These actions aim to prevent transmission and minimize economic losses.
5. Conclusions
This outbreak was likely driven by a high density of flies and the use of shared needles for intravenous oxytocin administration in lactating cows. The findings emphasize that the accurate diagnosis of trypanosomiasis requires the use of specific and sensitive methods. Although blood smear techniques are quick and practical for field conditions, their limitations in sensitivity and specificity can compromise disease detection. In contrast, PCR provides superior diagnostic accuracy, significantly reducing the risk of false-negative results in Brazilian cattle herds. Increased use of PCR in routine diagnostics could significantly improve disease control, management, and prevention of Trypanosoma vivax infections in livestock populations.
Author Contributions
Conceptualization, J.P.O.-F., A.S.B., and J.G.G.L.; methodology, R.K.T., J.P.O.-F., and A.S.B.; software, J.G.G.L.; validation, K.K., A.L.H.d.A., L.S.Z., and F.P.R.; formal analysis, J.G.G.L.; investigation, K.K., A.L.H.d.A., L.S.Z., and F.P.R.; resources, J.P.O.-F. and A.S.B.; data curation, J.G.G.L.; writing—original draft preparation, J.G.G.L.; writing—review and editing, L.S.Z., R.K.T., J.P.O.-F., A.S.B., and J.G.G.L.; visualization, J.P.O.-F., A.S.B., and J.G.G.L.; supervision, project administration and funding acquisition, J.P.O.-F. and A.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is an outbreak report, and the samples were collected during routine veterinary clinical visits to the farm.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data that supports the study findings are available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leger, M.; Vienne, M. Epizootie à trypanosomes chez les bovidés de la Guyane Française. Bull. Soc. Pathol. Exot. 1919, 12, 258–266. [Google Scholar] [CrossRef]
- Silva, R.A.; da Silva, J.A.; Schneider, R.C.; de Freitas, J.; Mesquita, D.; Mesquita, T.; Ramirez, L.; Rivera Dávila, A.M.; Pereira, M.E. Outbreak of trypanosomiasis due to Trypanosoma vivax (Ziemann, 1905) in bovines of the Pantanal, Brazil. Mem. Inst. Oswaldo Cruz 1996, 91, 561–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ventura, R.M.; Paiva, F.; Silva, R.A.; Takeda, G.F.; Buck, G.A.; Teixeira, M.M. Trypanosoma vivax: Characterization of the spliced-leader gene of a Brazilian stock and species-specific detection by PCR amplification of an intergenic spacer sequence. Exp. Parasitol. 2001, 99, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Osório, A.L.; Madruga, C.R.; Desquesnes, M.; Soares, C.O.; Ribeiro, L.R.; Costa, S.C. Trypanosoma (Duttonella) vivax: Its biology, epidemiology, pathogenesis, and introduction in the New World—A review. Mem. Inst. Oswaldo Cruz 2008, 103, 1–13. [Google Scholar] [CrossRef]
- Samoel, G.V.A.; Fernandes, F.D.; Roman, I.J.; Rodrigues, B.T.; Miletti, L.C.; Bräunig, P.; Guerra, R.R.; Sangioni, L.A.; Cargnelutti, J.F.; Vogel, F.S.F. Detection of anti-Trypanosoma spp. antibodies in cattle from southern Brazil. Braz. J. Vet. Parasitol. 2024, 33, e013723. [Google Scholar] [CrossRef]
- Batista, J.S.; Riet-Correa, F.; Teixeira, M.M.; Madruga, C.R.; Simões, S.D.; Maia, T.F. Trypanosomiasis by Trypanosoma vivax in cattle in the Brazilian semiarid: Description of an outbreak and lesions in the nervous system. Vet. Parasitol. 2007, 143, 174–181. [Google Scholar] [CrossRef]
