Human Anisakiosis Risk and Presence of Food-Spoiling Parasites Through the Consumption of the Atlantic Chub Mackerel, Scomber colias, Sold in Spanish Supermarkets
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Parasitological Procedures
2.2. Statistical Analysis
3. Results
3.1. Origin of the Fish Caught
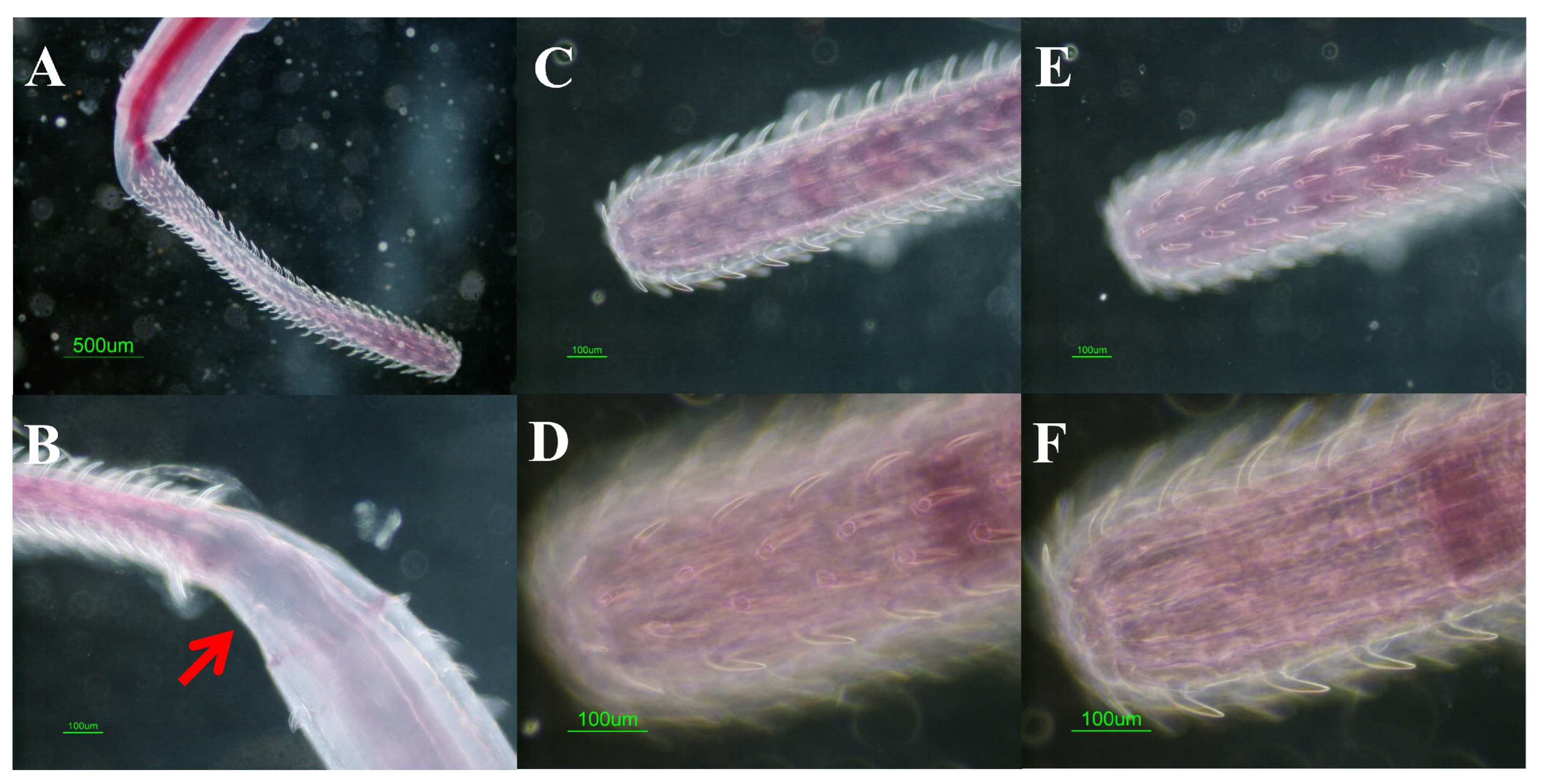
3.2. Parasitisation Sites of Anisakis Type I Larvae
3.3. Season of Capture
3.4. Fish Size
3.5. Days Passed After Capture
4. Discussion
4.1. Influence of the Origin of the Fish Caught
4.2. Influence of the Season of Capture
4.3. Influence of the Fish Size
4.4. Parasitisation Sites and Migration
4.5. Scomber colias vs. Scomber scombrus: Human Anisakiosis Risk Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MAGRAMA (Ministerio de Agricultura, Alimentación y Medio Ambiente). Ministerio de Agricultura, Alimentación y Medio Ambiente. El Mercado de la Caballa en España. 2017. Available online: https://www.mapa.gob.es/en/pesca/temas/mercados-economia-pesquera/informecaballa2017_tcm38-437224.pdf (accessed on 21 January 2025).
