Arthropod-Borne Zoonotic Parasitic Diseases in Africa: Existing Burden, Diversity, and the Risk of Re-Emergence
Abstract
1. Introduction
2. Review Methodology
3. Overview of Arthropod-Borne Parasitic Diseases in Africa
3.1. Human and Non-Human Primate Malaria
3.2. Human and Animal Trypanosomiasis
3.3. Human and Canine Leishmaniasis
3.4. Human and Animal Babesiosis
3.5. Theileriosis
3.6. Filarial Diseases
3.6.1. Human and Animal Onchocerciasis
3.6.2. Lymphatic Filariasis
3.6.3. Loiasis
3.6.4. Mansonellosis
3.6.5. Canine Heartworm Disease
3.6.6. Other Animal Filarial Diseases
Setaria Species
Dipetalonema and Acanthocheilonema Species
Litomosoides Species
3.7. Thelaziasis
3.8. Elaeophorosis
3.9. Emerging Parasitic Disease
Anthemosoma Garnhami
4. Current Knowledge of Prevalence and Diversity of Arthropod-Borne Parasitic Diseases in Africa and Its Implications in One Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Molyneux, D.H. Vector-Borne Parasitic Diseases--an Overview of Recent Changes. Int. J. Parasitol. 1998, 28, 927–934. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Zhang, L.; Rohr, J.; Cui, R.; Xin, Y.; Han, L.; Yang, X.; Gu, S.; Du, Y.; Liang, J.; Wang, X. Biological Invasions Facilitate Zoonotic Disease Emergences. Nat. Commun. 2022, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.E.; Tricarico, E.; Hassall, R.; Johns, C.A.; Roy, K.A.; Scalera, R.; Smith, K.G.; Purse, B.V. The Role of Invasive Alien Species in the Emergence and Spread of Zoonoses. Biol. Invasions 2023, 25, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Haines, A.; Kuruvilla, S.; Borchert, M. Bridging the Implementation Gap between Knowledge and Action for Health. Bull. World Health Organ. 2004, 82, 724–731, discussion 732. [Google Scholar]
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Smith, K.F.; Acevedo-Whitehouse, K.; Pedersen, A.B. The Role of Infectious Diseases in Biological Conservation. Anim. Conserv. 2009, 12, 1–12. [Google Scholar] [CrossRef]
- Molyneux, D.H. Control of Human Parasitic Diseases: Context and Overview. Adv. Parasitol. 2006, 61, 1–45. [Google Scholar] [CrossRef]
- Wilcox, B.A.; Gubler, D.J. Disease Ecology and the Global Emergence of Zoonotic Pathogens. Environ. Health Prev. Med. 2005, 10, 263–272. [Google Scholar] [CrossRef]
- Hudson, P.J.; Cattadori, I.M.; Boag, B.; Dobson, A.P. Climate Disruption and Parasite-Host Dynamics: Patterns and Processes Associated with Warming and the Frequency of Extreme Climatic Events. J. Helminthol. 2006, 80, 175–182. [Google Scholar] [CrossRef]
- Merino, S. Host–Parasite Interactions and Climate Change. In Effects of Climate Change on Birds; Dunn, P.O., Møller, A.P., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 187–198. ISBN 978-0-19-882426-8. [Google Scholar]
- Ahmed, A.; Ali, Y.; Elduma, A.; Eldigail, M.H.; Mhmoud, R.A.; Mohamed, N.S.; Ksiazek, T.G.; Dietrich, I.; Weaver, S.C. Unique Outbreak of Rift Valley Fever in Sudan, 2019. Emerg. Infect. Dis. 2020, 26, 3030–3033. [Google Scholar] [CrossRef]
- Ali, Y.; Siddig, E.E.; Mohamed, N.; Ahmed, A. Rift Valley Fever and Malaria Co-Infection: A Case Report. Clin. Case Rep. 2023, 11, e7926. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.T.; Valdivia, H.O.; de Oliveira, T.C.; Alves, J.M.P.; Duarte, A.M.R.C.; Cerutti-Junior, C.; Buery, J.C.; Brito, C.F.A.; de Souza, J.C.J.; Hirano, Z.M.B.; et al. Human Migration and the Spread of Malaria Parasites to the New World. Sci. Rep. 2018, 8, 1993. [Google Scholar] [CrossRef] [PubMed]
- Truc, P.; Grébaut, P.; Lando, A.; Makiadi Donzoau, F.; Penchenier, L.; Herder, S.; Geiger, A.; Vatunga, G.; Josenando, T. Epidemiological Aspects of the Transmission of the Parasites Causing Human African Trypanosomiasis in Angola. Ann. Trop. Med. Parasitol. 2011, 105, 261–265. [Google Scholar] [CrossRef]
- Ahmed, A.; Mohamed, N.S.; Siddig, E.E.; Algaily, T.; Sulaiman, S.; Ali, Y. The Impacts of Climate Change on Displaced Populations: A Call for Action. J. Clim. Change Health 2021, 3, 100057. [Google Scholar] [CrossRef]
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef]
- Rosenthal, J. Climate Change and the Geographic Distribution of Infectious Diseases. EcoHealth 2009, 6, 489–495. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Martens, P.; Hall, L. Malaria on the Move: Human Population Movement and Malaria Transmission. Emerg. Infect. Dis. 2000, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Schantz-Dunn, J.; Nour, N.M. Malaria and Pregnancy: A Global Health Perspective. Rev. Obstet. Gynecol. 2009, 2, 186–192. [Google Scholar] [PubMed]
- Mahanay, F.J.; Bashein, A.M.; EI-Buni, A.A.; Sheebah, A.; Annajar, B.B. Malaria in Illegal Immigrants in Southern Libya. Libyan J. Med. Sci. 2021, 5, 158–161. [Google Scholar] [CrossRef]
- Grande, R.; Antinori, S.; Meroni, L.; Menegon, M.; Severini, C. A Case of Plasmodium Malariae Recurrence: Recrudescence or Reinfection? Malar. J. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Mahmoud, D.M.; Hussein, H.M.; El Gozamy, B.M.R.; Thabet, H.S.; Hassan, M.A.; Meselhey, R.A.-A. Screening of Plasmodium Parasite in Vectors and Humans in Three Villages in Aswan Governorate, Egypt. J. Parasit. Dis. 2019, 43, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Saoud, M.; Ezzariga, N.; Benaissa, E.; Moustachi, A.; Lyagoubi, M.; Aoufi, S. Imported Malaria: 54 Cases Diagnosed at the Ibn Sina Hospital Center in Rabat, Morocco. Médecine Santé Trop. 2019, 29, 159–163. [Google Scholar]
- Nabah, K.; Mezzoug, N.; Aarab, A.; Oufdou, H.; Rharrabe, K. Epidemiological Profile of the Imported Malaria in the North Region of Morocco from 2014 to 2018. E3S Web Conf. 2021, 319, 01057. [Google Scholar] [CrossRef]
- Aoun, K.; Siala, E.; Tchibkere, D.; Zallagua, N.; Chahed, M.; Bouratbine, A. Imported Malaria in Tunisia: Consequences on the Risk of Resurgence of the Disease. Med. Trop. Rev. Corps Sante Colon. 2010, 70, 33–37. [Google Scholar]
- DePina, A.J.; Stresman, G.; Barros, H.S.B.; Moreira, A.L.; Dia, A.K.; Furtado, U.D.; Faye, O.; Seck, I.; Niang, E.H.A. Updates on Malaria Epidemiology and Profile in Cabo Verde from 2010 to 2019: The Goal of Elimination. Malar. J. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Aboobakar, S.; Tatarskv, A.; Cohen, J.M.; Bheecarry, A.; Boolaky, P.; Gopee, N.; Moonasar, D.; Phillips, A.A.; Kahn, J.G.; Moonen, B. Eliminating Malaria and Preventing Its Reintroduction: The Mauritius Case Study. Malar. J. 2012, 11, 1–2. [Google Scholar] [CrossRef]
- Bovet, P.; Gédéon, J.; Louange, M.; Durasnel, P.; Aubry, P.; Gauzere, B. Health Situation and Issues in the Seychelles in 2012. Med. Sante Trop. 2013, 23, 256–266. [Google Scholar] [CrossRef]
- Algeria: Epidemic—09-2024—South Algeria Malaria and Diphtheria (2024-10-04)—Algeria | ReliefWeb. Available online: https://reliefweb.int/report/algeria/algeria-epidemic-09-2024-south-algeria-malaria-and-diphtheria-2024-10-04 (accessed on 31 January 2025).
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H. A Global Map of Dominant Malaria Vectors. Parasit. Vectors 2012, 5, 1–11. [Google Scholar] [CrossRef]
- Singh, B.; Kim Sung, L.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.G.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A Large Focus of Naturally Acquired Plasmodium Knowlesi Infections in Human Beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef]
- Lalremruata, A.; Magris, M.; Vivas-Martínez, S.; Koehler, M.; Esen, M.; Kempaiah, P.; Jeyaraj, S.; Perkins, D.J.; Mordmüller, B.; Metzger, W.G. Natural Infection of Plasmodium Brasilianum in Humans: Man and Monkey Share Quartan Malaria Parasites in the Venezuelan Amazon. EBioMedicine 2015, 2, 1186–1192. [Google Scholar] [CrossRef]
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F.; et al. Outbreak of Human Malaria Caused by Plasmodium Simium in the Atlantic Forest in Rio de Janeiro: A Molecular Epidemiological Investigation. Lancet Glob. Health 2017, 5, e1038–e1046. [Google Scholar] [CrossRef] [PubMed]
- Zaw, M.T.; Lin, Z. Human Plasmodium Knowlesi Infections in South-East Asian Countries. J. Microbiol. Immunol. Infect. 2019, 52, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Abubakr, M.; Ali, Y.; Siddig, E.E.; Mohamed, N.S. Vector Control Strategy for Anopheles Stephensi in Africa. Lancet Microbe 2022, 3, e403. [Google Scholar] [CrossRef]
- Ahmed, A.; Irish, S.R.; Zohdy, S.; Yoshimizu, M.; Tadesse, F.G. Strategies for Conducting Anopheles Stephensi Surveys in Non-Endemic Areas. Acta Trop. 2022, 236, 106671. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Abdulai, A.; Mohammed, A.R.; Akuamoah-Boateng, Y.; Owusu-Asenso, C.M.; Sraku, I.K.; Yanney, S.A.; Malm, K.; Lobo, N.F. First Detection of Anopheles Stephensi in Ghana Using Molecular Surveillance. bioRxiv 2023. [Google Scholar] [CrossRef]
- Al-Eryani, S.M.; Irish, S.R.; Carter, T.E.; Lenhart, A.; Aljasari, A.; Montoya, L.F.; Awash, A.A.; Mohammed, E.; Ali, S.; Esmail, M.A. Public Health Impact of the Spread of Anopheles Stephensi in the WHO Eastern Mediterranean Region Countries in Horn of Africa and Yemen: Need for Integrated Vector Surveillance and Control. Malar. J. 2023, 22, 187. [Google Scholar] [CrossRef]
- Sinton, J.; Mulligan, H. A Critical Review of the Literature relating to the Identification of the Malarial Parasites recorded from Monkeys of the Families Cercopithecidae and Colobidae. Rec. Malar. Surv. India 1932, 3, 357–380. [Google Scholar]
- Poirriez, J.; Dei-Cas, E.; Landau, I. Further Description of Blood Stages of Plasmodium Petersi from Cercocebus Albigena Monkey. Folia Parasitol. 1994, 41, 168–172. [Google Scholar]
- Poirriez, J.; Baccam, D.; Dei-Cas, E.; Brogan, T.; Landau, I. Description de Plasmodium Petersi n. Sp. et Plasmodium Georgesi n. Sp., Parasites d’un Cercocebus Albigena Originaire de République Centrafricaine. Ann. Parasitol. Hum. Comp. 1993, 68, 203–210. [Google Scholar] [CrossRef]
- Prugnolle, F.; Ollomo, B.; Durand, P.; Yalcindag, E.; Arnathau, C.; Elguero, E.; Berry, A.; Pourrut, X.; Gonzalez, J.-P.; Nkoghe, D.; et al. African Monkeys Are Infected by Plasmodium Falciparum Nonhuman Primate-Specific Strains. Proc. Natl. Acad. Sci. USA 2011, 108, 11948–11953. [Google Scholar] [CrossRef]
- Escalante, A.A.; Freeland, D.E.; Collins, W.E.; Lal, A.A. The Evolution of Primate Malaria Parasites Based on the Gene Encoding Cytochrome b from the Linear Mitochondrial Genome. Proc. Natl. Acad. Sci. USA 1998, 95, 8124–8129. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, U.; Candolin, U. Behavioural Responses to Human-Induced Environmental Change. Biol. Rev. Camb. Philos. Soc. 2011, 86, 640–657. [Google Scholar] [CrossRef]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife-Livestock-Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Mewara, A.; Sreenivasan, P.; Khurana, S. Primate Malaria of Human Importance. Trop. Parasitol. 2023, 13, 73–83. [Google Scholar] [CrossRef]
- Imwong, M.; Madmanee, W.; Suwannasin, K.; Kunasol, C.; Peto, T.J.; Tripura, R.; von Seidlein, L.; Nguon, C.; Davoeung, C.; Day, N.P.J.; et al. Asymptomatic Natural Human Infections With the Simian Malaria Parasites Plasmodium Cynomolgi and Plasmodium Knowlesi. J. Infect. Dis. 2019, 219, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Prugnolle, F.; Durand, P.; Neel, C.; Ollomo, B.; Ayala, F.J.; Arnathau, C.; Etienne, L.; Mpoudi-Ngole, E.; Nkoghe, D.; Leroy, E.; et al. African Great Apes Are Natural Hosts of Multiple Related Malaria Species, Including Plasmodium Falciparum. Proc. Natl. Acad. Sci. USA 2010, 107, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Adler, S. Malaria in Chimpanzees in Sierra Leone. Ann. Trop. Med. Parasitol. 1923, 17, 13–18. [Google Scholar] [CrossRef]
- Faust, C.; Dobson, A.P. Primate Malarias: Diversity, Distribution and Insights for Zoonotic Plasmodium. One Health Amst. Neth. 2015, 1, 66–75. [Google Scholar] [CrossRef]
- Papagni, R.; Novara, R.; Minardi, M.L.; Frallonardo, L.; Panico, G.G.; Pallara, E.; Cotugno, S.; Ascoli Bartoli, T.; Guido, G.; De Vita, E.; et al. Human African Trypanosomiasis (Sleeping Sickness): Current Knowledge and Future Challenges. Front. Trop. Dis. 2023, 4, 1033. [Google Scholar] [CrossRef]
- Cox, F.E.G. History of Sleeping Sickness (African Trypanosomiasis). Infect. Dis. Clin. N. Am. 2004, 18, 231–245. [Google Scholar] [CrossRef]
- Sutherland, C.S.; Stone, C.M.; Steinmann, P.; Tanner, M.; Tediosi, F. Seeing beyond 2020: An Economic Evaluation of Contemporary and Emerging Strategies for Elimination of Trypanosoma Brucei Gambiense. Lancet Glob. Health 2017, 5, e69–e79. [Google Scholar] [CrossRef]
- Wanga, C.H. Cost Effective Control of Zoonotic African Trypanosomiasis in Kenya: Analysing Underreporting Factors and Modeling Prevalence in Busia Foci. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2015. [Google Scholar]
- Rock, K.S.; Stone, C.M.; Hastings, I.M.; Keeling, M.J.; Torr, S.J.; Chitnis, N. Mathematical Models of Human African Trypanosomiasis Epidemiology. Adv. Parasitol. 2015, 87, 53–133. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; DE Koning, H.P.; Barrett, M.P. The Animal Trypanosomiases and Their Chemotherapy: A Review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Tihon, E.; Imamura, H.; Dujardin, J.; Van Den Abbeele, J.; Van den Broeck, F. Discovery and Genomic Analyses of Hybridization between Divergent Lineages of Trypanosoma Congolense, Causative Agent of Animal African Trypanosomiasis. Mol. Ecol. 2017, 26, 6524–6538. [Google Scholar] [CrossRef]
- Cadioli, F.A.; de Athayde Barnabé, P.; Machado, R.Z.; Teixeira, M.C.A.; André, M.R.; Sampaio, P.H.; Fidélis Junior, O.L.; Teixeira, M.M.G.; Marques, L.C. First Report of Trypanosoma Vivax Outbreak in Dairy Cattle in São Paulo State, Brazil. Rev. Bras. Parasitol. Veterinária 2012, 21, 118–124. [Google Scholar] [CrossRef]
- McNamara, J.; Mohammed, G.; Gibson, W. Trypanosoma (Nannomonas) Godfreyi Sp. Nov. from Tsetse Flies in The Gambia: Biological and Biochemical Characterization. Parasitology 1994, 109, 497–509. [Google Scholar] [CrossRef]
- Nimpaye, H.; Njiokou, F.; Njine, T.; Njitchouang, G.; Cuny, G.; Herder, S.; Asonganyi, T.; Simo, G. Trypanosoma Vivax, T. Congolense “Forest Type” and T. Simiae: Prevalence in Domestic Animals of Sleeping Sickness Foci of Cameroon. Parasite J. Société Fr. Parasitol. 2011, 18, 171. [Google Scholar] [CrossRef]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B. The Genome of the African Trypanosome Trypanosoma Brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef]
- Johnson, P. A Case of Infection by Trypanosoma lewisi in a Child. Trans. R. Soc. Trop. Med. Hyg. 1933, 26, 467–468. [Google Scholar] [CrossRef]
- Janssen, J.; Wijers, D. Trypanosoma Simiae at the Kenya Coast. A Correlation between Virulence and the Transmitting Species of Glossina. Ann. Trop. Med. Parasitol. 1974, 68, 5–19. [Google Scholar] [CrossRef]
- Hutchinson, R.; Gibson, W. Rediscovery of Trypanosoma (Pycnomonas) Suis, a Tsetse-Transmitted Trypanosome Closely Related to T. Brucei. Infect. Genet. Evol. 2015, 36, 381–388. [Google Scholar] [CrossRef]
- Brotánková, A.; Fialová, M.; Čepička, I.; Brzoňová, J.; Svobodová, M. Trypanosomes of the Trypanosoma Theileri Group: Phylogeny and New Potential Vectors. Microorganisms 2022, 10, 294. [Google Scholar] [CrossRef]
- Wilson, S. Trypanosoma Uniforme-Trypanosoma Vivax Infections in Bovines and Trypanosoma Uniforme Infections in Goats and Sheep at Entebbe, Uganda. Parasitology 1949, 39, 198–208. [Google Scholar] [CrossRef]
- Brun, R.; Hecker, H.; Lun, Z.-R. Trypanosoma Evansi and T. Equiperdum: Distribution, Biology, Treatment and Phylogenetic Relationship (a Review). Vet. Parasitol. 1998, 79, 95–107. [Google Scholar] [CrossRef]
- Katsidzira, L.; Fana, G.T. Pitfalls in the Diagnosis of Trypanosomiasis in Low Endemic Countries: A Case Report. PLoS Negl. Trop. Dis. 2010, 4, e823. [Google Scholar] [CrossRef]
- Shereni, W.; Neves, L.; Argilés, R.; Nyakupinda, L.; Cecchi, G. An Atlas of Tsetse and Animal African Trypanosomiasis in Zimbabwe. Parasit. Vectors 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Boushaki, D.; Adel, A.; Dia, M.L.; Büscher, P.; Madani, H.; Brihoum, B.A.; Sadaoui, H.; Bouayed, N.; Issad, N.K. Epidemiological Investigations on Trypanosoma Evansi Infection in Dromedary Camels in the South of Algeria. Heliyon 2019, 5, e02086. [Google Scholar] [CrossRef]
- Bennoune, O.; Adili, N.; Amri, K.; Bennecib, L.; Ayachi, A. Trypanosomiasis of Camels (Camelus dromedarius) in Algeria: First Report. Vet. Res. Forum 2013, 4, 273–275. [Google Scholar]
- Medkour, H.; Laidoudi, Y.; Lafri, I.; Davoust, B.; Mekroud, A.; Bitam, I.; Mediannikov, O. Canine Vector-Borne Protozoa: Molecular and Serological Investigation for Leishmania spp., Trypanosoma spp., Babesia spp., and Hepatozoon spp. in Dogs from Northern Algeria. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100353. [Google Scholar] [CrossRef]
- Perich, P. Trypanosoma Rhodesiense African Human Trypanosomiasis Foci in Burundi (Vector: Glossina Morsitans). Historic and Present Aspects (Author’s Transl). Med. Trop. Rev. Corps Sante Colon. 1982, 42, 33–41. [Google Scholar]
- Soha, S.; SouaÃ, F.; Issaka, Y.A.K.; Jacques, D.T. African Animal Trypanosomosis in Cattle in Bnin: A Review. J. Vet. Med. Anim. Health 2019, 11, 115–122. [Google Scholar]
- Dobigny, G.; Gauthier, P.; Houéménou, G.; Dossou, H.; Badou, S.; Etougbétché, J.; Tatard, C.; Truc, P. Spatio-Temporal Survey of Small Mammal-Borne Trypanosoma Lewisi in Cotonou, Benin, and the Potential Risk of Human Infection. Infect. Genet. Evol. 2019, 75, 103967. [Google Scholar] [CrossRef]
- Sharma, S.; Losho, T.; Malau, M.; Mangate, K.; Linchwe, K.; Amanfu, W.; Motsu, T. The Resurgence of Trypanosomosis in Botswana. J. S. Afr. Vet. Assoc. 2001, 72, 232–234. [Google Scholar] [CrossRef]
- Kambire, R.; Lingue, K.; Courtin, F.; Sidibe, I.; Kiendrebeogo, D.; N’gouan, K.; Blé, L.; Kaba, D.; Koffi, M.; Solano, P. Human African Trypanosomiasis in Côte d’Ivoire and Burkina Faso: Optimization of Epidemiologic Surveillance Strategies. Parasite 2012, 19, 389–396. [Google Scholar] [CrossRef]
- Simo, G.; Mbida, J.A.M.; Eyenga, V.E.; Asonganyi, T.; Njiokou, F.; Grébaut, P. Challenges towards the Elimination of Human African Trypanosomiasis in the Sleeping Sickness Focus of Campo in Southern Cameroon. Parasit. Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef]
- Suh, P.; Njiokou, F.; Mamoudou, A.; Ahmadou, T.; Mouhaman, A.; Garabed, R. Bovine Trypanosomiasis in Tsetse-Free Pastoral Zone of the Far-North Region, Cameroon. J. Vector Borne Dis. 2017, 54, 263–269. [Google Scholar] [CrossRef]
- Simarro, P.P.; Cecchi, G.; Franco, J.R.; Paone, M.; Fèvre, E.M.; Diarra, A.; Postigo, J.A.R.; Mattioli, R.C.; Jannin, J.G. Risk for Human African Trypanosomiasis, Central Africa, 2000–2009. Emerg. Infect. Dis. 2011, 17, 2322. [Google Scholar] [CrossRef]
- Chappuis, F.; Lima, M.A.; Flevaud, L.; Ritmeijer, K. Human African Trypanosomiasis in Areas without Surveillance. Emerg. Infect. Dis. 2010, 16, 354. [Google Scholar] [CrossRef]
- Vourchakbe, J.; Zebaze, A.A.T.; Tagueu, S.K.; Kodindo, I.D.; Padja, A.B.; Simo, G. Diversity of Trypanosome Species in Small Ruminants, Dogs and Pigs from Three Sleeping Sickness Foci of the South of Chad. Parasitol. Int. 2023, 96, 102772. [Google Scholar] [CrossRef]
- Vourchakbé, J.; Tiofack, A.A.Z.; Kante, S.T.; Barka, P.A.; Simo, G. Prevalence of Pathogenic Trypanosome Species in Naturally Infected Cattle of Three Sleeping Sickness Foci of the South of Chad. PLoS ONE 2022, 17, e0279730. [Google Scholar] [CrossRef]
- Bemba, I.; Bamou, R.; Lenga, A.; Okoko, A.; Awono-Ambene, P.; Antonio-Nkondjio, C. Review of the Situation of Human African Trypanosomiasis in the Republic of Congo From the 1950s to 2020. J. Med. Entomol. 2022, 59, 421–429. [Google Scholar] [CrossRef]
- N’Djetchi, M.K.; Ilboudo, H.; Koffi, M.; Kaboré, J.; Kaboré, J.W.; Kaba, D.; Courtin, F.; Coulibaly, B.; Fauret, P.; Kouakou, L. The Study of Trypanosome Species Circulating in Domestic Animals in Two Human African Trypanosomiasis Foci of Cote d’Ivoire Identifies Pigs and Cattle as Potential Reservoirs of Trypanosoma Brucei Gambiense. PLoS Negl. Trop. Dis. 2017, 11, e0005993. [Google Scholar] [CrossRef]
- Lumbala, C.; Simarro, P.P.; Cecchi, G.; Paone, M.; Franco, J.R.; Kande Betu Ku Mesu, V.; Makabuza, J.; Diarra, A.; Chansy, S.; Priotto, G. Human African Trypanosomiasis in the Democratic Republic of the Congo: Disease Distribution and Risk. Int. J. Health Geogr. 2015, 14, 1–14. [Google Scholar] [CrossRef]
- Boodman, C.; Libman, M.; Ndao, M.; Yansouni, C.P. Case Report: Trypanosoma Brucei Gambiense Human African Trypanosomiasis as the Cause of Fever in an Inpatient with Multiple Myeloma and HIV-1 Coinfection. Am. J. Trop. Med. Hyg. 2019, 101, 123. [Google Scholar] [CrossRef]
- El-Sayed, S.A.E.-S.; El-Adl, M.A.; Ali, M.O.; Al-Araby, M.; Omar, M.A.; El-Beskawy, M.; Sorour, S.S.; Rizk, M.A.; Elgioushy, M. Molecular Detection and Identification of Babesia Bovis and Trypanosoma Spp. in One-Humped Camel (Camelus Dromedarius) Breeds in Egypt. Vet. World 2021, 14, 625. [Google Scholar] [CrossRef]
- Elhaig, M.M.; Selim, A.; Mahmoud, M.M.; El-Gayar, E.K. Molecular Confirmation of Trypanosoma Evansi and Babesia Bigemina in Cattle from Lower Egypt. Pak. Vet. J. 2016, 36, 409–414. [Google Scholar]
- Cordon-Obras, C.; Rodriguez, Y.F.; Fernandez-Martinez, A.; Cano, J.; Ndong-Mabale, N.; Ncogo-Ada, P.; Ndongo-Asumu, P.; Aparicio, P.; Navarro, M.; Benito, A. Molecular Evidence of a Trypanosoma Brucei Gambiense Sylvatic Cycle in the Human African Trypanosomiasis Foci of Equatorial Guinea. Front. Microbiol. 2015, 6, 765. [Google Scholar] [CrossRef]
- Cordon-Obras, C.; Berzosa, P.; Ndong-Mabale, N.; Bobuakasi, L.; Buatiche, J.; Ndongo-Asumu, P.; Benito, A.; Cano, J. Trypanosoma Brucei Gambiense in Domestic Livestock of Kogo and Mbini Foci (Equatorial guinea). Trop. Med. Int. Health 2009, 14, 535–541. [Google Scholar] [CrossRef]
- Martoglio, F. Trypanosomiasis of the Dromedary in Eritrea. Ann. D’igiene 1913, 23, 229–234. [Google Scholar]
- Domizio, G.D. A Trypanosomiasis (Gudhò) of Eritrean Dromedaries. Notes on Blood-Sucking Flies of the Colony of Eritrea. Clin. Vet. 1918, 17, 391–413. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19186300514 (accessed on 2 February 2025).