- Luckins, A.G.; Dwinger, R.H. Non-tsetse-transmitted animal trypanosomiasis. In The Trypanosomiases; Maudlin, I., Holmes, P.H., Miles, M.A., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 269–279. [Google Scholar] [CrossRef]
- Desquesnes, M. Livestock Trypanosomoses and Their Vectors in Latin America; OIE: Paris, France, 2004; p. 174. [Google Scholar]
- Garcia, H.A.; Rodrigues, A.C.; Rodrigues, C.M.F.; Bengaly, Z.; Minervino, A.H.H.; Riet-Correa, F.; Machado, R.D.; Paiva, F.; Batista, J.S.; Neves, L.; et al. Microsatellite analysis supports clonal propagation and reduced divergence of Trypanosoma vivax from asymptomatic to fatally infected livestock in South America compared to West Africa. Parasites Vectors 2014, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Bastos, T.S.A.; Faria, A.M.; Madrid, D.M.C.; Bessa, L.C.; Linhares, G.F.C.; Fidelis Junior, O.L.; Sampaio, P.H.; Cruz, B.C.; Cruvinel, L.B.; Nicaretta, J.E.; et al. First outbreak and subsequent cases of Trypanosoma vivax in the state of Goiás, Brazil. Braz. J. Vet. Parasitol. 2017, 26, 366–371. [Google Scholar] [CrossRef]
- Boulhosa, J. Informação Científica. Bol. Téc. Min. Agric. 1946, 21–26. [Google Scholar]
- Linhares, G.F.C.; Dias Filho, F.C.; Fernandes, P.R.; Duarte, S.C. Tripanossomíase em bovinos no município de Formoso do Araguaia, Tocantins (relato de caso). Ciênc. Anim. Bras. 2006, 7, 455–460. [Google Scholar]
- Batista, J.S.; Bezerra, F.S.B.; Lira, R.A.; Carvalho, J.R.G.; Rosado Neto, A.M.; Petri, A.A.; Teixeira, M.M.G. Aspectos clínicos, epidemiológicos e patológicos da infecção natural em bovinos por Trypanosoma vivax na Paraíba. Pesq. Vet. Bras. 2008, 28, 63–69. [Google Scholar] [CrossRef]
- Pereira, H.D.; Simões, S.V.D.; Souza, F.A.L.; Silveira, J.A.G.; Ribeiro, M.F.B.; Cadioli, F.A.; Sampaio, P.H. Aspectos clínicos, epidemiológicos e diagnóstico da infecção por Trypanosoma vivax em rebanho bovino no estado do Maranhão. Pesq. Vet. Bras. 2018, 38, 896–901. [Google Scholar] [CrossRef]
- Andrade Neto, A.Q.; Mendonça, C.L.; Souto, R.J.C.; Sampaio, P.H.; Junior, O.L.F.; André, M.R.; Machado, R.Z.; Afonso, J.A.B. Diagnostic, clinical, and epidemiological aspects of dairy cows naturally infected by Trypanosoma vivax in the states of Pernambuco and Alagoas, Brazil. Braz. J. Vet. Med. 2019, 41, e094319. [Google Scholar] [CrossRef]
- Silva, J.B.; Silva, B.M.; Silva, L.T.; Queiroz, W.C.C.; Coelho, M.R.; Silva, B.T.; Marcusso, P.F.; Baêta, B.A.; Machado, R.Z. First detection of Trypanosoma vivax in dairy cattle from the northwest region of Minas Gerais, Brazil. Arq. Bras. Med. Vet. Zootec. 2023, 75, 153–159. [Google Scholar] [CrossRef]
- Ng'ayo, M.O.; Njiru, Z.K.; Kenya, E.U.; Muluvi, G.M.; Osir, E.O.; Masiga, D.K. Detection of trypanosomes in small ruminants and pigs in western Kenya: Important reservoirs in the epidemiology of sleeping sickness? Kinetoplastid Biol. Dis. 2005, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.S.; Garcia Perez, H.A.; Costa, M.M.; França, R.T.; De Gasperi, D.; Zanette, R.A.; Amado, J.A.; Lopes, S.T.; Teixeira, M.M.; Monteiro, S.G. Horses naturally infected by Trypanosoma vivax in southern Brazil. Parasitol. Res. 2011, 108, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fetene, E.; Leta, S.; Regassa, F.; Büscher, P. Global distribution, host range and prevalence of Trypanosoma vivax: A systematic review and meta-analysis. Parasites Vectors 2021, 14, 80. [Google Scholar] [CrossRef]