- Illescas, J.L.; Bacho, O.; Ferrer, S. Pescados y Mariscos. Guía Práctica; Empresa Nacional Mercasa: Madrid, Spain, 2008; p. 211. [Google Scholar]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Sharun, K.; Emran, T.B.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Q. 2022, 42, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K.; Mehrdana, F. Effects of anisakid nematodes Anisakis simplex (s.l.), Pseudoterranova decipiens (s.l.) and Contracaecum osculatum (s.l.) on fish and consumer health. Food Waterborne Parasitol. 2016, 4, 13–22. [Google Scholar] [CrossRef]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.T.; Ansotegui, I.J.; Kennedy, M.W. Anisakis simplex: Dangerous-dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Daschner, A.; Perteguer, M.J.; Benito, A. Epidemiological scenario of anisakidosis in Spain based on associated hospitalizations: The tipping point of the iceberg. Clin. Inf. Dis. 2019, 69, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 43699. [Google Scholar] [CrossRef] [PubMed]
- Debenedetti, A.L.; Madrid, E.; Trelis, M.; Codes, F.J.; Gil-Gómez, F.; Sáez-Durán, S.; Fuentes, M.V. Prevalence and risk of anisakid larvae in fresh fish frequently consumed in Spain: An overview. Fishes 2019, 4, 13. [Google Scholar] [CrossRef]
- Llarena-Reino, M.; Piñeiro, C.; Antonio, J.; Outeriño, L.; Vello, C.; González, A.F.; Pascual, S. Optimization of the pepsin digestion method for anisakids inspection in the fishing industry. Vet. Parasitol. 2013, 191, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Berland, B. Identification of larval nematodes from fish. In Nematode Problems in North Atlantic Fish; Report from a Work-shop in Kiel, 3–4 April 1989; Möller, H., Ed.; International Council for Exploration of the Sea: Kiel, Germany, 1989; pp. 15–22. [Google Scholar]
- Grabda, J. Marine Fish Parasitology: An Outline; PWN-Polish Scientific Publisher: Warszawa, Poland, 1991; p. 306. [Google Scholar]
- De Oliveira-Rodrigues, H.; Noronha, D.; Carvalho-Varela, M. Alguns acantocéfalos de peixes do Oceano Atlántico, costa continental portuguesa e costa do norte da África. Mem. Inst. Oswaldo Cruz 1975, 73, 209–214. [Google Scholar] [CrossRef]
- Arandas-Rêgo, A. Rhadinorhynchus pristis (Rudolphi, 1802) acanthocephalan parasite of fishes, Scomber scombrus and S. japonicus. Some observations on the scanning electron microscope. Mem. Inst. Oswaldo Cruz 1987, 82, 287–288. [Google Scholar] [CrossRef]
- Monks, S. Phylogeny of the Acanthocephala based on morphological characters. Syst. Parasitol. 2001, 48, 81–116. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Murata, R.; Hosaka, M.; Araki, J. Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. Int. J. Food Microbiol. 2010, 137, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Bak, T.J.; Jeon, C.H.; Kim, J.H. Occurrence of anisakid nematode larvae in chub mackerel (Scomber japonicus) caught off Korea. Int. J. Food Microbiol. 2014, 191, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Cavallero, S.; D’Amelio, S.; Paggi, L.; García-Santamaría, M.T.; Borges-Perera, C.; Santos, M.J.; Khadem, M. Helminth parasites of the Atlantic chub mackerel, Scomber colias Gmelin, 1789 from Canary Islands, Central North Atlantic, with comments on their relations with other Atlantic regions. Acta Parasitol. 2011, 56, 98–104. [Google Scholar] [CrossRef]