- Gelaye, A.; Fesseha, H. Bovine Trypanosomiasis in Ethiopia: Epidemiology, Diagnosis and Its Economic Impact-a Review. Open Access J. Biog. Sci. Res. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Abera, A.; Mamecha, T.; Abose, E.; Bokicho, B.; Ashole, A.; Bishaw, T.; Mariyo, A.; Bogale, B.; Terefe, H.; Tadesse, H. Reemergence of Human African Trypanosomiasis Caused by Trypanosoma Brucei Rhodesiense, Ethiopia. Emerg. Infect. Dis. 2024, 30, 125. [Google Scholar] [CrossRef]
- Boundenga, L.; Mombo, I.M.; Augustin, M.-O.; Barthélémy, N.; Nzassi, P.M.; Moukodoum, N.D.; Rougeron, V.; Prugnolle, F. Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma Brucei Gambiense in Historical Foci of Human African Trypanosomiasis in Gabon. Pathogens 2022, 11, 992. [Google Scholar] [CrossRef]
- Iroungou, B.A.; Boundenga, L.; Guignali Mangouka, L.; Bivigou-Mboumba, B.; Nzenze, J.R.; Maganga, G.D. Human African Trypanosomiasis in Two Historical Foci of the Estuaire Province, Gabon: A Case Report. SAGE Open Med. Case Rep. 2020, 8, 2050313x20959890. [Google Scholar] [CrossRef]
- Ekloh, W.; Sunter, J.D.; Gwira, T.M. African Trypanosome Infection Patterns in Cattle in a Farm Setting in Southern Ghana. Acta Trop. 2023, 237, 106721. [Google Scholar] [CrossRef]
- Elliott, I.; Patel, T.; Shah, J.; Venkatesan, P. West-African Trypanosomiasis in a Returned Traveller from Ghana: An Unusual Cause of Progressive Neurological Decline. Case Rep. 2014, 2014, bcr2014204451. [Google Scholar] [CrossRef]
- Camara, O.; Camara, M.; Falzon, L.C.; Ilboudo, H.; Kaboré, J.; Compaoré, C.F.A.; Fèvre, E.M.; Büscher, P.; Bucheton, B.; Lejon, V. Performance of Clinical Signs and Symptoms, Rapid and Reference Laboratory Diagnostic Tests for Diagnosis of Human African Trypanosomiasis by Passive Screening in Guinea: A Prospective Diagnostic Accuracy Study. Infect. Dis. Poverty 2023, 12, 22. [Google Scholar] [CrossRef]
- Kivali, V.; Kiyong’a, A.N.; Fyfe, J.; Toye, P.; Fèvre, E.M.; Cook, E.A. Spatial Distribution of Trypanosomes in Cattle from Western Kenya. Front. Vet. Sci. 2020, 7, 554. [Google Scholar] [CrossRef]
- Remme, J.H.; Feenstra, P.; Lever, P.; Medici, A.C.; Morel, C.M.; Noma, M.; Ramaiah, K.; Richards, F.; Seketeli, A.; Schmunis, G. Tropical Diseases Targeted for Elimination: Chagas Disease, Lymphatic Filariasis, Onchocerciasis, and Leprosy. In Disease Control Priorities in Developing Countries; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Harley, G.W.; Miller, M.J. Human Trypanosomiasis in Northeastern Liberia. Am. J. Trop. Med. Hyg. 1955, 4, 249–253. [Google Scholar] [CrossRef]
- Mehlitz, D.; Gangpala, L. Sleeping Sickness in Liberia–a Historical Review. Sierra Leone J. Biomed. Res. 2017, 9, 38–46. [Google Scholar]
- Mehlitz, D. Trypanosome Infections in Domestic Animals in Liberia. Tropenmed. Parasitol. 1979, 30, 212–219. [Google Scholar]
- El Maghrbi, A.; Hosni, M. Detection of trypanosoma evansi in dromedary camels. Vet. Med. J. Giza 2008, 56, 277–283. [Google Scholar] [CrossRef]
- Rasoanoro, M.; Ramasindrazana, B.; Goodman, S.M.; Rajerison, M.; Randrianarivelojosia, M. A Review of Trypanosoma Species Known from Malagasy Vertebrates. Malagasy Natiora 2019, 13, 65–75. [Google Scholar]
- Rasoanoro, M.; Goodman, S.M.; Randrianarivelojosia, M.; Soarimalala, V.; Ramasindrazana, B. Trypanosoma Infection in Terrestrial Small Mammals from the Central Highlands of Madagascar. Malagasy Nat. 2022, 16, 134–142. [Google Scholar]
- Simarro, P.P.; Cecchi, G.; Paone, M.; Franco, J.R.; Diarra, A.; Ruiz, J.A.; Fèvre, E.M.; Courtin, F.; Mattioli, R.C.; Jannin, J.G. The Atlas of Human African Trypanosomiasis: A Contribution to Global Mapping of Neglected Tropical Diseases. Int. J. Health Geogr. 2010, 9, 1–18. [Google Scholar] [CrossRef]
- Marsela, M.; Hayashida, K.; Nakao, R.; Chatanga, E.; Gaithuma, A.K.; Naoko, K.; Musaya, J.; Sugimoto, C.; Yamagishi, J. Molecular Identification of Trypanosomes in Cattle in Malawi Using PCR Methods and Nanopore Sequencing: Epidemiological Implications for the Control of Human and Animal Trypanosomiases. Parasite 2020, 27, 46. [Google Scholar] [CrossRef]
- Frean, J.; Sieling, W.; Pahad, H.; Shoul, E.; Blumberg, L. Clinical Management of East African Trypanosomiasis in South Africa: Lessons Learned. Int. J. Infect. Dis. 2018, 75, 101–108. [Google Scholar] [CrossRef]
- Diakité, M.; Sacko, B.; Traore, C.; Diall, Y.G.; Sery, A.; Diarra, M.; Marico, O.; Sissoko, F.; Bengaly, S. Study of Bovine Trypanosomiasis in Mali: Case of the Kita Region. World J. Biol. Pharm. Health Sci. 2024, 20, 401–406. [Google Scholar] [CrossRef]
- Schwan, T.G.; Lopez, J.E.; Safronetz, D.; Anderson, J.M.; Fischer, R.J.; Maïga, O.; Sogoba, N. Fleas and Trypanosomes of Peridomestic Small Mammals in Sub-Saharan Mali. Parasit. Vectors 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Lamine, D.M. Epidemiology of Camel Trypanosomosis Due to Trypanosoma Evansi in Mauritania and Its Control Strategies for Sustainable Livestock Production. In Proceedings of the FAO/IAEA international symposium on sustainable improvement of animal production and health, Vienna, Austria, 8–11 June 2009. [Google Scholar]
- Desquesnes, M.; Holzmuller, P.; Lai, D.-H.; Dargantes, A.; Lun, Z.-R.; Jittaplapong, S. Trypanosoma Evansi and Surra: A Review and Perspectives on Origin, History, Distribution, Taxonomy, Morphology, Hosts, and Pathogenic Effects. BioMed Res. Int. 2013, 2013, 194176. [Google Scholar] [CrossRef]
- Rami, M.; Atarhouch, T.; Bendahman, M.; Azlaf, R.; Kechna, R.; Dakkak, A. Camel Trypanosomosis in Morocco: 2. A Pilot Disease Control Trial. Vet. Parasitol. 2003, 115, 223–231. [Google Scholar] [CrossRef]
- Specht, E. Prevalence of Bovine Trypanosomosis in Central Mozambique from 2002 to 2005. Onderstepoort J. Vet. Res. 2008, 75, 73–81. [Google Scholar] [CrossRef]
- Tsetse Control to Assist Livestock Production. Available online: https://www.fao.org/fishery/docs/CDrom/aquaculture/a0845t/volume2/docrep/field/383559.htm (accessed on 2 February 2025).
- Tatard, C.; Garba, M.; Gauthier, P.; Hima, K.; Artige, E.; Dossou, D.; Gagaré, S.; Genson, G.; Truc, P.; Dobigny, G. Rodent-Borne Trypanosoma from Cities and Villages of Niger and Nigeria: A Special Role for the Invasive Genus Rattus? Acta Trop. 2017, 171, 151–158. [Google Scholar] [CrossRef]
- Odebunmi, E.; Ibeachu, C.; Chukwudi, C.U. Prevalence of Human and Animal African Trypanosomiasis in Nigeria: A Scoping Review. medRxiv 2024. [Google Scholar] [CrossRef]
- Habeeb, I.F.; Chechet, G.D.; Kwaga, J.K. Molecular Identification and Prevalence of Trypanosomes in Cattle Distributed within the Jebba Axis of the River Niger, Kwara State, Nigeria. Parasit. Vectors 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Gashururu, S.R.; Maingi, N.; Githigia, S.M.; Gasana, M.N.; Odhiambo, P.O.; Getange, D.O.; Habimana, R.; Cecchi, G.; Zhao, W.; Gashumba, J. Occurrence, Diversity and Distribution of Trypanosoma Infections in Cattle around the Akagera National Park, Rwanda. PLoS Negl. Trop. Dis. 2021, 15, e0009929. [Google Scholar] [CrossRef]
- Gashururu, R.S.; Maingi, N.; Githigia, S.M.; Getange, D.O.; Ntivuguruzwa, J.B.; Habimana, R.; Cecchi, G.; Gashumba, J.; Bargul, J.L.; Masiga, D.K. Trypanosomes Infection, Endosymbionts, and Host Preferences in Tsetse Flies (Glossina Spp.) Collected from Akagera Park Region, Rwanda: A Correlational Xenomonitoring Study. One Health 2023, 16, 100550. [Google Scholar] [CrossRef]
- Figueiredo Moura Da Silva, E.L. Tropical Medicine behind Cocoa Slavery: A Campaign to Eradicate Sleeping Sickness in the Portuguese Colony of Príncipe Island, 1911–1914. Bull. Span. Port. Hist. Stud. 2020, 44, 28. [Google Scholar] [CrossRef]
- da Costa, B.F.B. Sleeping Sickness; A Record of Four Years’ War Against It in Principe, Portuguese West Africa; Baillière, Tindall and Cox: London, UK, 1916. [Google Scholar]
- Desquesnes, M.; Ravel, S.; Deschamps, J.-Y.; Polack, B.; Roux, F. Atypical Hyperpachymorph Trypanosoma (Nannomonas) Congolense Forest-Type in a Dog Returning from Senegal. Parasite 2012, 19, 239. [Google Scholar] [CrossRef]
- Seck, M.T.; Bouyer, J.; Sall, B.; Bengaly, Z.; Vreysen, M.J. The Prevalence of African Animal Trypanosomoses and Tsetse Presence in Western Senegal. Parasite 2010, 17, 257–265. [Google Scholar] [CrossRef]
- Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/data/gho/data/themes/topics/human-african-trypanosomiasis (accessed on 2 February 2025).
- Sudarshi, D.; Lawrence, S.; Pickrell, W.O.; Eligar, V.; Walters, R.; Quaderi, S.; Walker, A.; Capewell, P.; Clucas, C.; Vincent, A. Human African Trypanosomiasis Presenting at Least 29 Years after Infection—What Can This Teach Us about the Pathogenesis and Control of This Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2014, 8, e3349. [Google Scholar] [CrossRef]
- Dorward, D.C.; Payne, A. Deforestation, the Decline of the Horse, and the Spread of the Tsetse Fly and Trypanosomiasis (Nagana) in Nineteenth Century Sierra Leone. J. Afr. Hist. 1975, 16, 239–256. [Google Scholar] [CrossRef]
- Hassan-Kadle, A.A.; Ibrahim, A.M.; Nyingilili, H.S.; Yusuf, A.A.; Vieira, R.F. Parasitological and Molecular Detection of Trypanosoma spp. in Cattle, Goats and Sheep in Somalia. Parasitology 2020, 147, 1786–1791. [Google Scholar] [CrossRef]
- Latif, A.A.; Ntantiso, L.; De Beer, C. African Animal Trypanosomosis (Nagana) in Northern KwaZulu-Natal, South Africa: Strategic Treatment of Cattle on a Farm in Endemic Area. Onderstepoort J. Vet. Res. 2019, 86, 1–6. [Google Scholar] [CrossRef]
- Ruiz-Postigo, J.A.; Franco, J.R.; Lado, M.; Simarro, P.P. Human African Trypanosomiasis in South Sudan: How Can We Prevent a New Epidemic? PLoS Negl. Trop. Dis. 2012, 6, e1541. [Google Scholar] [CrossRef]
- Archibald, R. A Trypanosome of Cattle in the Southern Sudan. J. Comp. Pathol. Ther. 1912, 25, 292–297. [Google Scholar] [CrossRef]
- Mossaad, E.; Ismail, A.A.; Ibrahim, A.M.; Musinguzi, P.; Angara, T.E.; Xuan, X.; Inoue, N.; Suganuma, K. Prevalence of Different Trypanosomes in Livestock in Blue Nile and West Kordofan States, Sudan. Acta Trop. 2020, 203, 105302. [Google Scholar] [CrossRef]
- Hamill, L.C.; Kaare, M.T.; Welburn, S.C.; Picozzi, K. Domestic Pigs as Potential Reservoirs of Human and Animal Trypanosomiasis in Northern Tanzania. Parasit. Vectors 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Kargbo, A.; Ebiloma, G.U.; Ibrahim, Y.K.E.; Chechet, G.D.; Jeng, M.; Balogun, E.O. Epizootiology and Molecular Identification of Trypanosome Species in Livestock Ruminants in The Gambia. Acta Parasitol. 2022, 67, 130–142. [Google Scholar] [CrossRef]
- Hutchinson, M. The Epidemiology of Human Trypanosomiasis in British West Africa: II—The Gambia. Ann. Trop. Med. Parasitol. 1953, 47, 169–182. [Google Scholar] [CrossRef]
- Tchamdja, E.; Kulo, A.; Vitouley, H.; Batawui, K.; Bankolé, A.; Adomefa, K.; Cecchi, G.; Hoppenheit, A.; Clausen, P.; De Deken, R. Cattle Breeding, Trypanosomosis Prevalence and Drug Resistance in Northern Togo. Vet. Parasitol. 2017, 236, 86–92. [Google Scholar] [CrossRef]
- Talaki, E.; Dao, B.; Dayo, G.; Alfa, E.; N’Feide, T. Trypanosomoses Animales Dans La Plaine de Mô Au Togo. Int. J. Biol. Chem. Sci. 2014, 8, 2462–2469. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Hamida, T.B.; Dalgatova, Z.; Mahjoub, T.; Rejeb, A.; Dridi, W.; Gharbi, M. First Report of Surra (Trypanosoma Evansi Infection) in a Tunisian Dog. Parasite 2015, 22, 3. [Google Scholar] [CrossRef]
- Selmi, R.; Dhibi, M.; Ben Said, M.; Ben Yahia, H.; Abdelaali, H.; Ameur, H.; Baccouche, S.; Gritli, A.; Mhadhbi, M. Evidence of Natural Infections with Trypanosoma, Anaplasma and Babesia Spp. in Military Livestock from Tunisia. Trop. Biomed. 2019, 36, 742–757. [Google Scholar]
- Muhanguzi, D.; Mugenyi, A.; Bigirwa, G.; Kamusiime, M.; Kitibwa, A.; Akurut, G.G.; Ochwo, S.; Amanyire, W.; Okech, S.G.; Hattendorf, J. African Animal Trypanosomiasis as a Constraint to Livestock Health and Production in Karamoja Region: A Detailed Qualitative and Quantitative Assessment. BMC Vet. Res. 2017, 13, 1–13. [Google Scholar] [CrossRef]
- Katakura, K.; Lubinga, C.; Chitambo, H.; Tada, Y. Detection of Trypanosoma Congolense and T. Brucei Subspecies in Cattle in Zambia by Polymerase Chain Reaction from Blood Collected on a Filter Paper. Parasitol. Res. 1997, 83, 241–245. [Google Scholar] [CrossRef]
- Steverding, D. The History of Leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef]
- van Griensven, J.; Diro, E. Visceral Leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 309–322. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous Leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- David, C.V.; Craft, N. Cutaneous and Mucocutaneous Leishmaniasis. Dermatol. Ther. 2009, 22, 491–502. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; Musa, A.M.; Khalil, E.A.G.; el-Hassan, I.M.; el-Hassan, A.M. Post-Kala-Azar Dermal Leishmaniasis. Lancet Infect. Dis. 2003, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.; Hira, P.; Bhagwandeen, S.; Egere, J.; Versey, A. Kala-Azar in Zambia: First Report of Two Cases. Trans. R. Soc. Trop. Med. Hyg. 1976, 70, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Squarre, D.; Chambaro, H.M.; Hayashida, K.; Moonga, L.C.; Qiu, Y.; Goto, Y.; Oparaocha, E.; Mumba, C.; Muleya, W.; Bwalya, P. Autochthonous Leishmania Infantum in Dogs, Zambia, 2021. Emerg. Infect. Dis. 2022, 28, 888. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.; Boughoufalah, A.; Saegerman, C.; De Deken, R.; Bouchene, Z.; Soukehal, A.; Berkvens, D.; Boelaert, M. Epidemiology of Visceral Leishmaniasis in Algeria: An Update. PLoS ONE 2014, 9, e99207. [Google Scholar] [CrossRef]
- Status of Endemicity of Visceral Leishmaniasis. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/status-of-endemicity-of-visceral-leishmaniasis (accessed on 2 February 2025).
- Pratlong, F.; Debord, T.; Garnotel, E.; Garrabe, E.; Marty, P.; Raphenon, G.; Dedet, J. First Identification of the Causative Agent of Visceral Leishmaniasis in Djibouti: Leishmania Donovani. Ann. Trop. Med. Parasitol. 2005, 99, 21–25. [Google Scholar] [CrossRef]
- Jimenez, M.; Puente, S.; Gutierrez-Solar, B.; Martinez, P.; Alvar, J. Visceral Leishmaniasis in Angola Due to Leishmania (Leishmania) Infantum. Am. J. Trop. Med. Hyg. 1994, 50, 687–692. [Google Scholar] [CrossRef]
- Andre, L.; Sirol, J.; Le Vourch, C.; Labegorre, J.; Cochevelou, D. Sudanese Kala-Azar in West Africa (Author’s Transl). Med. Trop. Rev. Corps Sante Colon. 1978, 38, 435–442. [Google Scholar]
- Cagnard, V.; Lindrec, A. A Case of Visceral Leishmaniasis in Bangui, Central African Republic. Med. Trop. Rev. Corps Sante Colon. 1969, 29, 531–535. [Google Scholar]
- Eholié, S.; Tanon, A.; Folquet-Amorissani, M.; Doukouré, B.; Adoubryn, K.; Yattara, A.; Bissagnéné, E. Three New Cases of Visceral Leishmaniasis in Côte d’Ivoire. Bull. Soc. Pathol. Exot. 1990 2008, 101, 60–61. [Google Scholar]
- Ketema, H.; Weldegebreal, F.; Gemechu, A.; Gobena, T. Seroprevalence of Visceral Leishmaniasis and Its Associated Factors among Asymptomatic Pastoral Community of Dire District, Borena Zone, Oromia Region, Ethiopia. Front. Public Health 2022, 10, 917536. [Google Scholar] [CrossRef]
- Tournier, E. Note Sur Un Cas de Kala-Azar Infantile Observé Au Gabon. Bull. Soc. Pathol. Exot. 1920, 13, 175–176. [Google Scholar]
- Marlet, M.; Sang, D.; Ritmeijer, K.; Muga, R.; Onsongo, J.; Davidson, R. Emergence or Re-Emergence of Visceral Leishmaniasis in Areas of Somalia, Northeastern Kenya, and South-Eastern Ethiopia in 2000–2001. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Ruiz-Postigo, J.A.; Pita, J.; Lado, M.; Ben-Ismail, R.; Argaw, D.; Alvar, J. Visceral Leishmaniasis Outbreak in South Sudan 2009–2012: Epidemiological Assessment and Impact of a Multisectoral Response. PLoS Negl. Trop. Dis. 2014, 8, e2720. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Osman, H.A.; Muneer, M.S.; Samy, A.M.; Ahmed, A.; Mohammed, A.O.; Siddig, E.E.; Abdel Hamid, M.M.; Ali, M.S.; Omer, R.A.; et al. Identifying Asymptomatic Leishmania Infections in Non-Endemic Villages in Gedaref State, Sudan. BMC Res. Notes 2019, 12, 566. [Google Scholar] [CrossRef]
- Conteh, S.; Desjeux, P. Leishmaniasis in The Gambia. I. A Case of Cutaneous Leishmaniasis and a Case of Visceral Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 298–302. [Google Scholar] [CrossRef]
- de Campos, E.P.; Amedomé, A.A. Kala-Azar in Togo-West African. Presentation of a Clinic Case. Rev. Inst. Med. Trop. Sao Paulo 1979, 21, 29–32. [Google Scholar]
- Sentongo, E.; Ddumba, E.; Amandua, J.; Owor, R. Cutaneous Leishmaniasis in Uganda: Report of the First Case at Mulago National Referral and Teaching Hospital. 2012. Available online: http://makir.mak.ac.ug/handle/10570/928 (accessed on 2 February 2025).
