First Report of Troglotrema acutum Trematoda Parasitizing a European Polecat (Mustela putorius) in Bulgaria
Abstract
:1. Introduction
2. Material and Methods
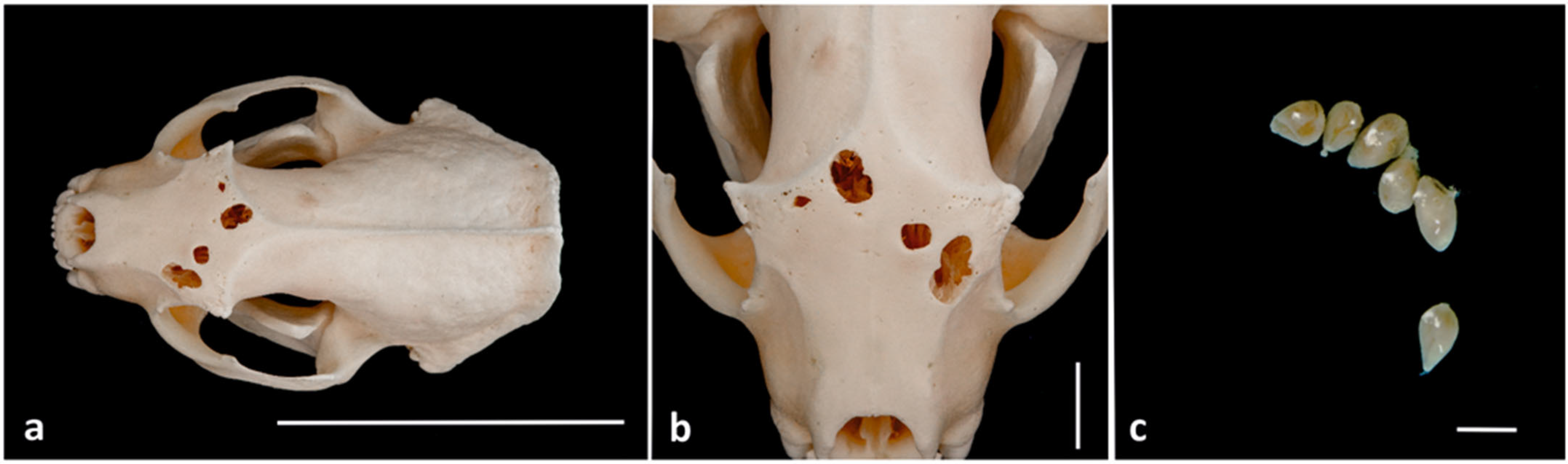
3. Results
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kierdorf, U.; Kierdorf, H.; Konjevie, D.; Lanzar, P. Remarks on cranial lesions in the European polecat (Mustela putorius) caused by helminth parasites. Vet. Arch. 2006, 76, S101–S109. [Google Scholar]
- Ribas, A.; Molina-Vacas, G.; Boadella, M.; Rodriguez-Teijeiro, J.D.; Fernandez-Cardo, R.; Arrizabalaga, A. First report of Troglotrema acutum (Digenea, Troglotrematidae) in the European badger Meles meles in the Iberian Peninsula and presumptive lesions caused in the host. J. Helminthol. 2012, 86, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Frantz, A.C.; Jenrich, J.; Müller, F. Dissections of fresh skulls confirm low prevalence of Troglotrema acutum (Trematoda: Troglotrematidae) in German badgers (Meles meles). Parasitol. Res. 2015, 114, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Heddergott, M.; Steffen, C.; Steinbach, P.; Frantz, A.C. First record of Troglotrema acutum (Trematoda, Troglotrematidae) in European polecats Mustela putorius from Luxembourg. Parasitol. Res. 2021, 120, 2659–2663. [Google Scholar] [CrossRef]
- Frantz, A.C.; Cantú Salazar, L.; Müller, F.; Steinbach, P.; Wittische, J.; Heddergott, M. Interactions of cranial helminths in the European polecat (Mustela putorius): Implications for host body condition. Int. J. Parasitol. Parasites Wildl. 2022, 18, 273–282. [Google Scholar] [CrossRef]
- Koubek, P.; Baruš, V.; Koubkova, B. Troglotrema acutum (Digenea) from carnivores in the Czech Republic. Helminthologia 2004, 41, 25–31. [Google Scholar]
- Vogel, H.; Voelker, J. Über den Lebenszyklus von Troglotrema acutum. Tropenmed. Parasitol. 1978, 29, 385–405. [Google Scholar]
- Skrjabin, K.I. Trematodes of Animals and Humans; Principles of Trematology; Izdatelstvo Akademii Nauk: Moscow, Rusia, 1949; Volume 3, p. 600. (In Russian) [Google Scholar]
- Yamaguti, S. Synopsis of Digenetic Trematodes of Vertebrates; Keigaku Publishing: Tokyo, Japan, 1971; Volumes I and II. [Google Scholar]
- Torres, J.; Feliu, C.; Miquel, J.; Casanova, J.C.; Garcia-Perea, R.; Gisbert, J. Helmitofauna de Mustela putorius Linnaeus, 1758 (Carnivora: Mustelidae) en la peninsula Iberica. Bol. Soc. Hist. Nat. Balear. 1996, 39, 155–165. [Google Scholar]
- Moniez, R. Sur un parasite (Distoma acutum F. S. Leuckart) qui vit dans l’os ethmoide et dans les sinus frontaux du putois. In Revue Biologique du Nord de la France; Le Bigot: Lille, France, 1890; Volume 2, p. 242. [Google Scholar]
- Artois, M.; Blancou, J.; Gerad, Y. Parasitisme du putois (Mustela putorius) par Troglotrema acutum. Rev. Med. Vet. Toulouse 1982, 133, 771–777. [Google Scholar]