- Costa, G.; Pontes, T.; Mattiucci, S.; D’Amélio, S. The occurrence and infection dynamics of Anisakis larvae in the black-scabbard fish, Aphanopus carbo, chub mackerel, Scomber japonicus, and oceanic horse mackerel, Trachurus picturatus from Madeira, Portugal. J. Helminthol. 2003, 77, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Abollo, E.; González, A.F.; Pascual, S. Scoring the parasite risk in highly-valuable fish species from southern ICES areas. Fish. Res. 2018, 202, 134–139. [Google Scholar] [CrossRef]
- Abattouy, N.; Valero, A.; Benajiba, M.H.; Lozano, J.; Martín-Sánchez, J. Anisakis simplex s.l. parasitization in mackerel (Scomber japonicus) caught in the North of Morocco—Prevalence and analysis of risk factors. Int. J. Food Microbiol. 2011, 150, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.; Castro, R.; Cavaleiro, F.; Rangel, L.; Palm, H.W. Comparison of anisakid infection levels between two species of Atlantic mackerel (Scomber colias and S. scombrus) off the Atlantic Portuguese coast. Sci. Mar. 2017, 81, 179–185. [Google Scholar] [CrossRef]
- Martin-Carrillo, N.; García-Livia, K.; Baz-González, E.; Abreu-Acosta, N.; Dorta-Guerra, R.; Valladares, B.; Foronda, P. Morphological and molecular identification of Anisakis spp. (Nematoda: Anisakidae) in commercial fish from the Canary Islands coast (Spain): Epidemiological data. Animals 2022, 12, 2634. [Google Scholar] [CrossRef] [PubMed]
- Piras, M.C.; Tedde, T.; Garippa, G.; Virgilio, S.; Sanna, D.; Farjallah, S.; Merella, P. Molecular and epidemiological data on Anisakis spp. (Nematoda: Anisakidae) in commercial fish caught off northern Sardinia (western Mediterranean Sea). Vet. Parasitol. 2014, 203, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Casti, D.; Scarano, C.; Piras, M.C.; Merella, P.; Muglia, S.; Piras, F.; Garippa, G.; Spanu, C.; De Santis, E.P.L. Occurrence of nematodes of the genus Anisakis in Mediterranean and Atlantic fish marketed in Sardinia. Ital. J. Food Saf. 2017, 6, 6185. [Google Scholar] [CrossRef] [PubMed]
- Chaligiannis, I.; Lalle, M.; Pozio, E.; Sotiraki, S. Anisakidae infection in fish of the Aegean Sea. Vet. Parasitol. 2012, 184, 362. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, G.Z.; Onuk, E.E.; Bolukbas, C.S.; Yardimci, B.; Gurler, A.T.; Acici, M.; Umur, S. Molecular identification of Anisakis species (Nematoda: Anisakidae) from marine fishes collected in Turkish waters. Vet. Parasitol. 2014, 201, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Mladineo, I.; Poljak, V. Ecology and genetic structure of zoonotic Anisakis spp. from Adriatic commercial fish species. Appl. Environ. Microbiol. 2014, 80, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, E.; Azzarito, L.; Di Taranto, P.; Mancini, M.E.; Normanno, G.; Didonna, A.; Faleo, S.; Occhiochiuso, G.; D’Attoli, L.; Pedarra, C.; et al. Prevalence of anisakid parasites in fish collected from Apulia region (Italy) and quantification of nematode larvae in flesh. Int. J. Food Microbiol. 2019, 292, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Ozuni, E.; Vodica, A.; Castrica, M.; Brecchia, G.; Curone, G.; Agradi, S.; Miraglia, D.; Menchetti, L.; Balzaretti, C.M.; Andoni, E. Prevalence of Anisakis larvae in different fish species in southern Albania: Five-year monitoring (2016–2020). Appl. Sci. 2021, 11, 11528. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Cavallero, S.; Zanzani, S.A.; Olivieri, E.; Malandra, R.; Ranghieri, V.; D’Amelio, S.; Manfredi, M.T. Anisakis sp. and Hysterothylacium sp. larvae in anchovies (Engraulis encrasicolus) and chub mackerel (Scomber colias) in the Mediterranean Sea: Molecular identification and risk factors. Food Control 2017, 80, 366–373. [Google Scholar] [CrossRef]
- Cavallero, S.; El Sherif, R.A.; Pizzarelli, A.; El Fituri, A.A.; El Showhdi, M.; Benmosa, F.; D’Amelio, S. Occurrence of Anisakis and Hysterothylacium nematodes in Atlantic chub mackerels from Libyan coasts. Helminthologia 2019, 56, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Arandas-Rêgo, A.; Carvalho-Varela, M.; Mendonça, M.M.; Afonso-Roque, M.M. Helminofauna da sarda (Scomber scombrus L. ) peixe da costa continental Portuguesa. Mem. Inst. Oswaldo Cruz 1985, 80, 97–100. [Google Scholar] [CrossRef]
- Costa, G.; Pontes, T.; Arandas-Rêgo, A. Prevalence, intensity and abundance of Rhadinorhynchus pristis (Acanthocephala, Rhadinorhynchidae) in chub mackerel, Scomber japonicus (Pisces, Scombridae) from Madeira Island. Acta Parasitol. 2004, 49, 41–44. [Google Scholar]
- Debenedetti, A.L.; Codes, F.; Laza, S.; Hernández, S.; Madrid, E.; Trelis, M.; Fuentes, M.V. Ascaridoid nematodes in horse mackerel, Trachurus trachurus, sold in Spanish supermarkets—Factors able to diminish consumer risk. Fish. Res. 2020, 230, 105669. [Google Scholar] [CrossRef]
- Madrid, E.; Gil, F.; García, M.; Debenedetti, Á.L.; Trelis, M.; Fuentes, M.V. Potential risk analysis of human anisakiasis through the consumption of mackerel, Scomber scombrus, sold at Spanish supermarkets. Food Control 2016, 66, 300–305. [Google Scholar] [CrossRef]
- Arthur, J.R.; Margolis, L.; Whitaker, D.J.; McDonald, T.E. A quantitative study of economically important parasites of walleye pollock (Theragra chalcogramma) from British Columbian waters and effects of post-mortem handling on their abundance in the musculature. Can. J. Fish. Aquat. Sci. 1982, 39, 710–726. [Google Scholar] [CrossRef]
- Boily, F.; Marcogliese, D.J. Geographical variations in abundance of larval anisakine nematodes in Atlantic cod (Gadus morhua) and American plaice (Hippoglossoides platessoides) from the Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 1995, 52, 105–115. [Google Scholar] [CrossRef]
- Højgaard, D.P. Impact of temperature, salinity and light on hatching of eggs of Anisakis simplex (Nematoda, Anisakidae), isolated by a new method, and some remarks on survival of larvae. Sarsia 1998, 83, 21–28. [Google Scholar] [CrossRef]
- Smith, J.W.; Wootten, R. Anisakis and anisakiasis. Adv. Parasitol. 1978, 16, 93–163. [Google Scholar] [CrossRef] [PubMed]
- Levsen, A.; Svanevik, C.S.; Cipriani, P.; Mattiucci, S.; Gay, M.; Hastie, L.C.; Buchmann, K.; Højgaard, D.P.; González, A.F.; Pascual, S.; et al. A survey of zoonotic nematodes of commercial key fish species from major European fishing grounds Introducing the FP7 PARASITE exposure assessment study. Fish. Res. 2018, 202, 4–21. [Google Scholar] [CrossRef]