- Mihoubi, I.; Picot, S.; Hafirassou, N.; de Monbrison, F. Cutaneous Leishmaniasis Caused by Leishmania Tropica in Algeria. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1157–1159. [Google Scholar] [CrossRef]
- Rapp, C.; Imbert, P.; Darie, H.; Simon, F.; Gros, P.; Debord, T.; Roue, R. Liposomal Amphotericin B Treatment of Cutaneous Leishmaniasis Contracted in Djibouti and Resistant to Meglumine Antimoniate. Bull. Soc. Pathol. Exot. 2003, 96, 209–211. [Google Scholar]
- Cortes, S.; Pereira, A.; Vasconcelos, J.; Paixão, J.P.; Quivinja, J.; Afonso, J.D.M.; Cristóvão, J.M.; Campino, L. PO 8505 Leishmaniasis in Angola–an Emerging Disease? BMJ Glob. Health 2019, 4. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Fraga, J.; Blanco, O.; González, D.; Monzote, L.; Soong, L.; Capó, V. Imported Leishmaniasis Cases in Cuba (2006–2016): What Have We Learned. Trop. Dis. Travel Med. Vaccines 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Zida, A.; Sawadogo, P.; Guiguemdé, K.; Soulama, I.; Chanolle, T.; Traoré, S.; Sangaré, I.; Bamba, S. Cutaneous Leishmaniasis in Burkina Faso: Epidemiological Evolution of a Vector-Borne Disease Locally Called “Ouaga 2000 Disease”: A Minireview. Niger. J. Parasitol. 2023, 44, 253. [Google Scholar] [CrossRef]
- Ngouateu, O.B.; Kollo, P.; Ravel, C.; Dereure, J.; Kamtchouing, P.; Same-Ekobo, A.; von Stebut, E.; Maurer, M.; Dondji, B. Clinical Features and Epidemiology of Cutaneous Leishmaniasis and Leishmania Major/HIV Co-Infection in Cameroon: Results of a Large Cross-Sectional Study. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kassa-Kelembho, E.; Kobangue, L.; Huerre, M.; Morvan, J. First Cases of Imported Cutaneous Leishmaniasis in Bangui Central African Republic: Efficacy of Metronidazole. Med. Trop. Rev. Corps Sante Colon. 2003, 63, 597–600. [Google Scholar]
- Morissi-Denissio, N.M.C.I.; Falmata, L.G.; Peggy, M.G.; Dieu, D.K.; Benedicte, Y.M.; Ornelle, K.I.; Kongbele, D.; Zengouin, E.; Kobangue, L. Epidemiological, Clinical and Treatment Profile of Leishmaniasisin Birao, Central African Republic. Clin. Dermatol. Open Access J. 2025, 10, 1–3. [Google Scholar] [CrossRef]
- Demba Kodindo, I.; Baïndaou, G.; Tchonfinet, M.; Ngamada, F.; Ndjékoundadé, A.; Moussa Djibrine, M.; Mahmout Nahor, N.; Kérah Hinzoumbé, C.; Saada, D.; Seydou, D. Retrospective Study of Cutaneous Leishmaniasis in the District Hospital of Am Timan, Chad. Bull. Société Pathol. Exot. 2015, 108, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Sekangue Obili, G.; Bidounga Lembe, D.P.; Boumba Anicet Atandi, A.; Pouki, F.; Nganga, F.; Ossibi Ibara, B.R. Molecular Diagnosis of the First Cases of leishmania Tropica-Cutaneous—Leishmaniasis in Elementary School Pupils in the Tchiamba-Nzassi health district in Pointe Noire, Republic of Congo. Afr. J. Parasitol. 2024, 9, 001–005. [Google Scholar]
- Diabaté, A.; Fukaura, R.; Terashima-Murase, C.; Vagamon, B.; Yotsu, R.R. Case Report: Cutaneous Leishmaniasis-A Hidden Disease in Côte d’Ivoire. Am. J. Trop. Med. Hyg. 2024, 111, 950–952. [Google Scholar] [CrossRef]
- Mpia Elenge, D. From Burden of the Disease to the Access to Care for Treatment: Case of Leishmaniasis in the Democratic Republic of Congo. ResearchGate 2015. [Google Scholar] [CrossRef]
- Samy, A.M.; Doha, S.A.; Kenawy, M.A. Ecology of Cutaneous Leishmaniasis in Sinai: Linking Parasites, Vectors and Hosts. Mem. Inst. Oswaldo Cruz 2014, 109, 299–306. [Google Scholar] [CrossRef]
- Zanger, P.; Kötter, I.; Raible, A.; Gelanew, T.; Schönian, G.; Kremsner, P.G. Case Report: Successful Treatment of Cutaneous Leishmaniasis Caused by Leishmania Aethiopica with Liposomal Amphothericin B in an Immunocompromised Traveler Returning from Eritrea. Am. J. Trop. Med. Hyg. 2011, 84, 692. [Google Scholar] [CrossRef]
- van Henten, S.; Adriaensen, W.; Fikre, H.; Akuffo, H.; Diro, E.; Hailu, A.; Van der Auwera, G.; van Griensven, J. Cutaneous Leishmaniasis Due to Leishmania Aethiopica. EClinicalMedicine 2018, 6, 69–81. [Google Scholar] [CrossRef]
- Akuffo, R.; Sanchez, C.; Chicharro, C.; Carrillo, E.; Attram, N.; Mosore, M.-T.; Yeboah, C.; Kotey, N.K.; Boakye, D.; Ruiz-Postigo, J.-A. Detection of Cutaneous Leishmaniasis in Three Communities of Oti Region, Ghana. PLoS Negl. Trop. Dis. 2021, 15, e0009416. [Google Scholar] [CrossRef]
- Boakye, D.; Wilson, M.; Kweku, M. A Review of Leishmaniasis in West Africa. Ghana Med. J. 2005, 39, 94. [Google Scholar]
- Sabbatani, S.; Calzado, A.I.; Feero, A.; Goudlaby, A.L.; Borghl, V.; Zanchetta, C.; Varnler, O. Atypical Leishmaniasis in an HIV-2-Seropositive Patient from Guinea-Bissau. Aids 1991, 5, 889–900. [Google Scholar] [CrossRef]
- Ngere, I.; Gufu Boru, W.; Isack, A.; Muiruri, J.; Obonyo, M.; Matendechero, S.; Gura, Z. Burden and Risk Factors of Cutaneous Leishmaniasis in a Peri-Urban Settlement in Kenya, 2016. PLoS ONE 2020, 15, e0227697. [Google Scholar] [CrossRef]
- Amro, A.; Gashout, A.; Al-Dwibe, H.; Zahangir Alam, M.; Annajar, B.; Hamarsheh, O.; Shubar, H.; Schönian, G. First Molecular Epidemiological Study of Cutaneous Leishmaniasis in Libya. PLoS Negl. Trop. Dis. 2012, 6, e1700. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, P.; Ponnighaus, J.; Chavula, D.; Lucas, S. Two Cases of Cutaneous Leishmaniasis in Malawi. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Doumbia, S.; Keita, S.; Sethi, A. Cutaneous Leishmaniasis in Mali. Dermatol. Clin. 2011, 29, 75–78. [Google Scholar] [CrossRef]
- Status of Endemicity of Cutaneous Leishmaniasis. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/status-of-endemicity-of-cutaneous-leishmaniasis (accessed on 2 February 2025).
- Kahime, K.; Boussaa, S.; Laamrani-El Idrissi, A.; Nhammi, H.; Boumezzough, A. Epidemiological Study on Acute Cutaneous Leishmaniasis in Morocco. J. Acute Dis. 2016, 5, 41–45. [Google Scholar] [CrossRef]
- Madede, B.; Maphosa, T.; Greyling, K.; Engelbrecht, J.; Kairinos, N. An Unexpected Encounter: Cutaneous Leishmaniasis in Wound Care. Wound Health S. Afr. 2024, 17, 48–50. [Google Scholar] [CrossRef]
- Develoux, M.; Blanc, L.; Garba, S.; Mamoudou, H.D.; Warter, A.; Ravisse, P. Cutaneous Leishmaniasis in Niger. Am. J. Trop. Med. Hyg. 1990, 43, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Bukar, A.; Denue, B.A.; Gadzama, G.B.; Ngadda, H.A. Cutaneous Leishmaniasis: Literature Review and Report of Two Cases from Communities Devastated by Insurgency in North-East Nigeria. Glob. J. Med. Public Health 2015, 4, 1–8. [Google Scholar]
- Diadie, S.; Diatta, B.; Ndiaye, M.; Seck, N.; Diallo, S.; Niang, S.; Dieng, M. Cutaneous Leishmaniasis in Senegal: A Series of 38 Cases at the Aristide Le Dantec University Hospital in Dakar. Med. Sante Trop. 2018, 28, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Gordon, W.; Emms, M. Cutaneous Leishmaniasis in Southern Africa. S. Afr. Med. J. 1979, 56, 1113. [Google Scholar]
- Grove, S. Cutaneous Leishmaniasis in South West Africa. S. Afr. Med. J. 1970, 44, 206–207. [Google Scholar]
- Elamin, E.; Guizani, I.; Guerbouj, S.; Gramiccia, M.; El Hassan, A.; Di Muccio, T.; Taha, M.; Mukhtar, M. Identification of Leishmania Donovani as a Cause of Cutaneous Leishmaniasis in Sudan. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 54–57. [Google Scholar] [CrossRef]
- Ashford, R.W. Cutaneous Leishmaniasis: Strategies for Prevention. Clin. Dermatol. 1999, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bousslimi, N.; Aoun, K.; Ben-Abda, I.; Ben-Alaya-Bouafif, N.; Raouane, M.; Bouratbine, A. Epidemiologic and Clinical Features of Cutaneous Leishmaniasis in Southeastern Tunisia. Am. J. Trop. Med. Hyg. 2010, 83, 1034. [Google Scholar] [CrossRef]
- Padovese, V.; Terranova, M.; Toma, L.; Barnabas, G.A.; Morrone, A. Cutaneous and Mucocutaneous Leishmaniasis in Tigray, Northern Ethiopia: Clinical Aspects and Therapeutic Concerns. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 707–711. [Google Scholar] [CrossRef]
- El-Hassan, A.M.; Meredith, S.; Yagi, H.; Khalil, E.A.G.; Ghalib, H.; Abbas, K.; Zijlstra, E.; Kroon, C.; Schoone, G.; Ismail, A. Sudanese Mucosal Leishmaniasis: Epidemiology, Clinical Features, Diagnosis, Immune Responses and Treatment. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 647–652. [Google Scholar] [CrossRef]
- Mathison, B.A.; Bradley, B.T. Review of the Clinical Presentation, Pathology, Diagnosis, and Treatment of Leishmaniasis. Lab. Med. 2023, 54, 363–371. [Google Scholar] [CrossRef]
- Tegegne, B.; Alemu, G. Progress of Mucocutaneous Leishmaniasis to Drug Nonresponsive Diffuse Cutaneous Leishmaniasis in Ethiopia. A Case Report. Int. Med. Case Rep. J. 2020, 13, 551–555. [Google Scholar] [CrossRef]
- Scheufele, C.J.; Giesey, R.L.; Delost, G.R. The Global, Regional, and National Burden of Leishmaniasis: An Ecologic Analysis from the Global Burden of Disease Study 1990–2017. J. Am. Acad. Dermatol. 2021, 84, 1203–1205. [Google Scholar] [CrossRef]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. Adv. Parasitol. 2004, 57, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Bwangamoi, O.; Busayi, R.; Courtney, S. Cutaneous Leishmaniasis in a Calf in Zimbabwe. Zimb. Vet. J. 1995, 26, 144. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19970804550 (accessed on 2 February 2025).
- Dubey, J.; Bwangamoi, O.; Courtney, S.; Fritz, D. Leishmania-like Protozoan Associated with Dermatitis in Cattle. J. Parasitol. 1998, 84, 865–867. [Google Scholar] [CrossRef]
- Touhami, N.A.K.; Ouchene, N.; Ouchetati, I.; Naghib, I. Animal Leishmaniasis in Algeria: A Systematic Review and Meta-Analysis. Comp. Immunol. Microbiol. Infect. Dis. 2023, 93, 101930. [Google Scholar]
- Vilhena, H.; Granada, S.; Oliveira, A.C.; Schallig, H.D.; Nachum-Biala, Y.; Cardoso, L.; Baneth, G. Serological and Molecular Survey of Leishmania Infection in Dogs from Luanda, Angola. Parasit. Vectors 2014, 7, 1–4. [Google Scholar] [CrossRef]
- Sangare, I.; Dabiré, R.; Guiguemdé, R.; Fournet, F.; Price, H.; Djibougou, A.; Yaméogo, B.; Drabo, F.; Diabaté, A.; Banuls, A. First Detection of Leishmania Infantum in Domestic Dogs from Burkina Faso (West Africa). Res. J. Parasitol. 2016, 12, 27–32. [Google Scholar] [CrossRef]
- Hamad, I.; Forestier, C.-L.; Peeters, M.; Delaporte, E.; Raoult, D.; Bittar, F. Wild Gorillas as a Potential Reservoir of Leishmania Major. J. Infect. Dis. 2015, 211, 267–273. [Google Scholar] [CrossRef]
- Kirk, R. African Leishmaniasis. Cent. Afr. J. Med. 1956, 2, 199–203. [Google Scholar] [PubMed]
- Medkour, H.; Laidoudi, Y.; Athias, E.; Bouam, A.; Dizoé, S.; Davoust, B.; Mediannikov, O. Molecular and Serological Detection of Animal and Human Vector-Borne Pathogens in the Blood of Dogs from Côte d’Ivoire. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101412. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Shoulah, S.; Abdelhady, A.; Alouffi, A.; Alraey, Y.; Al-Salem, W.S. Seroprevalence and Risk Factors Associated with Canine Leishmaniasis in Egypt. Vet. Sci. 2021, 8, 236. [Google Scholar] [CrossRef]
- Gebremedhin, E.Z.; Sarba, E.J.; Tola, G.K.; Endalew, S.S.; Marami, L.M.; Melkamsew, A.T.; Presti, V.D.M.L.; Vitale, M. Prevalence and Risk Factors of Toxoplasma Gondii and Leishmania spp. Infections in Apparently Healthy Dogs in West Shewa Zone, Oromia, Ethiopia. BMC Vet. Res. 2021, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saf’ianova, V.; Goncharov, D.; Emel’ianova, L. The Serological Examination of the Population for Leishmaniasis and the Detection of Leishmania in Rodents in the Republic of Guinea. Med. Parazitol. 1992, 42–46. Available online: https://pubmed.ncbi.nlm.nih.gov/1435540/ (accessed on 2 February 2025).
- Williams, A.O.; Mutinga, J.M.; Rodgers, M.R. Leishmaniasis in a Domestic Goat in Kenya. Mol. Cell. Probes 1991, 5, 319–325. [Google Scholar] [CrossRef]
- Postigo, J.A.R. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int. J. Antimicrob. Agents 2010, 36 (Suppl. 1), S62–S65. [Google Scholar] [CrossRef]
- Buck, G.; Courdurier, J.; Dorel, R.; Quesnel, J. First Case of Canine Leishmaniasis in Madagascar. Bull. Soc. Pathol. Exot. Fil. 1951, 44, 428–430. [Google Scholar]
- Johansson, S. General Health Conditions in the Dog Population of Lilongwe. Examensarbete 2015, 34. Available online: http://stud.epsilon.slu.se (accessed on 2 February 2025).
- Echchakery, M.; Chicharro, C.; Boussaa, S.; Nieto, J.; Carrillo, E.; Sheila, O.; Moreno, J.; Boumezzough, A. Molecular Detection of Leishmania Infantum and Leishmania Tropica in Rodent Species from Endemic Cutaneous Leishmaniasis Areas in Morocco. Parasit. Vectors 2017, 10, 1–8. [Google Scholar] [CrossRef]
- El-Mouhdi, K.; Boussaa, S.; Chahlaoui, A.; Fekhaoui, M. Prevalence and Risk Factors of Canine Leishmaniasis in Morocco: A Systematic Review and Meta-Analysis. J. Parasit. Dis. 2022, 46, 967–987. [Google Scholar] [CrossRef]
- World Health Organization; UNEP United Nations Environment Programme; World Organisation for Animal Health. One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment; World Health Organization: Geneva, Switzerland, 2022; ISBN 92-4-005913-X. [Google Scholar]
- Adediran, O.A.; Kolapo, T.U.; Uwalaka, E.C. Seroprevalence of Canine Leishmaniasis in Kwara, Oyo and Ogun States of Nigeria. J. Parasit. Dis. 2016, 40, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Faye, B.; Banuls, A.-L.; Bucheton, B.; Dione, M.; Bassanganam, O.; Hide, M.; Dereure, J.; Choisy, M.; Ndiaye, J.; Konate, O. Canine Visceral Leishmaniasis Caused by Leishmania Infantum in Senegal: Risk of Emergence in Humans? Microbes Infect. 2010, 12, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Van der Lugt, J.; Carlyon, J.F.; De Waal, D.T. Cutaneous Leishmaniasis in a Sheep. J. S. Afr. Vet. Assoc. 1992, 63, 74–77. [Google Scholar]
- Sixl, W.; Sebek, Z.; Reinthaler, F.; Mascher, F. Investigations of Wild Animals as Leishmania Reservoir in South Sudan. J. Hyg. Epidemiol. Microbiol. Immunol. 1987, 31, 483–485. [Google Scholar] [PubMed]
- Dereure, J.; El-Safi, S.H.; Bucheton, B.; Boni, M.; Kheir, M.M.; Davoust, B.; Pratlong, F.; Feugier, E.; Lambert, M.; Dessein, A. Visceral Leishmaniasis in Eastern Sudan: Parasite Identification in Humans and Dogs; Host-Parasite Relationships. Microbes Infect. 2003, 5, 1103–1108. [Google Scholar] [CrossRef]
- Desjeux, P.; Bryan, J.H.; Martin-Saxton, P. Leishmaniasis in The Gambia. 2. A Study of Possible Vectors and Animal Reservoirs, with the First Report of a Case of Canine Leishmaniasis in The Gambia. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 143–148. [Google Scholar] [CrossRef]
- Chargui, N.; Haouas, N.; Gorcii, M.; Messaidi, F.A.; Zribi, M.; Babba, H. Increase of Canine Leishmaniasis in a Previously Low-Endemicity Area in Tunisia. Parasite 2007, 14, 247–251. [Google Scholar] [CrossRef]
- Richardson, U. A Probable Case of Equine Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1926, 19, 411. [Google Scholar] [CrossRef]
- Dixit, B.; Kumar, R.; Dixit, A.K.; Singh, A.K. Risk Factors Associated with Parasitic Diseases in Dogs and Cats. In Principles and Practices of Canine and Feline Clinical Parasitic Diseases; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 19–30. [Google Scholar]
- Si, W.; Fang, C.; Liu, C.; Yin, M.; Xu, W.; Li, Y.; Yan, X.; Shen, Y.; Cao, J.; Sun, J. Why Is Babesia Not Killed by Artemisinin like Plasmodium? Parasit. Vectors 2023, 16, 193. [Google Scholar] [CrossRef]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia Divergens, a Bovine Blood Parasite of Veterinary and Zoonotic Importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.D.; Sajid, M.S.; Pascoe, E.L.; Foley, J.E. Detection of Babesia Odocoilei in Humans with Babesiosis Symptoms. Diagnostics 2021, 11, 947. [Google Scholar] [CrossRef]
- Singh, P.; Lonardi, S.; Liang, Q.; Vydyam, P.; Khabirova, E.; Fang, T.; Gihaz, S.; Thekkiniath, J.; Munshi, M.; Abel, S. Babesia Duncani Multi-Omics Identifies Virulence Factors and Drug Targets. Nat. Microbiol. 2023, 8, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Vydyam, P.; Fang, T.; Estrada, K.; Gonzalez, L.M.; Grande, R.; Kumar, M.; Chakravarty, S.; Berry, V.; Ranwez, V. Insights into the Evolution, Virulence and Speciation of Babesia MO1 and Babesia Divergens through Multiomics Analyses. Emerg. Microbes Infect. 2024, 13, 2386136. [Google Scholar] [CrossRef]
- Brown, W.C.; Norimine, J.; Knowles, D.P.; Goff, W.L. Immune Control of Babesia Bovis Infection. Vet. Parasitol. 2006, 138, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Buening, G.; Green, T.; Carson, C. In Vitro Cultivation of Babesia Bigemina. Am. J. Vet. Res. 1985, 46, 416–420. [Google Scholar] [CrossRef]
- Camacho, A.; Guitian, F.; Pallas, E.; Gestal, J.; Olmeda, A.; Habela, M.; Telford Iii, S.; Spielman, A. Theileria (Babesia) Equi and Babesia Caballi Infections in Horses in Galicia, Spain. Trop. Anim. Health Prod. 2005, 37, 293–302. [Google Scholar] [CrossRef]
- Farwell, G.; LeGrand, E.; Cobb, C. Clinical Observations on Babesia Gibsoni and Babesia Canis Infections in Dogs. J. Am. Vet. Med. Assoc. 1982, 180, 507–511. [Google Scholar] [CrossRef]
- Hong, S.-H.; Kim, S.-Y.; Song, B.G.; Roh, J.Y.; Cho, C.R.; Kim, C.-N.; Um, T.-H.; Kwak, Y.G.; Cho, S.-H.; Lee, S.-E. Detection and Characterization of an Emerging Type of Babesia Sp. Similar to Babesia Motasi for the First Case of Human Babesiosis and Ticks in Korea. Emerg. Microbes Infect. 2019, 8, 869–878. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Work, T.M.; Rameyer, R.A. Molecular Phylogeny of Babesia Poelea from Brown Boobies (Sula Leucogaster) from Johnston Atoll, Central Pacific. J. Parasitol. 2006, 92, 423–425. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Vanstreels, R.E.; Shock, B.C.; Purdee, M.; Horne, E.C.; Peirce, M.A.; Parsons, N.J. Molecular Characterization of Babesia Peircei and Babesia Ugwidiensis Provides Insight into the Evolution and Host Specificity of Avian Piroplasmids. Int. J. Parasitol. Parasites Wildl. 2017, 6, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, M.J.; Greiner, E.; Tseng, F.S.; Garner, M.M.; Nordhausen, R.W.; Ziccardi, M.H.; Borjesson, D.L.; Zabolotzky, S. Description of Novel Babesia Species and Associated Lesions from Common Murres (Uria Aalge) from California. J. Parasitol. 2009, 95, 1183–1188. [Google Scholar] [CrossRef]
- Friedhoff, K.T. Transmission of Babesia. In Babesiosis of Domestic Animals and Man; CRC Press: Boca Raton, FL, USA, 2018; pp. 23–52. [Google Scholar]
- Knapp, K.L.; Rice, N.A. Human Coinfection with Borrelia Burgdorferi and Babesia Microti in the United States. J. Parasitol. Res. 2015, 2015, 587131. [Google Scholar] [CrossRef] [PubMed]
- Vyas, J.M.; Telford, S.R.; Robbins, G.K. Treatment of Refractory Babesia Microti Infection with Atovaquone-Proguanil in an HIV-Infected Patient: Case Report. Clin. Infect. Dis. 2007, 45, 1588–1590. [Google Scholar] [CrossRef]
- Krause, P.J. Babesiosis Diagnosis and Treatment. Vector-Borne Zoonotic Dis. 2003, 3, 45–51. [Google Scholar] [CrossRef]
- Smith, R.P.; Hunfeld, K.-P.; Krause, P.J. Management Strategies for Human Babesiosis. Expert Rev. Anti Infect. Ther. 2020, 18, 625–636. [Google Scholar] [CrossRef]
- Wray, K.; Musuka, G.; Trees, A.; Jongejan, F.; Smeenk, I.; Kelly, P. Babesia Bovis and B. Bigemina DNA Detected in Cattle and Ticks from Zimbabwe by Polymerase Chain Reaction. J. S. Afr. Vet. Assoc. 2000, 71, 21–24. [Google Scholar]
- Kiouani, A.; Azzag, N.; Tennah, S.; Ghalmi, F. Infection with Babesia Canis in Dogs in the Algiers Region: Parasitological and Serological Study. Vet. World 2020, 13, 1351. [Google Scholar] [CrossRef]
- Palomar, A.M.; Molina, I.; Bocanegra, C.; Portillo, A.; Salvador, F.; Moreno, M.; Oteo, J.A. Old Zoonotic Agents and Novel Variants of Tick-Borne Microorganisms from Benguela (Angola), July 2017. Parasit. Vectors 2022, 15, 140. [Google Scholar] [CrossRef]
- Nyabongo, L.; Kanduma, E.G.; Bishop, R.P.; Machuka, E.; Njeri, A.; Bimenyimana, A.V.; Nkundwanayo, C.; Odongo, D.O.; Pelle, R. Prevalence of Tick-Transmitted Pathogens in Cattle Reveals That Theileria Parva, Babesia Bigemina and Anaplasma Marginale Are Endemic in Burundi. Parasit. Vectors 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Adehan, S.B.; Biguezoton, A.; Dossoumou, A.; Assogba, M.N.; Adehan, R.; Adakal, H.; Mensah, G.A.; Madder, M. Blood Survey of Babesia Spp and Theileria Spp in Monos Cattle, Benin. Afr. J. Agric. Res. 2016, 11, 1266–1272. [Google Scholar]
- McDermid, K.R.; Snyman, A.; Verreynne, F.J.; Carroll, J.P.; Penzhorn, B.L.; Yabsley, M.J. Surveillance for Viral and Parasitic Pathogens in a Vulnerable African Lion (Panthera Leo) Population in the Northern Tuli Game Reserve, Botswana. J. Wildl. Dis. 2017, 53, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ringo, A.E.; Moumouni, P.F.A.; Thekisoe, O.; Suzuki, H.; Xuan, X. Molecular Detection of Selected Tick-Borne Hemo-Parasites in Small Ruminants from Seno and Oudalan Provinces, Burkina Faso. J. Protozool. Res. 2023, 33, 1–17. [Google Scholar]
- Checa, R.; Peteiro, L.; Pérez-Hernando, B.; de la Morena, M.; Cano, L.; López-Suárez, P.; Barrera, J.P.; Estévez-Sánchez, E.; Sarquis, J.; Fernández-Cebrián, B. High Serological and Molecular Prevalence of Ehrlichia Canis and Other Vector-Borne Pathogens in Dogs from Boa Vista Island, Cape Verde. Parasit. Vectors 2024, 17, 374. [Google Scholar] [CrossRef]
- Mbitkebeyo, R.C.P.; Manchang, K.T.; Raï, C.; Tasse, G.C. Prevalence and Distribution of Tick-Borne Hemoparasites in Cattle from the Noun and Ndé Divisions of the West Region, Cameroon. Open J. Vet. Med. 2024, 14, 193–202. [Google Scholar] [CrossRef]
- Haynes, E.; Garrett, K.B.; Grunert, R.K.; Bryan, J.A.; Sidouin, M.; Oaukou, P.T.; Ngandolo, B.N.R.; Yabsley, M.J.; Cleveland, C.A. Surveillance of Tick-Borne Pathogens in Domestic Dogs from Chad, Africa. BMC Vet. Res. 2024, 20, 417. [Google Scholar] [CrossRef] [PubMed]
- Boucher, F.; Moutroifi, Y.; Peba, B.; Ali, M.; Moindjie, Y.; Ruget, A.-S.; Abdouroihamane, S.; Kassim, A.M.; Soulé, M.; Charafouddine, O. Tick-Borne Diseases in the Union of the Comoros Are a Hindrance to Livestock Development: Circulation and Associated Risk Factors. Ticks Tick-Borne Dis. 2020, 11, 101283. [Google Scholar] [CrossRef]
- Grace, R.; Kouassi, P.; Achi, Y.L.; Dosso, M.; Kgomotso, P. Detection and Distribution of Anaplasma Marginale, Babesia Bovis, and Theileria Annulata in Côte d’Ivoire. J. Parasitol. Vector Biol. 2023, 15, 1–11. [Google Scholar]
- Ilunga, A.K.; Inkale, C.B.; Kilara, T.; Woto, I.; Kabengele, G.K.; Bongenya, B.I.; Buassa, B.B.; Nyembue, D.T.; Kabengele, B.O.; Kamangu, E.N. Blood Safety in the Democratic Republic of the Congo: Literature Review. Open J. Blood Dis. 2023, 13, 102–120. [Google Scholar] [CrossRef]
- Menshawy, S.M. A Review on Bovine Babesiosis in Egypt. Egypt. Vet. Med. Soc. Parasitol. J. EVMSPJ 2020, 16, 8–19. [Google Scholar] [CrossRef][Green Version]
- Ramos, E.P. Misdiagnosis of Babesiosis as Malaria, Equatorial Guinea, 2014. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Conti, G. Bovine Anaplasmosis in Eritrea. Arch. Ital. Sci. Med. Colon. 1936, 17, 302–303. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19392201297 (accessed on 2 February 2025).