- Suganuma, K.; Acosta, T.J.; Valinotti, M.F.R.; Sanchez, A.R.; Mossaad, E.; Elata, A.; Inoue, N. First molecular survey of animal trypanosomes in Paraguayan horses. Vet. Parasitol. Reg. Stud. Rep. 2022, 27, 100664. [Google Scholar] [CrossRef]
- Cadioli, F.A.; Barnabé, P.A.; Machado, R.Z.; Teixeira, M.C.A.; André, M.R.; Sampaio, P.H.; Fidélis Junior, O.L.; Teixeira, M.M.G.; Marques, L.C. First report of Trypanosoma vivax outbreak in dairy cattle in São Paulo state, Brazil. Braz. J. Vet. Parasitol. 2012, 21, 118–124. [Google Scholar] [CrossRef]
- Hoare, C.A. The Trypanosomes of Mammals: A Zoological Monograph; Blackwell Scientific: Oxford, UK, 1972; p. 749. [Google Scholar]
- Dávila, A.M.R.; Ramirez, L.; Silva, R.A.M.S. Morphological and biometrical differences among Trypanosoma vivax isolates from Brazil and Bolivia. Mem. Inst. Oswaldo Cruz 1997, 92, 357–358. [Google Scholar] [CrossRef][Green Version]
- Cortez, A.P.; Rodrigues, A.C.; Garcia, H.A.; Neves, L.; Batista, J.S.; Bengaly, Z.; Paiva, F.; Teixeira, M.M. Cathepsin L-like genes of Trypanosoma vivax from Africa and South America—Characterization, relationships and diagnostic implications. Mol. Cell. Probes 2009, 23, 44–51. [Google Scholar] [CrossRef]
- Jain, N.C. Essentials of Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1993; pp. 76–250. [Google Scholar]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Katunguka-Rwakishaya, E.; Murray, M.; Holmes, P.H. The influence of supplementation with cotton seed cake on the resistance of Ugandan goats to primary and secondary challenges with Trypanosoma congolense and on their response to treatment. Vet. Parasitol. 1997, 70, 67–76. [Google Scholar] [CrossRef]
- Dagnachew, S.; Bezie, M. Review on Trypanosoma vivax. Afr. J. Basic Appl. Sci. 2015, 7, 41–64. [Google Scholar]
- Holland, W.G.; Claes, F.; My, L.N.; Thanh, N.G.; Tam, P.T.; Verloo, D.; Büscher, P.; Goddeeris, B.; Vercruysse, J. A comparative evaluation of parasitological tests and a PCR for Trypanosoma evansi diagnosis in experimentally infected water buffaloes. Vet. Parasitol. 2001, 97, 23–33. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, A.; Singh, A.; Srivastava, A.; Singh, R. Assessment of diagnostic efficacy of various methods in detection of Trypanosoma evansi infection in buffaloes. Buffalo Bull. 2017, 36, 147–154. [Google Scholar]
- Schenk, M.A.M.; Mendonça, C.L.; Madruga, C.R.; Kohayagawa, A.; Araújo, F.R. Avaliação clínico-laboratorial de bovinos Nelore infectados experimentalmente com Trypanosoma vivax. Pesq. Vet. Bras. 2001, 21, 157–161. [Google Scholar] [CrossRef]
- Fidelis Junior, O.L.; Sampaio, P.H.; Machado, R.Z.; André, M.R.; Marques, L.C.; Cadioli, F.A. Evaluation of clinical signs, parasitemia, hematologic and biochemical changes in cattle experimentally infected with Trypanosoma vivax. Braz. J. Vet. Parasitol. 2016, 25, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, H. Studies on Trypanosoma vivax. IX. Morphological differences in strains and their relation to pathogenicity. Ann. Trop. Med. Parasitol. 1953, 47, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.J.; Lainson, R. Trypanosoma vivax in Brazil. Ann. Trop. Med. Parasitol. 1972, 66, 25–32. [Google Scholar] [CrossRef]
- Guerra, R.M.S.N.; Feitosa Júnior, A.B.; Santos, H.P.; Abreu-Silva, A.L.; Santos, A.C.G. Biometry of Trypanosoma vivax found in a calf in the state of Maranhão, Brazil. Ciênc. Rural 2008, 38, 833–835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).