- Madrid, E.; Galán-Puchades, M.T.; Fuentes, M. Risk analysis of human anisakidosis through the consumption of the blue whiting, Micromesistius poutassou, sold at Spanish supermarkets. Foodborne Pathog. Dis. 2012, 9, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.V.; Madrid, E.; Cuesta, C.; Gimeno, C.; Baquedano-Rodríguez, M.; Soriano-Sánchez, I.; Bolívar, A.M.; Sáez-Durán, S.; Trelis, M.; Debenedetti, Á.L. Anisakid nematodes and potential risk of human anisakiasis through the consumption of hake, Merluccius spp., sold fresh in Spanish supermarkets. Pathogens 2022, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.V.; Madrid, E.; Meliá, L.V.; Casañ, F.; Sáez-Durán, S.; Trelis, M.; Debenedetti, Á.L. Nematode parasites of the European pilchard, Sardina pilchardus (Walbaum, 1792): A genuine human hazard? Animals 2022, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Strømnes, E.; Andersen, K. “Spring rise” of whaleworm (Anisakis simplex; Nematoda, Ascaridoidea) third stage larvae in some fish species from Norwegian waters. Parasitol. Res. 2000, 86, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K. The life cycle of Anisakis simplex: A review. In Intestinal Anisakiasis in Japan; Ishikura, H., Kikuchi, K., Eds.; Springer: Tokyo, Japan, 2010; pp. 31–40. [Google Scholar]
- Smith, J.W. The abundance of Anisakis simplex L3 in the body-cavity and flesh of marine teleosts. Int. J. Parasitol. 1984, 14, 491–495. [Google Scholar] [CrossRef]
- Cipriani, P.; Acerra, V.; Bellisario, B.; Sbaragli, G.L.; Cheleschi, R.; Nascetti, G.; Mattiucci, S. Larval migration of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy, Engraulis encrasicolus: Implications to seafood safety. Food Control 2016, 59, 148–157. [Google Scholar] [CrossRef]
- Šimat, V.; Miletić, J.; Bogdanović, T.; Poljak, V.; Mladineo, I. Role of biogenic amines in the post-mortem migration of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) larvae into fish fillets. Int. J. Food Microbiol. 2015, 214, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Young, P.C. The relationship between the presence of larval anisakine nematodes in cod and marine mammals in British home waters. J. Appl. Ecol. 1972, 9, 459–485. [Google Scholar] [CrossRef]
- Cruz, C.; Barbosa, C.; Saraiva, A. Distribution of larval anisakids in blue whiting off Portuguese fish market. Helminthologia 2007, 44, 21–24. [Google Scholar] [CrossRef]
- Abollo, E.; Gestal, C.; Pascual, S. Anisakis infestation in marine fish and cephalopods from Galician waters: An updated perspective. Parasitol. Res. 2001, 87, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Bušelić, I.; Botić, A.; Hrabar, J.; Stagliĉić, N.; Cipriani, P.; Mattiucci, S.; Mladineo, I. Geographic and host size variations as indicators of Anisakis pegreffii infection in European pilchard (Sardina pilchardus) from the Mediterranean Sea: Food safety implications. Int. J. Food Microbiol. 2018, 266, 126–132. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Re-evaluation of certain aspects of the EFSA Scientific Opinion of April 2010 on risk assessment of parasites in fishery products, based on new scientific data. Part 1: ToRs1 3. EFSA J. 2024, 22, e8719. [Google Scholar] [CrossRef] [PubMed]
| Number of Days | Atlantic | Mediterranean |
|---|---|---|
| 1 | 23 | 26 |