- Ledger, K.J.; Beati, L.; Wisely, S.M. Survey of Ticks and Tick-Borne Rickettsial and Protozoan Pathogens in Eswatini. Pathogens 2021, 10, 1043. [Google Scholar] [CrossRef]
- Fesseha, H.; Mathewos, M.; Eshetu, E.; Tefera, B. Babesiosis in Cattle and Ixodid Tick Distribution in Dasenech and Salamago Districts, Southern Ethiopia. Sci. Rep. 2022, 12, 6385. [Google Scholar] [CrossRef] [PubMed]
- Kenguele, M.; Meye, B.; Ndong, T.; Mickala, P. Prevalence of Haemoparasites among Blood Donors Attending the Regional Hospital Center of Franceville (Southern Gabon). J. Infect. Epidemiol. 2022, 8, 270. [Google Scholar]
- Makouloutou-Nzassi, P.; Nze-Nkogue, C.; Makanga, B.K.; Longo-Pendy, N.M.; Bourobou, J.A.B.; Nso, B.C.B.B.; Akomo-Okoue, E.F.; Mbazoghe-Engo, C.-C.; Bangueboussa, F.; Sevidzem, S.L. Occurrence of Multiple Infections of Rodents with Parasites and Bacteria in the Sibang Arboretum, Libreville, Gabon. Vet. World 2024, 17, 2506. [Google Scholar] [CrossRef] [PubMed]
- Nimako-Boateng, M.; Boakye, O.; Bediako, O.; Asare, D.; Emikpe, B. Prevalence of Haemoparasites and Effects on Blood Parameters of Horses in the Ashanti Region of Ghana. Niger. J. Parasitol. 2022, 43, 253–259. [Google Scholar] [CrossRef]
- Tomassone, L.; Pagani, P.; De Meneghi, D. Detection of Babesia Caballi in Amblyomma Variegatum Ticks (Acari: Ixodidae) Collected from Cattle in the Republic of Guinea. Parassitologia 2005, 47, 247. [Google Scholar]
- Githaka, N.W.; Bishop, R.P.; Šlapeta, J.; Emery, D.; Nguu, E.K.; Kanduma, E.G. Molecular Survey of Babesia Parasites in Kenya: First Detailed Report on Occurrence of Babesia Bovis in Cattle. Parasit. Vectors 2022, 15, 161. [Google Scholar] [CrossRef]
- Mahlobo, S.I.; Zishiri, O.T. A Descriptive Study of Parasites Detected in Ticks of Domestic Animals in Lesotho. Vet. Parasitol. Reg. Stud. Rep. 2021, 25, 100611. [Google Scholar] [CrossRef]
- EZELDIN, N. Incidence of Theileriosis, Babesiosis and Anaplasmosis in Cattle in Tripoli-Libya. Vet. Med. J. Giza 2008, 56, 71–82. [Google Scholar]
- Ranaivoson, H.C.; Héraud, J.-M.; Goethert, H.K.; Telford, S.R.; Rabetafika, L.; Brook, C.E. Babesial Infection in the Madagascan Flying Fox, Pteropus Rufus É. Geoffroy, 1803. Parasit. Vectors 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chatanga, E.; Maganga, E.; Mohamed, W.M.A.; Ogata, S.; Pandey, G.S.; Abdelbaset, A.E.; Hayashida, K.; Sugimoto, C.; Katakura, K.; Nonaka, N. High Infection Rate of Tick-Borne Protozoan and Rickettsial Pathogens of Cattle in Malawi and the Development of a Multiplex PCR for Babesia and Theileria Species Identification. Acta Trop. 2022, 231, 106413. [Google Scholar] [CrossRef] [PubMed]
- Diakite, M.; SACKO, B.; Sidibe, F.; Bengaly, S.; Sidibe, S. Study of the Prevalence of Bovine Babesiosis with Babesia Bovis and Babesia Bigemina Isolated in the Livestock of Bamako and Its Peri-Urban Area between 2018 and 2023. Int. J. Vet. Sci. Anim. Husb. 2024, 9, 805–807. [Google Scholar]
- Lee, G.K.C.; Ignace, J.A.E.; Robertson, I.D.; Irwin, P.J. Canine Vector-Borne Infections in Mauritius. Parasit. Vectors 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Rhalem, A.; Sahibi, H.; Lasri, S.; Johnson, W.C.; Kappmeyer, L.S.; Hamidouch, A.; Knowles, D.P.; Goff, W.L. Validation of a Competitive Enzyme-Linked Immunosorbent Assay for Diagnosing Babesia Equi Infections of Moroccan Origin and Its Use in Determining the Seroprevalence of B. Equi in Morocco. J. Vet. Diagn. Investig. 2001, 13, 249–251. [Google Scholar] [CrossRef]
- Martins, T.M.; Pedro, O.C.; Caldeira, R.A.; do Rosário, V.E.; Neves, L.; Domingos, A. Detection of Bovine Babesiosis in Mozambique by a Novel Seminested Hot-Start PCR Method. Vet. Parasitol. 2008, 153, 225–230. [Google Scholar] [CrossRef]
- Matheus, E.K.; Oosthuizen, J.; Mbajiorgu, C.A.; Oguttu, J.W. Prevalence of Babesiosis in Sanga Cattle in the Ohangwena Region of Namibia. Indian J. Anim. Res. 2019, 53, 110–114. [Google Scholar] [CrossRef]
- Ogo, N.; Lawal, A.; Okubanjo, O.; Kamani, J.; Ajayi, O. Current Status of Canine Babesiosis and the Situation in Nigeria: A Review. Niger. Vet. J. 2011, 32, 69–78. [Google Scholar]
- Bazarusanga, T. The Epidemiology of Theileriosis in Rwanda and Implications for Control. Ph.D Thesis, Ghent University, Gent, Belgium, 2008. [Google Scholar]
- Paling, R.; Mpangala, C.; Luttikhuizen, B.; Sibomana, G. Exposure of Ankole and Crossbred Cattle to Theileriosis in Rwanda. Trop. Anim. Health Prod. 1991, 23, 203–214. [Google Scholar] [CrossRef]
- Gueye, A.; Mbengue, M.; Diouf, A. Ticks and Hemoparasitic Diseases in Cattle in Senegal. IV. The Southern Sudan Area. Rev. Elev. Med. Vet. Pays Trop. 1990, 42, 517–528. [Google Scholar] [CrossRef]
- Peirce, M. Nuttallia França, 1909 (Babesiidae) Preoccupied by Nuttallia Dall, 1898 (Psammobiidae): A Re-Appraisal of the Taxonomic Position of the Avian Piroplasms. Int. J. Parasitol. 1975, 5, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Ibrahim, A.M.; Mohamed, R.H.; Aden, H.H. Preliminary Assessment of Goat Piroplasmosis in Benadir Region, Somalia. Open J. Vet. Med. 2013, 3, 273–276. [Google Scholar] [CrossRef]
- Oosthuizen, M.C.; Zweygarth, E.; Collins, N.E.; Troskie, M.; Penzhorn, B.L. Identification of a Novel Babesia Sp. from a Sable Antelope (Hippotragus Niger Harris, 1838). J. Clin. Microbiol. 2008, 46, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Earle, R.; Huchzermeyer, F.W.; Bennett, G.F.; Brassy, J.J. Babesia Peircei Sp. Nov. from the Jackass Penguin. Afr. Zool. 1993, 28, 88–90. [Google Scholar]
- Kivaria, F.M.; Kapaga, A.M.; Mbassa, G.K.; Mtui, P.F.; Wani, R.J. Epidemiological Perspectives of Ticks and Tick-Borne Diseases in South Sudan: Cross-Sectional Survey Results. Onderstepoort J. Vet. Res. 2012, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Antunes, S.; Galindo, R.C.; do Rosário, V.E.; De la Fuente, J.; Domingos, A.; El Hussein, A.M. Prevalence and Genetic Diversity of Babesia and Anaplasma Species in Cattle in Sudan. Vet. Parasitol. 2011, 181, 146–152. [Google Scholar] [CrossRef]
- Bloch, E.M.; Kasubi, M.; Levin, A.; Mrango, Z.; Weaver, J.; Munoz, B.; West, S.K. Babesia Microti and Malaria Infection in Africa: A Pilot Serosurvey in Kilosa District, Tanzania. Am. J. Trop. Med. Hyg. 2018, 99, 51. [Google Scholar] [CrossRef]
- Coultous, R.M.; McDonald, M.; Raftery, A.G.; Shiels, B.R.; Sutton, D.G.; Weir, W. Analysis of Theileria Equi Diversity in The Gambia Using a Novel Genotyping Method. Transbound. Emerg. Dis. 2020, 67, 1213–1221. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Gharbi, M.; Mhadhbi, M.; Mabrouk, W.; Ayari, B.; Nasfi, I.; Jedidi, M.; Sassi, L.; Rekik, M.; Darghouth, M.A. Prevalence of Piroplasms in Small Ruminants in North-West Tunisia and the First Genetic Characterisation of Babesia Ovis in Africa. Parasite 2014, 21, 23. [Google Scholar] [CrossRef]
- Muhanguzi, D.; Matovu, E.; Waiswa, C. Prevalence and Characterization of Theileria and Babesia Species in Cattle under Different Husbandry Systems in Western Uganda. Int. J. Anim. Vet. Adv. 2010, 2, 51–58. [Google Scholar]
- Nalubamba, K.S.; Hankanga, C.; Mudenda, N.B.; Masuku, M. The Epidemiology of Canine Babesia Infections in Zambia. Prev. Vet. Med. 2011, 99, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Yongabi, K.A.; Chia-Garba, M. Incidence of Babessia Infections Causing Pyrexia of Unknown Origin (PUO) amongst HIV/AIDS Patients in Cameroon. Am. J. Res. Commun. 2014, 2, 88–96. [Google Scholar]
- Michael, S.; Morsy, T.; Montasser, M. A Case of Human Babesiosis (Preliminary Case Report in Egypt). J. Egypt. Soc. Parasitol. 1987, 17, 409–410. [Google Scholar]
- Rodriguez, O.; Isabel, M.; Dias, R.; Rodriguez, P. Report on Infection with Babesia Bovis (Babes, 1888) in the Human Population of the Popular Republic of Mozambique. Rev. Cub. Cienc. Vet. 1984, 15, 41–50. [Google Scholar]
- Ahmad, M.M.; Mohammed, Y.; Jiya, N.M.; Jibrin, B.; Zainu, S.M.; Legbo, J.F.; Abubakar, F.; Jimoh, A.K. Fatal Human Babesiosis in a Nine-Year Old Nigerian Girl. Int. J. Trop. Dis. Health 2020, 41, 26–30. [Google Scholar] [CrossRef]
- Bush, J.; Isaäcson, M.; Mohamed, A.; Potgieter, F.; Waal, D. de Human Babesiosis-a Preliminary Report of 2 Suspected Cases in Southern Africa. S. Afr. Med. J. 1990, 78, 699. [Google Scholar]
- Owusu, I.A. Detection of Zoonotic Babesia Species in Greater Accra, Ghana. Doctoral Dissertation, University of Ghana, Accra, Ghana, 2015. [Google Scholar]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular Protozoan Parasites of Wild and Domestic Ruminants Transmitted by Ixodid Ticks. Parasitology 2004, 129, S271–S283. [Google Scholar] [CrossRef]
- Nene, V.; Kiara, H.; Lacasta, A.; Pelle, R.; Svitek, N.; Steinaa, L. The Biology of Theileria Parva and Control of East Coast Fever–Current Status and Future Trends. Ticks Tick-Borne Dis. 2016, 7, 549–564. [Google Scholar] [CrossRef]
- Gul, N.; Ayaz, S.; Gul, I.; Adnan, M.; Shams, S.; Akbar, N. Tropical Theileriosis and East Coast Fever in Cattle: Present, Past and Future Perspective. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 1000–1018. [Google Scholar]
- Surve, A.A.; Hwang, J.Y.; Manian, S.; Onono, J.O.; Yoder, J. Economics of East Coast Fever: A Literature Review. Front. Vet. Sci. 2023, 10, 1239110. [Google Scholar] [CrossRef] [PubMed]
- Gachohi, J.; Skilton, R.; Hansen, F.; Ngumi, P.; Kitala, P. Epidemiology of East Coast Fever (Theileria Parva Infection) in Kenya: Past, Present and the Future. Parasit. Vectors 2012, 5, 1–13. [Google Scholar] [CrossRef]
- Norval, R.A.I.; Perry, B.D.; Young, A. The Epidemiology of Theileriosis in Africa; Academic Press: London, UK, 1992; ISBN 0-12-521740-4. [Google Scholar]
- Zeroual, F.; Saidani, K.; Righi, S.; Simion, V.E.; Mellah, A.; Kourtel, S.; Benakhla, A. An Overview on Tropical Theileriosis in Algeria. Romanian J. Vet. Med. Pharmacol. 2022, 7, 90. [Google Scholar]
- Kubelová, M.; Mazancová, J.; Široký, P. Theileria, Babesia, and Anaplasma Detected by PCR in Ruminant Herds at Bié Province, Angola. Parasite 2012, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, A.S.; Zannou, O.M.; Biguezoton, A.S.; Kouassi, P.Y.; Belem, A.; Farougou, S.; Oosthuizen, M.; Saegerman, C.; Lempereur, L. Cattle Ticks and Associated Tick-Borne Pathogens in Burkina Faso and Benin: Apparent Northern Spread of Rhipicephalus Microplus in Benin and First Evidence of Theileria Velifera and Theileria Annulata. Ticks Tick-Borne Dis. 2021, 12, 101733. [Google Scholar] [CrossRef]
- Binta, M.; Losho, T.; Allsopp, B.; Mushi, E. Isolation of Theileria Taurotragi and Theileria Mutans from Cattle in Botswana. Vet. Parasitol. 1998, 77, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Atuhaire, D.K.; Muleya, W.; Mbao, V.; Niyongabo, J.; Nyabongo, L.; Nsanganiyumwami, D.; Salt, J.; Namangala, B.; Musoke, A.J. Molecular Characterization and Population Genetics of Theileria Parva in Burundi’s Unvaccinated Cattle: Towards the Introduction of East Coast Fever Vaccine. PLoS ONE 2021, 16, e0251500. [Google Scholar] [CrossRef]
- Silatsa, B.A.; Simo, G.; Githaka, N.; Kamga, R.; Oumarou, F.; Keambou Tiambo, C.; Machuka, E.; Domelevo, J.; Odongo, D.; Bishop, R. First Detection of Theileria Parva in Cattle from Cameroon in the Absence of the Main Tick Vector Rhipicephalus Appendiculatus. Transbound. Emerg. Dis. 2020, 67, 68–78. [Google Scholar] [CrossRef]
- Uilenberg, G. Existence of Haematoxenus Veliferus (Sporozoa, Theileriidae) in Central African Republic [and Chad]. Presence of Haematoxenus Sp. in African Buffalo. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1970, 23, 455–456. [Google Scholar] [CrossRef]
- De Deken, R.; Martin, V.; Saido, A.; Madder, M.; Brandt, J.; Geysen, D. An Outbreak of East Coast Fever on the Comoros: A Consequence of the Import of Immunised Cattle from Tanzania? Vet. Parasitol. 2007, 143, 245–253. [Google Scholar] [CrossRef]
- Amzati, G.S.; Djikeng, A.; Odongo, D.O.; Nimpaye, H.; Sibeko, K.P.; Muhigwa, J.-B.B.; Madder, M.; Kirschvink, N.; Marcotty, T. Genetic and Antigenic Variation of the Bovine Tick-Borne Pathogen Theileria Parva in the Great Lakes Region of Central Africa. Parasit. Vectors 2019, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Carpano, M. A Piroplasm of the Parvum Type (Genus Theileria) in a Gazelle in Eritrea. Clin. Vet. 1913, 36, 254–256. [Google Scholar]
- Gebrekidan, H.; Hailu, A.; Kassahun, A.; Rohoušová, I.; Maia, C.; Talmi-Frank, D.; Warburg, A.; Baneth, G. Theileria Infection in Domestic Ruminants in Northern Ethiopia. Vet. Parasitol. 2014, 200, 31–38. [Google Scholar] [CrossRef]
- Mangombi, J.B.; N’dilimabaka, N.; Lekana-Douki, J.-B.; Banga, O.; Maghendji-Nzondo, S.; Bourgarel, M.; Leroy, E.; Fenollar, F.; Mediannikov, O. First Investigation of Pathogenic Bacteria, Protozoa and Viruses in Rodents and Shrews in Context of Forest-Savannah-Urban Areas Interface in the City of Franceville (Gabon). PLoS ONE 2021, 16, e0248244. [Google Scholar] [CrossRef]
- Addo, S.O.; Bentil, R.E.; Baako, B.O.A.; Addae, C.A.; Behene, E.; Asoala, V.; Mate, S.; Oduro, D.; Dunford, J.C.; Larbi, J.A. First Record of Babesia and Theileria Parasites in Ticks from Kassena-Nankana, Ghana. Med. Vet. Entomol. 2023, 37, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Diallo, T.; Singla, L.; Sumbria, D.; Kaur, P.; Bal, M. Conventional and Molecular Diagnosis of Haemo-Protozoan Infections in Cattle and Equids from Republic of Guinea and India. Indian J. Anim. Res. 2018, 52, 1206–1211. [Google Scholar] [CrossRef]
- Rosa, F.; Crespo, M.; Dias, J. Some Ectoparasites and Protozoans in Bovines from the Republic of Guinea Bissau. Garcia Orta 1998, 22, 59–61. [Google Scholar]
- King’ori, E.; Obanda, V.; Chiyo, P.I.; Soriguer, R.C.; Morrondo, P.; Angelone, S. Molecular Identification of Ehrlichia, Anaplasma, Babesia and Theileria in African Elephants and Their Ticks. PLoS ONE 2019, 14, e0226083. [Google Scholar] [CrossRef]
- Uilenberg, G. Haematoxenus Veliferus, Hématozoaire Des Bovins à Madagascar: Note Complémentaire. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux 1965, 18, 429–433. Available online: http://revues.cirad.fr/index.php/REMVT/index (accessed on 2 February 2025). [CrossRef][Green Version]
- Wymann, M.N. Calf Mortality and Parasitism in Periurban Livestock Production in Mali. Doctoral Dissertation, University of Basel, Basel, Switszerland, 2005. [Google Scholar]
- d’Oliveira, C.; Van der Weide, M.; Jacquiet, P.; Jongejan, F. Detection of Theileria Annulata by the PCR in Ticks (Acari: Ixodidae) Collected from Cattle in Mauritania. Exp. Appl. Acarol. 1997, 21, 279–291. [Google Scholar] [CrossRef]
- Spitalska, E.; Riddell, M.; Heyne, H.; Sparagano, O.A. Prevalence of Theileriosis in Red Hartebeest (Alcelaphus Buselaphus Caama) in Namibia. Parasitol. Res. 2005, 97, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Dipeolu, O. Prevalence of Theileria Schizonts in the Domestic Ruminants in Nigeria and the Identification and Bionomics of the Vector. In Advances in the Control of Theileriosis; Springer: Dordrecht, The Netherlands, 1981; pp. 88–93. [Google Scholar]
- David, O.-F.S.; Goria, K.P.; Abraham, D.G.A. Haemoparasite Fauna of Domestic Animals in Plateau State, North Central Nigeria. Bayero J. Pure Appl. Sci. 2018, 11, 156–161. [Google Scholar] [CrossRef]
- Malbrant, R. Piroplasmoses in the French Congo. Bull. Société Pathol. Exot. 1938, 31, 599–603. [Google Scholar]
- Addah, L. Pathological Constraints to the Improvement of Dairy Production Potential in Tick-Infested Tropical Areas: The Case of Sao Tome and Principe. Bull. Anim. Health Prod. Afr. 1987, 35, 181–184. [Google Scholar]
- Gueye, A.; Mbengue, M.; Diouf, A. Présence de Theileria Velifera Au Sénégal. Rev. D’élevage Médecine Vét. Pays Trop. 1987, 40, 117–118. [Google Scholar] [CrossRef]
- Pabs-Garnon, L.; Foley, V. Caprine Theileriasis in Sierra Leone: First Recorded Cases. Vet. Rec. 1974, 94, 603. [Google Scholar] [CrossRef]
- Matjila, P.T.; Leisewitz, A.; Oosthuizen, M.; Jongejan, F.; Penzhorn, B. Detection of a Theileria Species in Dogs in South Africa. Vet. Parasitol. 2008, 157, 34–40. [Google Scholar] [CrossRef]
- Abaker, I.A.; Salih, D.A.; El Haj, L.M.; Ahmed, R.E.; Osman, M.M.; Ali, A.M. Prevalence of Theileria Annulata in Dairy Cattle in Nyala, South Darfur State, Sudan. Vet. World 2017, 10, 1475. [Google Scholar] [CrossRef]
- Ekpetsi Bouka, C.; Batawui, K.; Napala, A.; Bastiaensen, P.; Faye, N.; Hendrickx, G. Parasitoses Des Veaux Dans La Région Septentrionale Du Togo. Rev. D’élevage Médecine Vét. Pays Trop. 2001, 54, 17–27. [Google Scholar] [CrossRef]
- Kabi, F.; Masembe, C.; Muwanika, V.; Kirunda, H.; Negrini, R. Geographic Distribution of Non-Clinical Theileria Parva Infection among Indigenous Cattle Populations in Contrasting Agro-Ecological Zones of Uganda: Implications for Control Strategies. Parasit. Vectors 2014, 7, 414. [Google Scholar] [CrossRef]
- Nuttall, P.A. Climate Change Impacts on Ticks and Tick-Borne Infections. Biologia 2022, 77, 1503–1512. [Google Scholar] [CrossRef]
- Kamyingkird, K.; Sayed-Ahmed, M.Z.; Rizk, M.A. Treatment of Tick-Borne Diseases: Current Status, Challenges, and Global Perspectives. Front. Pharmacol. 2024, 15, 1366988. [Google Scholar] [CrossRef] [PubMed]
