Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA
Abstract
1. Introduction
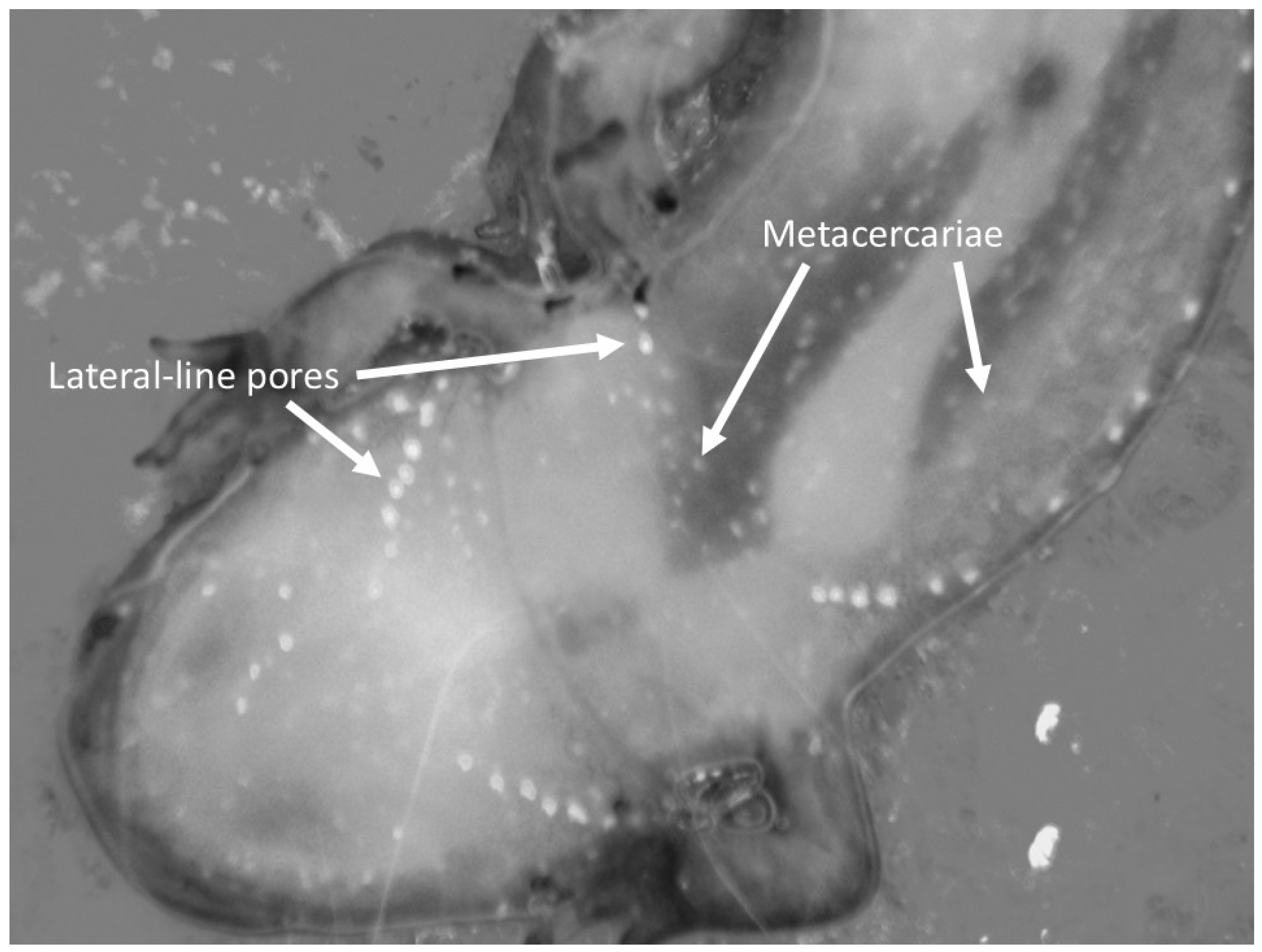
2. Materials and Methods
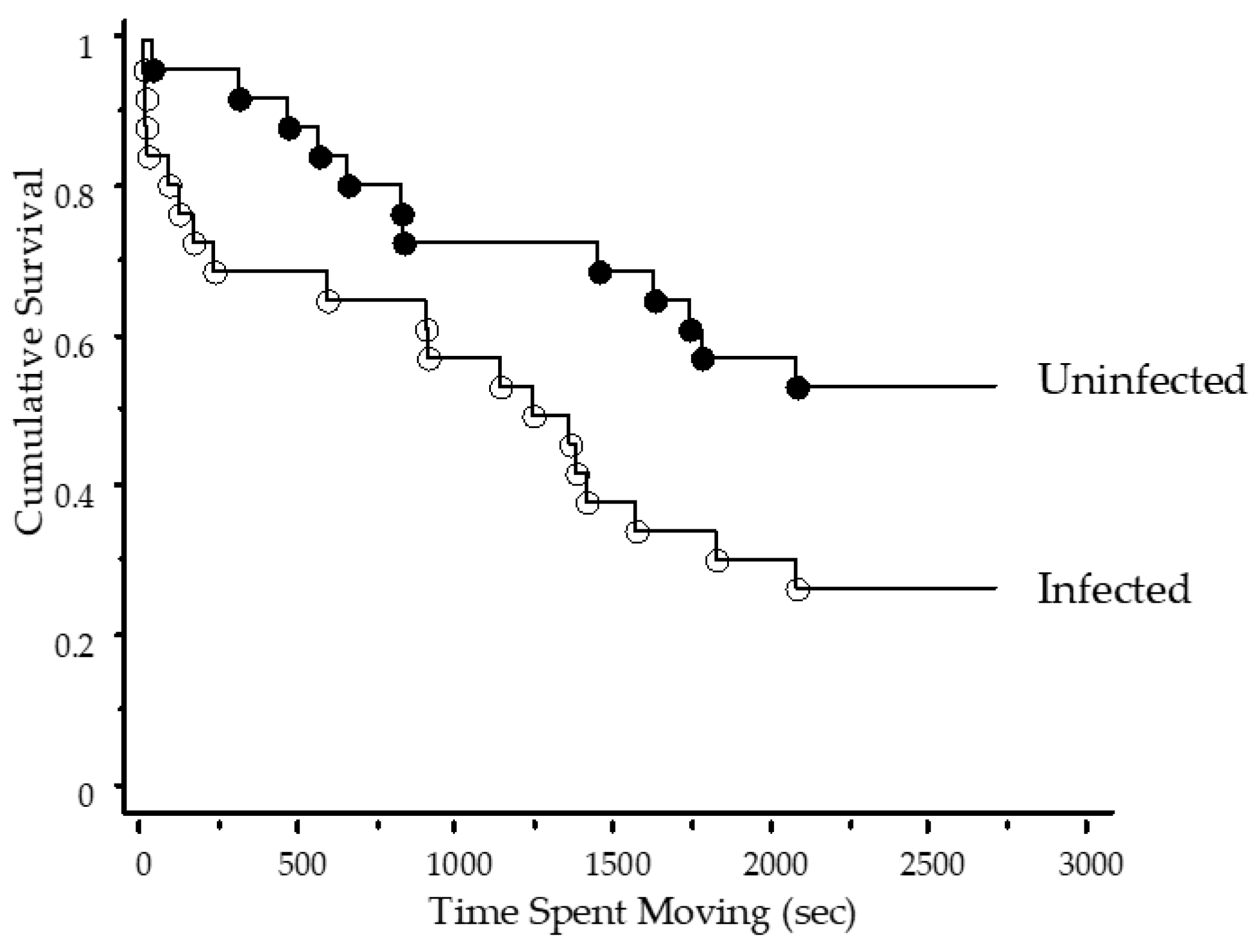
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez-Caballero, I.; Garcia-Longoria, L.; Gomez-Mestre, I.; Marzal, A. The Adaptive Host Manipulation Hypothesis: Parasites Modify the Behaviour, Morphology, and Physiology of Amphibians. Diversity 2022, 14, 739. [Google Scholar] [CrossRef]
- Goodman, B.A.; Johnson, P.T.J. Disease and the Extended Phenotype: Parasites Control Host Performance and Survival through Induced Changes in Body Plan. PLoS ONE 2011, 6, e20193. [Google Scholar] [CrossRef]
- Binning, S.A.; Shaw, A.K.; Roche, D.G. Parasites and Host Performance: Incorporating Infection into Our Understanding of Animal Movement. Integr. Comp. Biol. 2017, 57, 267–280. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological Annihilation via the Ongoing Sixth Mass Extinction Signaled by Vertebrate Population Losses and Declines. Proc. Natl. Acad. Sci. USA 2017, 114, 6089–6096. [Google Scholar] [CrossRef]
- Hussain, Q.A.; Pandit, A.K. Global Amphibian Declines: A Review. Int. J. Biodivers. Conserv. 2012, 4, 348–357. [Google Scholar]
- Stopper, G.F.; Hecker, L.; Franssen, R.A.; Sessions, S.K. How Trematodes Cause Limb Deformities in Amphibians. J. Exp. Zool. (Mol. Dev. Evol.) 2002, 294, 252–263. [Google Scholar] [CrossRef]
- Dubois, A. Rostand’s Anomaly P in Palaearctic Green Frogs (Pelophylax) and Similar Anomalies in Amphibians. Mertensiella 2017, 25, 49–56. [Google Scholar]
- Henle, K.; Dubois, A.; Vershinin, V. A Review of Anomalies in Natural Populations of Amphibians and Their Potential Causes. Studies on Anomalies in Natural Populations of Amphibians. Mertensiella 2017, 25, 57–164. [Google Scholar]
- Svinin, A.O.; Bashinskiy, I.W.; Litvinchuk, S.N.; Ermakov, O.A.; Ivanov, A.Y.; Neymark, L.A.; Vedernikov, A.A.; Osipov, V.V.; Drobot, G.P.; Dubois, A. Strigea robusta Causes Polydactyly and Severe Forms of Rostand’s Anomaly P in Water Frogs. Parasit. Vectors 2020, 13, 381. [Google Scholar] [CrossRef]
- Svinin, A.O.; Matushkina, K.A.; Dedukh, D.V.; Bashinskiy, I.V.; Ermakov, O.A.; Litvinchuk, S.N. Strigea robusta (Digenea: Strigeidae) Infection Effects on the Gonadal Structure and Limb Malformation in Toad Early Development. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 675–686. [Google Scholar] [CrossRef]