| 2 | 21 | 19 |
| 3 | 26 | 24 |
| 4 | 19 | 9 |
| 5 | 22 | 20 |
| 6 | 12 | 13 |
| 7 | 2 | 11 |
| 8 | --- | 3 |
| Origin of Capture | Total Ascaridoid | Anisakis Type I | Contracaecum spp. | Hysterothylacium spp. | Rhadinorhynchus pristis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | P% (CI 95%) | n | P% (CI 95%) | n | P% (CI 95%) | n | P% (CI 95%) | n | P% (CI 95%) | |
| Atlantic (n = 125) | 52 | 41.6 (38.6–44.6) | 52 | 41.6 (38.6–44.6) | - | - | 2 | 1.6 (0.8–2.4) | 45 | 36.0 (33.1–38.9) |
| Mediterranean (n = 125) | 26 | 20.8 (18.4–23.3) | 21 | 16.8 (14.5–19.1) | 3 | 2.4 (1.5–3.3) | 3 | 2.4 (1.5–3.3) | 21 | 16.8 (14.5–19.1) |
| Origin of Capture | Total Ascaridoid | Anisakis Type I | Contracaecum spp. | Hysterothylacium spp. | Rhadinorhynchus pristis |
|---|---|---|---|---|---|
| mI ± SD (range) | mI ± SD (range) | mI ± SD (range) | mI ± SD (range) | mI ± SD (range) | |
| Atlantic | 14.3 ± 18.2 (1–95) | 14.1 ± 18.1 (1–95) | - | 4.5 ± 3.5 (2–7) | 3.2 ± 3.7 (1–15) |
| Mediterranean | 2.2 ± 2.2 (1–9) | 2.4 ± 2.3 (1–9) | 1.0 ± 0 (1–3) | 1.0 ± 0 (1) | 8.8 ± 10.6 (1–44) |
| Origin of Capture | Viscera | Flesh | Autumn | Winter–Spring | ||||
|---|---|---|---|---|---|---|---|---|
| n | P% (CI 95%) | n | P% (CI 95%) | n | P% (CI 95%) | n | P% (CI 95%) | |
| Atlantic | 51 | 40.8 (37.8–43.8) | 12 | 9.6 (7.8–11.4) | 42 | 40.8 (37.5–44.0) | 10 | 45.5 (38.3–52.6) |
| Mediterranean | 18 | 14.4 (12.3–16.5) | 3 | 2.4 (1.5–3.3) | 10 | 19.6 (15.9–23.4) | 11 | 14.9 (12.1–17.7) |
| Origin of Capture | Viscera | Flesh | Autumn | Winter-Spring |
|---|---|---|---|---|
| mI ± SD (range) | mI ± SD (range) | mI ± SD (range) | mI ± SD (range) | |
| Atlantic | 13.9 ± 17.9 (1–93) | 2.0 ± 1.4 (1–5) | 6.9 ± 19.0 (1–95) | 0.8 ± 1.9 (1–7) |
| Mediterranean | 2.7 ± 2.5 (1–9) | 1.0 ± 0 (1) | 0.8 ± 2.8 (1–9) | 0.2 ± 0.4 (1–2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, M.V.; Royo, I.; Tellols, A.; Madrid, E.; Debenedetti, Á.L.; Sáez-Durán, S.; Trelis, M. Human Anisakiosis Risk and Presence of Food-Spoiling Parasites Through the Consumption of the Atlantic Chub Mackerel, Scomber colias, Sold in Spanish Supermarkets. Parasitologia 2025, 5, 37. https://doi.org/10.3390/parasitologia5030037
Fuentes MV, Royo I, Tellols A, Madrid E, Debenedetti ÁL, Sáez-Durán S, Trelis M. Human Anisakiosis Risk and Presence of Food-Spoiling Parasites Through the Consumption of the Atlantic Chub Mackerel, Scomber colias, Sold in Spanish Supermarkets. Parasitologia. 2025; 5(3):37. https://doi.org/10.3390/parasitologia5030037
Chicago/Turabian StyleFuentes, Màrius Vicent, Irina Royo, Alba Tellols, Elena Madrid, Ángela Lilia Debenedetti, Sandra Sáez-Durán, and María Trelis. 2025. "Human Anisakiosis Risk and Presence of Food-Spoiling Parasites Through the Consumption of the Atlantic Chub Mackerel, Scomber colias, Sold in Spanish Supermarkets" Parasitologia 5, no. 3: 37. https://doi.org/10.3390/parasitologia5030037
APA StyleFuentes, M. V., Royo, I., Tellols, A., Madrid, E., Debenedetti, Á. L., Sáez-Durán, S., & Trelis, M. (2025). Human Anisakiosis Risk and Presence of Food-Spoiling Parasites Through the Consumption of the Atlantic Chub Mackerel, Scomber colias, Sold in Spanish Supermarkets. Parasitologia, 5(3), 37. https://doi.org/10.3390/parasitologia5030037