- Cambra-Pellejà, M.; Gandasegui, J.; Balaña-Fouce, R.; Muñoz, J.; Martínez-Valladares, M. Zoonotic Implications of Onchocerca Species on Human Health. Pathogens 2020, 9, 761. [Google Scholar] [CrossRef]
- Bergua, A.; Hohberger, B.; Held, J.; Muntau, B.; Tannich, E.; Tappe, D. Human Case of Onchocerca Lupi Infection, Germany, August 2014. Eurosurveillance 2015, 20, 21099. [Google Scholar] [CrossRef]
- Uni, S.; Fukuda, M.; Otsuka, Y.; Hiramatsu, N.; Yokobayashi, K.; Takahashi, H.; Murata, S.; Kusatake, K.; Morita, E.; Maruyama, H. New Zoonotic Cases of Onchocerca Dewittei Japonica (Nematoda: Onchocercidae) in Honshu, Japan. Parasit. Vectors 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Wesołowska, M.; Zając-Pytrus, H.; Masny, A.; Pytrus, W.; Knysz, B.; Golab, E.; Sałamatin, R. Onchocerca Jakutensis Ocular Infection in Poland: A New Vector-Borne Human Health Risk? Parasit. Vectors 2020, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.W.; Neafie, R.C.; McLean, M.; Markman, A.W. Zoonotic Onchocerciasis of the Shoulder: A Case Report. JBJS 2002, 84, 627–629. [Google Scholar] [CrossRef]
- Burr Jr, W.E.; Brown, M.F.; Eberhard, M.L. Zoonotic Onchocerca (Nematoda: Filarioidea) in the Cornea of a Colorado Resident. Ophthalmology 1998, 105, 1494–1497. [Google Scholar] [CrossRef]
- Kirk, R. African Onchocerciasis. Cent. Afr. J. Med. 1959, 5, 233–242. [Google Scholar]
- Van den Berghe, L.; Chardome, M.; Peel, E. The Filarial Parasites of the Eastern Gorilla in the Congo. J. Helminthol. 1964, 38, 349–368. [Google Scholar] [CrossRef]
- Gustavsen, K.; Hopkins, A.; Sauerbrey, M. Onchocerciasis in the Americas: From Arrival to (near) Elimination. Parasit. Vectors 2011, 4, 205. [Google Scholar] [CrossRef] [PubMed]
- Bwangamoi, O.; Obwolo, M.; Joshua, R.; Chigarisano, J. A Preliminary Report on the Finding of Onchocerca Ochengi in Cattle in Zimbabwe. Zimb. Vet. J. 1993, 24, 107–109. [Google Scholar]
- Zouré, H.G.; Noma, M.; Tekle, A.H.; Amazigo, U.V.; Diggle, P.J.; Giorgi, E.; Remme, J.H. The Geographic Distribution of Onchocerciasis in the 20 Participating Countries of the African Programme for Onchocerciasis Control:(2) Pre-Control Endemicity Levels and Estimated Number Infected. Parasit. Vectors 2014, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Sole, G.; Accorsi, S.; Cresveaux, H.; Remme, J.; Walsh, F.; Hendrickx, J. Distribution and Severity of Onchocerciasis in Southern Benin, Ghana and Togo. Acta Trop. 1992, 52, 87–97. [Google Scholar] [CrossRef]
- Bussiéras, J.; Amégee, E.; Bain, O. Les Onchocercoses Des Bovins Togolais a O. Dukei et O. Dermata. Rev. D’élevage Médecine Vét. Pays Trop. 1974, 27, 189–194. [Google Scholar] [CrossRef]
- Prost, A.; Nebout, M.; Rougemont, A. Lepromatous Leprosy and Onchocerciasis. Br. Med. J. 1979, 1, 589. [Google Scholar] [CrossRef]
- Neary, J.M.; Trees, A.J.; Ekale, D.D.; Tanya, V.N.; Hetzel, U.; Makepeace, B.L. Onchocerca Armillata Contains the Endosymbiotic Bacterium Wolbachia and Elicits a Limited Inflammatory Response. Vet. Parasitol. 2010, 174, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Wanji, S.; Kengne-Ouafo, J.A.; Esum, M.E.; Chounna, P.W.; Tendongfor, N.; Adzemye, B.F.; Eyong, J.E.; Jato, I.; Datchoua-Poutcheu, F.R.; Kah, E. Situation Analysis of Parasitological and Entomological Indices of Onchocerciasis Transmission in Three Drainage Basins of the Rain Forest of South West Cameroon after a Decade of Ivermectin Treatment. Parasit. Vectors 2015, 8, 1–21. [Google Scholar] [CrossRef]
- Koudou, B.G.; Kouakou, M.-M.; Ouattara, A.F.; Yeo, S.; Brika, P.; Meite, A.; Aba, E.; King, C.L.; Kouakou, R.; Weil, G.J. Update on the Current Status of Onchocerciasis in Côte d’Ivoire Following 40 Years of Intervention: Progress and Challenges. PLoS Negl. Trop. Dis. 2018, 12, e0006897. [Google Scholar] [CrossRef]
- Prince-Guerra, J.L.; Cama, V.A.; Wilson, N.; Thiele, E.A.; Likwela, J.; Ndakala, N.; wa Muzinga, J.M.; Ayebazibwe, N.; Ndjakani, Y.D.; Pitchouna, N.A. Comparison of PCR Methods for Onchocerca Volvulus Detection in Skin Snip Biopsies from the Tshopo Province, Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2018, 98, 1427. [Google Scholar] [CrossRef]
- Radwan, A.; Ahmed, N.; Elakabawy, L.; Ramadan, M.; Elmadawy, R. Prevalence and Pathogenesis of Some Filarial Nematodes Infecting Donkeys in Egypt. Vet. World 2016, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Chodnik, K. Aortic Onchocerciasis Due to Onchocerca Armillata in Cattle in Ghana, with Special Reference to the Morphology of the Parasite. Ann. Trop. Med. Parasitol. 1957, 51, 216–224. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Onchocerciasis Control in Guinea: Achievements and Prospects After OCP. 2002. Available online: https://iris.who.int/bitstream/handle/10665/342983/10665274421Oncho-Guinee-eng.pdf?sequence=1&isAllowed=y (accessed on 2 February 2025).
- Gracio, A.S.; Shelley, A.; Charalambous, M.; Lowry, C.; Gracio, M.; Forte, J.; Raybould, J.; Molyneux, D.; Nhaque, A. Onchocerciasis in Guinea Bissau, West Africa. Parasite 1994, 1, S6–S7. [Google Scholar] [CrossRef]
- Burnham, G.M. Onchocerciasis in the Thyolo Highlands of Malawi. Malawi Med. J. 1992, 8. Available online: https://journals.co.za/doi/pdf/10.10520/AJA19957262_66 (accessed on 2 February 2025).
- Séchan, Y. Developpement d’onchocerques Animales Chez Le Vecteur de l’onchocercose Humaine Simulium Sirbanum Vajime et Dunbar, 1975 (Diptera: Simuliidae) En Zone Subsahelienne Du Mali Afrique de l’Ouest. Trav. Doc. ORSTOM 1984, 178, 234. [Google Scholar]
- Noormahomed, E.V.; Akrami, K.; Mascaró-Lazcano, C. Onchocerciasis, an Undiagnosed Disease in Mozambique: Identifying Research Opportunities. Parasit. Vectors 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Ogbogu, V.; Bablis, J.; Ajanusi, O. Prevalence of Microfilariae in Cattle at Slaughter in Zaria, Nigeria. Vet. Parasitol. 1990, 36, 171–175. [Google Scholar] [CrossRef]
- Wright, F. Onchocerca Volvulus Causing Abscesses. J. Trop. Med. Hyg. 1972, 75, 140–141. [Google Scholar]
- Vassiliades, G.; Delbove, P.; Bain, O. Onchocercoses Bovines Au Sénégal. Note Préliminaire. Rev. D’élevage Médecine Vét. Pays Trop. 1983, 36, 351–353. [Google Scholar]
- Trees, A.; McCall, P.; Davies, J. On the Possibility of Bovine Onchocerca Species Infecting Simulium Damnosum Sl in the Forest Zone of Sierra Leone: I. Parasitological Aspects. Ann. Trop. Med. Parasitol. 1989, 83, 595–601. [Google Scholar] [CrossRef]
- El-Massry, A.A.; Derbala, A. Evidence of Onchocerca fasciata (Filaroidea: Onchocercidae) in Camels (Camelus dromedarius): I-Prevalence, Nodular Lesions Appearance and Parasite Morphology. Vet. Parasitol. 2000, 88, 305–312. [Google Scholar] [CrossRef] [PubMed]
- De Kock, G.; Snyman, P. Occurrence of Onchocerca in South Africa; Government Printer and Stationery Office: Pretoria, South Afica, 1928. [Google Scholar]
- Wani, M. Oncocerciasis. South Sudan Med. J. 2008, 1. Available online: https://www.ajol.info/index.php/ssmj/article/view/132341 (accessed on 2 February 2025).
- Hussein, H.; El Mannan, A.A.; El Sinnary, K. Onchocerca Armillata Railliet and Henry, 1909 and Onchocerca Gutturosa (Neumann, 1910) in Camels (Camelus dromedarius L.) in the Sudan. Vet. Res. Commun. 1988, 12, 475–480. [Google Scholar] [CrossRef]
- Mtei, B.; Sanga, H. Aortic Onchocercosis and Elaeophorosis in Traditional TSZ-Cattle in Tabora (Tanzania): Prevalence and Pathology. Vet. Parasitol. 1990, 36, 165–170. [Google Scholar] [CrossRef]
- Denke, A. The Prevalence of Onchocerca spp. in Cattle in Northern Togo in 1979. Trop. Med. Parasitol. Off. Organ Dtsch. Tropenmedizinische Ges. Dtsch. Ges. Tech. Zusammenarbeit GTZ 1986, 37, 46–48. [Google Scholar]
- Otranto, D.; Dantas-Torres, F.; Cebeci, Z.; Yeniad, B.; Buyukbabani, N.; Boral, O.B.; Gustinelli, A.; Mounir, T.; Mutafchiev, Y.; Bain, O. Human Ocular Filariasis: Further Evidence on the Zoonotic Role of Onchocerca Lupi. Parasit. Vectors 2012, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Lehucher, P. Premier Cas d’onchocercose Cutanée Observ En Tunisie. Arch. L’Institut Pasteur Tunis 1940, 29, 105–112. [Google Scholar]
- Wildenburg, G.; Plenge-Bönig, A.; Renz, A.; Fischer, P.; Büttner, D.W. Distribution of Mast Cells and Their Correlation with Inflammatory Cells around Onchocerca gutturosa, O. tarsicola, O. ochengi, and O. flexuosa. Parasitol. Res. 1997, 83, 109–120. [Google Scholar] [CrossRef]
- Kipp, W.; Kasoro, S.; Burnham, G. Onchocerciasis and Epilepsy in Uganda. Lancet 1994, 343, 183–184. [Google Scholar] [CrossRef]
- Beaver, P.; Hira, P.; Patel, B. Onchocerciasis in Zambia: Report of O. Volvulus in a Child and Its Differentiation from O. Dukei in Cattle. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 162–166. [Google Scholar] [CrossRef]
- Onchocerciasis. Available online: https://www.who.int/data/gho/data/themes/topics/onchocerciasis (accessed on 6 February 2025).
- Lymphatic Filariasis (Elephantiasis). Available online: https://www.who.int/data/gho/data/themes/topics/lymphatic-filariasis (accessed on 8 February 2025).
- Hawking, F. The Distribution of Bancroftian Filariasis in Africa. Bull. World Health Organ. 1957, 16, 581. [Google Scholar] [PubMed]
- Paulo, R. Epidemiology of Human Filarial Infections in Angola Revealed by Rapid Surveys Coupled with Serological and Molecular Assays. Doctoral Thesis, Liverpool School of Tropical Medicine, Liverpool, UK, 2020. [Google Scholar]
- Price, E. Endemic Elephantiasis of the Lower Legs in Rwanda and Burundi. Trop. Geogr. Med. 1976, 28, 283–290. [Google Scholar]
- Boko-Collins, P.M.; Ogouyemi-Hounto, A.; Adjinacou-Badou, E.G.; Gbaguidi-Saizonou, L.; Dossa, N.I.; Dare, A.; Ibikounle, M.; Zoerhoff, K.L.; Cohn, D.A.; Batcho, W. Assessment of Treatment Impact on Lymphatic Filariasis in 13 Districts of Benin: Progress toward Elimination in Nine Districts despite Persistence of Transmission in Some Areas. Parasit. Vectors 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Botswana On the Search Mode for Lymphatic Filariasis (LF) in the Country | OMS | Bureau régional pour l’Afrique. Available online: https://www.afro.who.int/countries/botswana/news/botswana-search-mode-lymphatic-filariasis-lf-country (accessed on 7 February 2025).
- Coulibaly, S.; Sawadogo, S.P.; Hien, A.S.; Nikièma, A.S.; Sangaré, I.; Rabila, B.; Koala, L.; Bougouma, C.; Bougma, R.W.; Ouedraogo, G.A. Malaria and Lymphatic Filariasis Co-Transmission in Endemic Health Districts in Burkina Faso. Adv. Entomol. 2021, 9, 155–175. [Google Scholar] [CrossRef]
- World Health Organization. World Health Report-2000. Geneva, WHO, 2000. Lymphatic Filariasis. 2000. Available online: https://iris.who.int/bitstream/handle/10665/231510/WER7620_149-154.PDF (accessed on 2 February 2025).
- Nana-Djeunga, H.C.; Tchatchueng-Mbougua, J.B.; Bopda, J.; Mbickmen-Tchana, S.; Elong-Kana, N.; Nnomzo’o, E.; Akame, J.; Tarini, A.; Zhang, Y.; Njiokou, F. Mapping of Bancroftian Filariasis in Cameroon: Prospects for Elimination. PLoS Negl. Trop. Dis. 2015, 9, e0004001. [Google Scholar] [CrossRef]
- Brumpt, L.; Sang, H.; Jaeger, G. Blood and Intestinal Parasitism in 2 Central African Villages. Bull. Soc. Pathol. Exot. Filiales 1972, 65, 263–270. [Google Scholar]
- Bregani, E.R.; Balzarini, L.; Mbaïdoum, N.; Rovellini, A. Prevalence of Filariasis in Symptomatic Patients in Moyen Chari District, South of Chad. Trop. Doct. 2007, 37, 175–177. [Google Scholar] [CrossRef]
- Chesnais, C.B.; Missamou, F.; Pion, S.D.; Bopda, J.; Louya, F.; Majewski, A.C.; Fischer, P.U.; Weil, G.J.; Boussinesq, M. A Case Study of Risk Factors for Lymphatic Filariasis in the Republic of Congo. Parasit. Vectors 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Charafoudine, H.; Pesson, B. Bancroft’s Filariasis in Anjouan (Comoro Islands). Bull. Société Pathol. Exot. Ses Fil. 1986, 79, 229–236. [Google Scholar]
- Brahima, D.; Alain Didier, A.; Gonat Serge Pacôme, D.; Nguiessan Alphonse, A.; Abdoul, K.; Jean-Marie, D.M.I. A Rare Case of Ovarian Filariasis in Abidjan. Case Rep. Pathol. 2016, 2016, 4075162. [Google Scholar] [CrossRef][Green Version]
- Kelly-Hope, L.A.; Thomas, B.C.; Bockarie, M.J.; Molyneux, D.H. Lymphatic Filariasis in the Democratic Republic of Congo; Micro-Stratification Overlap Mapping (MOM) as a Prerequisite for Control and Surveillance. Parasit. Vectors 2011, 4, 1–13. [Google Scholar] [CrossRef]
- Harb, M.; Faris, R.; Gad, A.; Hafez, O.; Ramzy, R.; Buck, A.A. The Resurgence of Lymphatic Filariasis in the Nile Delta. Bull. World Health Organ. 1993, 71, 49. [Google Scholar] [PubMed]
- Eritrea | ESPEN. Available online: https://espen.afro.who.int/countries/eritrea (accessed on 8 February 2025).
- Shiferaw, W.; Kebede, T.; Graves, P.M.; Golasa, L.; Gebre, T.; Mosher, A.W.; Tadesse, A.; Sime, H.; Lambiyo, T.; Panicker, K. Lymphatic Filariasis in Western Ethiopia with Special Emphasis on Prevalence of Wuchereria Bancrofti Antigenaemia in and around Onchocerciasis Endemic Areas. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Biritwum, N.-K.; Yikpotey, P.; Marfo, B.K.; Odoom, S.; Mensah, E.O.; Asiedu, O.; Alomatu, B.; Hervie, E.T.; Yeboah, A.; Ade, S. Persistent ‘Hotspots’ of Lymphatic Filariasis Microfilaraemia despite 14 Years of Mass Drug Administration in Ghana. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Champetier de Ribes, G.; Ranaivoson, G.; Radoerimanana, R.; Rabeson, D. Bancroftian Filariasis in Madagascar: Persistent Endemicity. Med. Trop. Rev. Corps Sante Colon. 2000, 60, 141–145. [Google Scholar]
- Coulibaly, Y.I.; Dembele, B.; Diallo, A.A.; Konaté, S.; Dolo, H.; Coulibaly, S.Y.; Doumbia, S.S.; Soumaoro, L.; Coulibaly, M.E.; Bockarie, M.J. The Impact of Six Annual Rounds of Mass Drug Administration on Wuchereria Bancrofti Infections in Humans and in Mosquitoes in Mali. Am. J. Trop. Med. Hyg. 2015, 93, 356. [Google Scholar] [CrossRef]
- Huehns, E. Filariasis in Mauritius. Trans. R. Soc. Trop. Med. Hyg. 1953, 47, 549–555. [Google Scholar] [CrossRef]
- Plucinski, M.M.; Candrinho, B.; Chambe, G.; Muchanga, J.; Muguande, O.; Matsinhe, G.; Mathe, G.; Rogier, E.; Doyle, T.; Zulliger, R. Multiplex Serology for Impact Evaluation of Bed Net Distribution on Burden of Lymphatic Filariasis and Four Species of Human Malaria in Northern Mozambique. PLoS Negl. Trop. Dis. 2018, 12, e0006278. [Google Scholar] [CrossRef]
- Badia-Rius, X.; Adamou, S.; Taylor, M.J.; Kelly-Hope, L.A. Morbidity Hotspot Surveillance: A Novel Approach to Detect Lymphatic Filariasis Transmission in Non-Endemic Areas of the Tillabéry Region of Niger. Parasite Epidemiol. Control 2023, 21, e00300. [Google Scholar] [CrossRef]
- Olanrewaju, J.; Shamsudin, A.; Shamsuddeen, Y.; Orinya, A.; Bello, K.; Musa, A.; Usman, A.; Joseph, G.; Balogun, E. Diagnosis of Filariasis: A Case Report of a Reemerging Neglected Tropical Disease. Ger. J. Microbiol. 2024, 4, 25–31. [Google Scholar]
- Fraga de Azevedo, J.; Pinhao, R.; Meira, M.; Gardette, M. Bancroftian and Malayan Filariasis in Overseas Portuguese Territories. An. Esc. Nac. Saude Publica Med. Trop. 1969, 3, 3–9. [Google Scholar]
- Wilson, N.O.; Badara Ly, A.; Cama, V.A.; Cantey, P.T.; Cohn, D.; Diawara, L.; Direny, A.; Fall, M.; Feeser, K.R.; Fox, L.M. Evaluation of Lymphatic Filariasis and Onchocerciasis in Three Senegalese Districts Treated for Onchocerciasis with Ivermectin. PLoS Negl. Trop. Dis. 2016, 10, e0005198. [Google Scholar] [CrossRef] [PubMed]
- Nuti, M.; Ferrari, J.; Au, A. Seroepidemiology of Bancroftian Filariasis in the Seychelles Islands. Tropenmed. Parasitol. 1982, 33, 25–27. [Google Scholar] [PubMed]
- Dome, M.; Ansumana, R.; Covington, A.L.; Rebollo, M.P.; Sesay, S.; Jacobsen, K.H.; de Souza, D.K.; Koudou, B.G.; Michael, E.; Bockarie, M.J. Lymphedema in a 7-Year-Old Boy Infected with Wuchereria Bancrofti in Sierra Leone: A Case Report. Acta Trop. 2014, 134, 13–16. [Google Scholar] [CrossRef]
- Senkwe, M.N.; Berta, K.K.; Logora, S.M.Y.; Sube, J.; Bidali, A.; Abe, A.; Onyeze, A.; Pita, J.; Rumunu, J.; Maleghemi, S. Prevalence and Factors Associated with Transmission of Lymphatic Filariasis in South Sudan: A Cross-Sectional Quantitative Study. Pan Afr. Med. J. 2022, 42, 9. [Google Scholar]
- Kirk, R. Filariasis in the Sudan. Bull. World Health Organ. 1957, 16, 593. [Google Scholar]
- Simonsen, P.; Niemann, L.; Meyrowitsch, D. Wuchereria Bancrofti in Tanzania: Microfilarial Periodicity and Effect of Blood Sampling Time on Microfilarial Intensities. Trop. Med. Int. Health 1997, 2, 153–158. [Google Scholar] [CrossRef]
- Onapa, A.W.; Simonsen, P.E.; Pedersen, E.M.; Okello, D.O. Lymphatic Filariasis in Uganda: Baseline Investigations in Lira, Soroti and Katakwi Districts. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 161–167. [Google Scholar] [CrossRef]
- Matapo, B.B.; Mpabalwani, E.M.; Kaonga, P.; Simuunza, M.C.; Bakyaita, N.; Masaninga, F.; Siyumbwa, N.; Siziya, S.; Shamilimo, F.; Muzongwe, C. Lymphatic Filariasis Elimination Status: Wuchereria Bancrofti Infections in Human Populations after Five Effective Rounds of Mass Drug Administration in Zambia. Trop. Med. Infect. Dis. 2023, 8, 333. [Google Scholar] [CrossRef]
- Loiasis | ESPEN. Available online: https://espen.afro.who.int/diseases/loiasis (accessed on 8 February 2025).