- Koprivnikar, J.; Marcogliese, D.J.; Rohr, J.R.; Orlofske, S.A.; Raffel, T.R.; Johnson, P.T.J. Macroparasite Infections of Amphibians: What Can They Tell Us? Ecohealth 2012, 9, 342–360. [Google Scholar] [CrossRef]
- Bower, D.S.; Brannelly, L.A.; McDonald, C.A.; Webb, R.J.; Greenspan, S.E.; Vickers, M.; Gardner, M.G.; Greenlees, M.J. A Review of the Role of Parasites in the Ecology of Reptiles and Amphibians. Austral Ecol. 2018, 44, 433–448. [Google Scholar] [CrossRef]
- Shen, X.; Liang, D.; Chen, M.; Mao, R.; Wake, D.B.; Zhang, P. Enlarged Multilocus Data Set Provides Surprisingly Younger Time of Origin for the Plethodontidae, the Largest Family of Salamanders. Syst. Biol. 2016, 65, 66–81. [Google Scholar] [CrossRef]
- Feder, M.E. Integrating the Ecology and Physiology of Plethodontid Salamanders. Herpetologica 1983, 39, 291–310. [Google Scholar]
- Falcón-Ordaz, J.; Windfield-Pérez, J.C.; Mendoza-Garfias, B.; Parra-Olea, G.; de León, P.-P. Cosmocerca acanthurum n. sp. (Nematoda: Cosmocercidae) in Pseudoeurycea leprosa and Chiropterotriton orculus from the Transmexican Volcanic Belt, Central Mexico, with a Checklist of the Helminth Parasites of Plethodontid Salamanders. Zootaxa 2007, 1434, 27–49. [Google Scholar] [CrossRef]
- Pyron, R.A.; Beamer, D.A. Nomenclatural Solutions for Diagnosing ‘Cryptic’ Species using Molecular and Morphological Data Facilitate a Taxonomic Revision of the Black-bellied Salamanders (Urodela, Desmognathus ‘quadramaculatus’) from the Southern Appalachian Mountains. Bionomina 2022, 27, 1–43. [Google Scholar] [CrossRef]
- Austin, R.M., Jr.; Camp, C.D. Larval Development of Black-Bellied Salamanders, Desmognathus quadramaculatus, in Northeastern Georgia. Herpetologica 1992, 48, 313–317. [Google Scholar]
- Bruce, R.C. Life History Variation in the Salamander Desmognathus quadramaculatus. Herpetologica 1988, 44, 218–227. [Google Scholar]
- Bruce, R.C.; Castanet, J.; Francillon-Vieillot, H. Skeletochronological Analysis of Variation in Age Structure, Body Size, and Life History in Three Species of Desmognathine Salamanders. Herpetologica 2002, 58, 181–193. [Google Scholar] [CrossRef]