- Muller, A. Les Infestations a Skrjabingylus spp. Chez les Mustelides de l’Est de la France. Ph.D. Thesis, Ecole Normale Superieure de Lyon (ENV), Lyon, France, 1989. [Google Scholar]
- Torres, J.; Miquel, J.; Founier, P.; Founier-Chambrillon, C.; Liberge, M.; Fons, R.; Feliu, C. Helminth communities of the autochthonous mustelids Mustela lutreola and M. putorius and the introduced Mustela vison in south-western France. J. Helminthol. 2008, 82, 349–355. [Google Scholar] [CrossRef]
- Müller, F.; Heddergott, M. Befall des Europäischen Iltis Mustela putorius (Mustelidae) mit Troglotrema acutum (Trematoda) und Skrjabingylus nasicola (Nematoda) in der Region Osthessen und der angrenzenden Rhön (Bayern, Hessen und Thüringen). Beitr. Jagd Wildforsch. 2009, 34, 367–383. [Google Scholar]
- Heddergott, M.; Müller, F. First record of Troglotrema acutum (Trematoda: Troglotrematidae) from a pine marten Martes martes in Germany. J. Parasit. Dis. 2020, 44, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wegelin, H. Merkwürdige Nasenparasiten des Iltis, Putorius foetorius Cuv. Mitt. Thurgau. Nat. Forsch. Ges. 1930, 28, 159–166. [Google Scholar]
- Baer, J.G. Quelques helminthes rares ou peu connus du putois. Rev. Suisse Zool. 1931, 38, 313–334. [Google Scholar]
- Mermod, C.; Debrot, S.; Marchesi, P.; Weber, J.M. Le putois (Mustela putorius L.) en Suisse romande. Rev. Suisse Zool. 1983, 90, 847–856. [Google Scholar] [CrossRef]
- Schumacher, S. Distoma acutum, ein Parasit in den Nebenhöhlen der Nase des Iltis. Osterreich. Waidw. 1929, 2, 362–364. [Google Scholar]
- Kerschagl, W. Jahresbericht über die Untersuchungen kranken Wildes im Jahre 1930. St. Hubertus 1931, 17, 54–56. [Google Scholar]
- Kerschagl, W. Jahresbericht über die Untersuchungen kranken Wildes im Jahre 1932. St. Hubertus 1933, 19, 51–53. [Google Scholar]
- Duscher, G.G.; Harl, J.; Fuehrer, H.P. Evidence of Troglotrema acutum and Skrjabingylus sp. coinfection in a polecat from Lower Austria. Helminthologia 2015, 52, 63–66. [Google Scholar] [CrossRef]
- Marconcini, A.; Tasselli, E. Trematodi e nematodi reperiti nella puzzola (Putorius putorius) in Toscana. Ann. Fac. Med. Vet. Pisa Univ. 1969, 22, 203–216. [Google Scholar]
- Mituch, J.; Hovorka, J.; Hovorka, I.; Vilagiova, I. Helminty masožrave zveri (Carnivora) v modelovom uzemi Tatranskeho Narodneho parku. Folia Venatoria 1992, 22, 191–200. [Google Scholar]
- Demuth, J.; Hromada, M.; Kraeczyk, A.J.; Malecha, A.W.; Tobolka, M.; Tryjanowski, P. Cranial lesions caused by helminth parasites and morphological traits in the European polecat Mustela putorius. Helminthologia 2009, 46, 85–89. [Google Scholar] [CrossRef]
- Ullrich, K. Über das Vorkommen von seltenen oder wenig bekannten Parasiten der Säugetiere und Vögel in Böhmen und Mähren. Prag. Arch. Tiermed. 1930, 10, 19–43. [Google Scholar]
- Stanĕk, M. Dalši nalezy cizopashnych červů u šelem na uzemi ČSSR. Zool. Listy 1963, 12, 359–362. [Google Scholar]
- Svatoš, I. Doplňky k helmintofaunĕ nĕkterych volnĕ žijicich šelem. Zool. Listy 1993, 12, 173–175. [Google Scholar]