- Lalaoui, A.; Nassih, H.; Rabiy El Qadiry, N.M.; Bourahouat, A.; Sab, I.A.; Berradi, S.; Moutaj, R. Subconjunctival Loa Loa Worm: A First Native Case Report in a Moroccan Child. Arch. Clin. Med. Case Rep. 2020, 4, 898–902. [Google Scholar] [CrossRef]
- Boussinesq, M.; Gardon, J. Prevalences of Loa Loa Microfilaraemia throughout the Area Endemic for the Infection. Ann. Trop. Med. Parasitol. 1997, 91, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Massougbodji, A.; Sadeler, B.-C.; Ottesen, E.A.; Nutman, T.B. Loiasis in Endemic and Nonendemic Populations: Immunologically Mediated Differences in Clinical Presentation. J. Infect. Dis. 1991, 163, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D. Loiasis. In Hunter’s Tropical Medicine and Emerging Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 823–826. [Google Scholar]
- Antinori, S.; Schifanella, L.; Million, M.; Galimberti, L.; Ferraris, L.; Mandia, L.; Trabucchi, G.; Cacioppo, V.; Monaco, G.; Tosoni, A. Imported Loa Loa Filariasis: Three Cases and a Review of Cases Reported in Non-Endemic Countries in the Past 25 Years. Int. J. Infect. Dis. 2012, 16, e649–e662. [Google Scholar] [CrossRef] [PubMed]
- Labrigui, M.; Layat, H.; Bouikhif, M.; Ziad, F.; Khalil, Z.; Jerroundi, Z.; Zahid, H.; Ouzzif, Z. Fortuitous Discovery of a Microfilaria of the Genus Loa Loa during a Routine Blood Smear at the Hematology Laboratory of the Mohamed V Military Instruction Hospital in Rabat: A Case Report. Access Microbiol. 2024, 7, 000895-v1. [Google Scholar] [CrossRef]
- El Haouri, M.; Erragragui, Y.; Sbai, M.; Alioua, Z.; El Mellouki, W.; Sedrati, O. Cutaneous Filariasis Loa Loa: 26 Moroccan Cases of Importation. Ann. Dermatol. Venereol. 2001, 128, 899–902. [Google Scholar]
- Mediannikov, O.; Ranque, S. Mansonellosis, the Most Neglected Human Filariasis. New Microbes New Infect. 2018, 26, S19–S22. [Google Scholar] [CrossRef]
- Clarke, V.; Harwin, R.M.; MacDonald, D.F.; Green, C.A.; Rittey, D.A.W. Filariasis: Dipetalonema Perstans Infections in Rhodesia. Cent. Afr. J. Med. 1971, 17, 1–11. [Google Scholar]
- Casaca, V. Contribuicao Para o Estudo Da Filariase Bancrofti Em Angola. Inst. Hig. Med. Trop. Lisb. 1966, 23, 127–132. [Google Scholar]
- Van Oye, E.; Pierquin, L. Les Filarioses Humaines Au Congo et Au Ruanda-Urundi. Esquisse Historique. Brux Med. 1961, 41, 39–53. [Google Scholar]
- Pfister, R. Résultats d’une Enquête Sur Les Porteurs de Microfilaire En Afrique Occidentale Française. Bull. Soc. Pathol. Exot. 1954, 47, 408–411. [Google Scholar]
- Kyelem, D.; Sanou, S.; Boatin, B.A.; Medlock, J.; Coulibaly, S.; Molyneux, D. Impact of Long-Term Ivermectin (Mectizan®) on Wuchereria Bancrofti and Mansonella Perstans Infections in Burkina Faso: Strategic and Policy Implications. Ann. Trop. Med. Parasitol. 2003, 97, 827–838. [Google Scholar] [CrossRef]
- Wanji, S.; Tendongfor, N.; Esum, M.; Ndindeng, S.; Enyong, P. Epidemiology of Concomitant Infections Due to Loa Loa, Mansonella Perstans, and Onchocerca Volvulus in Rain Forest Villages of Cameroon. Med. Microbiol. Immunol. 2003, 192, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Okelo, G.; Kyobe, J.; Gatiri, G. Mansonella Streptocerca in the Central African Republic. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 464. [Google Scholar] [CrossRef]
- Bregani, E.R.; Ceraldi, T.; Rovellini, A.; Ghiringhelli, C. Case Report: Intraocular Localization of Mansonella Perstans in a Patient from South Chad. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 654. [Google Scholar] [CrossRef]
- Noireau, F.; Carme, B.; Apembet, J.; Gouteux, J.-P. Loa Loa and Mansonella Perstans Filariasis in the Chaillu Mountains, Congo: Parasitological Prevalence. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.; Fain, A.; Maertens, K. Enquête Sur Les Filarioses Humaines Dans La Région de Bwamanda Au Nord-Ouest Du Zaïre. Ann. Soc. Belge. Méd. Trop. 1982, 62, 315–342. [Google Scholar]
- Abo-Aziza, F.A.; Hendawy, S.H.; Abdullah, H.H.; El Namaky, A.; Laidoudi, Y.; Mediannikov, O. Emergent and Neglected Equine Filariosis in Egypt: Species Diversity and Host Immune Response. Pathogens 2022, 11, 979. [Google Scholar] [CrossRef]
- Yoboue, A.C. Molecular Epidemiology of Mansonella Perstans on Bioko Island: Identification of Risk Factors, Co-Infection with Malaria and Loa Loa and Impact in Pregnant Women. Doctoral Dissertation, University of Basel, Basel, Switzerland, 2023. [Google Scholar]
- Endeshaw, T.; Kebede, A.; Aseffa, S. Observation of Blood Microfilariae during Human Trypanosomiasis Survey in Gambella, South West Ethiopia. Ethiop. J. Health Dev. 1997, 11. Available online: https://www.ejhd.org/index.php/ejhd/article/view/1020 (accessed on 2 February 2025).
- Mourembou, G.; Fenollar, F.; Lekana-Douki, J.B.; Ndjoyi Mbiguino, A.; Maghendji Nzondo, S.; Matsiegui, P.B.; Zoleko Manego, R.; Ehounoud, C.H.B.; Bittar, F.; Raoult, D. Mansonella, Including a Potential New Species, as Common Parasites in Children in Gabon. PLoS Negl. Trop. Dis. 2015, 9, e0004155. [Google Scholar] [CrossRef]
- Awadzi, K.; Hero, M.; Opoku, O.; Büttner, D.; Gilles, H. The Chemotherapy of Onchocerciasis. XV. Studies with Albendazole. Trop. Med. Parasitol. Off. Organ Dtsch. Tropenmedizinische Ges. Dtsch. Ges. Tech. Zusammenarbeit GTZ 1991, 42, 356–360. [Google Scholar]
- Simonsen, P.E.; Onapa, A.W.; Asio, S.M. Mansonella Perstans Filariasis in Africa. Acta Trop. 2011, 120, S109–S120. [Google Scholar] [CrossRef] [PubMed]
- Oram, R. Filariasis on the North Nyasa Lake Shore. Cent. Afr. J. Med. 1958, 4, 99–103. [Google Scholar]
- Keiser, P.B.; Coulibaly, Y.I.; Keita, F.; Traore, D.; Diallo, A.; Diallo, D.A.; Semnani, R.T.; Doumbo, O.K.; Traore, S.F.; Klion, A.D. Clinical Characteristics of Post-Treatment Reactions to Ivermectin/Albendazole for Wuchereria Bancrofti in a Region Co-Endemic for Mansonella Perstans. Am. J. Trop. Med. Hyg. 2003, 69, 331–335. [Google Scholar] [CrossRef]
- Pinhao, R. Incidência de Filarioses No Vale Do Zambeze. An. Inst. Med. Trop. 1961, 18, 15–18. [Google Scholar]
- Anosike, J.C.; Nwoke, B.E.; Onwuliri, C.O.; Obiukwu, C.E.; Duru, A.F.; Nwachukwu, M.I.; Ukaga, C.N.; Uwaezuoke, J.C.; Uduji, O.S.; Amajuoyi, O.U. Prevalence of Parasitic Diseases among Nomadic Fulanis of South-Eastern Nigeria. Ann. Agric. Environ. Med. 2004, 11, 221–225. Available online: https://pubmed.ncbi.nlm.nih.gov/15627328/ (accessed on 2 February 2025).
- De Azevedo, J.F. As Filariases Na Ilha Do Principe. Anais Inst. Med. Trop. 1960, 17, 621–639. [Google Scholar]
- Hocquet, P.; Lariviere, M.; Camerlynck, P.; Diallo, S. Contribution a l’etude de La Repartition Des Filarioses Humaines Au Senegal: Enquetes Dans La Region de Damantan (Senegal Oriental) et La Region Cotiere de Casamance. Bull. Soc. Med. Afr. Noire Lang. Fr. 1964, 9, 398–405. [Google Scholar] [PubMed]
- Gbakima, A.; Sahr, F. Filariasis in the Kaiyamba Chiefdom, Moyamba District Sierra Leone: An Ep Demiological and Clinical Study. Public Health 1996, 110, 169–174. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Rodari, P.; Salas-Coronas, J.; Bottieau, E.; Salvador, F.; Soriano-Pérez, M.J.; Cabeza-Barrera, M.I.; Van Esbroeck, M.; Treviño, B.; Buonfrate, D. A Large Case Series of Travel-Related Mansonella Perstans (Vector-Borne Filarial Nematode): A TropNet Study in Europe. J. Travel Med. 2022, 29, taac048. [Google Scholar] [CrossRef]
- Jordan, P. Microfilarial, Density and Infection Rates of Wuchereria Bancrofti and Acanthocheilonema Perstans in the Southern Province of Tanganyika Territory. Ann. Trop. Med. Parasitol. 1955, 49, 42–53. [Google Scholar] [CrossRef]
- Knight, R. Current Status of Filarial Infections in The Gambia. Ann. Trop. Med. Parasitol. 1980, 74, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Key, H.; Albrecht, W.; Heuschkel, C.; Soboslay, P.; Banla, M.; Görgen, H. Efficacy of Ivermectin in the Treatment of Concomitant Mansonella Perstans Infections in Onchocerciasis Patients. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 227–229. [Google Scholar] [CrossRef]
- Asio, S.M.; Simonsen, P.E.; Onapa, A.W. Mansonella Perstans Filariasis in Uganda: Patterns of Microfilaraemia and Clinical Manifestations in Two Endemic Communities. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R. Filariasis in the Luangwa Basin. Med. J. Zamb. 1971, 5, 201–203. [Google Scholar]
- Ferreira, M.U.; Crainey, J.L.; Gobbi, F.G. The Search for Better Treatment Strategies for Mansonellosis: An Expert Perspective. Expert Opin. Pharmacother. 2023, 24, 1685–1692. [Google Scholar] [CrossRef]
- Rivasi, F.; Boldorini, R.; Criante, P.; Leutner, M.; Pampiglione, S. Detection of Dirofilaria (Nochtiella) Repens DNA by Polymerase Chain Reaction in Embedded Paraffin Tissues from Two Human Pulmonary Locations: Case Report. Apmis 2006, 114, 566–573. [Google Scholar] [CrossRef]
- Genchi, C.; Kramer, L.H. The Prevalence of Dirofilaria Immitis and D. Repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef] [PubMed]
- Meriem-Hind, B.-M.; Mohamed, M. Prevalence of Canine Dirofilaria Immitis Infection in the City of Algiers, Algeria. Afr. J. Agric. Res. 2009, 4, 1097–1100. [Google Scholar]
- Elnazer, M. Human Ectopic Infection with Dirofilaria Immitis (Leydi, 1865) Representing First Record from Egypt. Egypt. Med. J. 1994, 11, 435–438. [Google Scholar]
- Awadalla, H.; Bayoumi, D.; Ibrahim, I. The First Case Report of Suspected Human Dirofilariasis in the Eyelid of a Patient from Alexandria. J. Egypt. Soc. Parasitol. 1998, 28, 941–943. [Google Scholar]
- Al-Kappany, Y.; Lappin, M.; Kwok, O.; Abu-Elwafa, S.; Hilali, M.; Dubey, J. Seroprevalence of Toxoplasma Gondii and Concurrent Bartonella Spp., Feline Immunodeficiency Virus, Feline Leukemia Virus, and Dirofilaria Immitis Infections in Egyptian Cats. J. Parasitol. 2011, 97, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Ziadi, S.; Trimèche, M.; Mestiri, S.; Mokni, M.; Trabelsi, A.; Ben Abdelkader, A.; Ben Said, M.; Ben Hadj Hamida, F.; Korbi, S. La Dirofilariose Sous-Conjonctivale Humaine. A Propos de Deux Cas Tunisiens [Human Subconjunctival Dirofilariasis: Two Tunisian Case Studies]. J. Fr. Ophtalmol. 2005, 28, 773. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.; Akande, F.; Adenubi, O. Canine Dirofilariasis: A Case Report and Review of the Literature. Folia Vet. 2020, 64, 75–81. [Google Scholar] [CrossRef]
- Moodley, K.; Govind, C.N.; Peer, A.K.; van der Westhuizen, M.; Parbhoo, D.; Sun, L.M.; du Plessis, D.C.; Frean, J.A. First Detection of Human Dirofilariasis in South Africa. Infect. Dis. Rep. 2015, 7, 5726. [Google Scholar] [CrossRef]
- Motsi, T.R. Prevalence of Dirofilaria Repens in Dogs in Potchefstroom and Mahikeng, South Africa. Master’s Thesis, University of Pretoria (South Africa), Pretoria, South Africa, 2023. [Google Scholar]
- Goldsmid, J.; Bettiol, S. A Probable Case of Subcutaneous Dirofilariasis Acquired in Zimbabwe. Ann. ACTM Int. J. Trop. Travel Med. 2002, 3, 16. [Google Scholar]
- Schwan, E.; Durand, D. Canine Filariosis Caused by Dirofilaria Immitis in Mozambique: A Small Survey Based on the Identification of Microfilariae: Research Communication. J. S. Afr. Vet. Assoc. 2002, 73, 124–126. [Google Scholar] [CrossRef]
- Magayuka, S. Development of Filarial Parasites in Mosquitos in North-East Tanzania. Bull. World Health Organ. 1973, 49, 110. [Google Scholar]
- Ntesang, K. Gastrointestinal and Filarial Helminth Infections of Domestic Dogs in Gaborone Botswana; University of Pretoria (South Africa): Pretoria, South Africa, 2016; ISBN 979-8-3809-7058-7. [Google Scholar]
- Pereira, C.; Almeida, C.; Malta, M.; Vilaça, R.; Payo-Puente, P. First Report of Dirofilaria Immitis in the Republic of Cape Verde. Vet. Parasitol. 2013, 192, 290–291. [Google Scholar] [CrossRef]
- Idrissi, H.; Khatat, S.E.H.; Duchateau, L.; Kachani, M.; Azrib, R.; Sahibi, H. Canine Cardiopulmonary Nematodes in Morocco: Prevalence of Dirofilaria Immitis and Report of the First Autochthonous Infection with Angiostrongylus Vasorum. Moroc. J. Agric. Sci. 2022, 3, 117–123. [Google Scholar]
- Ribeiro, M.F. Study of the Prevalence of Canine Cardiopulmonary Dirofilariosis in the City of São Tomé, Democratic Republic of São Tomé and Príncipe. Master’s Thesis, Universidade de Lisboa (Portugal), Lisboa, Portugal, 2024. [Google Scholar]
- Haynes, E.; Cleveland, C.A.; Garrett, K.B.; Grunert, R.K.; Bryan II, J.A.; Sidouin, M.; Oaukou, P.T.; Ngandolo, B.N.R.; Yabsley, M.J. Characterization of the Genetics and Epidemiology of Brugia Sp. in Domestic Dogs in Chad, Africa. Vet. Parasitol. Reg. Stud. Rep. 2022, 35, 100784. [Google Scholar] [CrossRef]
- BWANGAMOI, O.; Frank, H. The Incidence and Pathology of Dirofilaria Immitis Infection in Dogs in Nairobi. J. Small Anim. Pract. 1970, 11, 293–300. [Google Scholar] [CrossRef]
- Bruijning, C. Human Dirofilariasis: A Report of the First Case of Ocular Dirofilariasis in the Netherlands and a Review of the Literature. Trop. Geogr. Med. 1981, 33, 295–305. [Google Scholar] [PubMed]
- Davoust, B.; Normand, T.; Bourry, O.; Dang, H.; Leroy, E.; Bourdoiseau, G. Epidemiological Survey on Gastro-Intestinal and Blood-Borne Helminths of Dogs in North-East Gabon: Research Communication. Onderstepoort J. Vet. Res. 2008, 75, 359–364. [Google Scholar] [CrossRef]
- Abdulkadir, M.; Kebede, I.A. A Review of Canine Dirofilariasis and Its Zoonotic Importance. WJ Health Med. 2024, 2, 1018. [Google Scholar]
- Nehra, A.K.; Kumari, A.; Moudgil, A.D.; Vohra, S. Parasites in the Cardiovascular System. In Organ-Specific Parasitic Diseases of Dogs and Cats; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–88. [Google Scholar]
- McCall, J.W.; Varloud, M.; Hodgkins, E.; Mansour, A.; DiCosty, U.; McCall, S.; Carmichael, J.; Carson, B.; Carter, J. Shifting the Paradigm in Dirofilaria Immitis Prevention: Blocking Transmission from Mosquitoes to Dogs Using Repellents/Insecticides and Macrocyclic Lactone Prevention as Part of a Multimodal Approach. Parasit. Vectors 2017, 10, 75–85. [Google Scholar] [CrossRef]
- Panaitescu, D.; Preda, A.; Bain, O.; Vasile-Bugarin, A. Four Cases of Human Filariosis Due to Setaria Labiatopapillosa Found in Bucharest, Romania. Roum. Arch. Microbiol. Immunol. 1999, 58, 203–207. [Google Scholar]
- Ţălu, S.; Ştefănuţ, A.; Mihalca, A.; Coroiu, Z. Subconjunctival Infestation with Setaria. Helminthologia 2012, 49, 119–121. [Google Scholar] [CrossRef]
- Siriyasatien, P.; Intayot, P.; Sawaswong, V.; Preativatanyou, K.; Wacharapluesadee, S.; Boonserm, R.; Sor-Suwan, S.; Ayuyoe, P.; Cantos-Barreda, A.; Phumee, A. Description of Potential Vectors of Zoonotic Filarial Nematodes, Brugia Pahangi, Setaria Digitata, and Setaria Labiatopapillosa in Thai Mosquitoes. Heliyon 2023, 9, e13255. [Google Scholar] [CrossRef] [PubMed]
- Nabie, R.; Spotin, A.; Rouhani, S. Subconjunctival Setariasis Due to Setaria Equina Infection; a Case Report and a Literature Review. Parasitol. Int. 2017, 66, 930–932. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Bhadwal, M.; Khajuria, J.; Gupta, A. Ocular Setariosis in Horses: A Case Study. J. Vet. Parasitol. 2006, 20, 183–184. [Google Scholar]
- Rhee, J.K.; Choi, E.Y.; Park, B.K.; Jang, B.G. Application of Scanning Electron Microscopy in Assessing the Prevalence of Some Setaria Species in Korean Cattle. Korean J. Parasitol. 1994, 32, 1–6. [Google Scholar] [CrossRef]
- Deli, C.; Sobrero, R. Nematodes of Domestic Ruminants in Somalia. Parassitologia 1966, 8, 29–44. [Google Scholar]
- Brengues, J.; Gidel, R. Research on Setaria Labiatopapillosa (Perroncito, 1882) in Western Africa. II. Dynamics of This Filariasis under Natural Conditions. Ann. Parasitol. Hum. Comp. 1972, 47, 597–611. [Google Scholar] [CrossRef]
- McFadzean, J.A. Setarial Infections in the Gambia, British West Africa. Ann. Trop. Med. Parasitol. 1955, 49, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.H.; Amanzougaghene, N.; Dahmana, H.; Louni, M.; Raoult, D.; Mediannikov, O. Multiple Vector-Borne Pathogens of Domestic Animals in Egypt. PLoS Negl. Trop. Dis. 2021, 15, e0009767. [Google Scholar] [CrossRef]
- Shoho, C.; Sachs, R. On Two Filarioid Worms, Setaria Labiatopapillosa and Pseudofilaria Giraffae n. Sp., from the East African Giraffe. Tropenmed. Parasitol. 1975, 26, 489–493. [Google Scholar]
- Shoho, C. On Setaria Spp;(Nematoda, Filarioidea, Setariidae) from the Peritoneal Cavity of Equine Spp.: Two New Sub-Species, Setaria Equina Theilerae from Wild Zebra of Africa, and Setaria Equina Dafaallai from Horse and Donkey of Southern Sahara Area (Author’s Transl). Ann. Parasitol. Hum. Comp. 1976, 51, 589–599. [Google Scholar]
- Scialdo-Krecek, R.C.; Reinecke, R.; Biggs, H. Studies on the Parasites of Zebras. III. Nematodes of the Mountain Zebra from the Farm” Kelpie” and the Namib-Naukluft Park, South West Africa/Namibia. Onderstepoort J. Vet. Res. 1983, 50, 283–290. [Google Scholar]
- Watermeyer, R.; Putterill, J.F.; Boomker, J.; Kuzmin, Y.; Junker, K. Redescription of Setaria Graberi Shoho in Troncy, Graber & Thal, 1976 (Nematoda: Filarioidea) Based on Specimens from Redunca Arundinum (Bovidae) in South Africa. Parasite 2013, 20, 43. [Google Scholar]
- Condy, J. & H.R. Filariasis in Rhodesian Wild Life. Cent. Afr. J. Med. 1970, 16, 249–251. [Google Scholar]
- Sachs, R.; Sachs, C. A Survey of Parasitic Infestation of Wild Herbivores in the Serengeti Region in Northern Tanzania and the Lake Rukwa Region in Southern Tanzania. Bull. Epizoot. Dis. Afr. 1968, 16, 455–472. [Google Scholar] [PubMed]
- Troncy, P.; Graber, M.; Thal, J. Study of a Collection of Setaria from African Ruminants. Bulletin de l’Institut Fondamental d’Afrique Noire 1976, 4, 808–831. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19800868090 (accessed on 2 February 2025).