- Belden, L.K.; Peterman, W.E.; Smith, S.A.; Brooks, L.R.; Benfield, E.F.; Black, W.P.; Yang, Z.; Wojdak, J.M. Metagonimoides oregonensis (Heterophyidae: Digenea) Infection in Pleurocerid Snails and Desmognathus quadramaculatus Salamander Larvae in Southern Appalachian Streams. J. Parasitol. 2012, 98, 760–767. [Google Scholar] [CrossRef]
- Burns, W.C.; Pratt, I. The Life Cycle of Metagonimoides oregonensis Price (Trematoda: Heterophyidae). J. Parasitol. 1953, 39, 60–69. [Google Scholar] [CrossRef]
- Wyderko, J.A.; Benfield, E.F.; Maerz, J.C.; Cecala, K.C.; Belden, L.K. Variable Infection of Stream Salamanders in the Southern Appalachians by the Trematode Metagonimoides oregonensis (family: Heterophyidae). Parasitol. Res. 2015, 114, 3159–3165. [Google Scholar] [CrossRef]
- Goater, T.M.; Esch, G.W.; Bush, A.O. Helminth Parasites of Sympatric Salamanders: Ecological Concepts at Infracommunity, Component and Compound Community Levels. Am. Midl. Nat. 1987, 118, 289–300. [Google Scholar] [CrossRef]
- Camp, C.D.; Jones, D.; Phillips, J.; Brock, T.L.; Wooten, J.A. Differential Infection of Two Sympatric, Cryptic Species of Appalachian Salamander (Genus Desmognathus) by the Trematode Metagonimoides oregonensis. Comp. Parasitol. 2021, 88, 183–186. [Google Scholar] [CrossRef]
- Ward, J.K.; Hoare, D.J.; Couzin, I.D.; Broom, M.; Krause, J. The Effects of Parasitism and Body Length on Positioning within Wild Fish Shoals. J. Anim. Ecol. 2002, 71, 10–14. [Google Scholar] [CrossRef]
- Kework, C.A.; Ash, J.A.; Camp, C.D. Factors Affecting Infection Levels in the Salamander Host Desmognathus amphileucus by a Digenetic Trematode Within Appalachian Headwater Streams. Herpetologica 2024, 80, 112–116. [Google Scholar] [CrossRef]
- Camp, C.D. The Status of the Black-Bellied Salamander (Desmognathus quadramaculatus) as a Predator of Heterospecific Salamanders in Appalachian Streams. J. Herpetol. 1997, 31, 613–616. [Google Scholar] [CrossRef]
- Marsh, D.M.; Thakur, K.A.; Bulka, K.C.; Clarke, L.B. Dispersal and Colonization through Open Fields by a Terrestrial, Woodland Salamander. Ecology 2004, 85, 3396–3405. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.; Ly, A.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. JASP: Graphical Statistical Software for Common Statistical Designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Etikan, İ.; Abubakar, S.; Alkassim, R. The Kaplan Meier Estimate in Survival Analysis. Biom. Biostat. Int. J. 2017, 5, 00128. [Google Scholar] [CrossRef]