- Mituch, J. Helmintofauna masozravcov na Slovensku a v CSSR. Folia Venatoria 1972, 2, 161–171. [Google Scholar]
- Rajsky, D.; Porubčansky, S. Rozširenie cicavice nosnej Troglotrema acutum u lasicovitych šeliem na Slovensku. Veterinařstvi 1989, 39, 78–79. [Google Scholar]
- Vasarhelyi, S. Distoma acutum Leuckart von Ungarn. Zool 1941, 135, 265–270. [Google Scholar]
- Müller, F.; Heddergott, M. Nachweismethodik für einen Befall von Musteliden mit Troglotrema acutum (Trematoda) und Skrjabingylus spp. (Nematoda). Beitr. Jagd Wildforsch. 2009, 34, 385–390. [Google Scholar]
- Heddergott, M. First record of Skrjabingylus petrowi (Nematoda: Metastrongyloidea) in a pine marten (Martes martes) in Germany. Eur. J. Wildl. Res. 2009, 55, 543–546. [Google Scholar] [CrossRef]
- Leuckart, F.S. Zoologische Bruckstücke. III. Helminthologische Beiträge; Forgotten Books: Freiburg, Germany, 1842; p. 60. [Google Scholar]
- Heddergott, M.; Pohl, D.; Steinbach, P.; Cantú Salazar, L.; Müller, F.; Frantz, A.C. Determinants and effects of sinus worm Skrjabingylus nasicola (Nematoda: Metastrongyloidae) infestation in invasive American mink Neovison vison in Germany. Parasitol. Res. 2016, 115, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Glöer, P.; Georgiev, D. Bulgaria, a hot spot of biodiversity (Gastropoda: Rissooidea)? J. Conchol. 2011, 40, 489. [Google Scholar]
- Klupiec, P. Die Helminthenfauna des Iltis (Mustela putorius L.) in seinem nordwestdeutschen Verbreitungsgebiet. Ph.D. Thesis, Veterinarmedizinische Hochschule, Hannover, Germany, 2001. [Google Scholar]
- Pohl, L. Über das Vorkommen von Distoma acutum Leuck. bei Putorius putorius L. Jena Z. Naturw. 1912, 48, 563–568. [Google Scholar]
- Olt, A. Untersuchungen von Iltisköpfen auf Parasiten in der Nasen-und Stirnhöhle. Dtsch. Jagerztg. 1929, 93, 83–84. [Google Scholar]
- Lehmensick, R. Über die Veränderungen am Iltis Schädel durch den Befall mit Troglotrema acutum. Z. Parasitenkd. 1942, 12, 659–664. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heddergott, M. First Report of Troglotrema acutum Trematoda Parasitizing a European Polecat (Mustela putorius) in Bulgaria. Parasitologia 2024, 4, 369-374. https://doi.org/10.3390/parasitologia4040032
Heddergott M. First Report of Troglotrema acutum Trematoda Parasitizing a European Polecat (Mustela putorius) in Bulgaria. Parasitologia. 2024; 4(4):369-374. https://doi.org/10.3390/parasitologia4040032
Chicago/Turabian StyleHeddergott, Mike. 2024. "First Report of Troglotrema acutum Trematoda Parasitizing a European Polecat (Mustela putorius) in Bulgaria" Parasitologia 4, no. 4: 369-374. https://doi.org/10.3390/parasitologia4040032
APA StyleHeddergott, M. (2024). First Report of Troglotrema acutum Trematoda Parasitizing a European Polecat (Mustela putorius) in Bulgaria. Parasitologia, 4(4), 369-374. https://doi.org/10.3390/parasitologia4040032