- Crespo, M. A Preliminary Study of the Nematoda of Cattle from the Republic of Guinea-Bissau. Garcia Orta Ser. Zool. 1998, 22, 63–67. [Google Scholar]
- Achi, Y.; Zinsstag, J.; Yeo, N.; Dea, V.; Dorchies, P. Gastrointestinal Nematodes of Cattle in the Savannah Area of Côte-d’Ivoire: An Abattoir Survey. Revue Méd. Vét. 2003, 154, 105–112. [Google Scholar]
- Mrifag, R.; Lemrabott, M.A.; El Kharrim, K.; Belghyti, D.; Basco, L.K. Setaria Labiatopapillosa (Filarioidea, Nematoda) in Moroccan Cattle: Atypical Localization and Morphological Characterization of Females and Microfilariae by Light and Scanning Electron Microscopy. Parasitol. Res. 2021, 120, 911–918. [Google Scholar] [CrossRef]
- Anderson, R.C. The Life Cycles of Dipetalonematid Nematodes (Filarioidea, Dipetalonematidae): The Problem of Their Evolution. J. Helminthol. 1957, 31, 203–224. [Google Scholar] [CrossRef]
- Notarnicola, J.; Jiménez, F.A.; Gardner, S.L. A New Species of Dipetalonema (Filarioidea: Onchocercidae) from Ateles Chamek from the Beni of Bolivia. J. Parasitol. 2007, 93, 661–667. [Google Scholar] [CrossRef]
- Martin, T.; Collins, G. Prevalence of Dirofilaria Immitis and Dipetalonema Reconditum in Greyhounds. Aust. Vet. J. 1985, 62, 159–163. [Google Scholar] [CrossRef]
- Kommu, S.; Fathima, L.; Murthy, G.S. Clinical Report on Canine Filariosis Due to Dipetalonema Reconditum. Pharma Innov. 2017, 6, 1019. [Google Scholar]
- Conga, D.F.; Mayor, P.; Furtado, A.P.; Giese, E.G.; Santos, J.N. dos Occurrence of Dipetalonema Gracile in a Wild Population of Woolly Monkey Lagothrix Poeppiigii in the Northeastern Peruvian Amazon. Rev. Bras. Parasitol. Veterinária 2018, 27, 154–160. [Google Scholar] [CrossRef]
- Zárate-Rendón, D.A.; Salazar-Espinoza, M.N.; Catalano, S.; Sobotyk, C.; Mendoza, A.P.; Rosenbaum, M.; Verocai, G. Molecular Characterization of Dipetalonema Yatesi from the Black-Faced Spider Monkey (Ateles Chamek) with Phylogenetic Inference of Relationships among Dipetalonema of Neotropical Primates. Int. J. Parasitol. Parasites Wildl. 2022, 17, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, R.; Azizi, S.; Nourollahifard, S.; Imani, M.; Kermani, R.S.; Hassanzadeh, S. Histopathologic and Histomorphometric Evaluation of Dipetalonema Evansi Infection in Camel Testicular Tissue. J. Parasit. Dis. 2021, 45, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Thean, J.; Maini, R. Dipetalonema Reconditum in the Human Eye. Br. J. Ophthalmol. 2001, 85, 1384. [Google Scholar] [CrossRef] [PubMed]
- Bruley, M.; Duron, O. Multi-Locus Sequence Analysis Unveils a Novel Genus of Filarial Nematodes Associated with Ticks in French Guiana. Parasite 2024, 31, 14. [Google Scholar] [CrossRef]
- Eberhard, M.L. Dipetalonema (Cercopithifilaria) Kenyensis Subgen. et Sp. n.(Nematoda: Filarioidea) from African Baboons, Papio Anubis. J. Parasitol. 1980, 66, 551–554. [Google Scholar] [CrossRef]
- Bobade, P.; Ojebuoboh, P.; Akinboade, O. A Case of Canine Filariasis Due to Dipetalonema Reconditum (Grassi 1889) in Nigeria. J. Small Anim. Pract. 1981, 22, 201–206. [Google Scholar] [CrossRef]
- Hashem, M. Parasitological, blood cellular and biochemical studies on filariasis of dogs. Vet. Med. J. Giza 2007, 55, 991–1003. [Google Scholar]
- Minnaar, W.N.; Krecek, R. Helminths in Dogs Belonging to People in a Resource-Limited Urban Community in Gauteng, South Africa. Onderstepoort J. Vet. Res. 2001, 68, 111–117. [Google Scholar]
- Van Heerden, J. Disease and Mortality of Captive Wild Dogs Lycaon Pictus. S. Afr. J. Wildl. Res.-24-Mon. Delayed Open Access 1986, 16, 7–11. [Google Scholar]
- Siwila, J.; Mwase, E.T.; Nejsum, P.; Simonsen, P.E. Filarial Infections in Domestic Dogs in Lusaka, Zambia. Vet. Parasitol. 2015, 210, 250–254. [Google Scholar] [CrossRef]
- Bwangamoi, O.; Isyagi, A. The Incidence of Filariasis and Babesiosis in Dogs in Uganda. Bull. Epizoot. Afrique 1973, 21, 33–37. [Google Scholar]
- Guerrero, R.; Martin, C.; Gardner, S.L.; Bain, O. New and Known Species of Litomosoides (Nematoda: Filarioidea): Important Adult and Larval Characters and Taxonomic Changes. Comp. Parasitol. 2002, 69, 177–195. [Google Scholar] [CrossRef]
- Bain, O.; Casiraghi, M.; Martin, C.; Uni, S. The Nematoda Filarioidea: Critical Analysis Linking Molecular and Traditional Approaches. Parasite 2008, 15, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Petit, G.; Schulz-Key, H.; Taylor, D.; Bain, O.; Goff, L.L.; Hoffmann, W.; Petit, G.; Schulz-Key, H.; Taylor, D.; et al. Litomosoides Sigmodontis in Mice: Reappraisal of an Old Model for Filarial Research. Parasitol. Today 2000, 16, 387–389. [Google Scholar] [CrossRef]
- Junker, K.; Barbuto, M.; Casiraghi, M.; Martin, C.; Uni, S.; Boomker, J.; Bain, O. Litomosa Chiropterorum Ortlepp, 1932 (Nematoda: Filarioidea) from a South African Miniopterid: Redescription, Wolbachia Screening and Phylogenetic Relationships with Litomosoides. Parasite 2009, 16, 43–50. [Google Scholar] [CrossRef]
- Ramasindrazana, B.; Dellagi, K.; Lagadec, E.; Randrianarivelojosia, M.; Goodman, S.M.; Tortosa, P. Diversity, Host Specialization, and Geographic Structure of Filarial Nematodes Infecting Malagasy Bats. PLoS ONE 2016, 11, e0145709. [Google Scholar] [CrossRef]
- Priyadarshini, D.R. Thelaziasis. In Textbook of Parasitic Zoonoses; Springer: Singapore, 2022; pp. 557–564. [Google Scholar]
- Patnool, R.B.; Mohammad, B.D.; Nagendra, V.H.; Prajapati, B.G.; Muhamadkazim, M.K.; Ponnusankar, S. Epidemiology and Current Treatment Trends in “Thelaziasis”. In Rising Contagious Diseases: Basics, Management, and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 396–410. [Google Scholar]
- Otašević, S.; Trenkic, B.M.; Tasić, A.; Petrović, A.; Petrović, V. Thelazia Callipaeda and Eye Infections. Acta Fac. Medicae Naissensis 2014, 31, 171–176. [Google Scholar] [CrossRef]
- Trenkić, M.; Tasić-Otašević, S.; Bezerra-Santos, M.A.; Stalević, M.; Petrović, A.; Otranto, D. Prevention of Thelazia Callipaeda Reinfection among Humans. Emerg. Infect. Dis. 2023, 29, 843. [Google Scholar] [CrossRef]
- Ryu, J.S.; Im, K.I.; Byun, Y.J.; Kim, S.C. A Case of Human Thelaziasis in Korea. Kisaengchunghak Chapchi 1987, 25, 83–84. [Google Scholar] [CrossRef]
- Hong, S.T.; Lee, S.H.; Shim, Y.B.; Choi, J.S.; Choe, J.K. A Human Case of Thelaziasis in Korea. Korean J. Parasitol. 1981, 19, 76–80. [Google Scholar] [CrossRef]
- Subba, S.H.; Shenga, D.O.; Sharma, T.; Choden, T.; Bhutia, K.D. Human Ocular Thelaziasis: First Case Report from Sikkim. Academia 2020. [Google Scholar] [CrossRef]
- Singh, T.; Singh, K.N. Thelaziasis: Report of Two Cases. Br. J. Ophthalmol. 1993, 77, 528. [Google Scholar] [CrossRef]
- Koyama, Y.; Ohira, A.; Kono, T.; YONEYAMA, T.; SHIWAKU, K. Five Cases of Thelaziasis. Br. J. Ophthalmol. 2000, 84, 439. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhang, P.; Li, F.; Li, Y.; Liu, Z.; Li, X. Thelaziasis in an Urban Woman in Beijing: A Case Report and Literature Review. BMC Ophthalmol. 2024, 24, 514. [Google Scholar] [CrossRef] [PubMed]
- do Vale, B.; Lopes, A.P.; da Conceição Fontes, M.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Systematic Review on Infection and Disease Caused by Thelazia Callipaeda in Europe: 2001–2020. Parasite 2020, 27, 52. [Google Scholar] [CrossRef]
- Marek, P. Thelaziosis in Tanzania. Vet. Rec. 1967, 80, 718. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19670802955 (accessed on 2 February 2025).
- Munang’andu, H.M.; Chembensofu, M.; Siamudaala, V.M.; Munyeme, M.; Matandiko, W. Thelazia Rhodesii in the African Buffalo, Syncerus Caffer, in Zambia. Korean J. Parasitol. 2011, 49, 91. [Google Scholar] [CrossRef]
- Gretillat, S.; Touré, S. First Studies on the Epidemiology of Bovine Thelaziasis and Determination of the Vector in West Africa. Comptes Rendus Hebdo-Madiares Des Seances De L’academie Des Sci. 1970, 270D, 239–241. [Google Scholar]
- Toure, S.; Vassiliades, G. Ocular Thelaziasis in Catte in Senegal. Bull. L’office Int. Epizooties 1971, 76, 711–716. [Google Scholar]
- Alemneh, T.; Dagnachew, S. Thelazia Species (Eyeworms) Infection in Cattle: Prevalence, Species Identification, Seasonal Dynamics and Its Ocular Effects in South Gondar Zone, Northwest Ethiopia. Vet. Parasitol. Reg. Stud. Rep. 2025, 61, 101254. [Google Scholar] [CrossRef]
- Round, M. The Helminth Parasites of Domesticated Animals in Kenya. J. Helminthol. 1962, 36, 375–449. [Google Scholar] [CrossRef] [PubMed]
- Ikeme, M. Kerato-Conjunctiv-Itis in Cattle in the Plateau Area of Northern Nigeria. A Study of Thelazia Rhodesii as a Possible Aetiological Agent. Bull. Epizoot. Dis. Afr. 1967, 15, 363–367. [Google Scholar]
- Schwartz, H.J.; Dioli, M.; Stimmelmayr, R.; Walsh, M. The One-Humped Camel (Camelus Dromedarius) in Eastern Africa. A Pictorial Guide to Diseases, Health Care and Management; Verlag Joseph Margraf: Weikersheim, Germany, 1992; ISBN 3-8236-1218-2. [Google Scholar] [CrossRef]
- Chartier, C.; Eboma, K. Ocular Thelaziasis of Cattle in Ituri (Zaire): Epidemiology and Clinical Studies. Rev. Med. Vet. 1988, 139, 1053–1058. [Google Scholar]
- Walker, M.L.; Becklund, W.W. Occurrence of a Cattle Eyeworm, Thelazia Gulosa (Nematoda: Thelaziidae), in an Imported Giraffe in California and T. Lacrymalis in a Native Horse in Maryland. J. Parasitol. 1971, 57, 1362–1363. [Google Scholar] [CrossRef] [PubMed]
- Bindernagel, J.A. Thelazia Rhodesi (Nematoda: Spiruroidea) in African Buffalo in Uganda, East Africa. J. Parasitol. 1972, 58, 594. [Google Scholar] [CrossRef]
- Grunenwald, C.M.; Butler, E.; Wünschmann, A.; Armien, A.G.; Carstensen, M.; Hildebrand, E.; Moon, R.D.; Gerhold, R.W. Emergence of the Arterial Worm Elaeophora Schneideri in Moose (Alces Alces) and Tabanid Fly Vectors in Northeastern Minnesota, USA. Parasit. Vectors 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Railliet, A.; Henry, A. Nematodes Vasculicoles Des Bovins Annamites. Bull. Sociiti Pathol. Exot. 1912, 5, 115–118. [Google Scholar]