- Muñoz, G.B.; Rebolledo, M.; Landaeta, M.F. Effects of Metacercariae of Prosorhynchoides sp. (Trematoda: Bucephalidae) on the Swimming Ability and Blood Parameters of the Intertidal Fish Girella laevifrons (Osteichthyes: Kyphosidae). Exp. Parasitol. 2023, 246, 108473. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.D.; Dickinson, T.E. Ribeiroia ondatrae Causes Limb Abnormalities in a Canadian Amphibian Community. Can. J. Zool. 2012, 90, 808–814. [Google Scholar] [CrossRef]
- Marvin, G.A. Effect of Caudal Autotomy on Aquatic and Terrestrial Locomotor Performance in Two Desmognathine Salamander Species. Copeia 2010, 2010, 468–474. [Google Scholar] [CrossRef]
- Landberg, T.; Azizi, E. Ontogeny of Escape Swimming Performance in the Spotted Salamander. Funct. Ecol. 2010, 24, 576–587. [Google Scholar] [CrossRef]
- Ijspeert, A.J.; Crespi, A.; Ryczko, D.; Cabelguen, J. From Swimming to Walking with a Salamander Robot Driven by a Spinal Cord Model. Science 2007, 315, 1416–1420. [Google Scholar] [CrossRef]
- Wright, S. Isolation by Distance. Genetics 1942, 38, 114–138. [Google Scholar] [CrossRef]
- Slatkin, M. Isolation by Distance in Equilibrium and Non-equilibrium populations. Evolution 1993, 47, 264–279. [Google Scholar] [CrossRef]
- Baptestini, E.M.; de Aguiar, M.A.M.; Bar-Yam, Y. Conditions for Neutral Speciation via Isolation by Distance. J. Theor. Biol. 2013, 335, 51–56. [Google Scholar] [CrossRef]
- Cabe, P.R.; Page, R.B.; Hanlon, T.J.; Aldrich, M.E.; Connors, L.; Marsh, D.M. Fine-scale Population Differentiation and Gene Flow in a Terrestrial Salamander (Plethodon cinereus) Living in Continuous Habitat. Heredity 2007, 98, 53–60. [Google Scholar] [CrossRef]
- Staub, N.L.; Brown, C.W.; Wake, D.B. Patterns of Growth and Movements in a population of Ensatina eschscholtzii platensis (Caudata: Plethodontidae) in the Sierra Nevada. California. J. Herpetol. 1995, 29, 593–599. [Google Scholar] [CrossRef]
- Bonett, R.M.; Kozak, K.H.; Vieites, D.R.; Bare, A.; Wooten, J.A.; Trauth, S.E. The Importance of Comparative Phylogeography in Diagnosing Introduced Species: A Lesson from the Seal Salamander, Desmognathus monticola. BMC Ecol. 2007, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Solano, I.; Jockusch, E.L.; Wake, D.B. Extreme Population Subdivision Throughout a Continuous Range: Phylogeography of Batrachoseps attenuatus (Caudata: Plethodontidae) in Western North America. Mol. Ecol. 2007, 16, 4335–4355. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Snodgrass, J.W.; Gasparich, G.E. The Importance of Terrestrial Dispersal for Connectivity among Headwater Salamander Populations. Ecosphere 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Radomski, T.; Hantak, M.M.; Brown, A.D.; Kuchta, S.R. Multilocus Phylogeography of Eastern Red-Backed Salamanders (Plethodon cinereus): Cryptic Appalachian Diversity and Postglacial Range Expansion. Herpetologica 2020, 76, 61–73. [Google Scholar] [CrossRef]
- Dillon, R.T., Jr.; Robinson, J.D. The Snails the Dinosaurs Saw: Are the Pleurocerid Populations of the Older Appalachians a Relict of the Paleozoic Era? J. N. Am. Benthol. Soc. 2009, 28, 1–11. [Google Scholar] [CrossRef]
- Zemmer, S.A.; Wyderko, J.; Da Silva Neto, J.; Cedillos, I.; Clay, L.; Benfield, E.F.; Belden, L.K. Seasonal and Annual Variation in Trematode Infection of Stream Snail Elimia proxima in the Southern Appalachian Mountains of Virginia. J. Parasitol. 2017, 103, 213–220. [Google Scholar] [CrossRef]
- Karvonen, A.; Seehausen, O. The Role of Parasitism in Adaptive Radiations—When Might Parasites Promote and When Might They Constrain Ecological Speciation? Int. J. Ecol. 2012, 2012, 280169. [Google Scholar] [CrossRef]
- Bracamonte, S.E.; Hofmann, M.J.; Lozano-Martín, C.; Eizaguirre, C.; Barluenga, M. Divergent and Non-parallel Evolution of MHC IIB in the Neotropical Midas Cichlid Species Complex. BMC Ecol. Evol. 2022, 22, 41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camp, C.; Vaca-Nava, A.; Bowen, A. Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA. Parasitologia 2024, 4, 375-381. https://doi.org/10.3390/parasitologia4040033
Camp C, Vaca-Nava A, Bowen A. Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA. Parasitologia. 2024; 4(4):375-381. https://doi.org/10.3390/parasitologia4040033
Chicago/Turabian StyleCamp, Carlos, Alexia Vaca-Nava, and Addison Bowen. 2024. "Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA" Parasitologia 4, no. 4: 375-381. https://doi.org/10.3390/parasitologia4040033
APA StyleCamp, C., Vaca-Nava, A., & Bowen, A. (2024). Effect of Trematode Metacercarial Infection on Walking in Larval Salamanders in the Southern Appalachian Mountains, USA. Parasitologia, 4(4), 375-381. https://doi.org/10.3390/parasitologia4040033