- Anderson, R.C.; Chabaud, A.G.; Willmott, S.; Bureaux, C.A. CIH Keys to the Nematode Parasites of Vertebrates; Commonwealth Agricultural Bureaux Farnham Royal: Slough, UK, 1974; Volume 1. [Google Scholar]
- Pence, D. Elaeophorosis in Wild Ruminants. Bull. Soc. Vector Ecol. 1991, 16, 149–160. [Google Scholar]
- Huchzermeyer, F.; Penrith, M.; Elkan, P. Multifactorial Mortality in Bongos and Other Wild Ungulates in the North of the Congo Republic. Onderstepoort J. Vet. Res. 2001, 68, 263–269. [Google Scholar]
- Solismaa, M.; Laaksonen, S.; Nylund, M.; Pitkänen, E.; Airakorpi, R.; Oksanen, A. Filarioid Nematodes in Cattle, Sheep and Horses in Finland. Acta Vet. Scand. 2008, 50, 1–8. [Google Scholar] [CrossRef]
- Landau, I.; Boulard, Y.; Housin, R. Anthemosoma Garnhami Ngn Sp., 1st Dactylosomidae Known in Mammals. Comptes Rendus Hebd. Seances Acad. Sci. Ser. Sci. Nat. 1969, 268, 873–875. [Google Scholar]
- Kreier, J.; Baker, J.; Kreier, J.; Baker, J. Piroplasms. In Parasitic Protozoa; Springer: Dordrecht, The Netherlands, 1987; pp. 187–202. [Google Scholar]
- Landau, I. Études Au Laboratoire Sur Anthemosoma Garnhami Landau, Boulard, Houin 1969. J. Parasitol. 1970, 56, 199. [Google Scholar]
- Chavatte, J.-M.; Karadjian, G.; Landau, I. Half a Century after Its Discovery, New Insights on Anthemosoma Garnhami (Sporozoa, Piroplasmida): Morphology, Molecular Characterisation and Phylogenetic Position. Parasitol. Res. 2018, 117, 3917–3925. [Google Scholar] [CrossRef]
- Gunders, A. Anthemosoma sp. Isolated from Aethomys-Namaquensis in Namibia; Bureau Scientific publ: Pretoria, South Africa, 1985; Volume 81, p. 48. [Google Scholar]
- Stead, D.; du Plessis, D.; Sun, L.M.; Frean, J. Anthemosoma Garnhami in an HIV-Infected Man from Zimbabwe Living in South Africa. Emerg. Infect. Dis. 2021, 27, 1991–1993. [Google Scholar] [CrossRef]
- Wong, M.L.; Zulzahrin, Z.; Vythilingam, I.; Lau, Y.L.; Sam, I.-C.; Fong, M.Y.; Lee, W.-C. Perspectives of Vector Management in the Control and Elimination of Vector-Borne Zoonoses. Front. Microbiol. 2023, 14, 1135977. [Google Scholar] [CrossRef]
- Hay, S.I.; Battle, K.E.; Pigott, D.M.; Smith, D.L.; Moyes, C.L.; Bhatt, S.; Brownstein, J.S.; Collier, N.; Myers, M.F.; George, D.B. Global Mapping of Infectious Disease. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120250. [Google Scholar] [CrossRef] [PubMed]
- Akande, T.; Musa, I. Epidemiology of Malaria in Africa. Afr. J. Clin. Exp. Microbiol. 2005, 6, 107–111. [Google Scholar]
- Wamwiri, F.N.; Changasi, R.E. Tsetse Flies (Glossina) as Vectors of Human African Trypanosomiasis: A Review. BioMed Res. Int. 2016, 2016, 6201350. [Google Scholar] [CrossRef]
- Ready, P.D. Biology of Phlebotomine Sand Flies as Vectors of Disease Agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Pryce, J.; Medley, N.; Choi, L. Indoor Residual Spraying for Preventing Malaria in Communities Using Insecticide-treated Nets. Cochrane Database Syst. Rev. 2022, 1, CD012688. [Google Scholar]
- Karunaratne, S.H.P.P.; Silva, W.A.P.P.D.; Weeraratne, T.C.; Surendran, S.N. Insecticide Resistance in Mosquitoes: Development, Mechanisms and Monitoring. Ceylon J. Sci. 2018, 47, 299–309. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.; Bergoin, M.; Parker, A.G.; Maniania, N.K.; Vlak, J.M.; Bourtzis, K.; Boucias, D.G.; Aksoy, S. Improving Sterile Insect Technique (SIT) for Tsetse Flies through Research on Their Symbionts and Pathogens. J. Invertebr. Pathol. 2013, 112, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent Reservoirs of Future Zoonotic Diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Courtenay, O. Transmission, Reservoir Hosts and Control of Zoonotic Visceral Leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.E.; Mubanga, J.; Fevre, E.M.; Picozzi, K.; Eisler, M.C.; Thomas, R.; Welburn, S.C. Characterisation of the Wildlife Reservoir Community for Human and Animal Trypanosomiasis in the Luangwa Valley, Zambia. PLoS Negl. Trop. Dis. 2011, 5, e1211. [Google Scholar] [CrossRef]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of Environmental Change on Emerging Parasitic Diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- Gebre-Michael, T.; Malone, J.; McNally, K. Use of Geographic Information Systems in the Development of Prediction Models for Onchocerciasis Control in Ethiopia. Parassitologia 2005, 47, 135–144. [Google Scholar]
- Elagali, A.; Ahmed, A.; Makki, N.; Ismail, H.; Ajak, M.; Alene, K.A.; Weiss, D.J.; Mohammed, A.A.; Abubakr, M.; Cameron, E.; et al. Spatiotemporal Mapping of Malaria Incidence in Sudan Using Routine Surveillance Data. Sci. Rep. 2022, 12, 14114. [Google Scholar] [CrossRef]
- Abubakr, M.; Sami, H.; Mahdi, I.; Altahir, O.; Abdelbagi, H.; Mohamed, N.S.; Ahmed, A. The Phylodynamic and Spread of the Invasive Asian Malaria Vectors, Anopheles Stephensi, in Sudan. Biology 2022, 11, 409. [Google Scholar] [CrossRef]
- Xu, M.; Cao, C.; Li, Z.; Zhao, L. Editorial: Application of Spatial Information Technology in Infectious Disease Surveillance. Front. Public Health 2024, 12, 1435397. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.C.; Rodrigues, J.J.P.C.; de la Torre Díez, I.; López-Coronado, M.; Saleem, K. Mobile-Health: A Review of Current State in 2015. J. Biomed. Inform. 2015, 56, 265–272. [Google Scholar] [CrossRef]
- Liyew, B.; Tarekegn, G.E.; Kassew, T.; Tsegaye, N.; Asfaw, M.G.; Tilahun, A.D.; Tadesse, A.Z.; Alamneh, T.S. Individual and Community-Level Factors of Treatment-Seeking Behaviour among Caregivers with Febrile Children in Ethiopia: A Multilevel Analysis. PLoS ONE 2022, 17, e0264707. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.A.; AbdElbagi, H.; Fathelrahman, O.; Ahmed, A.E.; Ali, M.S.A.; Ahmed, M.A.; Osman, D.I.; Ali, Y.; Abubakr, M.; Siddig, E.E.; et al. Bridging the Knowledge Gap: Associations between Malaria Infections, Personally Used Prevention Measures, and Risk Factors in Al Gezira State, Sudan. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Siddig, E.E.; Ahmed, A.; Ahmed, E.S.; Mohammed, M.A.; Kunna, E.; El-Sadig, S.M.; Ali, Y.; Hassan, R.A.; Ali, E.T.; Mohamed, N.S. Knowledge and Attitudes towards Cervical Cancer Prevention among Women in Khartoum State, Sudan. Womens Health 2023, 19, 17455057231166286. [Google Scholar] [CrossRef]
- Ruppel, A.; Halim, M.I.; Kikon, R.; Mohamed, N.S.; Saebipour, M.R. Could COVID-19 Be Contained in Poor Populations by Herd Immunity Rather than by Strategies Designed for Affluent Societies or Potential Vaccine (s)? Glob. Health Action 2021, 14, 1863129. [Google Scholar] [CrossRef]
- Ahmed, A.; Elbashir, A.; Mohamed, A.A.; Alim, A.A.; Mubarak, A.; Abdelrahman, D.; Mohammed, E.; Mohamed, N.S.; Elaagip, A.H.; Zarroug, I.M. Socioeconomic Impacts of Elimination of Onchocerciasis in Abu-Hamed Focus, Northern Sudan: Lessons after Elimination. BMC Res. Notes 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Ahmed, A.; Siddig, E.E.; Mohamed, N.S. The Role of Integrated Programs in the Prevention of COVID-19 in a Humanitarian Setting. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 193–196. [Google Scholar] [CrossRef]
- Ahmed, A. Urgent Call for a Global Enforcement of the Public Sharing of Health Emergencies Data: Lesson Learned from Serious Arboviral Disease Epidemics in Sudan. Int. Health 2020, 12, 238–240. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Ali, Y.; Siddig, E.E.; Ahmed, A. Assessment of the COVID-19 Surveillance System in Sudan: Performance, Limitations, and Recommendations. Am. J. Trop. Med. Hyg. 2024, 111, 1093–1096. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Ali, Y.; Abdalrahman, S.; Ahmed, A.; Siddig, E.E. The Use of Cholera Oral Vaccine for Containment of the 2019 Disease Outbreak in Sudan. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From “One Medicine” to “One Health” and Systemic Approaches to Health and Well-Being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S. Chapter 271—Zoonotic Agents, Arthropod-Borne. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 1065–1068. ISBN 978-0-12-374144-8. [Google Scholar]
- Waldetensai, A.; Abose, E.; Getachew, A.; Tadesse, H.; Meharenet, B.; Eukubay, A.; Kinde, S.; Kassahun, A.; Gonfa, G.; Kore, K.; et al. Exploring the Factors Driving the Recurrence of Human African Trypanosomiasis in Ethiopia after Three Decades. Ethiop. J. Public Health Nutr. EJPHN 2024, 7, 41–51. [Google Scholar] [CrossRef]
- Cantey, P.T.; Weeks, J.; Edwards, M.; Rao, S.; Ostovar, G.A.; Dehority, W.; Alzona, M.; Swoboda, S.; Christiaens, B.; Ballan, W.; et al. The Emergence of Zoonotic Onchocerca Lupi Infection in the United States--A Case-Series. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 778–783. [Google Scholar] [CrossRef]
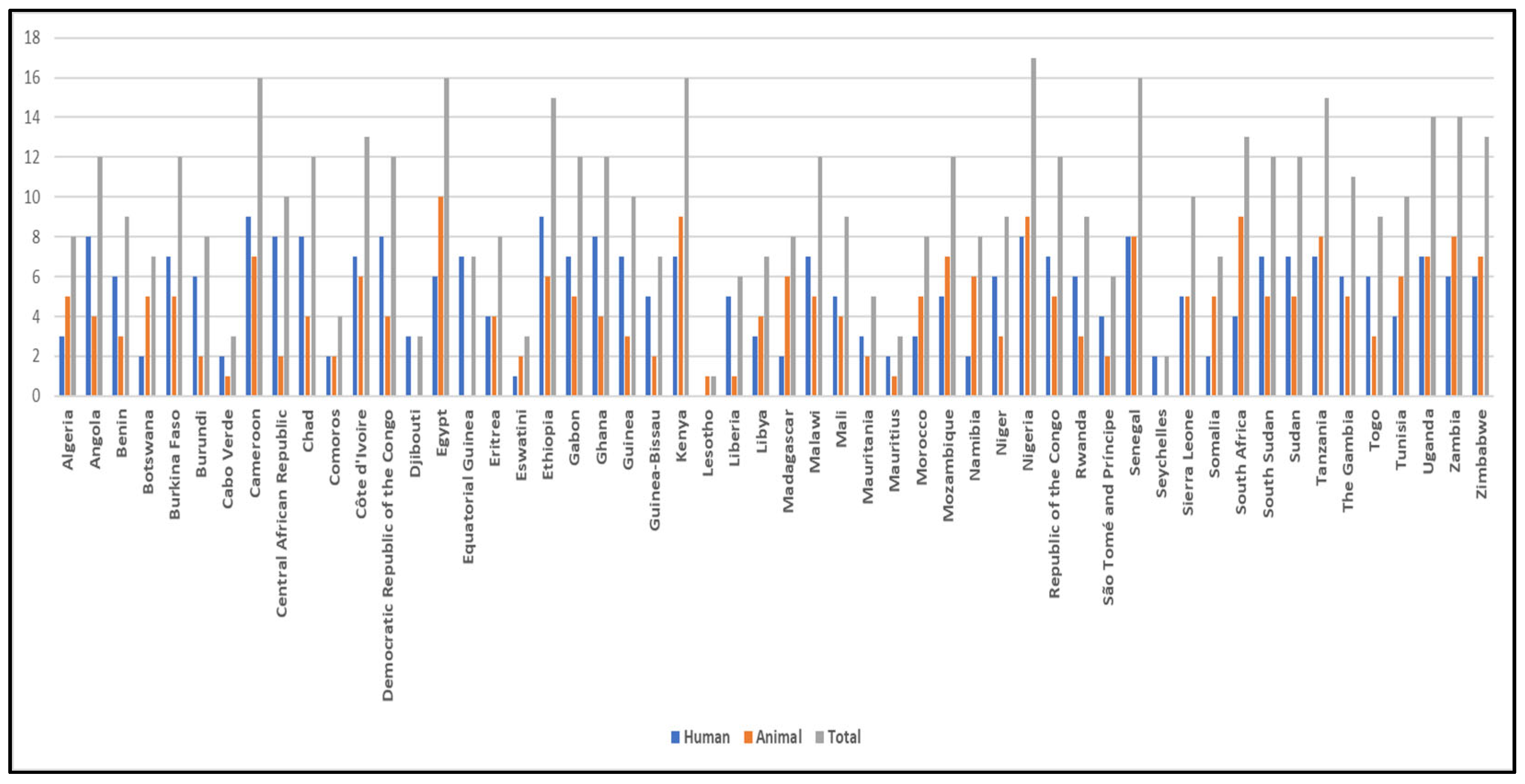
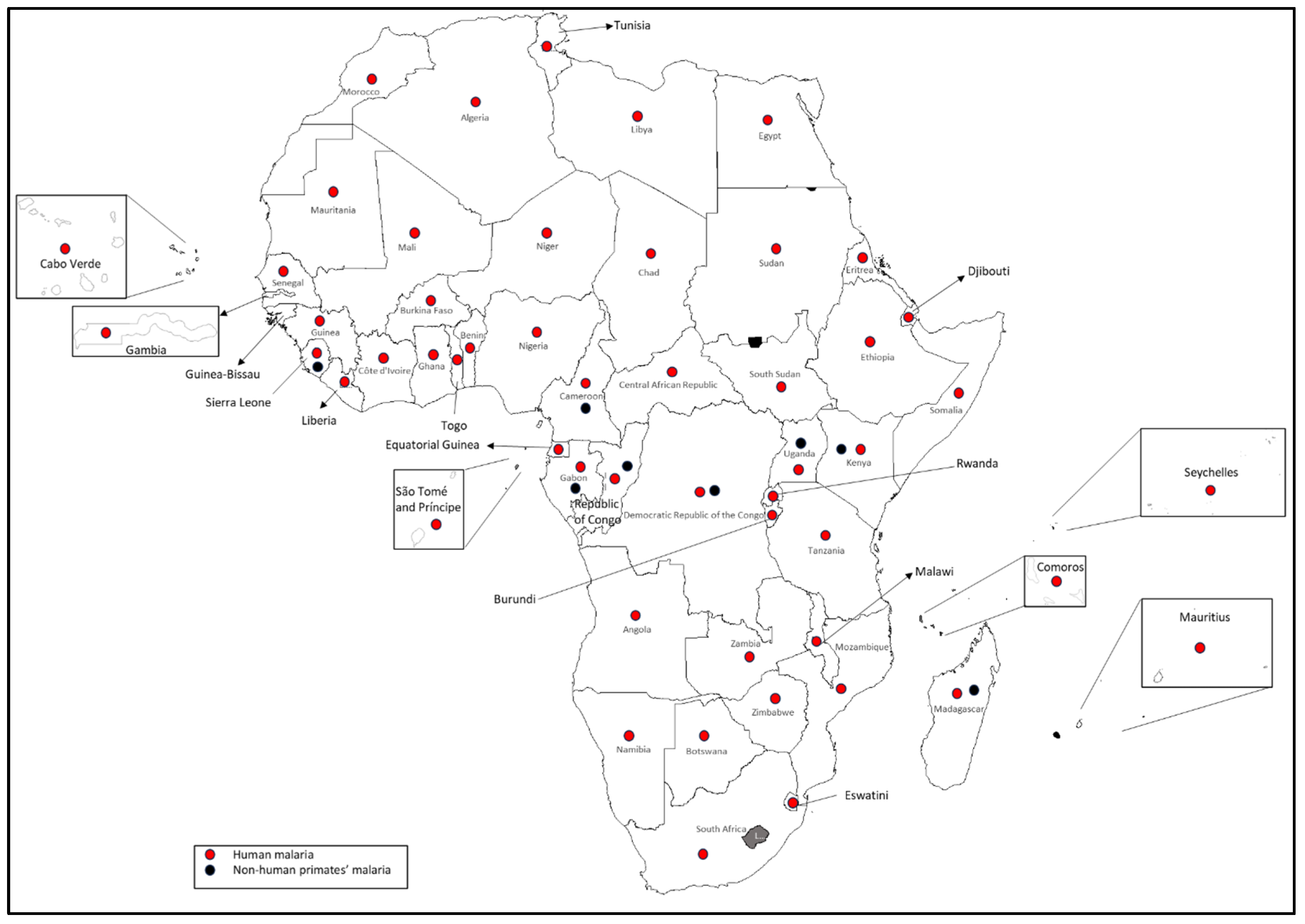
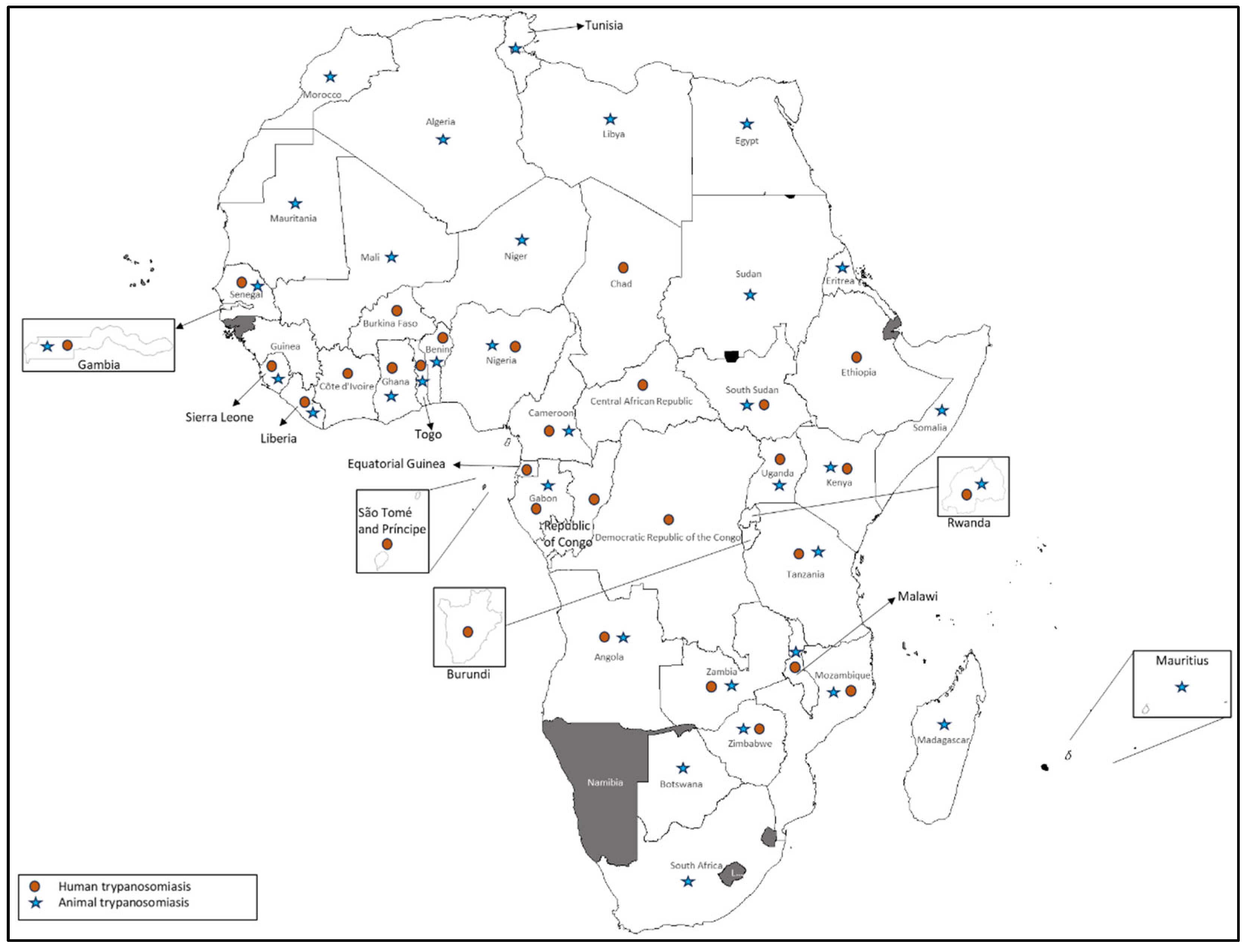
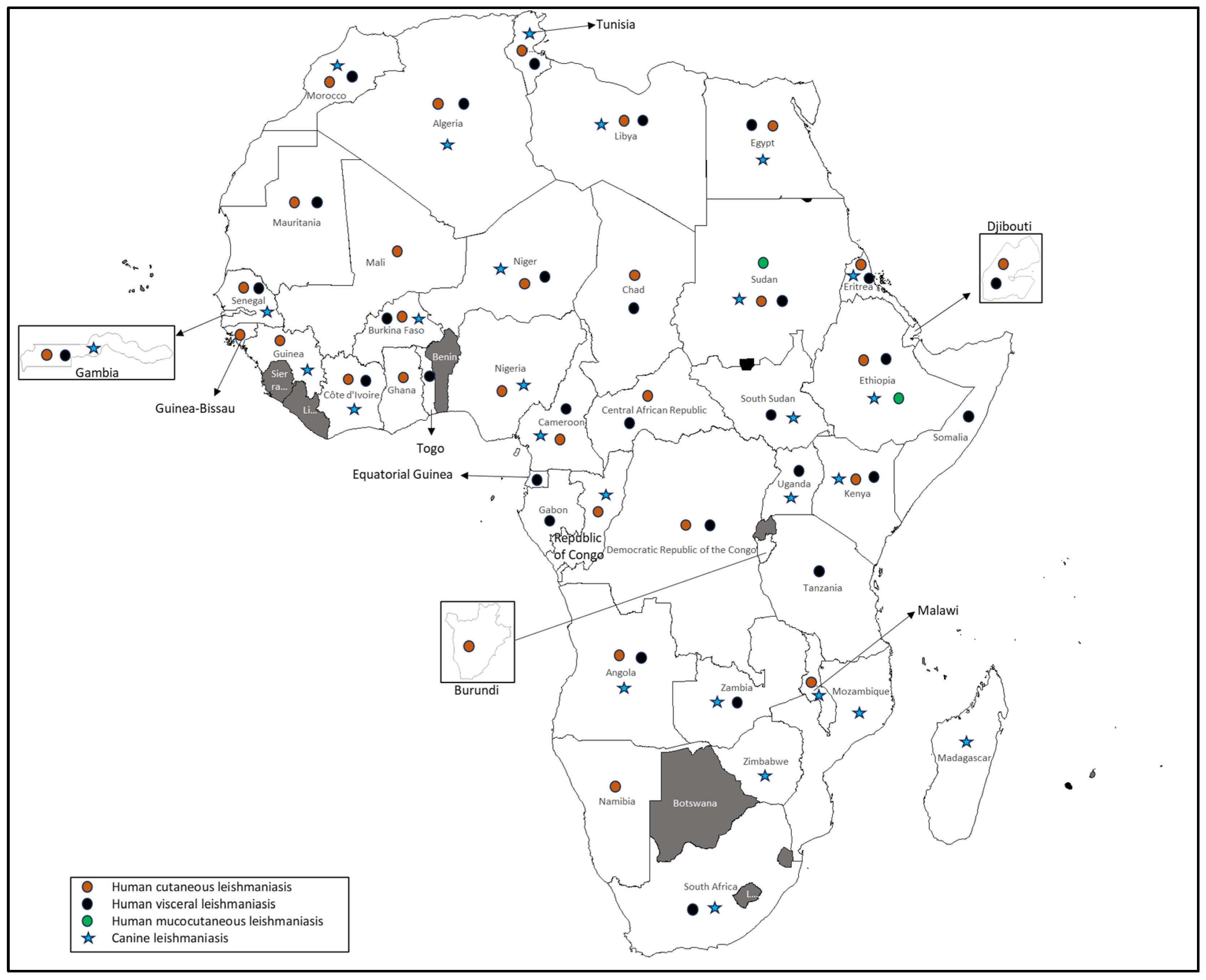

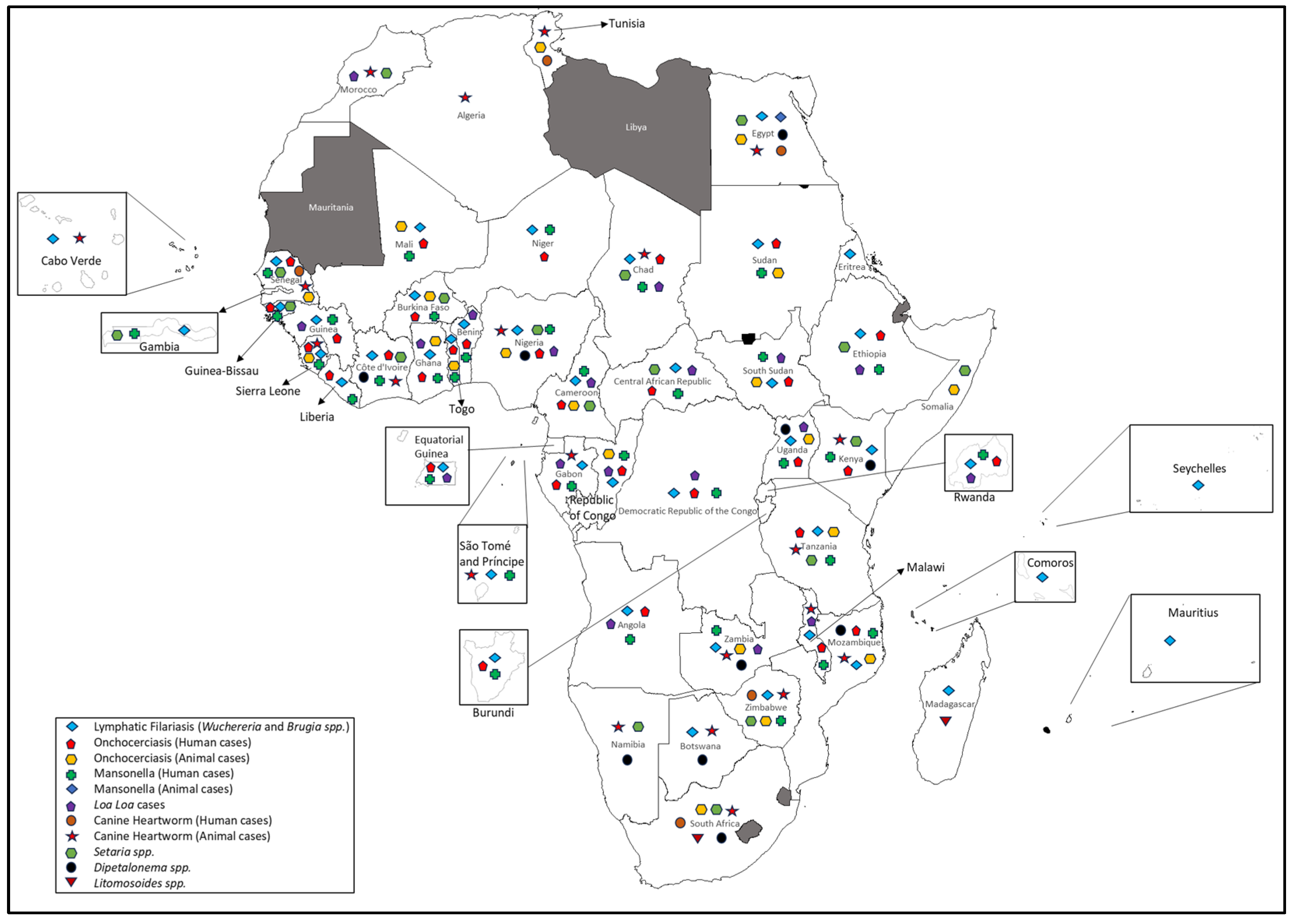
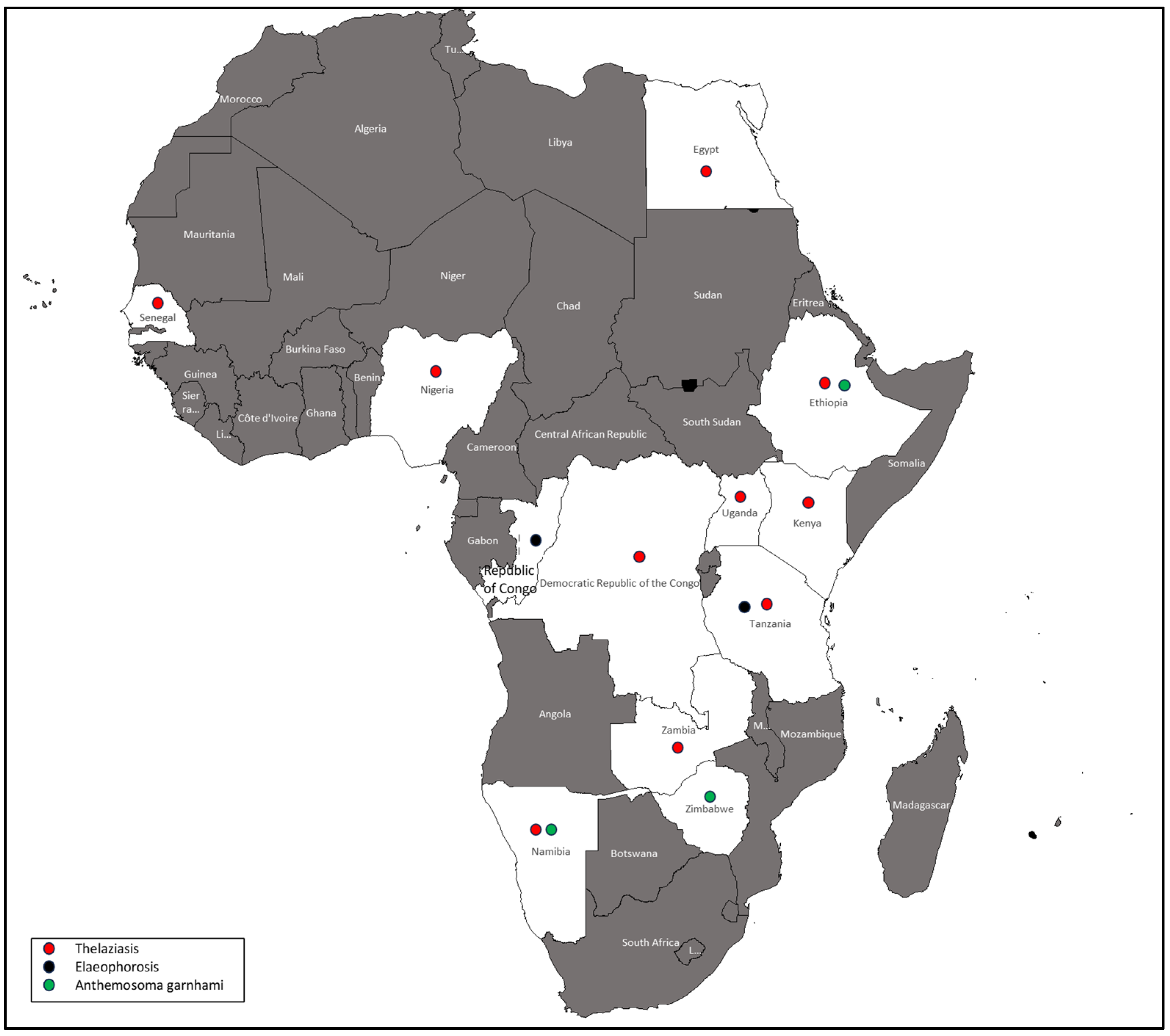
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Siddig, E.E.; Mohamed, N.S. Arthropod-Borne Zoonotic Parasitic Diseases in Africa: Existing Burden, Diversity, and the Risk of Re-Emergence. Parasitologia 2025, 5, 29. https://doi.org/10.3390/parasitologia5030029
Ahmed A, Siddig EE, Mohamed NS. Arthropod-Borne Zoonotic Parasitic Diseases in Africa: Existing Burden, Diversity, and the Risk of Re-Emergence. Parasitologia. 2025; 5(3):29. https://doi.org/10.3390/parasitologia5030029
Chicago/Turabian StyleAhmed, Ayman, Emmanuel Edwar Siddig, and Nouh Saad Mohamed. 2025. "Arthropod-Borne Zoonotic Parasitic Diseases in Africa: Existing Burden, Diversity, and the Risk of Re-Emergence" Parasitologia 5, no. 3: 29. https://doi.org/10.3390/parasitologia5030029
APA StyleAhmed, A., Siddig, E. E., & Mohamed, N. S. (2025). Arthropod-Borne Zoonotic Parasitic Diseases in Africa: Existing Burden, Diversity, and the Risk of Re-Emergence. Parasitologia, 5(3), 29. https://doi.org/10.3390/parasitologia5030029








